Abstract
Cavernous sinus syndrome is a rare entity in oncology reported only in occasional case reports. Optimal therapy is thus poorly defined with rapidly progressive disease dominating the picture. Management includes prompt diagnosis, attempts at stabilization of cranial nerve function, and aggressive control of central pain syndrome. Here, we report cavernous sinus syndrome secondary to the original squamous cell carcinoma of the lung. With common presenting causes of this syndrome being infection, thrombosis or tumor, it might seem that metastatic tumor would be expected in a patient with a cancer diagnosis. What was not so expected was the extremely rapid progression from mild headache and mild trigeminal neuralgia with negative-contrast head CT to a massive, destructive lesion involving several skull bones and skull base, only 3 weeks later. In addition, the patient was severely immunosuppressed at the completion of induction chemotherapy. Infectious processes, although unlikely, were considered, as aggressive cancer therapy (including high-dose steroids and radiation therapy) had no impact on this disease. Despite accurate localization, the aggressive nature of this disease with massive bone destruction and dural thickening limited any chance of a durable control. We discuss the process of evaluation, diagnosis and treatment of symptoms and the importance of a team approach to best palliate these unfortunate patients.
Key words: Non-small cell lung cancer, Sphenoid bone metastasis, Central pain syndrome, Cranial nerve examination, Cancer progression and bone metastasis, Cavernous sinus syndrome
Introduction
Despite being a rare phenomenon, cavernous sinus syndrome is of great clinical importance due to its high propensity for developing into a life-threatening disorder [1, 2]. The cavernous sinus contains the carotid artery, its sympathetic plexus, and the oculomotor nerves [cranial nerve (CN) III, IV, and VI]. In addition, the ophthalmic branch and occasionally the maxillary