Abstract
As part of the 2nd Berlin BedRest Study (BBR2-2), we investigated the pattern of muscle atrophy of the postero-lateral hip and hamstring musculature during prolonged inactivity and the effectiveness of two exercise countermeasures. Twenty-four male subjects underwent 60 days of head-down tilt bedrest and were assigned to an inactive control (CTR), resistive vibration exercise (RVE), or resistive exercise alone (RE) group. Magnetic resonance imaging (MRI) of the hip and thigh was taken before, during, and at end of bedrest. Volume of posterolateral hip and hamstring musculature was calculated, and the rate of muscle atrophy and the effect of countermeasure exercises were examined. After 60 days of bedrest, the CTR group showed differential rates of muscle volume loss (F = 21.44; P ≤ 0.0001) with fastest losses seen in the semi-membranosus, quadratus femoris and biceps femoris long head followed by the gluteal and remaining hamstring musculature. Whole body vibration did not appear to have an additional effect above resistive exercise in preserving muscle volume. RE and RVE prevented and/or reduced muscle atrophy of the gluteal, semi-membranosus, and biceps femoris long head muscles. Some muscle volumes in the countermeasure groups displayed faster recovery times than the CTR group. Differential atrophy occurred in the postero-lateral hip musculature following a prolonged period of unloading. Short-duration high-load resistive exercise during bedrest reduced muscle atrophy in the mono-articular hip extensors and selected hamstring muscles. Future countermeasure design should consider including isolated resistive hamstring curls to target this muscle group and reduce the potential for development of muscle imbalances.
Keywords: muscle atrophy, magnetic resonance imaging, microgravity, whole body vibration
the development of appropriate countermeasure exercises to combat the effects of human spaceflight on the musculoskeletal system (21, 31, 33) continues to be a prime consideration for space agencies worldwide. Bedrest is a common model used to simulate the effects of space travel on the human body (40); however, it is also a good model to understand changes that occur in the muscles during prolonged levels of reduced physical activity (16).
Although a number of studies have examined the effects of bedrest on the lower limb and lumbar spine musculature (1–3, 9, 12, 30, 31, 44, 50, 57), limited information is known regarding the effects of prolonged inactivity on the muscles of the hip. The hip musculature is considered to play an integral role in control and stabilization of the hip joint during locomotion and in facilitation of upright stance (34–36). Furthermore, few works have examined the time course of recovery in the musculature after bedrest. An understanding of the recovery process of the muscles is also essential to help guide rehabilitation protocols. Thus the first aim of this work is to investigate the effect of bedrest and subsequent recovery of the muscles of the hip region.
Previous studies examining the effects of spaceflight and bedrest on the trunk and lower limb musculature have demonstrated that not all muscles within a region are affected to the same extent, with the degree of muscle atrophy found to differ between muscle synergists. During unloading, greater atrophy has been reported in the vastii muscles compared with their synergist, rectus femoris (1, 3, 58), with varying degrees of atrophy also documented in the three heads of triceps surae, soleus, and gastrocnemius medials and lateralis (12). Ground-based unloading studies have found decreases in muscle force and power to exceed reductions in muscle size in the hip (14), knee (14, 39, 52, 58), and calf extensors (30). If similar changes were to occur in the postero-lateral hip musculature, this may create muscle force imbalances in this region. Muscle force imbalances have been reported to be associated with increased risk of pain and/or injury in the hip (41), back (47), and lower limb (15, 22). Thus another aim of this study was to examine differential atrophy among synergists of the postero-lateral hip-hamstring muscle complex.
A further consideration is the development of appropriate countermeasure exercises against such changes at the hip. Thus far, development for countermeasures against unloading has focused largely on the lower limb muscles with little information available on the hip musculature. High load resistive countermeasure exercises have been shown to be effective at reducing/preventing muscle atrophy in the lower limb during bedrest (1–4, 13, 49, 52, 53, 58); however, effects have been inconsistent and often limited to a select number of muscles. Therefore, the second aim of this work is to evaluate the effectiveness of two different countermeasure exercises, resistive exercise and resistive exercise with the addition of whole body vibration, in protecting the hip musculature against atrophy during prolonged inactivity.
Resistive whole body vibration (WBV) is thought to provide additional stimulus to muscles during training with reported effects including increased muscle strength, power, and flexibility (17, 19, 48, 50). It has been proposed that vibration applied directly to muscle or tendon facilitates muscle contraction via the excitation of primary and secondary muscle spindle endings (18) and leads to a greater recruitment of motoneuron pools and subsequent increased muscle contraction via the tonic vibration reflex (48). WBV applied at the feet is transmitted to the lower limbs during loading (51); hence, it is possible that resistive WBV could be more effective in reducing muscle atrophy than resistive exercise alone. Since no studies to date have investigated this theory on the muscles of the hip, we wish to examine this as part of our countermeasure exercise protocol.
This study aimed to investigate: 1) the pattern of muscle atrophy that occurs in the postero-lateral muscles of the hip during 60 days of prolonged bedrest, in addition to the rate of muscle recovery up to 6 mo post-bedrest and 2) the effectiveness of two exercise countermeasures, resistive exercise and resistive vibration exercise, in protecting against muscle atrophy at the hip.
METHODS
Subjects and bedrest protocol.
Twenty-four medically and psychologically healthy males were recruited for the 2nd Berlin Bedrest Study (BBR2-2), conducted at the Charité Campus Benjamin Franklin in Berlin, Germany, by the Centre for Muscle and Bone Research. The protocol of the BBR2-2 has been described in detail elsewhere (11). In brief, however, subjects underwent 60 days of 6° head-down tilt bedrest (HDT), were required to perform all activities, including hygiene, in the head-down tilt position, and were discouraged from moving excessively or unnecessarily. Twenty-four hour nursing care and video surveillance permitted monitoring of subjects' activities. The Charité Universitätsmedizin Berlin ethics committee approved this study. Subjects gave their informed, written consent before participating in the study and were aware that they were permitted to withdraw from the study at any time.
Subjects were randomized to three different groups: one that performed resistive exercises with whole body vibration during bedrest (RVE; n = 7), one that performed resistive exercise only (RE; n = 8), and one that performed no exercise and served as a control group (CTR; n = 9). For medical reasons unrelated to the current study, one RE subject dropped out of the study after HDT30 (day 30 of bedrest). At R+90 (day 90 of recovery) and R+180 (day 180 of recovery), one RE and one CTR subject did not attend scanning.
The sample size estimates of the BBR2-2 were based on bone parameters [specifically distal tibia bone mineral content (10)] and were not powered to the muscle volume measurements considered in the present investigations. Since there is limited data on the hip musculature with countermeasures in bedrest in similar detail as in the present study, it was difficult to conduct a sensitivity analysis for this study. Thus we consider the present work to be an exploratory study for the comparison between RE and RVE. For all comparisons, particularly between the RVE and RE groups, where no significant differences are found, the findings should be considered in light of the fact that a meaningful effect size of vibration may not be able to be detected for the hip musculature given the present study design.
