Abstract

The neurotransmitter dopamine plays important roles in modulating cognitive, affective, and motor functions. Dysregulation of dopaminergic neurotransmission is thought to be involved in the pathophysiology of several psychiatric and neurological disorders, including schizophrenia, Parkinson’s disease and drug abuse. Dopaminergic systems are regulated by cholinergic, especially muscarinic, input. Not surprisingly, increasing evidence implicates muscarinic acetylcholine receptor-mediated pathways as potential targets for the treatment of these disorders classically viewed as “dopamine based”. There are five known muscarinic receptor subtypes (M1 to M5). Due to their overlapping expression patterns and the lack of receptor subtype-specific ligands, the roles of the individual muscarinic receptors have long remained elusive. During the past decade, studies with knockout mice lacking specific muscarinic receptor subtypes have greatly advanced our knowledge of the physiological roles of the M1–M5 receptors. Recently, new ligands have been developed that can interact with allosteric sites on different muscarinic receptor subtypes, rather than the conventional (orthosteric) acetylcholine binding site. Such agents may lead to the development of novel classes of drugs useful for the treatment of psychosis, drug abuse, and Parkinson’s disease. The present review highlights recent studies carried out using muscarinic receptor knockout mice and new subtype-selective allosteric ligands to assess the roles of M1, M4, and M5 receptors in various central processes that are under strong dopaminergic control. The outcome of these studies opens new perspectives for the use of novel muscarinic drugs for several severe disorders of the central nervous system.
Keywords: Muscarinic receptors, schizophrenia, drug abuse, Parkinson’s disease, knockout mice, allosteric modulators
Acetylcholine activates two families of receptors: nicotinic receptors, which are ligand-gated cation channels and participate in rapid postsynaptic neurotransmission, and muscarinic receptors, which are G-protein coupled receptors and play roles in modulating the activity of many circuits within the central nervous system (CNS).1 Due to the wide distribution of muscarinic receptors in the CNS and their involvement in many important neuronal functions, these receptors have long been viewed as possible targets for the treatment of various conditions such as Alzheimer’s disease, schizophrenia, Parkinson’s disease, and drug abuse.2−4
Preclinical and clinical evidence suggests that the cholinergic and dopaminergic systems operate in a dynamic balance and that a disruption of this balance may lead to neurological and psychiatric disorders.5,6 The first observation supporting this concept was made in 1867 when Jean-Martin Charcot found that the muscarinic receptor antagonist scopolamine could improve symptoms in Parkinson’s disease patients.7 It was later discovered that muscarinic receptor antagonists could also alleviate parkinsonian symptoms induced by dopamine D2 receptor antagonists used as antipsychotics.8 However, antimuscarinic agents have also been reported to induce psychotic symptoms.9−12 Thus, the cholinergic and dopaminergic systems have long been regarded as balancing, or opposing, each other. More recent findings revealed that muscarinic receptor stimulation can both interfere with and enhance dopamine signaling, depending on the receptor subtype and brain region under investigation (as described below).
Muscarinic Acetylcholine Receptor Subtypes
Five different muscarinic receptor subtypes (M1 to M5) have been cloned (for reviews, see Langmead et al.,3 Wess13). The amino acids lining the conventional acetylcholine binding site appear to be identical in the five receptor subtypes. M1, M3, and M5 receptors preferentially couple to Gαq proteins, resulting in the activation of phospholipase Cβ and the subsequent release of calcium from intracellular stores and the stimulation of protein kinase C. M2 and M4 receptors couple predominantly to Gαi/o proteins to inhibit adenylate cyclase, causing a decrease in intracellular cAMP levels. Activation of Giβγ subunits through M2 and M4 receptor stimulation also modulates various ion channels including voltage-gated calcium channels as well as inwardly rectifying potassium channels.14,15
Different experimental approaches have shown that muscarinic receptors are present in many regions of the CNS.13,16−19 The M1, M4 and M5 receptors are predominantly expressed in the CNS, while the M2 and M3 receptor subtypes are widely distributed in both the CNS and peripheral tissues.2,15 In the forebrain, including the striatum, the M1 and M4 receptors are the most abundantly expressed muscarinic receptors, whereas the expression of M2 and M3 receptors is moderate20−24 and the density of M5 receptors is low.19,25
In the present review, we will summarize recent work suggesting that central M1, M4, and M5 receptors represent promising new targets for the treatment of various CNS disorders including schizophrenia, drug abuse, and Parkinson’s disease.
