Abstract
Lung cancer incidence continues to rise and is the number one cause of cancer death in both men and women worldwide with projected 221,130 new cases and 156,940 deaths in the United States in 2011.1 Non-small cell lung cancer (NSCLC) represents more than 85% of the cases with most patients having either locally advanced or metastatic disease at the time of initial diagnosis, and approximately 60%–70% of them have an adenocarcinoma histologic subtype. In the last three years, we have seen several advances in the management of NSCLC, with several factors playing an important role in the treatment decision making process. Maintenance therapy has been added to the algorithm of NSCLC management and Pemetrexed has been studied as single agent or in combination in this setting with recent studies showing safety and improved progression free survival (PFS) and/or overall survival (OS), still the disease for the most part has a dismal outcome. More research work needs to be done to identify which patients truly benefit from these approaches, and to whom we should offer maintenance or switch maintenance vs. close observation.
Keywords: Pemetrexed, maintenance, lung cancer
Introduction
Lung cancer incidence continues to rise and is the number one cause of cancer death in both men and women worldwide with a projected 221,130 new cases and 156,940 deaths in the United States in 2011.1 Non-small cell lung cancer (NSCLC) represents more than 85% of the cases with most patients having either locally advanced or metastatic disease at the time of initial diagnosis, and approximately 60%–70% of them have an adenocarcinoma histologic subtype. Several studies have shown that 4 to 6 cycles of chemotherapy given as frontline therapy offer a survival advantage.2–6 For many decades, the standard of care was a “watch and wait” approach after initial therapy, and then to offer NSCLC patients second-line treatment at progression of disease meanwhile patient continues having a good performance status. To date, three agents are well established as second-line treatment for lung cancer: docetaxel, pemetrexed, and erlotinib.
The fact that approximately 40 percent of those patients who received first line chemotherapy are eligible for second line treatment starts to question if second line should be moved upfront (immediately after finishing 4 or 6 cycles of induction therapy). There were some attempts to prove this concept; however, the side effects of conventional chemotherapies outweighed their benefits without a survival advantage. The advent of more tolerable drugs with a better toxicity profile and clinical efficacy such as pemetrexed and erlotinib caught the interest of researchers to revisit the issue of maintenance therapy in NSCLC patients. Thus, two large randomized clinical trials were developed. The first one included pemetrexed as maintenance therapy for patients who had received 4 cycles of platinum-based doublets (none of them included pemetrexed) and attained stable disease or any objective response (JMEN trial).7 The second trial known as SATURN trial used erlotinib as maintenance therapy with similar inclusion criteria during the randomization.8 Because neither JMEN nor SATURN used pemetrexed or erlotinib during the initial therapy, the term “switch maintenance” has been coined to differentiate them from a truly maintenance therapy in which patient is kept on the same therapeutic agent received during the initial therapy. The later is the case for studies such as ECOG 4599, FLEX, and PARAMOUNT in which maintenance therapy involved single agent bevacizumab, cetuximab, and pemetrexed, respectively.9–11
ECOG 4599, FLEX, JMEN, and SATURN clinical trials met their primary endpoints, and all of them improved overall survival (OS). Nowadays, maintenance or switch maintenance are options that medical oncologists should discuss with their patients. The National Comprehensive Cancer Network recognizes bevacizumab, pemetrexed, erlotinib, and cetuximab as maintenance or switch maintenance (depending on the case) agents with category 1 or 2A designation. Other agents like gemcitabine and docetaxel have also been studied for the possibility of maintenance use.12–14 Although there were improvements in progression-free survival (PFS), no significant effect on OS was observed. In this article, we will discuss in depth the recent developed data as well as ongoing research with the antifolate agent known as pemetrexed.