branch of CN V traverse the cavernous sinus [3]. Signs and symptoms from inflammation to involvement include orbital or facial pain, orbital swelling and chemosis (secondary to ophthalmic vein occlusion), fever, oculomotor neuropathy, and acute trigeminal neuropathy. Cavernous sinus tumors are among the most common causes of cavernous sinus syndrome. Tumors may be primary, or invade from adjacent structures, as in lateral extension of pituitary tumors, or metastases [4, 5].
Here, we present a patient with squamous cell lung cancer, having no metastasis on initial diagnosis, developing cavernous sinus syndrome as a site of first recurrence.
Case Report
We present a 59-year-old white female diagnosed six months earlier with non-small cell lung cancer (stage IIIB). She presented with slightly enlarged mediastinal nodes and pericardial fluid cytology positive for malignancy. Her cancer therapy consisted of platinum/Taxol-based induction chemotherapy.
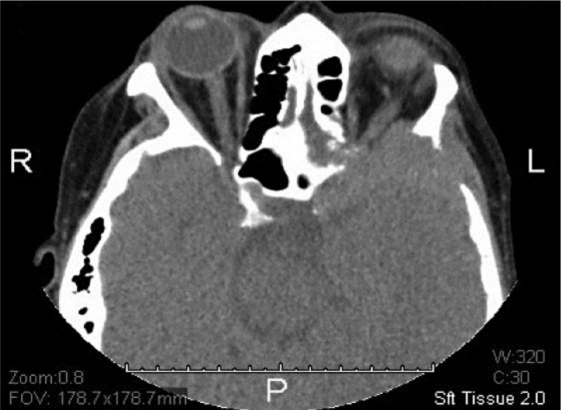
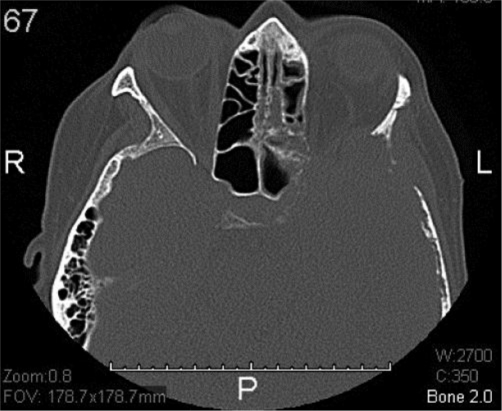
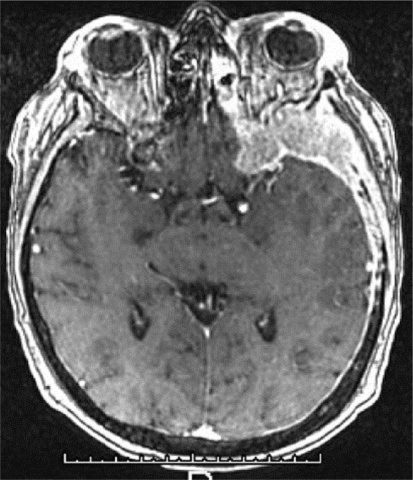
At the end of induction therapy, she presented with a 2-week history of progressively worsening headache, hearing loss and severe facial pain, all unilateral, left sided, and resembling acute trigeminal neuralgia. (Head CT with contrast was normal 3 weeks prior during workup for these milder symptoms.) Current physical exam showed left-sided facial sensitivity to light touch, moderate retraction of the left eardrum, and subtle weakness of the left lateral rectus muscle (VI) (see fig. 1, fig. 2). Head CT with contrast was repeated. Imaging now demonstrated massive destruction of the left sphenoid bone with extension to the left mastoid and left frontal bone (roof of left orbit) without any brain involvement (see fig. 3, fig. 4). MRI confirmed these findings, with encasement of several CN of the left cavernous sinus (fig. 5, fig. 6).
Fig. 1.
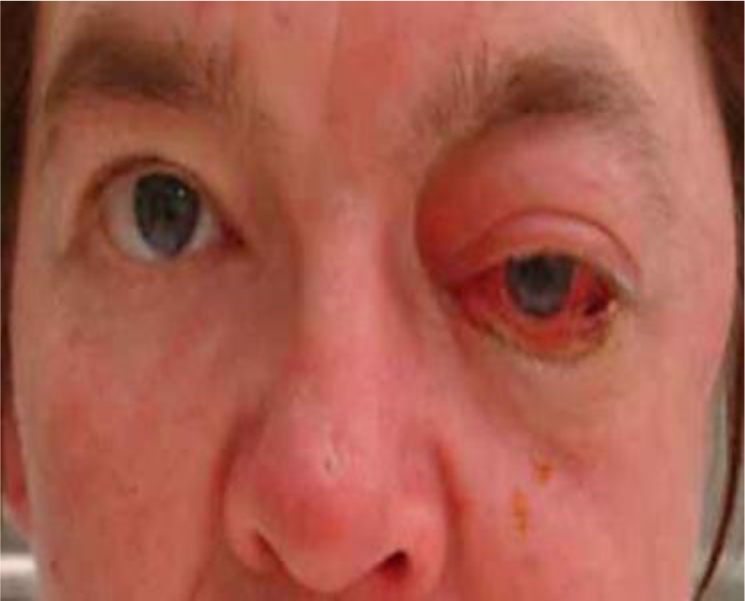
Physical findings in our patient.
Fig. 2.