Localization and Function of Central M1 Receptors
The M1 receptor subtype is expressed throughout the forebrain, including the neocortex, dorsal striatum, nucleus accumbens (NAcc), and hippocampus.17,18,20−22,26 M1 receptors have been implicated in many functions of the CNS. For example, pharmacological and genetic studies support a role of M1 receptors in cognitive functions like learning and memory, especially in the acquisition phase (see Robinson et al.27 for review). In the striatum, M1 receptors are coexpressed with D2 dopamine receptors by GABAergic projection neurons,20,21 suggesting that activation of M1 receptors may oppose D2 receptor-mediated neuronal inhibition.5
Localization and Function of Central M4 Receptors
In rodents, the M4 receptor is highly expressed in the dorsal striatum, NAcc, neocortex, and hippocampus.17,26 The expression levels decrease caudally toward the diencephalon and mesencephalon and are lowest in the metencephalon, that is, pons and cerebellum.25 The M4 receptor is the most highly expressed muscarinic receptor in the striatum, where it is present on medium spiny GABAergic output neurons (MSNs).22,28 The M4 receptor subtype is also localized on cholinergic interneurons, where it acts as an autoreceptor.29 M4 receptor-expressing MSNs usually coexpress dopamine D1 receptors.20,21,30 Yan et al.28 have shown that the M4 receptor is five times more abundant on D1 receptor-expressing MSNs in the striatonigral direct pathway than on D2 receptor-expressing MSNs in the striatopallidal indirect pathway. Since M4 receptor activation inhibits D1 receptor-stimulated cAMP formation,31 it is likely that this interaction is of physiological relevance for the regulation of striatal function.
Localization and Function of Central M5 Receptors
The M5 receptor is expressed at relatively low levels in the CNS but has been detected in the cerebral cortex, striatum, hippocampus, thalamus-hypothalamus, midbrain, pons, medulla, and cerebellum.19,25 M5 receptor mRNA is the only muscarinic receptor mRNA that has been identified in dopaminergic neurons of the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc).20,32 In these regions, it is coexpressed with D2 dopamine receptor mRNA, which has led to the suggestion that the M5 receptor might play a role in modulating dopaminergic neurotransmission.20 Studies with M5 receptor-deficient mice (M5–/– mice) also revealed a role of this receptor subtype in cerebrovascular function.33,34
Muscarinic Receptor Knockout Mice
For many years, studies of the roles of the central muscarinic cholinergic system have been complicated by the overlapping expression patterns of the M1–M5 receptors and the lack of receptor subtype-specific ligands. To overcome these obstacles, we, as well as other laboratories, have developed M1–M5 receptor knockout mice.2 More recently, we employed Cre-lox technology to delete specific muscarinic receptors in a cell type- or brain region-specific fashion. For example, we recently generated mutant mice (D1-M4–/–mice) lacking M4 receptors only in D1 receptor-expressing neurons.35 In the following, we will review recent behavioral studies carried out with M1–/–, M4–/–, M5–/–, and D1-M4–/– mice.