Mechanism of action
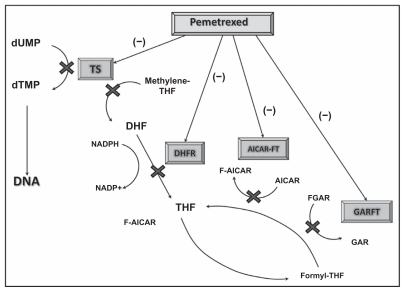
Pemetrexed, a multitargeted antifolate compound, works by inhibiting multiple enzymes involved in folate metabolism and DNA synthesis. Pemetrexed enters cells via the reduced folate carrier, and binds to folate receptor-α with a very high affinity, similar to that of folic acid. Intracellularly, pemetrexed is polyglutamated to the active pentaglutamide by a reaction catalyzed by folylpolyglutamate synthase. The enzymes inhibited by pemetrexed include thymidylate synthase (TS), dihydrofolate reductase (DHFR), glycinamide ribonucleotide formyltransferase (GARFT) and aminoimidazole carboxamide ribonucleotide formyltransferase. Inhibition of TS results in decreased thymidine production necessary for DNA synthesis. In addition, pemetrexed inhibits DHFR as well as GARFT which is a folate-dependent enzyme involved in purine synthesis (Fig. 1).15–17,27,28 Contrary to methotrexate, a potent antifolate that inhibits mainly DHFR, pemetrexed the major site of action is TS.16 The therapeutic benefit of pemetrexed in lung cancer when compared to other malignancies may also be explained because of the low as baseline expression of the TS gene and protein in adenocarcinomas of the lung.20
Figure 1.
Pemetrexed mechanism of action.
Almost 80% of pemetrexed is bound to plasma proteins and it is rapidly eliminated by urinary excretion with a half-life of 3.5 hours. Plasma clearance is diminished in patients with reduced renal function resulting in an increased systemic exposure; pharmacokinetics are not affected by diminished liver function due to limited hepatic metabolism.29
Clinical Studies
Background on the use of pemetrexed
Pemetrexed was approved in 2004 as a single agent for second line treatment of patients with advanced NSCLC (all histologic subtypes) after Hanna et al conducted a large randomized phase III trial of pemetrexed vs. docetaxel in this clinical setting.18 In this study patients received pemetrexed 500 mg/m2 or docetaxel 75 mg/m2 every 21 days. Cycles were repeated until disease progression, unacceptable toxicity, or if decided by the patient or the investigator. The two regimens were found to have analogous efficacy endpoints, including OS (median 8.3 vs. 7.9 months for pemetrexed and docetaxel, respectively) and 1-year survival (29.7% in both arms). The overall response rate (ORR) (9.1% vs. 8.8%) and stable disease (SD) rates (45.8% vs. 46.4%) were similar for pemetrexed and docetaxel arms, respectively. Other measurements such as PFS, median time to response, and duration of response were not statistically different among both arms. Of note, time to treatment failure was longer with pemetrexed (2.3 vs. 2.1 months; P = 0.046).18 Although comparable in terms of efficacy, grade 3 and 4 hematologic toxicities had a lower incidence on the pemetrexed arm when compared with docetaxel: neutropenia (5.3% vs. 40.2%), febrile neutropenia (1.9% vs. 12.7%) and neutropenia with infection (0.0% vs. 3.3%); these differences were obvious once the study was amended, and patients received supplementation with folic acid and vitamin B-12. Anemia and thrombocytopenia rates were similar in both arms. Cullen et al studied a higher dose of pemetrexed at 900 mg/m2 vs. its standard dose in a randomized phase III trial.19 This study found no statistical difference in efficacy between the two doses, with a median survival of 6.7 vs. 6.9 months, PFS of 2.6 vs. 2.8 months, ORR of 7.1% vs. 4.3% (P = 0.1616) and stable disease (SD) rate 50.6% vs. 53.1% for P500 and P900, respectively. Conversely, more toxicity was encountered with P900 dosing.