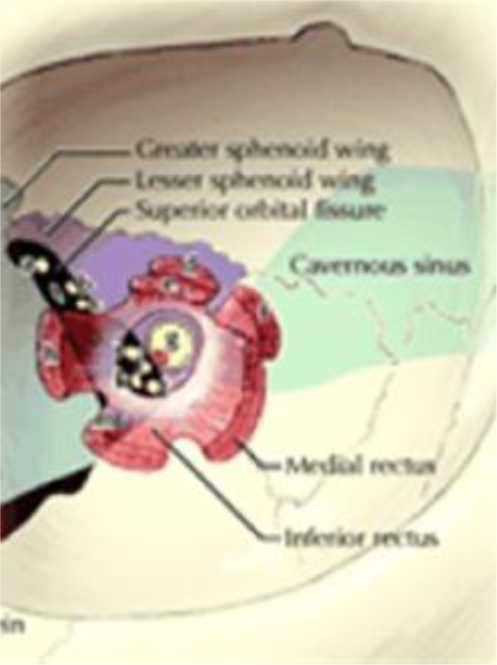
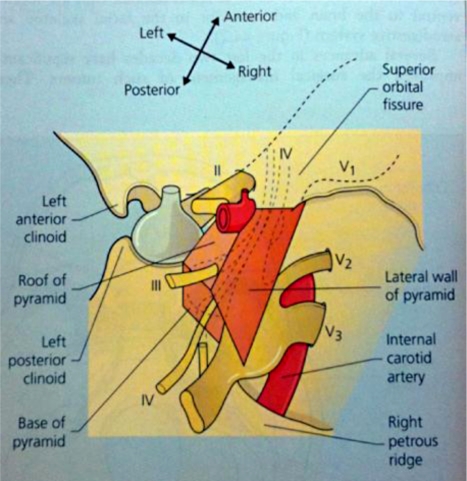
Correlation with cranial nerve and cavernous sinus anatomy.
Fig. 3.
CT showing soft tissue density surrounding the left sphenoid bone with complete cortical destruction and invasion of local structures.
Fig. 4.
CT (bone window) showing massive destruction of the sphenoid bone with extension to the left mastoid and frontal bone (roof of orbit).
Fig. 5.
Illustration of several cranial nerves (III–VI) as they pierce the dura mater to enter the cavernous sinus. Note: The ophthalmic nerve (CN II) does not travel through the cavernous sinus. Visual symptoms are usually a secondary event due to impaired venous drainage from the eye itself leading to either retinal edema or severely increased intraocular pressures.
Fig. 6.
MRI-confirmed soft-tissue component extending into the left cavernous sinus and along the dura. There was no intracranial metastatic disease.
Pain control was extremely difficult due to acute CN involvement and infiltration of the cavernous sinus as well as intractable nausea.
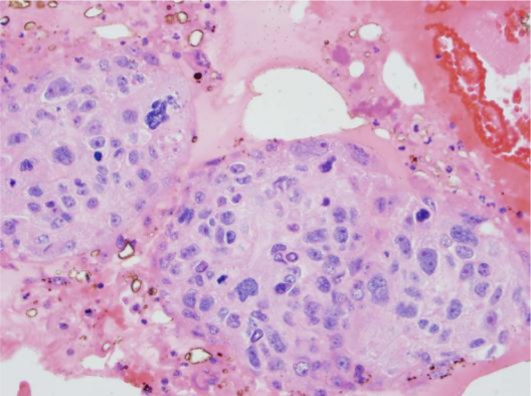
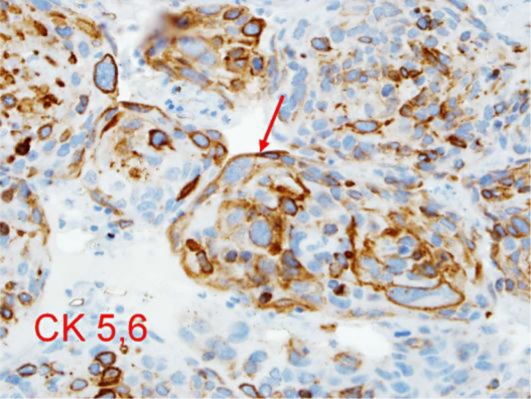
Our patient began urgent whole brain irradiation (1,500 cGy at 300 cGy per day over 5 days) for presumed aggressive metastatic disease. Due to a lack of visible response to therapy, progressive CN dysfunction, and a rapid decline in vision, neurosurgical stereotactic biopsy was performed to rule out an infectious process as opposed to a neoplasm. Frozen sections revealed squamous cell cancer, consistent with original cytology, with little or no radiation effect (see fig. 7, fig. 8).