The use of constitutive knockout mice as tools to study normal muscarinic receptor function may be complicated by compensatory changes in the expression patterns of other muscarinic receptor subtypes or downstream signaling molecules. However, many studies have shown that the inactivation of one particular muscarinic receptor species usually has little effect on the expression levels of the remaining muscarinic receptor subtypes.18
Allosteric Modulators of Muscarinic Receptors
For reasons already outlined above, the development of orthosteric muscarinic ligands endowed with a high degree of muscarinic receptor subtype selectivity remains a very challenging task.36 To circumvent this problem, medicinal chemists have redirected their efforts toward developing compounds that act at less conserved allosteric binding sites present on the extracellular surface of different muscarinic receptor subtypes. “First-generation” allosteric ligands targeting the M1 or M4 receptor lacked efficacy and the physiochemical properties required for in vivo use.37−40 More recently, M1, M4, and M5 receptor-selective allosteric ligands useful for in vivo studies have been developed (see Bridges et al.41 for review). The emergence of these new highly subtype-selective ligands has intensified interest in developing new classes of muscarinic drugs for clinical use.3,36,40,42,43
Centrally active allosteric (or “ectopic”) agonists and positive allosteric modulators (PAMs) with high selectivity for the M1 receptor have been generated recently. These agents include the allosteric M1 agonists TBPB, VU0184670, and VU0357017 and the M1 PAMs VU0405652 and BQCA.43−48 TBPB was found to attenuate amphetamine-induced locomotor activity in rats.46 In addition, we recently tested TBPB, VU0357017, and BQCA in a cocaine discrimination procedure and TBPB in a chronic intravenous cocaine self-administration procedure. In these studies, all agents attenuated cocaine’s effects.49,50 While some gastrointestinal side effects were observed after treatment of mice with BQCA, which is poorly brain-penetrant, no adverse effects were observed with the more brain-penetrant ligands TBPB and VU0357017.49,50
Centrally active PAMs of the M4 receptor currently include LY2033298,42 VU0152099, VU0152100,51 VU0152129 (initially named 13k,) and VU0359509 (initially named 21o).41,52 Consistent with findings in M4–/– mice, LY2033298 dose-dependently attenuated apomorphine-induced deficits in prepulse inhibition of the startle response.42 Moreover, VU0152099 and VU0152100 potently attenuated amphetamine-induced hyperlocomotion in rats.51 To obtain M4 receptor PAMs with increased metabolic stability and improved physiochemical properties, Kennedy et al.52 developed VU0152129 and VU0359509.41 However, these compounds displayed a more moderate attenuation of amphetamine-induced hyperlocomotion in rats, most likely due to low potency at rat M4 receptors.52 Since the action of M4 receptor PAMs requires the presence of endogenous acetylcholine it is likely that such agents may not cause major motor side effects in clinical use. Consistent with this notion, VU0152100 did not affect performance in the rotarod test adversely.51 Thus, data obtained with both M4–/– mice and M4 receptor PAMs support the concept that central M4 receptors represent an attractive drug target for the treatment of various CNS disorders.2,3,50,53
Recently, selective allosteric ligands targeting the M5 receptor have also been developed.54,55 Future in vivo studies with this new class of compounds should reveal whether drug-induced modulation of M5 receptor activity may have therapeutic potential.
Muscarinic Receptors and Schizophrenia
Post mortem studies have consistently shown widespread decreases in the levels of muscarinic receptors in brains from patients suffering from schizophrenia.56−59 Specifically, it has been reported that M1 and M4 receptor levels are decreased in striatal areas, hippocampus and prefrontal cortex of schizophrenic patients.60−64 Altered M1 receptor function has also been described in a subset of schizophrenic patients suffering from “muscarinic receptor-deficit schizophrenia”.65 The detection of anti-M1 antibodies in schizophrenic patients suggests that an autoimmune response may contribute to muscarinic receptor dysfunction in schizophrenia.66,67 These findings, together with the known localization of M1 and M4 receptors in brain areas relevant for psychosis, suggested that the M1 and/or M4 receptor may represent a new target for the treatment of psychosis including schizophrenia. This notion is supported by a considerable body of preclinical evidence.68−73
Receptor localization, pharmacological and genetic studies converge to support a role for M1 receptors in cognitive functions (see Robinson et al.27 for review). Interestingly, Bymaster et al.75 speculated that M1 (and/or M4) agonists may improve cognitive function in schizophrenia and other CNS disorders, largely based on data with the M1/M4 receptor-preferring muscarinic agonist xanomeline (see below).