Exercise countermeasures.
Details of the countermeasure exercise programme have been published in detail elsewhere (11). In brief, however, exercise maneuvers were chosen to target those load-bearing regions of the body that are most affected by bedrest (i.e., lower-quadrant and lumbar region). Both countermeasure groups performed their exercise on the Galileo Space exercise device (Novotec Medical, Pforzheim, Germany; Fig. 1), with the device switched off for the resistive exercise only group. Training was performed 3 days/wk during the HDT phase. After a short warm-up, the following exercises were performed: bilateral squats (∼75–80% of pre-bedrest maximum voluntary contraction; in RVE group vibration frequency 24 Hz, amplitude 3.5–4 mm, peak acceleration ∼8.7 g where g = 9.81 ms−2), single leg heel raises (∼1.3 times body weight; in RVE group vibration frequency 26 Hz, amplitude 3.5–4 mm, peak acceleration ∼10.2 g), double leg heel raises (∼1.8 times body weight; in RVE group vibration frequency 26 Hz, amplitude 3.5–4 mm, peak acceleration ∼10.2 g), back and heel raises (performing hip and lumbar spine extension against gravity with ankle dorsiflexion, with ∼1.5 times body weight applied at the shoulders; in RVE group vibration frequency 16 Hz, amplitude 3.5–4 mm, acceleration ∼3.9 g). The RVE group performed the same exercises as the RE group except that whole body vibration was applied. Note that the acceleration parameters stated refer to the acceleration of the platform itself; effective accelerations on the subject are much lower. The maximum resulting ground reaction forces transmitted to the feet of the subjects result in effective acceleration at the feet in the order of 0.7 g.
Fig. 1.
Countermeasure exercise. This figure depicts a training subject performing a bilateral squat. Bilateral squats were included in the countermeasure exercise program to target the hip musculature, in particular the gluteal and hamstring muscles, which contract concentrically during the ascension phase of the squat. Other exercises included single and bilateral heel raises and back extension maneuvers. Both the resistance exercise only (RE) and resistance exercise with whole body vibration (RVE) groups performed their exercises on the Galileo Space exercise device (Novotec Medical, Pforzheim, Germany), with the RE group performing their exercises without vibration. Subjects were positioned in head-down tilt on a moveable board with their feet placed on either side of the Galileo platform. Shoulder pads and hand grips ensured subjects remained in the desired position and enabled application of force via the vibrating platform. A pneumatic system generated the force, applied through the moveable board, against which the subject was required to resist and move (via the shoulder pads and hand grips). A sport scientist supervised all exercises to ensure correct performance, and a monitor positioned in the subjects field of view provided visual feedback regarding exercise motion and speed and the subjects actual and target exercise position.
Magnetic resonance imaging protocol.
Baseline magnetic resonance (MR) scanning was conducted 8 or 9 days before bed rest (BDC −8/9), at mid-bedrest (BR27/28), and at end bedrest (HDT55/56). Post-bedrest scans were conducted on days 14, 90, and 180 of recovery (R+14, R+90, R+180). Before baseline and post-bedrest MRI scanning, subjects remained horizontal, lying for at least 2 h to allow time for shift of body fluids from the extremities (20). During the head-down tilt bedrest phase, beds were placed in the horizontal position 2 h before MR scanning to ensure comparability to pre- and post-head down tilt data.
Subjects were positioned on the scanning bed supine with their knees and hips supported in slight flexion by a pillow under the knee. Sandbags were used to support their legs in neutral rotation (kneecaps oriented to the ceiling and first metatarsal of the foot oriented vertically). Using a 1.5-T Avanto MR system (Siemens, Erlangen, Germany), transverse MR images were acquired from the iliac crest to the inferior-most portion of the gluteus maximus with a second sequence overlapping the gluteus maximus and extending to the knee joint line (slice thickness = 6 mm; interslice distance = 0.6 mm, TR = 8,000 ms, TE = 36 ms, FA = 150°, field of view: 400 × 294 mm interpolated to 320 × 224 pixels). Typically, 39–43 images from the hip and 63–69 images from the thigh were obtained during scanning, although for taller subjects additional images were added to ensure the region of interest was captured. Images were stored for offline analysis.
Image measurements.
One operator (TM) performed all image measurements. To ensure operator blinding to study time point and subject group, each data set was assigned a random numeric code (http://www.random.org). ImageJ (version 1.38x, http://rsb.info.nih.gov/ij/) was used for MR image analysis. Cross-sectional areas (CSA) of the following muscles in the hip (Fig. 2) were measured in each image for both the left and right sides: upper gluteus maximus (UGM), lower gluteus maximus (LGM), gluteus medius (GMED), gluteus minimus (GMIN), obturator internus (OBT_INT), obturator externus (OBT_EXT), quadratus femoris (QUAD_FEM), piriformis (PIRI), semi-membranosus (SEMI_M), semi-tendinosus (SEMI_T), biceps femoris long head (BF_LH), and biceps femoris short head (BF_SH). The gluteus maximus was divided into upper and lower portions at the point of greatest CSA of the femoral head (28), since these have been shown to have functional differences (8, 28, 36, 59). Due to difficulties in defining muscle borders, the superior and inferior gemelli muscles were grouped together with the obturator internus. These muscles have often been proposed to have a common embryological origin (8), function, and anatomy (55).
Fig. 2.
Hip and thigh muscle image measurements. Example images from the proximal hip (A), distal hip (B) and mid thigh (C). Thirty-four to 39 images were acquired from the iliac crest to the inferior-most portion of the gluteus maximus for the hip musculature. A second sequence of 63–69 images were acquired overlapping the inferior-most portion of the gluteus maximus and extending to the knee joint line to capture the hamstring musculature. The cross-sectional area (when present) of the muscles: upper gluteus maximus (UGM), lower gluteus maximus (LGM), gluteus medius (GMED), gluteus minimus (GMIN), obturator internus (OBT_INT), obturator externus (OBT_EXT), quadratus femoris (QUAF_FEM), piriformis (PIRI), biceps femoris short head (BF_SH), biceps femoris long head (BF_LH), semi-membranosus (SEMI_M), and semi-tendinosus (SEMI_ T) were measured in each image. Note the bony landmarks: iliac crest (IC), femoral head (FH), and ischial tuberosity (IT).
Further data processing and statistical analyses.
To efficiently check for any errors in image measurement, another operator (D. L. Belavý), also blinded to study time point and subject group, plotted data from each muscle for every scanning date for a particular subject. Changes in muscle volume compared with muscle volume at baseline and relative amount (percentage) of muscle atrophy were calculated. Muscle volume was calculated by interpolating muscle cross-sectional areas.
Statistical analysis focused on two aspects: 1) the effect of bedrest in the inactive control group only and 2) the impact of countermeasures on muscle changes in the bedrest and recovery phases. To examine the effect of inactivity alone, the following process was used.