Peterson et al analyzed all patients enrolled into the original phase III study from Hanna and compared the outcomes based on histology and treatment delivered. This analysis revealed patients with nonsquamous histology had a longer OS time on pemetrexed than on docetaxel (HR, 0.78; P = 0.047), whereas patients with squamous histology had a shorter OS time on pemetrexed than on docetaxel (HR, 1.56; P = 0.018). Similarly, patients with nonsquamous histology had a longer PFS time on pemetrexed than on docetaxel (HR, 0.82; P = 0.076). As seen with OS, patients with squamous histology had a shorter PFS time on pemetrexed than on docetaxel (HR, 1.40; P = 0.046).
In another landmark publication, Scagliotti et al compared cisplatin/pemetrexed vs. cisplatin/ gemcitabine in the first-line setting with a pre-planned analyses for efficacy based on histology. In this study, 1725 chemonaïve patients with Stage IIIB or IV NSCLC were randomized to receive either cisplatin 75 mg/m2 on day 1 plus gemcitabine 1250 mg/m2 on days 1 and 8 or cisplatin 75 mg/m2 plus pemetrexed at 500 mg/m2 both on day 1 only. Both chemotherapy regimens were administered in a 3-week cycle for up to six cycles. The OS was identical at 10.3 months for both cisplatin/gemcitabine and cisplatin/ pemetrexed, but there was a significant survival differences according to tumor histologic subtype. Patients with adenocarcinoma and large-cell histology had superior OS with the cisplatin/pemetrexed arm in comparison with cisplatin/gemcitabine (12.6 vs. 10.9 months in adenocarcinoma) and 10.4 vs. 6.7 months (in large cell histology).21 In this study, cisplatin/gemcitabine was superior than cisplatin/pemetrexed in patients diagnosed with squamous cell histology (10.8 vs. 9.4 months; (P = 0.05). (Table 1). Thus, based on these 2 studies, pemetrexed found a change in its package insert when the US Food and Drug Administration (FDA) limited its use for non-squamous NSCLC histologies only in 2008.
Table 1.
Efficacy of pemetrexed in the treatment of NSCLC.
| Authors | Chemotherapy regimen | Overall survival | Overall response rate | Stable disease | Progression free survival |
|---|---|---|---|---|---|
| Hanna et al18 | Pemetrexed | 8.3 months | 9.1% | 45.8% | 2.9 months |
| vs. | vs. | vs. | vs. | vs. | |
| docetaxel | 7.9 months | 8.8% | 46.4% | 2.9 months | |
| Cullen et al19 | High-dose pemetrexed | 6.7 months | 7.1% | 50.6% | 2.6 months |
| vs. | vs. | vs. | vs. | vs. | |
| Standard dose pemetrexed | 6.9 months | 4.3% | 53.1% | 2.8 months | |
| Scagliotti et al21 | Cisplatin/pemetrexed | 10.3 months | 30.6% | Not reported | 4.8 months |
| vs. | vs. | vs. | vs. | ||
| Cisplatin/gemcitabine | 10.3 months | 28.2% | 5.1 months | ||
| Ciuleanu et al7 | Maintenance pemetrexed | 13.4 months | 6.8% | 51.7% | 4.3 months |
| vs. | vs. | vs. | vs. | vs. | |
| Best supportive care | 10.6 months | 1.8% | 33.3% | 2.6 months |
Maintenance pemetrexed as single agent
Once the pemetrexed indication by the US FDA was restricted to non-squamous cell histology and approved to be used also as first-line therapy, the next step was to study this compound in the maintenance setting. In this regard, Ciuleanu et al tested this drug as maintenance treatment in the JMEN trial.7 In this study, patients were treated with 4 cycles of platinum-based therapy (6 different doublets were used; none included pemetrexed). Those patients who attained complete response (CR), partial response (PR) or SD during induction were randomized to continue maintenance therapy with either pemetrexed plus best supportive care (BSC) or placebo plus BSC. A total of 663 patients with stage IIIB/IV NSCLC and ECOG PS 0–1 were enrolled into this multicenter, randomized, placebo-control trial. Patients were randomly assigned in a 2:1; patients with squamous and non-squamous histology were included with approximately two third of patients presenting with the latter. The final results of this trial revealed a statistically significant median OS in favor of the pemetrexed group in the overall population (13.4 vs. 10.6 months; P = 0.012).7 This difference was more pronounced for non-squamous NSCLC (15.5 vs. 10.3 months; P < 0.0001) (Table 2). Moreover, for patients who had adenocarcinoma histology, the median OS was a striking 16.8 months.7 Furthermore, to confirm pemetrexed-histology selectivity, those patients who had squamous cell carcinoma did much better on placebo than pemetrexed (10.8 vs. 9.9 months). Treatment-related adverse events (AEs) were more common in those treated with pemetrexed when compared to placebo, with fatigue and neutropenia being the most common side effects. Nonetheless, the maintenance therapy was well tolerated. The differences found in survival based on histologic subtypes in the JMEN trial confirmed those from the two large phase III trials previously discussed. Based on this study, the US FDA approved pemetrexed as maintenance therapy for advanced non-squamous NSCLC in 2009.