Fig. 7.
HE. 40×. Nests of squamous cell carcinoma show marked nuclear pleomorphism and ample eosinophilic cytoplasm.
Fig. 8.
Immunohistochemical stain. 40×. Nests of squamous cell carcinoma are immunoreactive for high-molecular-weight cytokeratin CK 5,6.
Despite high-dose steroids and radiation treatments, CN symptoms rapidly progressed with loss of CN III through VIII, all left sided. Repeat MRI showed progressive disease along the base of the skull, filling the left cavernous sinus with almost complete encasement of the left optic nerve. After only 2 weeks from the initial cranial findings, the patient was placed on hospice and PCA pump for pain control.
Discussion
The impressive nature of rapidly progressing disease and bone destruction, albeit from aggressive malignancy, almost resembled an infectious process and was unresponsive to any oncological therapy offered.
Our patient's central pain syndrome was difficult to manage [6, 7]. Pain is typically constant, though it may be moderate to severe in intensity, and is often made worse by touch, movement, emotions, and temperature changes – usually cold temperatures. Individuals experience one or more types of pain sensation, the most prominent being burning. Acute central pain syndrome related to malignancy often begins shortly after the causative injury or damage, and is often difficult to manage.
Causes of cavernous sinus thrombosis often include infection, but less frequent etiologies include aneurysm of the carotid artery, carotid-cavernous fistula, meningioma, nasopharyngeal carcinoma, idiopathic granulomatous disorders, and tumors – with only two reported cases of metastasis secondary from lung squamous cell carcinoma [8, 9]. Since the two cavernous sinuses communicate, involvement of one may extend to the other. Early diagnosis is essential and treatment depends on the etiology. For infectious causes, broad spectrum antibiotics, drainage of the abscess and culture are essential for eradication of the offending organism. Anticoagulation may help in cases of primary thrombosis, especially in younger patients. In cases where the underlying etiology is secondary to metastasis or tumor, the treatment becomes more complex. Effective treatment for such cases has not been well studied nor is it substantially effective [10, 11].
When cancer metastasizes to bone, considerable pain and deregulated bone remodeling occur, greatly diminishing the possibility of a cure [12, 13]. Metastasizing tumor cells mobilize and sculpt the bone microenvironment to enhance tumor growth and promote bone invasion. This presentation of the development of cavernous sinus syndrome is exceptionally unique, and sphenoid bone metastasis arising from established primary lung or other malignancies is itself rare. None of the published literature addresses the optimal management course or its subsequent impact on the quality of life of patients with sphenoid bone metastasis [14, 15, 16].
Specialists involved in the management of our patient's diagnosis included those from the following fields: radiation therapy/oncology, radiology, ophthalmology, neurosurgery, pathology, and pain management. The patient experienced a rapid deterioration from fully active, with only mildly symptomatic headaches, to suffering from utterly debilitating central pain syndrome and ultimately death in a matter of 3 weeks. With modern imaging techniques, we are often able to correlate physical exam findings with early cavernous sinus involvement. In the case presented here, we could see the limitations of CT until the tumor mass had enlarged to a thickness relative to dural structures, and at that time bone destruction was evident as well, aiding in the diagnosis. In addition, MRI allows better soft-tissue resolution of the critical structures in this area of the skull base to establish the recurrence. This case will hopefully aid clinicians in evaluating cancer response and being aware of the ‘sanctuary site’ of the central nervous system to many active systemic chemotherapeutic agents [17, 18, 19].