1) Using raw muscle volume, linear-mixed effects models (44), with subsequent ANOVA, were constructed with a main effect of “study date” to evaluate changes within each muscle in the CTR group subjects over all measurement dates.
2) Subsequently, to facilitate comparisons between muscles and to determine which muscles atrophied the most/fastest in the CTR group, the fractional changes in muscle volume at HDT27/28 and HDT55/56 compared with baseline were calculated. Nonlinear mixed effects models (44) were then used to compute an exponential decay model e[k·(HDT week)], where k is the time constant for muscle volume loss and HDT week is the week of head-down tilt bedrest. In this model, a more negative k value indicates a faster rate of muscle volume loss. (Similar results were achieved when linear models were used, but since it is to be expected that muscle atrophy occurs in a nonlinear fashion, the use of an exponential decay model was more appropriate.)
To examine the effect of countermeasures, the following process was used.
3) Using raw muscle volume data, the bedrest (HDT27/28, HDT55/56 vs. BDC) and recovery (R+14, R+90, R+180 vs. HDT55/56) phases were examined separately. Linear-mixed effects models, with subsequent ANOVA, were built with main effects of study date and group with their two-way interaction.
4) If the group × studydate interaction from this first model showed a P value of <0.05, a further model, considering only the RVE and RE groups, was constructed in a similar fashion to determine whether there was a difference in response between the two countermeasure groups.
For all statistical models, random effects for each subject and side-of-body within subject and, where necessary, allowances for heterogeneity of variance (such as due to group or study date) were modeled. Due to the number of muscles examined and number of comparisons performed, an alpha level of 0.01 was used for statistical significance for the “study date” main effect, for “group × study date” interaction, and for between-muscle comparisons on ANOVA; P values for these model terms being <0.05 but >0.01 were considered trends. For analysis of changes during and after bedrest, since multiple measurement sessions were undertaken on the same subjects, a Bonferroni adjustment was not performed; rather we looked for consistent “significant” differences across time points. Subject age, height, and weight had little influence on the findings if they were incorporated in the models as linear covariates and were hence excluded from the analyses presented here. Where raw muscle volume data was used in statistical analyses, using percentage change in muscle volume data yielded similar results; however, these additional analyses are not shown. The R statistical environment (version 2.10.1, http://www.r-project.org) was used for all analyses.
RESULTS
In addition to the RE subject that dropped out after the HDT27/28 measurement, two further subjects, one from the RE and one from the CTR group, did not return for the R+90 and R+180 data collections. No differences existed in baseline muscle volume for any muscles between groups (F ≤ 2.2 for all; P ≥ 0.14 for all).
Effect of bedrest on muscle volume (CTR group only).
Tables 1–3 represent the percentage changes in muscle volume across all groups during the course of the study. With the exception of obturator externus (study date: F = 2.0, P = 0.086), piriformis (study date: F = 2.3, P = 0.054) and obturator internus (study date: F = 3.1, P = 0.014), analysis of CTR group only data showed strong effects for changes in volume across the muscles examined over the course of the study (study date: F ≥ 3.8 for all; P ≤ 0.004 for all).
Table 1.
Hamstring musculature: percentage change in muscle volume during bedrest and recovery in CTR, RE, and RVE groups
| Day of Bedrest/Recovery |
||||||
|---|---|---|---|---|---|---|
| Group | BDC | HDT27/28 | HDT55/56 | R + 14 | R + 90 | R + 180 |
| Biceps femoris long head | ||||||
| CTR | 235.1 (26.2) | −9.6 (5.9)%‡ | −17.7 (5.8)%‡ | −9.1 (6.8)%‡ | −3.1 (5.7)% | −1.1 (5.6)% |
| RE | 245.6 (54.3) | −9.3 (4.0)%‡ | −13.1 (3.9)%‡ | −5.3 (4.9)%† | 4.1 (5.3)% | 1.8 (4.8)% |
| RVE | 209.9 (24.0) | −10.2 (5.9)%‡ | −13.6 (5.4)%‡ | −3.2 (5.5)% | 4.4 (5.7)%* | 2.4 (5.8)% |
| Biceps femoris short head | ||||||
| CTR | 140.1 (29.7) | 0.4 (10.1)% | −6.5 (11.2)% | 3.2 (10.2)% | 4.4 (5.7)%* | 1.2 (9.8)% |
| RE | 134.8 (29.9) | 0.6 (6.5)% | −2.7 (6.8)% | 3.2 (6.2)% | 3.9 (6.0)% | 3.0 (5.7)% |
| RVE | 128.8 (35.4) | 0.8 (9.1)% | −2.7 (9.0)% | 3.1 (8.7)% | 3.4 (9.2)% | 2.5 (9.2)% |
| Semi-membranosus | ||||||
| CTR | 300.4 (42.8) | −10.6 (5.5)%‡ | −18.1 (4.9)%‡ | −7.7 (5.5)%‡ | −2.1 (5.3)% | 0.0 (5.2)% |
| RE | 292.1 (56.0) | −10.0 (4.1)%‡ | −12.8 (4.1)%‡ | −4.6 (4.0)%† | 2.9 (3.6)% | 1.8 (4.1)% |
| RVE | 256.1 (43.9) | −11.2 (6.3)%‡ | −14.1 (6.1)%‡ | −2.2 (5.8)% | 3.9 (6.0)% | 3.5 (5.5)% |
| Semi-tendinosus | ||||||
| CTR | 220.9 (30.0) | −2.0 (5.8)% | −5.2 (5.8)%† | −1.8 (6.1)% | 0.5 (6.9)% | 1.0 (6.8)% |
| RE | 236.1 (55.9) | −2.0 (4.6)% | −5.3 (5.0)%† | 1.0 (6.8)% | 5.0 (5.6)%* | 1.1 (6.3)% |
| RVE | 207.7 (59.5) | −0.4 (9.7)% | −2.4 (9.4)% | 5.6 (8.8)% | 7.6 (8.6)%* | 3.9 (8.8)% |
BDC, baseline data; HDT27/28, head-down-tilt bedrest day 27/28; HDT 55/56, head-down-tilt bedrest day 55/56; R + 14, day 14 of recovery; R + 90, day 90 of recovery; R + 180, day 180 of recovery; CTR, control; RE, resistive exercise; RVE, resistive vibration exercise. At BDC, values are means (SD) muscle volume (in ml). Subsequent to BDC, values are means (SD) percentage change compared with BDC. Significant difference of the percentage change in muscle volume compared with baseline (BDC):
P < 0.05;
P < 0.01;
P < 0.001.
Table 2.