Table 2.
Efficacy measures according to treatment group in the JMEN trial (Pemetrexed vs. Placebo as maintenance therapy).
| Outcome value | Pemetrexed (N = 441) |
Placebo (N = 222) |
P value |
|---|---|---|---|
| Response rate (% of patients) | 6.8 | 1.8 | 0.05 |
| Median overall survival (months) | 13.4 | 10.6 | 0.012 |
| Median progression free survival (months) | 4.3 | 2.6 | <0.0001 |
Nevertheless, JMEN did not answer the question if maintenance pemetrexed was effective once the patient has been exposed to this compound in the initial 4 cycles of therapy. Thus, there was skepticism among clinicians if continuing pemetrexed after 4 cycles of cisplatin/pemetrexed can match the results from the JMEN study in terms of PFS and OS. Hence, the PARAMOUNT trial was designed and in the 2011 American Society of Clinical Oncology (ASCO) meeting, investigators presented the preliminary results of this important study.11 The PARAMOUNT trial is a phase III randomized double blinded study looking at maintenance pemetrexed plus BSC vs. placebo plus BSC in patients with advanced non-squamous NSCLC after induction with pemetrexed plus cisplatin. A total of 939 patients received four cycles of pemetrexed and cisplatin every three weeks; those patient who had not progressed and who had preserved performance status were randomized in a 2:1 ratio to receive either maintenance premetrexed plus BSC (n = 359) or placebo plus BSC (n = 180). Pemetrexed continuation resulted in a reduction in the risk of progression of 36% (P = 0.00025), median PFS was 4.1 vs. 2.8 months in favor of pemetrexed (P = 0.00006) (Table 3) and, disease control rate (DCR) was 71.8 vs. 59.6% also in favor of pemetrexed arm (P = 0.009). Serious AEs were more common in the pemetrexed arm compared to the placebo group with 8.9 vs. 2.8%. The rate of discontinuation due to AEs was higher in the pemetrexed arm than placebo group (5.3% vs. 3.3%).
Table 3.
Reported progression-free survival and response rates from PARAMOUNT trial.
| Pemetrexed + BSC (N = 359) |
Placebo + BSC (N = 180) |
|
|---|---|---|
| PFS (months) | 3.9 (3.0–4.2) | 2.6 (2.2–2.9) |
| Response rates n (%)* | 9 (2.9) | 0 (0.6) |
Note:
Response rates after randomization.