Xanomeline: An M1/M4 Receptor Preferring Muscarinic Agonist
In a large randomized, placebo-controlled, double-blind clinical trial on Alzheimer’s patients, xanomeline, an M1/M4 receptor-preferring muscarinic agonist, displayed a robust effect against psychosis-like behaviors.75 A smaller clinical trial with xanomeline in schizophrenic patients supported these initial findings.76 Xanomeline also improved cognitive performance in some tests (e.g., verbal learning, short-term memory), supporting the notion that M1 and/or M4 agonists may prove useful in the treatment of cognitive deficits in schizophrenia that are typically poorly managed by existing medications.75,76 In both clinical studies, xanomeline administration was associated with significant gastrointestinal and other side effects,75,76 precluding further development of the drug for clinical use. Recent studies with M4–/– and M1–/– mice suggest that the antipsychotic effects of xanomeline are mediated primarily through M4 receptors.50,77
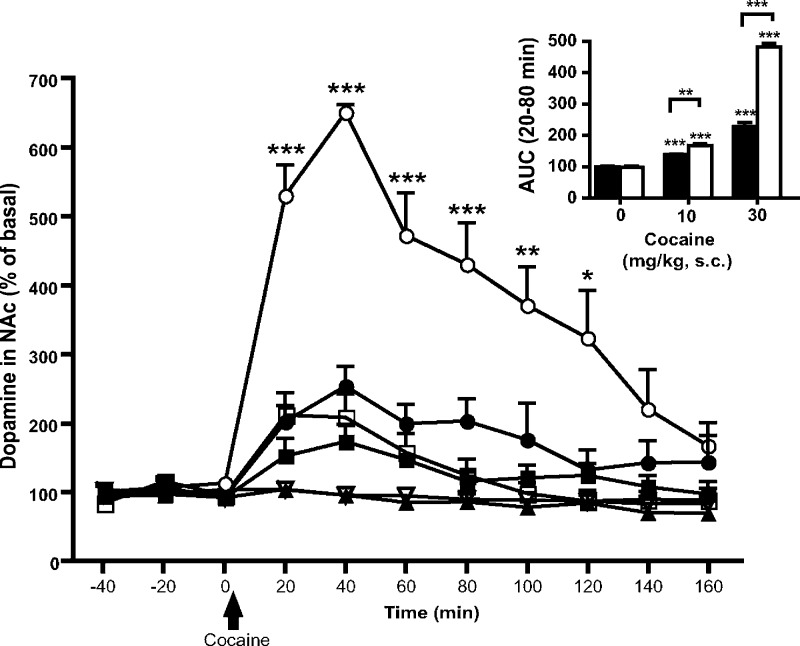
Interestingly, M1–/– mice display a phenotype that is similar to that seen in animal models of psychosis, including hyperactivity, increased striatal dopamine release, certain cognitive deficits, and an elevated response to amphetamine.78−80 Moreover, in several studies M4–/– mice displayed increased locomotion following the administration of selective D1, nonselective, and indirect dopamine receptor agonists.74,81,82 In vivo microdialysis studies revealed that M4–/– mice exhibit elevated basal dopamine release in the NAcc and enhanced dopamine release in response to psychostimulants (amphetamine and phencyclidine).83 In addition, M4–/– mice have increased basal acetylcholine tonus in the midbrain, consistent with the established role of M4 receptors as autoreceptors inhibiting acetylcholine release.83 However, in studies with M4–/– mice that had been extensively backcrossed (mouse genetic background: C57BL/6NTac), we did not find any difference in basal dopamine release in the NAcc between M4–/– mice and wildtype (WT) littermates.84 In line with previous findings, backcrossed M4–/– mice showed an increased dopamine efflux in the NAcc in response to cocaine (Figure 1).84
Figure 1.
Exaggerated cocaine-induced increases in dopamine in the nucleus accumbens of M4–/– mice. Cocaine-induced increases in extracellular dopamine in M4–/– (white symbols/bars) and M4+/+ (black symbols/bars) mice were measured by in vivo microdialysis in freely moving animals in the nucleus accumbens after s.c. administration of cocaine 10 mg/kg (squares), 30 mg/kg (circles), or vehicle (triangles) Cocaine (30 mg/kg) caused a greater increase in extracellular dopamine in M4–/– mice (open circles) compared to WT mice (filled circles). Inset shows dopamine as % baseline, area under the curve (AUC) from 20 to 80 min (***p < 0.001, **p < 0.01, *p < 0.05). [Reprinted with permission from Psychopharmacology.]84
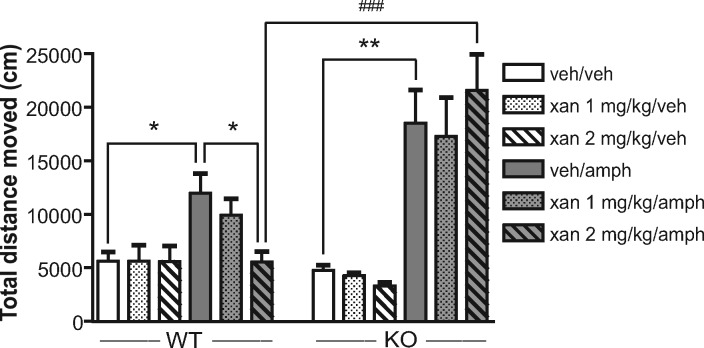
To investigate the functional role in regulation of dopaminergic neurotransmission of the subpopulation of M4 receptors present on D1 receptor-expressing neurons, we generated mice that lack M4 receptors only in D1 receptor-expressing cells (D1-M4–/– mice).35 Similar to the whole-body M4–/– mice, these mice also displayed a “dopamine hypersensitivity phenotype” with increased hyperlocomotion in response to dopamine agonists and enhanced sensitization to psychostimulants, a process thought to reflect adaptive changes induced by drug abuse (Figure 2).35 Moreover, we found that the antipsychotic-like effects of xanomeline were also almost completely abolished in D1-M4–/– mice (Figure 3).85 Taken together, these data suggest that a subpopulation of M4 receptors present on D1 receptor-expressing neurons plays an important role in regulating central dopaminergic neurotransmission and that these receptors are of key importance in mediating the antipsychotic-like effect of xanomeline.