Gluteal musculature: percentage change in muscle volume during bedrest and recovery in CTR, RE, and RVE groups
| Day of BedRest/Recovery |
||||||
|---|---|---|---|---|---|---|
| Group | BDC | HDT27/28 | HDT55/56 | R + 14 | R + 90 | R + 180 |
| Gluteus minimus | ||||||
| CTR | 124.8 (16.0) | −11.3 (4.4)%‡ | −11.2 (4.2)%‡ | −5.5 (3.8)%‡ | −1.6 (4.2)% | −2.7 (4.5)% |
| RE | 112.5 (21.6) | −3.7 (4.6)% | −5.4 (4.0)%‡ | −2.5 (4.6)% | 2.2 (3.6)% | 7.1 (7.6)%* |
| RVE | 112.6 (19.5) | −9.4 (6.8)%‡ | −5.6 (6.3)%* | 0.8 (6.2)% | 2.5 (5.8)% | 1.6 (5.7)% |
| Gluteus medius | ||||||
| CTR | 373.1 (41.9) | −2.2 (2.8)%* | −3.7 (2.8)%‡ | −1.0 (2.7)% | −0.1 (2.5)% | −0.2 (3.1)% |
| RE | 363.0 (75.4) | −1.9 (2.7)% | 0.0 (3.1)% | 1.9 (2.9)% | 2.7 (2.8)%* | 2.1 (3.4)% |
| RVE | 373.6 (43.3) | −4.8 (5.1)%* | −3.6 (6.2)% | −1.2 (5.3)% | 0.6 (5.6)% | 1.0 (5.7)% |
| Lower gluteus maximus | ||||||
| CTR | 573.3 (128.6) | −1.9 (5.3)% | −5.7 (4.9)%‡ | −2.7 (5.3)% | −0.9 (3.9)% | −2.4 (3.8)% |
| RE | 609.5 (113.3) | 6.0 (4.8)%† | 8.2 (5.6)%‡ | 7.4 (5.6)%‡ | 7.4 (5.7)%† | 3.2 (6.1)% |
| RVE | 510.5 (58.2) | 1.2 (5.7)% | 7.0 (6.0)%† | 5.8 (6.2)%* | 4.6 (5.5)%* | 6.8 (8.5)%* |
| Upper gluteus maximus | ||||||
| CTR | 397.4 (37.9) | −8.5 (6.9)%‡ | −9.9 (7.2)%‡ | −7.4 (7.3)%† | −2.7 (5.3)% | −1.0 (6.2)% |
| RE | 398.2 (87.8) | −0.9 (5.5)% | 2.5 (7.6)% | 2.6 (7.9)% | 2.5 (4.9)% | 5.3 (5.2)%* |
| RVE | 397.4 (52.2) | −1.6 (5.6)% | −4.3 (5.3)%* | −2.0 (5.7)% | 6.7 (6.6)%† | 0.5 (6.6)% |
At BDC, values are means (SD) muscle volume (in ml). Subsequent to BDC, values are means (SD) percentage change compared with BDC. Significant difference of the percentage change in muscle volume compared with baseline (BDC):
P < 0.05;
P < 0.01;
P < 0.001.
Table 3.
Thigh external rotators: percentage change in muscle volume during bedrest and recovery in CTR, RE, and RVE groups
| Day of BedRest/Recovery |
||||||
|---|---|---|---|---|---|---|
| Group | BDC | HDT27/28 | HDT55/56 | R + 14 | R + 90 | R + 180 |
| Obturator externus | ||||||
| CTR | 73.3 (13.9) | 0.6 (12.1)% | −4.3 (8.9)% | −1.7 (9.6)% | 0.4 (6.7)% | 0.6 (7.2)% |
| RE | 77.4 (21.2) | −2.8 (10.1)% | −1.2 (11.0)% | 4.1 (12.8)% | 5.1 (12.3)% | 1.4 (12.4)% |
| RVE | 68.5 (11.8) | −6.3 (7.1)%* | −3.7 (6.3)% | −2.6 (5.2)% | 0.2 (7.0)% | 0.7 (5.1)% |
| Obturator internus | ||||||
| CTR | 80.3 (12.4) | −0.6 (5.1)% | −3.0 (5.9)% | 3.3 (5.3)% | −2.5 (5.4)% | 1.4 (5.0)% |
| RE | 82.1 (10.4) | 4.3 (4.5)%* | 8.3 (5.9)%‡ | 6.4 (5.0)%† | 2.8 (5.2)% | 5.5 (5.8)%* |
| RVE | 75.9 (5.5) | −0.9 (8.1)% | −0.2 (7.4)% | 3.8 (7.8)% | 1.3 (7.8)% | 5.4 (8.3)% |
| Quadratus femoris | ||||||
| CTR | 46.3 (13.0) | −9.8 (12.2)%* | −18.1 (13.3)%‡ | −10.0 (11.4)%* | −4.5 (9.8)% | −3.6 (9.8)% |
| RE | 41.4 (9.4) | −5.5 (8.1)% | −8.2 (9.4)%* | −4.0 (9.5)% | 1.0 (7.5)% | −2.8 (8.1)% |
| RVE | 43.7 (13.6) | −9.5 (7.2)%† | −14.4 (9.8)%‡ | −2.9 (6.8)% | 7.3 (8.5)%* | 3.9 (10.0)% |
| Piriformis | ||||||
| CTR | 44.3 (10.4) | −0.6 (8.2)% | −2.0 (6.7)% | 3.1 (6.4)% | −2.4 (5.2)% | −0.6 (5.1)% |
| RE | 43.2 (4.4) | 12.1 (9.1)%† | 12.9 (9.2)%‡ | 7.5 (11.8)% | 10.2 (10.0)%* | 5.4 (8.5)% |
| RVE | 43.9 (6.8) | 0.2 (7.1)% | 1.9 (7.2)% | 6.8 (8.4)%* | 5.0 (8.2)% | 4.0 (7.7)% |
At BDC, values are means (SD) muscle volume (in ml). Subsequent to BDC, values are means (SD) percentage change compared with BDC. Significant difference of the percentage change in muscle volume compared with baseline (BDC):
P < 0.05;
P < 0.01;
P < 0.001.
At the end of bedrest, the greatest losses in muscle volume were observed in the quadratus femoris (−18.1% at HDT 55/56: t ≥ −2.4; P ≤0.02), semi-membranosus (−18.1% at HDT 55/56: t ≥ −5.7; P ≤ 0.0001), and biceps femoris long head (−17.7% at HDT 55/56: t ≥ −4.9; P ≤ 0.0001 for both). The following muscles also demonstrated significant losses in muscle volume (P ≤ 0.0090 for all): gluteus minimus (−11.2%), upper gluteus maximus (−9.9%), lower gluteus maximus (−5.7%), semi-tendinosus (−5.2%), and gluteus medius (−3.7%). Nonsignificant changes in muscle volume were observed in the biceps femoris short head, obturator internus, obturator externus, and piriformis during bedrest (P ≥ 0.13 for all).