Maintenance pemetrexed in combination with another agent
The safety and efficacy of pemetrexed plus bevacizumab has been evaluated in a phase II study conducted by Sandler and colleagues.23 The authors treated 120 non-squamous NSCLC patients who had unresectable or recurrent disease. The patients were randomized to receive docetaxel or pemetrexed plus placebo (arm 1), docetaxel or pemetrexed plus bevacizumab (arm 2), or bevacizumab plus erlotinib (arm 3). AEs prompting drug discontinuation were greater in the chemotherapy containing arms (24% in the chemotherapy alone arm and 28% in the chemotherapy and bevacizumab arm) compared to 13% in the bevacizumab plus erlotinib arm. The most common non hematologic AE was fatigue in 65% of patients. There were no grade 5 neutropenias. The median PFS times for chemotherapy alone, bevacizumab-chemotherapy, and bevacizumab-erlotinib were 3.0, 4.8, and 4.4 months, respectively. Median OS times were 8.6, 12.6, and 13.7 months for the chemotherapy alone, bevacizumab-chemotherapy, and bevacizumab-erlotinib arms, whereas the 1-year survival rates were 33.1%, 53.8%, and 57.4%, respectively. This phase II study suggested that bevacizumab enhanced the activity of chemotherapy and was safe to use in combination with pemetrexed. Also, in another phase II trial, Patel et al studied this combination plus carboplatin during induction phase followed by maintenance pemetrexed and bevacizumab.24 In this trial, 50 patients were enrolled with a median follow-up of 13.0 months and a median number of treatment cycles of seven (1 to 51). Thirty patients (60%) completed ≥six treatment cycles, and nine (18%) completed ≥18 treatment cycles. Among the 49 patients assessable for response, the ORR was 55% (95% CI, 41% to 69%). Median PFS and OS rates were 7.8 months and 14.1 months, respectively. Grade 3/4 hematologic toxicity in up to 8% of patients included anemia, neutropenia, and thrombocytopenia. Grade 3/4 non hematologic toxicities occurred in a small group of patients and included proteinuria, venous thrombosis, arterial thrombosis, fatigue, infection, nephrotoxicity, and diverticulitis. There was no grade 3 or greater hemorrhagic events or hypertension cases.
Based on the safety data observed with the combination of pemetrexed and bevacizumab as well as its efficacy on non-squamous NSCLC histology, several clinical trials were launched. Some of them have recently reported their preliminary results, and others are still ongoing. Among these studies, AVAPERL1 by Barlesi et al initially reported its safety data at the 2011 ASCO meeting.25 Herein, the trial is looking at bevacizumab alone or in combination with pemetrexed as maintenance therapy after first line treatment with cisplatin, pemetrexed and bevacizumab in chemonaive patients diagnosed with metastatic or recurrent NSCLC. The safety analysis reported that most AEs were grade 1 or 2. The most common grade 3 or more AEs were neutropenia, embolism, hypertension, anemia, fatigue, diarrhea, nausea, vomiting, hyperglycemia, pneumonia, and dyspnea. Serious AEs included pneumonia, dyspnea, embolism, pulmonary embolism, neutropenia, renal failure and nausea (Table 4). In the 2011 European Society of Medical Oncology (ESMO) meeting, Dr. Barlesi et al, presented the preliminary efficacy results of this trial. Primary endpoint is PFS and secondary endpoints are OS, ORR, DCR, and duration of response. The study met its primary endpoint by reporting a statistically difference in PFS between the two groups: 10.2 months (cisplatin/pemetrexed/ bevacizumab followed by maintenance bevacizumab/ pemetrexed) vs. 6.6 months (same induction therapy followed by single agent bevacizumab as maintenance therapy) (HR: 0.5, P < 0.001).30 From the randomization, PFS was 7.4 vs. 3.7 months in favor of maintenance pemetrexed/bevacizumab (HR: 0.48, P < 0.001). OS is still immature.
Table 4.
Safety data comparing bevacizumab/pemetrexed vs. bevacizumab alone as maintenance therapy. AVAPERL1 Trial.