Figure 2.
Increased amphetamine-induced hyperlocomotion in D1-M4–/– mice. To induce behavioral sensitization repeated injections of amphetamine (amph; 2 mg/kg, s.c.) were paired with exposure of the mice to activity test cages for 1 h per day. After an initial saline injection at day 0, D1-M4–/– mice (black) and control floxed littermates (white) were divided into two groups that received either saline (triangles) or amphetamine (circles) for 6 days. In D1-M4–/– mice, amphetamine-induced hyperlocomotion was significantly greater on days 4 and 5 as compared to day 1 (#p < 0.05, ###p < 0.001). In control mice, amphetamine injections resulted in a clear trend toward enhanced locomotor responses on days 2–5; however, this effect did not reach statistical significance. The repeated amphetamine injections generally induced higher levels of hyperlocomotion in D1-M4–/– mice, reaching significance on days 4 and 5 (*p < 0.05, p < 0.01). After a 13-day drug- and test-free period, all mice were injected with amphetamine on day 20 and retested. Amphetamine pretreated D1-M4–/– mice showed a significantly increased locomotor response (###p < 0.001 versus amphetamine-pretreated control mice). The observed hyperlocomotion could not be ascribed to context conditioning (day 21). [Reprinted with permission Journal of Neuroscience.]35
Figure 3.
Lack of attenuation of amphetamine-induced hyperlocomotion by xanomeline in D1-M4–/– mice. The effect of xanomeline (xan) on amphetamine (amph)-induced hyperlocomotion was measured after coadministration of xanomeline, vehicle (veh), and/or amphetamine (2 mg/kg, s.c.) for 2 h in an open field arena. Amphetamine induced a significant increase in locomotor activity (measured as total distance moved) in both genotypes (*p < 0.05, **p < 0.01 vs vehicle). In floxed control mice, 2 mg/kg xanomeline reversed the amphetamine-induced hyperlocomotion, but had no effect in D1-M4–/– mice (###p < 0.001 vs WT). [Reprinted with permission from Journal of Neuroscience.]85
In summary, the findings reviewed above suggest that selective allosteric M4 receptor agonists or M4 receptor PAMs may prove beneficial in the treatment of schizophrenia. It remains to be established whether these agents are endowed with a more favorable side effect profile than xanomeline.