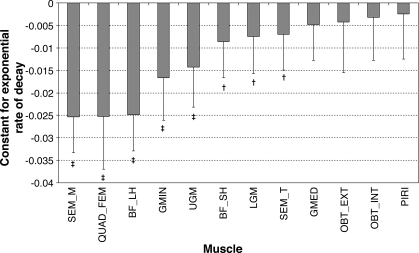
To facilitate easier comparison between muscles, the rates of muscle volume change during bedrest were calculated (see Fig. 3). ANOVA showed that rates of muscle atrophy differed between muscles (F = 20.1; P < 0.0001). Table 4 displays data comparing the rates of muscle atrophy between muscles. Interestingly, this data reveals that a number of synergistic muscles atrophies at significantly different rates. From the hamstring muscle group, semi-membranosus atrophied at a significantly faster rate than its synergists, biceps femoris short head, and semi-tendinosus (P < 0.0001 for both), with the latter demonstrating a faster rate of atrophy than biceps femoris short head (P < 0.0001). Within the gluteal muscle group, gluteus minimus atrophied at a faster rate than both gluteus medius and lower gluteus maximus (P < 0.007), whereas upper gluteus maximus demonstrated a trend toward faster atrophy than both lower gluteus maximus and gluteus medius (P < 0.0383 for both). Concerning the muscles involved in hip rotation, quadratus femoris atrophied significantly faster than all other rotator muscles (P < 0.0001 for all).
Fig. 3.
Estimates of rates of volume loss (exponential rates of decay) in the postero-lateral hip muscles during bedrest. Values are means and SD estimates of the time constant k in the fitted exponential decay model e[k·(BRx−1)] (where BRx is the xth day of bedrest). More negative time constants indicate faster loss of muscle volume during bedrest. BF_LH, biceps femoris long head; BF_SH, biceps femoris short head; GMED, gluteus medius; GMIN, gluteus minimus; LGM, lower gluteus maximus; OBT_EXT, obturator externus; OBT_INT, obturator internus; PIRI, piriformis; QUAD_FEM, quadratus femoris; SEMI_M, semi-mebranosus; SEMI_T, semi-tendinosus; UGM, upper gluteus maximus. Significant difference of the percentage change in muscle volume compared with zero: *P < 0.05; †P < 0.01; ‡P < 0.001 indicate. Otherwise P > 0.05.
Table 4.
Comparisons of rates of atrophy in the hip muscles
| Muscle | BF_LH | QF | SEMI_M | GMIN | UGM | BF_SH | LGM | SEMI_T | OBT_EXT | PIRI | OBT_INT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| QF | 0.91 | ||||||||||
| SEMI_M | 0.87 | 0.99 | |||||||||
| GMIN | 0.02 | 0.05 | 0.01 | ||||||||
| UGM | 0.001 | 0.01 | 0.0007 | 0.51 | |||||||
| BF_SH | <0.0001 | <0.0001 | <0.0001 | 0.012 | 0.08 | ||||||
| LGM | <0.0001 | <0.0001 | <0.0001 | 0.008 | 0.04 | 0.7 | |||||
| SEMI_T | <0.0001 | <0.0001 | <0.0001 | 0.004 | 0.03 | 0.59 | 0.89 | ||||
| OBT_EXT | <0.0001 | <0.0001 | <0.0001 | 0.004 | 0.017 | 0.27 | 0.43 | 0.48 | |||
| PIRI | <0.0001 | <0.0001 | <0.0001 | 0.0003 | 0.002 | 0.09 | 0.17 | 0.2 | 0.68 | ||
| OBT_INT | <0.0001 | <0.0001 | <0.0001 | 0.0005 | 0.003 | 0.12 | 0.23 | 0.26 | 0.81 | 0.85 | |
| GMED | <0.0001 | <0.0001 | <0.0001 | 0.0005 | 0.004 | 0.19 | 0.39 | 0.44 | 0.89 | 0.51 | 0.64 |
Values are P values of direct comparisons between muscles of estimates of rates of volume loss. Differences of P < 0.01 are in bold. BF_LH, biceps femoris long head; BF_SH, biceps femoris short head; GMED, gluteus medius; GMIN, gluteus minimus; LGM, lower gluteus maximus; OBT_EXT, obturator externus; OBT_INT, obturator internus; PIRI, piriformis; QF, quadratus femoris; SEMI_M, semi-mebranosus; SEMI_T, semi-tendinosus; UGM, upper gluteus maximus.
Not only did rate of muscle atrophy differ within the mono-articular gluteal muscles and bi-articular hamstring muscles but also between these two groups, with the biceps femoris long head and semi-membranosus displaying a tendency toward a faster rate of atrophy than the gluteal muscles (P < 0.0234). Similar results were achieved with a linear percentage change per week model (data not shown).
Effect of countermeasures: gluteal musculature.
ANOVA results provided strong evidence for an effect of the countermeasure exercise on the volume of all of the gluteal muscles (F ≥ 4.6; P ≤ 0.02; Table 2). There were no significant differences between the two training groups (F ≤ 3.96; P ≥ 0.068). For the lower gluteus maximus, both countermeasure groups demonstrated significant increases in muscle volume at the end of bedrest (HDT 55/56) compared with a decrease in the CTR group. Muscle loss was attenuated in the upper gluteus maximus in both training groups up to mid-bedrest (HDT27/28); however, by end of bedrest there appeared to be a non-significant increase in muscle volume in the RE group and a significant decrease in muscle volume in RVE group, although the difference between the two training groups was not statistically significant. Countermeasure exercises reduced muscle loss in the gluteus minimus in both training groups compared with the CTR group. For the gluteus medius, although muscle volume loss was quite minor in the CTR group, countermeasure exercises appeared to prevent this in the RE group only.
Effect of countermeasures: hamstring musculature.
ANOVA demonstrated a strong effect for the countermeasure on muscle volume in semi-membranosus and biceps femoris long head (F ≥ 9.04; P ≤ 0.0005; Table 1), but not for the semi-tendinosus and biceps femoris short head (F ≤ 1.86; P ≥ 0.18). There was no difference between the two countermeasure groups for any of the hamstring muscles (F ≤ 0.67; P ≥ 0.43). In the semi-membranosus and biceps femoris long head, atrophy was reduced in both training groups, although this effect was not complete.
Effect of countermeasures: hip rotators.
The quadratus femoris was the only hip rotator to demonstrate a significant loss of muscle volume throughout bedrest; however, according to ANOVA, there was very weak statistical evidence for an effect of the countermeasure (F ≤ 3.4; P ≥ 0.053; Table 3). Significant muscle hypertrophy was seen in the RE group for piriformis and obturator internus (F ≥ 4.9; P ≤ 0.01), but there was some statistical evidence of difference in the RVE group for piriformis only (F ≥ 6.9; P ≤ 0.02).
Recovery of the musculature after bedrest.
In the CTR group (Tables 1–3), at R+14, muscles still significantly decreased in volume compared with baseline included: quadratus femoris (−10%), biceps femoris long head (−9.2%), semi-membranosus (−7.7%), upper gluteus maximus (−7.4%), and gluteus minimus (−5.5%) (P ≤ 0.04). By R+90, across all groups, the volume of these muscles was no longer significantly reduced compared with baseline.