| Bevacizumab maintenance (N = 110) |
Bevacizumab/pemetrexed maintenance (N = 117) |
|
|---|---|---|
| Number of AE any grade | 867 | 1134 |
| Number and (%) of patients with AE | 102 (93%) | 110 (94%) |
| Number of AE grade ≥3 | 55 | 44 |
| Number and (%) of patients with grade ≥3 AE | 38 (35%) | 45 (38%) |
| % of patients with AE by phase | ||
| First line | 82 | 86 |
| Maintenance | 65 | 72 |
| % of patients with AE by intensity | ||
| Grade 1/2 | 85/73 | 90/74 |
| Grade 3/4 | 31/4 | 37/6 |
| Grade 5 | 3 | 2 |
Another very interesting trial which recently completed accrual is the PointBreak study.26 In this trial, Patel et al are currently looking into two different treatment approaches; both of them include a maintenance phase. This is an open phase III randomized clinical trial. Patients with advanced non-squamous NSCLC are randomized to receive either 4 cycles of carboplatin/pemetrexed/bevacizumab induction followed by pemetrexed and bevacizumab maintenance (as described by Patel et al, J Clin Oncol 2009)24 or carboplatin/paclitaxel/bevacizumab induction followed by bevacizumab alone (as described by Sandler et al, NEJM 2006). The primary objective of this study is OS and secondary endpoints were response rate, DCR, PFS, and time to progressive disease, safety and quality of life will also be evaluated.
Conclusion
In the last three years, we have seen several advances in the management of NSCLC, and now we have several factors playing an important role in the treatment decision making process. Maintenance therapy has been added to the algorithm of NSCLC management. Although all these recent studies have improved PFS and/or OS, the disease for the most part still have a dismal outcome. Some patients do very well while other progress with our best therapies and the use of a clinical biomarker such as histology subtype to select treatment. Thus, more research work needs to be done to identify which patients truly benefit from these approaches, and to whom we should offer maintenance or switch maintenance vs. close observation.
Several predictive biomarkers are still on validation through large phase III clinical trials. Today, the only predictive molecular biomarkers that we have to personalize our NSCLC therapies are epidermal growth factor receptor (EGFR) mutation and the presence of EML4/ALK mutation. In the case of pemetrexed, TS has been proposed as a potential predictive biomarker, but its utility in this regard is under investigation. There is no question that if we are able to predict a major response on the tumor, the likelihood that we may impact PFS and OS will also be higher. Thus far, histology has become a clinical biomarker to select therapy in NSCLC management for agents such as pemetrexed (due to its efficacy) and bevacizumab (due to safety and efficacy factor). But, we can do much better than that, and proofs of it are the development of other therapies based on the expression of certain tumor markers; a discussion that is beyond the scope of this review.
Pemetrexed has gone through many changes in the last 3 years since its approval back in 2004. The safety profile of this drug has made possible its study in the maintenance setting and the possibility to combine it with other efficacious drugs such as bevacizumab in this setting. We are eager to know the final results of PARAMOUNT and PointBreak. The latest large phase 3 trial reporting a positive outcome is AVAPERL1 (ESMO, Stockholm, Sweden, September 2011). The Eastern Cooperative Oncology Group has also joined to this task: to define the best induction therapy and maintenance approach for non-squamous NSCLC. ECOG 5508 will usecarboplatin/paclitaxel/ bevacizumab (4 cycles) as initial therapy, and then patients who attain objective response or stable disease will be randomized into three arms: pemetrexed alone, bevacizumab alone, and the combination of these two agents. We hope that altogether will give us and our patients a final answer in terms of the best initial and best maintenance approach for advanced non-squamous NSCLC patients.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Socinski MA, Schell MJ, Peterman A, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV Non-Small Cell Lung Cancer. J Clin Oncol. 2002:1335–43. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]
- 3.Barata FJ, Parente B, Teixeira E, et al. Optimal duration of chemotherapy in non-small-cell lung cancer: multicenter, randomized, prospective clinical trial comparing 4 vs. 6 cycles of carboplatin and gemcitabine: P2–235. J Thorac Oncol. 2007;2(Suppl 4):S666. [Google Scholar]
- 4.Park JO, Kim S-W, Ahn JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non-small-cell lung cancer. J Clin Oncol. 2007:5233–9. doi: 10.1200/JCO.2007.10.8134. [DOI] [PubMed] [Google Scholar]