References
- 1.Yildiza O, et al. Facial nerve palsy: an unusual presenting feature of small cell lung cancer. Case Rep Oncol. 2011;4:35–38. doi: 10.1159/000324182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weilbaecher KN, et al. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiwana MS, et al. Non-small cell lung cancer (NSCLC) presenting as isolated facial nerve palsy from metastasis to temporal bone. Indian J Clin Med. 2010;1:3–6. [Google Scholar]
- 4.Rengachery SS, Ellenbogen RG. Principles of Neurosurgery, ed 2. Edinburgh: Elsevier Mosby; 2005. pp. 700–704. [Google Scholar]
- 5.Keane JR. Cavernous sinus syndrome analysis of 151 cases. Arch Neurol. 1996;53:967–971. doi: 10.1001/archneur.1996.00550100033012. [DOI] [PubMed] [Google Scholar]
- 6.Moeller AR. Vascular compression of cranial nerves. II. Pathophysiology. Neurol Res. 1999;21:439–443. [PubMed] [Google Scholar]
- 7.Martin LA, Hagen NA. Neuropathic pain in cancer patients: mechanisms, syndromes, and clinical controversies. J Pain Symptom Manage. 1997;14:99–117. doi: 10.1016/s0885-3924(97)00009-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Lee HK, Park JK, Choi CG, Suh DC. Cavernous sinus syndrome: clinical features and differential diagnosis with MR imaging. AJR Am J Roentgenol. 2003;181:583–590. doi: 10.2214/ajr.181.2.1810583. [DOI] [PubMed] [Google Scholar]
- 9.Ogundiya DA, Keith DA, Mirowski J. Cavernous sinus thrombosis and blindness as complications of an odontogenic infection: report of a case and review of literature. J Oral Maxillofac Surg. 1989;47:1317–1321. doi: 10.1016/0278-2391(89)90733-7. [DOI] [PubMed] [Google Scholar]
- 77.Gilman S, et al. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988;38:517. doi: 10.1212/wnl.38.4.517. [DOI] [PubMed] [Google Scholar]
- 11.Niazi A, et al. Septic thrombosis of the cavernous sinuses. Arch Intern Med. 2001;161:2671–2676. doi: 10.1001/archinte.161.22.2671. [DOI] [PubMed] [Google Scholar]
- 12.Dolenc VV. The Cavernous Sinus: A Multidisciplinary Approach to Vascular and Tumorous Lesions. Vienna: Springer-Verlag; 1987. pp. 30–43. [Google Scholar]
- 13.Julien J, et al. Cavernous sinus syndrome due to lymphoma. J Neurol Neurosurg Psychiatry. 1984;47:558–560. doi: 10.1136/jnnp.47.5.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supler ML, Friedman WA. Acute bilateral ophthalmoplegia secondary to cavernous sinus metastasis: a case report. Neurosurgery. 1992;31:783–786. doi: 10.1227/00006123-199210000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mefty O, Smith RR. Surgery of tumors invading the cavernous sinus. Surg Neurol. 1988;30:370–381. doi: 10.1016/0090-3019(88)90200-5. [DOI] [PubMed] [Google Scholar]
- 16.DeMonte F, Smith HK, Al-Mefty O, et al. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245–251. doi: 10.3171/jns.1994.81.2.0245. [DOI] [PubMed] [Google Scholar]
- 17.Postmus PE, Smit EF. Chemotherapy for brain metastases of lung cancer: a review. Ann Oncol. 1999;10:753–759. doi: 10.1023/a:1008318515795. [DOI] [PubMed] [Google Scholar]
- 18.Kasner SE, Galetta SL, Vaughn DJ. Cavernous sinus syndrome in Hodgkin's disease. J Neuroophthalmol. 1996;16:204–207. [PubMed] [Google Scholar]
- 19.Sharkawi E, Tumuluri K, Olver JM. Metastatic choriocarcinoma causing cavernous sinus syndrome. Br J Ophthalmol. 2006;90:654–655. doi: 10.1136/bjo.2005.086744. [DOI] [PMC free article] [PubMed] [Google Scholar]