Generally, the countermeasure groups displayed faster recovery of the muscles. According to ANOVA, there was some statistical evidence of this for the upper gluteus maximus, gluteus minimus, biceps femoris long head, semi-tendinosus (F ≥ 2.6 for all; P ≤0.04 for all), and lower gluteus maximus (F = 9.6; P = 0.001), which continued to display a degree of muscle hypertrophy throughout the recovery period. Of these, the upper gluteus maximus was the only muscle to demonstrate a significantly different effect for the two countermeasure groups (F = 5.8; P = 0.006) with the RVE group displaying a significant degree of hypertrophy at R+90 compared with the RE group and a reversal of this effect at R+180.
The post-bedrest rehabilitation program implemented evaluating two different exercise programs for the recovery of the lumbar spine (27) did not impact the recovery of the hip or thigh muscles examined in this paper (all P ≥ 0.04). Although ANOVA suggested a different response between the “specific motor control” and “trunk flexor strengthening” rehabilitation groups for the quadratus femoris muscle (P = 0.0069), this effect was largely due to a greater loss of quadratus femoris muscle volume at the end of bedrest in the “specific motor control” group before the beginning of the rehabilitation intervention (data not shown).
DISCUSSION
Although prior works have investigated the effects of unloading on the anterior hip (23), gluteus maximus (14, 57) and selected hamstring muscles (1, 3, 32, 33, 53), this study is, to our knowledge, the first work to investigate in detail the effect of bedrest on the muscles of the postero-lateral hip. Our main finding was that muscles of the postero-lateral hip atrophy at different rates with fastest rates of atrophy observed in the quadratus femoris, semi-membranosus and biceps femoris long head, followed by the gluteal and remaining hamstring musculature. The remaining hip rotators were not significantly affected by prolonged unloading. The second main finding was that high-load resistive exercise countermeasures (with and without vibration) incorporating squat exercises, reduced and/or prevented atrophy of the gluteal muscles and demonstrated a “significant” effect on semi-membranosus and biceps femoris long head; however, the effect size was relatively minor for these two muscles. Third, the addition of whole body vibration did not appear to have a supplementary effect in protecting against atrophy of the postero-lateral hip and hamstring musculature. Finally, muscle volume remained reduced 2 wk after bedrest but appeared completely recovered after 90 days of bedrest.
When we consider the activity of the postero-lateral hip musculature during loading and functional tasks, it is not surprising that, first, these muscles are affected by unloading and that, second, they respond differently to unloading and exercise stimuli. Regarding the hamstring muscles, a similar pattern of differential atrophy to that in the present study has been described in previous unloading studies (2, 3, 12), with semi-tendinosus and biceps femoris short head comparatively less affected than semi-membranosus and biceps femoris long head. The biarticular semi-membranosus and biceps femoris long head are active during the loading and swing phases of walking and running (36, 37, 46) and are thought to play an important role in providing hip extension force for propulsion. In contrast, the biceps femoris short head does not cross the hip joint and is thought to be more involved in knee flexion and control (37).
Although the gluteal muscles can act synergistically with the hamstring muscles, they do of course have different functions and, hence, demonstrate a different response to unloading/bedrest. Interestingly, our findings showed significantly greater atrophy in the upper gluteus maximus compared with the lower gluteus maximus. These two muscle portions are not only embryologically (59) and functionally different, with lower gluteus maximus thought to act primarily as a hip extensor and upper gluteus maximus as a hip abductor (28), but they also have been found to respond differently in hip pathology (25). We find two alternate explanations for the differential atrophy demonstrated in this study to be plausible. First, we cannot exclude the possibility that, for the purpose of muscle measurements, our division of the upper and lower gluteus maximus was imperfect. Second, although subjects were instructed to limit load bearing through their feet during bedrest, during changes in position, daily transfers, and use of bed pans, it is possible that one or both feet were partially loaded. Although relatively infrequent, these hip-extension movements may have activated the lower gluteus maximus sufficiently enough to maintain its muscle volume. Unfortunately, previous works in spaceflight and bedrest, which investigated the effect of unloading on the gluteal musculature, grouped the muscles together, making comparisons to our findings difficult. Interestingly, inconsistent responses of the gluteal muscles to unloading were reported in these studies, with 2.3% muscle volume loss documented after 35 days of bed rest (14) compared with 8% muscle volume after 17 days of spaceflight (57). These findings demonstrate that, although bedrest may generally represent physical inactivity, this does not mean that all muscles are inactive, and, moreover, it may be possible for increased usage of some muscles to occur.
An interesting finding from our study was that of quadratus femoris, which appears to have a unique response among the hip external rotators to unloading. Originating on the lateral border of the ischial tuberosity and inserting on the quadrate tubercle of the femur, this deep muscle of the hip (38) demonstrated the fastest rate of atrophy and greatest loss of muscle volume of all the muscles we examined. The current understanding of quadratus femoris and its function is limited, with research on this muscle in humans largely comprising of anatomical and embryological (7, 8, 61) studies. Case studies on injury of the quadratus femoris (6, 29, 42, 43, 60) may help us to understand that it is active in load bearing, thigh adduction, and external rotation and may also be involved in hamstring tears (6); however, further investigation is necessary to elucidate the role of this muscle in control of the hip joint and function.
Our findings of differential atrophy between different muscle groups and among muscle synergists, have important implications for exercise countermeasure development and rehabilitation of the muscles after loading. Data from modeling studies show that decreased force from the gluteal musculature during hip extension results in increased superior (35) and anterior (34) forces on the hip joint, with subsequent substitution and increased muscle activity in the semi-membranosus (34, 35), gluteus medius, vastii, and tensor fascia latae (35). Furthermore, research suggests that hip muscle force imbalances contribute to increased risk of hamstring tears (22) and hip and lower limb overuse injuries (41, 47). Hence, it is possible that the differential atrophy occurring in the muscles at the hip during bedrest could lead to altered joint loading and subsequent increased risk of injury in this area.
The countermeasure exercise program reduced/prevented muscle volume loss in the mono-articular gluteal musculature but had less effect on the bi-articular hamstring musculature. The strongest effect of the countermeasure was observed in lower gluteus maximus, which is strongly (55–60% MVIC) activated during squats (5) and hence resulted in the hypertrophic response observed in both training groups by end bedrest. The hamstring musculature, however, is only moderately activated during squats (3–30% MVIC) (5, 26, 56), and, as a result, this muscle group underwent significant atrophy in the training groups in our study. Isolated hamstring curls are reported to produce twice as much hamstring muscle activity (between 67 and 70% MVIC) as squats or leg press (5, 62) and, furthermore, facilitate more equal recruitment of semi-membranosus and biceps femoris (5, 24), with increased muscle activation with greater resistance (54). Future countermeasure exercise programs should consider including resisted isolated hamstring curls to help reduce muscle atrophy and potential development of muscle force imbalances in this area.