- 5.Soon Y, Stockler MR, Boyer M, Askie L. Duration of chemotherapy for advanced non-small-cell lung cancer: An updated systematic review and meta-analysis. J Clin Oncol. 2008;26(15S):8013. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Ciuleanu T, Brodowicz T, Zielinski, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non small cell lung cancer: a randomized, double blind, phase 3 study. Lancet. 2009:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 8.Capuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicenter, randomized, placebo-controlled phase 3 study. Lancet. 2010:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell-lung cancer (FLEX): an open label randomized phase III trial. Lancet. 2009:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares LG, De Marinis F, Dediu M, et al. PARAMOUNT: Phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo plus BSC immediately following induction treatment with pem plus cisplatin for advanced nonsquamous non small cell lung cancer(NSCLC) J Clin Oncol. 2011;29(suppl abst CRA7510) [Google Scholar]
- 12.Brodowicz T, Krzaowski M, Zwitter M, et al. Central European Cooperative Oncology Group CECOG. Cisplatin and gemcitabine fist-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006:155–63. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009:591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd FA, Dancey J, Ramlau R, et al. Prospective Randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 15.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006:1589–96. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]; The DISTAL 01 study. Br J Cancer. 2004:1996–2004. doi: 10.1038/sj.bjc.6602241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih C, Chen VJ, Gossett LS, et al. LY231514, a pyrolo [2,3-d] pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997:1116–23. [PubMed] [Google Scholar]
- 17.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007:404–17. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]
- 18.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 19.Cullen MH, Zatloukal P, Sorenson S, et al. A randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2008:939–45. doi: 10.1093/annonc/mdm592. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti G, Hanna N, Fosella F, et al. The differential efficacy of pemetrexed accosrding to NSCLC histology: A review of two phase III studies. The Oncologist. 2009:253–63. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 21.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy naive patients with advanced stage non-small-cell lung cancer. J Clin Oncol. 2008:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 22.Peterson P, Park K, Fossella F, et al. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC): P2-328. J Thorac Oncol. 2007;2(Suppl 4):s851. [Google Scholar]
- 23.Herbst RS, O’Neill VJ, Fehrenbacher, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non-small-cell lung cancer. J Clin Oncol. 2007:4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 24.Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009:3284–9. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 25.Barlesi F, Scherpereel A, Gervais R, et al. AVAPERL1 (MO22089): Maintenance (mtc) Bevacizumab (bev) with or without pemetrexed (pem) in patients with advanced nonsquamous non-small cell lung cancer treated with first-line bev-cisplatin-pem: Interim safety data. J Clin Oncol. 2011;29(suppl abst 7562) [Google Scholar]
- 26.Patel JD, Bonomi P, Socinski MA, et al. Treatment rationale and study design for the point break study: a randomized open label phase III study of pemetrexed/carboplatin/bavacizumab followed by maintenance pemetrexed/ bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2009:252–6. doi: 10.3816/CLC.2009.n.035. [DOI] [PubMed] [Google Scholar]
- 27.Adjei AA. Pharmacology and mechanism of action of pemetrexed. Clin Lung Cancer. 2004:S51–55. doi: 10.3816/clc.2004.s.003. [DOI] [PubMed] [Google Scholar]
- 28.Adjei AA. Pemetrexed (Alimta), A novel multitargeted antineoplastic agent. Clin Cancer Res. 2004:4276S. doi: 10.1158/1078-0432.CCR-040010. [DOI] [PubMed] [Google Scholar]
- 29.Rollins KD, Lindley C. Pemetrexed: a multitargeted antifolate. Clin Ther. 2005:1343–82. doi: 10.1016/j.clinthera.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Barlesi F, de Castro J, Dvornichenko V, et al. AVAPERL (MO22089): Final Efficacy Outcomes for Patients (pts) with Advanced Non-Squamous Non-Small Cell Lung Cancer (nsNSCLC) Randomised to Continuation Maintenance (mtc) with Bevacizumab (bev) or Bev + Pemetrexed (pem) after First-line (1L) Bev-cisplatin (cis)-pem Treatment (Tx) Eur J Cancer. 2011;47(Suppl 2):16(34LBA). [Google Scholar]