Whole body resistive vibration did not provide a significant additional effect above resistive exercise alone in ameliorating loss of muscle volume during bed rest. Due to limited existing data on the effects of bedrest on the hip musculature, it was not possible to perform a meaningful sensitivity analysis for the RE-RVE comparison; hence, low subject numbers may have prevented us from accurately assessing any potential additional effect(s) of vibration. Another possibility is that, although resistive vibration training has been reported to have positive effects on functional muscle parameters such as muscle strength, power, and flexibility (17, 19, 48, 50), muscle volume is a morphological parameter and, as such, may not be ideal to reflect additional effects of vibration.
Regarding recovery of the musculature after bedrest, the countermeasure groups appeared to recover more quickly than the control group. Not unexpectedly, the muscles most affected by bedrest took longer to recover, with significant reductions in volume evident in a number of muscles at 14 days post-bedrest. By R+90, recovery appeared complete for all muscles across all groups. Muscle injury and inflammation have been documented post-immobilization and have been found to be associated with increases in muscle volume (45). Hence, we cannot be certain that inflammation-induced fluid changes masked a lack of recovery of muscle volume in the initial recovery scans. Future unloading studies could reduce this uncertainty by monitoring recovery of musculature at more frequent intervals and/or using muscle biopsies for comparison of muscle fiber cross-sectional area and muscle volume measurements.
In conclusion, the present study found that differential rates of atrophy occur between both functional and synergistic muscle groups of the postero-lateral hip. The fastest rates of muscle volume loss were seen in the quadratus femoris, semi-membranosus and biceps femoris long head, followed by the gluteal and remaining hamstring musculature. Short-duration, high-load, resistive exercises, performed 3 days/wk were sufficient to preserve muscle volume of the mono-articular gluteal muscles during bedrest but were less effective in preserving muscle volume of the bi-articular hamstring muscles involved in hip extension and knee flexion. The addition of whole body vibration to resistive exercise did not appear to have a supplementary effect in reducing muscle volume loss during bedrest on the hip muscles we studied, although limited subject numbers may have played a role in this finding. Recovery of muscle volume changes after bedrest took longer than 2 wk but appeared complete by 90 days post-bedrest. Future countermeasure exercise programs should consider including hamstring-specific exercises, such as isolated, resisted hamstring curls, to target a wider range of muscles in the hip and minimize the risk of the development of muscle force imbalances in this region.
GRANTS
The 2nd Berlin Bed-Rest Study (BBR2-2) was supported by grant 14431/02/NL/SH2 from the European Space Agency and grant 50WB0720 from the German Aerospace Centre (DLR). The BBR2-2 was also sponsored by Novotec Medical, Charité Universitätsmedizin Berlin, Siemens, Osteomedical Group, Wyeth Pharma, Servier Deutschland, P&G, Kubivent, Seca, Astra-Zeneka, and General Electric. T. Miokovic was supported by grant 50WB0720 from the German Aerospace Center (DLR). D. L. Belavý was supported by a postdoctoral fellowship from the Alexander von Humboldt Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the subjects who participated in the study. Michael Russmann and Gerd Wolynski are thanked for assistance in operating the MR scanner over the course of the study.
REFERENCES
- 1. Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand 172: 269–278, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Akima H, Kubo K, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Leg-press resistance training during 20 days of 6 degrees head-down-tilt bed rest prevents muscle deconditioning. Eur J Appl Physiol 82: 30–38, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Akima H, Ushiyama J, Kubo J, Fukuoka H, Kanehisa H, Fukunaga T. Effect of unloading on muscle volume with and without resistance training. Acta Astronaut 60: 728–736, 2007 [Google Scholar]
- 4. Alkner BA, Tesch PA. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand 181: 345–357, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Andersen LL, Magnusson SP, Nielsen M, Haleem J, Poulsen K, Aagaard P. Neuromuscular activation in conventional therapeutic exercises and heavy resistance exercises: implications for rehabilitation. Phys Ther 86: 683–697, 2006 [PubMed] [Google Scholar]
- 6. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during slow-speed stretching: clinical, magnetic resonance imaging, and recovery characteristics. Am J Sports Med 35: 1716–1724, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Aung HH, Sakamoto H, Akita K, Sato T. Anatomical study of the obturator internus, gemelli and quadratus femoris muscles with special reference to their innervation. Anat Rec 263: 41–52, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Bardeen RC. Development and variation of the nerves and musculature of the inferior extremity and of the neighboring regions of the trunk in man. Am J Anat 6: 263–390, 1907 [Google Scholar]
- 9. Belavý DL, Armbrecht G, Richardson CA, Felsenberg D, Hides JA. Muscle Atrophy and Changes in Spinal Morphology: Is the Lumbar Spine Vulnerable After Prolonged Bedrest? Spine 36: 137–145, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Belavý DL, Beller G, Armbrecht G, Perschel FH, Fitzner R, Bock O, Börst H, Degner C, Gast U, Felsenberg D. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bedrest. Osteoporosis International; DOI:10.1007/s00198-010-1371-6http://dx.doi.org/10.1007/s00198-101-1371-6 [DOI] [PubMed] [Google Scholar]
- 11. Belavý DL, Bock O, Börst H, Armbrecht G, Gast U, Degner C, Beller G, Heer M, de Haan A, Stegeman DF, Ceretteli P, Blottner D, Rittweger J, Gelfi C, Kornak U, Felsenberg D. The 2nd Berlin Bed-Rest Study: protocol and implementation. J Musculoskelet Neuronal Interact 10: 207–219, 2010 [PubMed] [Google Scholar]
- 12. Belavý DL, Miokovic T, Armbrecht G, Richardson CA, Rittweger J, Felsenberg D. Differential atrophy of the lower-limb musculature during prolonged bedrest. Eur J Appl Physiol 107: 489–499, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Belavý DL, Miokovic T, Armbrecht G, Rittweger J, Felsenberg D. Resistive vibration exercise reduces lower limb muscle atrophy during 56-day bedrest. J Musculoskelet Neuronal Interact 9: 225–235, 2009 [PubMed] [Google Scholar]
- 14. Berg HE, Eiken O, Miklavcic L, Mekjavic IB. Hip, thigh and calf muscle atrophy and bone loss after 5-week bedrest inactivity. Eur J Appl Physiol 99: 283–289, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Beukeboom C, Birmingham TB, Forwell L, Ohrling D. Asymmetrical strength changes and injuries in athletes training on a small radius curve indoor track. Clin J Sport Med 10: 245–250, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Booth FW, Gollnick PD. Effects of disuse on the structure and function of skeletal-muscle. Med Sci Sports Exerc 15: 415–420, 1983 [PubMed] [Google Scholar]
- 17. Bosco C, Cardinale M, Tsarpela O. Influence of vibration on mechanical power and electromyogram activity in human arm flexor muscles. Eur J Appl Physiol Occup Physiol 79: 306–311, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol 261: 695–711, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev 31: 3–7, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Conley MS, Foley JM, Ploutz-Snyder LL, Meyer RA, Dudley GA. Effect of acute head-down tilt on skeletal muscle cross-sectional area and proton transverse relaxation time. J Appl Physiol 81: 1572–1577, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Convertino VA. Neuromuscular aspects in development of exercise countermeasures. Physiologist 34: 125–128, 1991 [PubMed] [Google Scholar]
- 22. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med 36: 1469–1475, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Dilani Mendis M, Hides JA, Wilson SJ, Grimaldi A, Belavý DL, Stanton W, Felsenberg D, Rittweger J, Richardson C. Effect of prolonged bed rest on the anterior hip muscles. Gait Posture 30: 533–537, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Escamilla RF, Fleisig GS, Zheng N, Barrentine SW, Wilk KE, Andrews JR. Biomechanics of the knee during closed kinetic chain and open kinetic chain exercises. Med Sci Sports Exerc 30: 556–569, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Grimaldi A, Richardson C, Durbridge G, Donnelly W, Darnell R, Hides J. The association between degenerative hip joint pathology and size of the gluteus maximus and tensor fascia lata muscles. Man Ther 14: 611–617, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Gryzlo SM, Patek RM, Pink M, Perry J. Electromyographic analysis of knee rehabilitation exercises. J Orthop Sports Phys Ther 20: 36–43, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Hides JA, Lambrecht G, Richardson CA, Stanton WR, Armbrecht G, Pruett C, Damann V, Felsenberg D, Belavý DL. The effects of rehabilitation on the muscles of the trunk following prolonged bed rest. Eur Spine J. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jaegers S, Dantuma R, de Jongh HJ. Three-dimensional reconstruction of the hip muscles on the basis of magnetic resonance images. Surg Radiol Anat 14: 241–249, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Klinkert P, Jr, Porte RJ, de Rooij TP, de Vries AC. Quadratus femoris tendinitis as a cause of groin pain. Br J Sports Med 31: 348–349, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Blanc A, Gogia P, Schneider V, Krebs J, Schonfeld E, Evans H. Calf muscle area and strength changes after five weeks of horizontal bed rest. Am J Sports Med 16: 624–629, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Le Blanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol 89: 2158–2164, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Le Blanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviat Space Environ Med 66: 1151–1154, 1995 [PubMed] [Google Scholar]
- 33. Le Blanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact 1: 157–160, 2000 [PubMed] [Google Scholar]
- 34. Lewis CL, Sahrmann SA, Moran DW. Anterior hip joint force increases with hip extension, decreased gluteal force, or decreased iliopsoas force. J Biomech 40: 3725–3731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis CL, Sahrmann SA, Moran DW. Effect of position and alteration in synergist muscle force contribution on hip forces when performing hip strengthening exercises. Clin Biomech (Bristol, Avon) 24: 35–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyons K, Perry J, Gronley JK, Barnes L, Antonelli D. Timing and relative intensity of hip extensor and abductor muscle action during level and stair ambulation. An EMG study. Phys Ther 63: 1597–1605, 1983 [DOI] [PubMed] [Google Scholar]
- 37. Montgomery WH, 3rd, Pink M, Perry J. Electromyographic analysis of hip and knee musculature during running. Am J Sports Med 22: 272–278, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Moore KL, Dalley AF. Clinically Orientated Anatomy. Baltimore, MD: Lippincott Williams & Wilkins, 2006 [Google Scholar]
- 39. Mulder ER, Stegeman DF, Gerrits KH, Paalman MI, Rittweger J, Felsenberg D, de Haan A. Strength, size and activation of knee extensors followed during 8 weeks of horizontal bed rest and the influence of a countermeasure. Eur J Appl Physiol 97: 706–715, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Nicogossian AE, Dietlein LF. Microgravity simulation and analogues. In: Space Physiology and Medicine, edited by Nicogossian AE. Philadelphia, PA: Lea and Febiger, 1982, p. 240–248 [Google Scholar]
- 41. Niemuth PE, Johnson RJ, Myers MJ, Thieman TJ. Hip muscle weakness and overuse injuries in recreational runners. Clin J Sport Med 15: 14–21, 2005 [DOI] [PubMed] [Google Scholar]
- 42. O'Brien SD, Bui-Mansfield LT. MRI of quadratus femoris muscle tear: another cause of hip pain. Am J Roentgenol 189: 1185–1189, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Peltola K, Heinonen OJ, Orava S, Mattila K. Quadratus femoris muscle tear: an uncommon cause for radiating gluteal pain. Clin J Sport Med 9: 228–230, 1999 [PubMed] [Google Scholar]
- 44. Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. Berlin: Springer, 2000 [Google Scholar]
- 45. Ploutz-Snyder LL, Tesch PA, Hather BM, Dudley GA. Vulnerability to dysfunction and muscle injury after unloading. Arch Phys Med Rehabil 77: 773–777, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Prilutsky BI, Gregor RJ. Swing- and support-related muscle actions differentially trigger human walk-run and run-walk transitions. J Exp Biol 204: 2277–2287, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Renkawitz T, Boluki D, Grifka J. The association of low back pain, neuromuscular imbalance, and trunk extension strength in athletes. Spine J 6: 673–683, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol 108: 877–904, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D. Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36: 1019–1029, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Rittweger J, Just K, Kautzsch K, Reeg P, Felsenberg D. Treatment of chronic lower back pain with lumbar extension and whole-body vibration exercise: a randomized controlled trial. Spine 27: 1829–1834, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine 28: 2621–2627, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Schulze K, Gallagher P, Trappe S. Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc 34: 303–313, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97: 119–129, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Shields RK, Madhavan S, Gregg E, Leitch J, Petersen B, Salata S, Wallerich S. Neuromuscular control of the knee during a resisted single-limb squat exercise. Am J Sports Med 33: 1520–1526, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shinohara H. Gemelli and obturator internus muscles: different heads of one muscle? Anat Rec 243: 145–150, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Stuart MJ, Meglan DA, Lutz GE, Growney ES, An KN. Comparison of intersegmental tibiofemoral joint forces and muscle activity during various closed kinetic chain exercises. Am J Sports Med 24: 792–799, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 93: 463–468, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol 96: 1451–1458, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Tichy M, Grim M. Morphogenesis of the human gluteus maximus muscle arising from two muscle primordia. Anat Embryol (Berl) 173: 275–277, 1985 [DOI] [PubMed] [Google Scholar]
- 60. Willick SE, Lazarus M, Press JM. Quadratus femoris strain. Clin J Sport Med 12: 130–131, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Wilson JT. Abnormal distribution of the nerve to the quadratus femoris in man, with remarks on its significance. J Anat Physiol 23: 354–357, 1889 [PMC free article] [PubMed] [Google Scholar]
- 62. Wright GA, Delong TH, Gehlsen G. Electromyographic activity of the hamstrings during performance of the leg curl, stiff-leg deadlift, and back squat movements. J Str Cond Res 13: 168–174, 1999 [Google Scholar]