Muscarinic Receptors and Drug Abuse
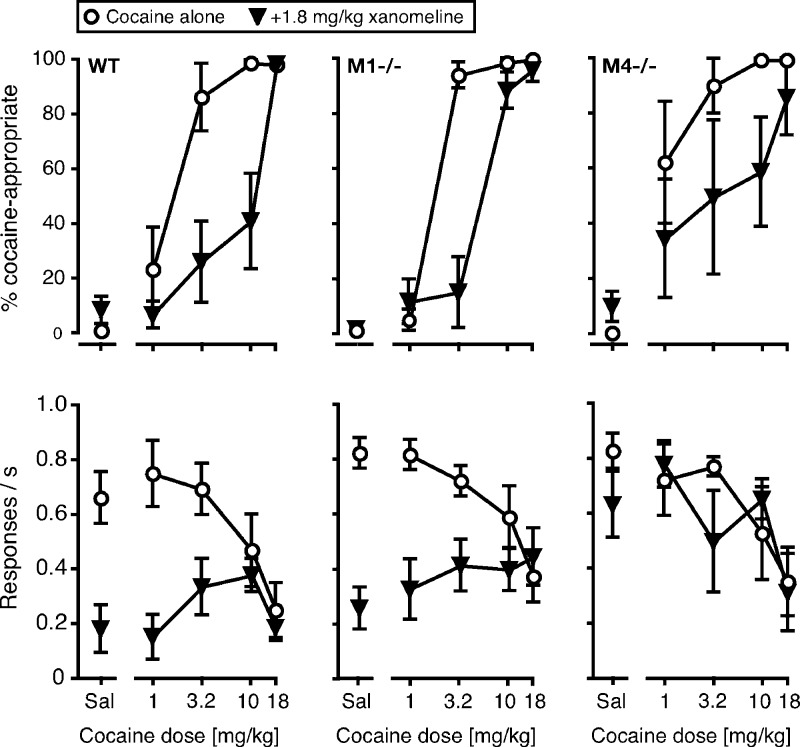
In M1–/– mice, both cocaine- and morphine-conditioned place preference was found to be reduced at low drug doses.86 It remains unclear whether this phenotype reflects altered rewarding effects of the drugs or altered acquisition of the context/reward association, that is, changes in cognition. So far, M1–/– mice have not been characterized in self-administration models, which would allow to address this question. However, the allosteric M1 agonist TBPB and the M1/M4 agonist xanomeline both reduced cocaine self-administration to saline levels in WT mice, without decreasing food-maintained behavior significantly.49 M1–/– mice and M1–/–M4–/– double knockout mice were successfully trained to discriminate cocaine from saline.49,50 Studies with these mutant mice confirmed that M1 receptor stimulation was necessary for the anticocaine effects of the allosteric M1 agonist VU0357017.50 Furthermore, we found that the ability of xanomeline to attenuate cocaine discrimination was blunted in the M1–/– mice (Figure 4).50 These findings suggest that selective M1 agonists may become useful clinically for treating psychostimulant addiction.
Figure 4.
Reduced attenuation of the discriminative stimulus of cocaine by xanomeline in both M1–/– and M4–/– mice. WT mice, M1–/– mice and M4–/– mice were trained to discriminate 10 mg/kg (i.p.) cocaine from saline in a standard drug discrimination procedure. Pretreatment with 1.8 mg/kg xanomeline (s.c.) produced a significant (8-fold) rightward shift in the cocaine dose–effect function in the WT mice. This effect was still significant, but blunted or more variable, in both M1–/– mice and M4–/– mice. Ordinates: % responses emitted on the cocaine-paired side (top panels); rate of responding (maintained by food), in responses per second (bottom panels). [Reprinted with permission from Psychopharmacology.]50
We also tested the potential involvement of M4 receptors in the reinforcing effects of drugs of abuse. We found that M4–/– mice self-administered more cocaine than WT mice, and worked harder to earn a cocaine injection than WT mice (Figure 5), suggesting that M4 receptors also play a role in modulating the reinforcing properties of cocaine.84 In addition, as observed with M1–/– mice, xanomeline was less effective in attenuating cocaine discrimination in M4–/– mice as compared to WT mice (Figure 4).50 These drug discrimination data suggest that combined stimulation of M1 and M4 receptors is likely to reduce cocaine’s abuse-related effects more effectively than activation of either of the two receptors alone. However, this hypothesis remains to be tested experimentally.
Figure 5.
Increased intravenous cocaine self-administration in M4–/– mice. Intravenous cocaine self-administration was measured under an FR 1 (A) and a PR (B) schedule of reinforcement in M4–/– mice (open symbols) and WT littermates (filled symbols). M4–/– mice exhibited higher response rates than WT mice at cocaine doses of 0.3 and 1.0 mg/kg/infusion under the FR 1 schedule. Under the PR schedule of reinforcement, M4–/– mice reached higher breaking points than WT mice at the 1.0 mg/kg per infusion dose (*p < 0.05, **p < 0.01, and ***p < 0.001 vs WT). [Reprinted with permission from Psychopharmacology.]84
The activity of midbrain dopaminergic neurons projecting to the NAcc is believed to mediate the reinforcing effects of drugs of abuse.87 In intact animals, electrical stimulation of the laterodorsal tegmental nucleus (LDT) produces both acute and prolonged dopamine release in the NAcc.88,89 Interestingly, the prolonged release was absent in M5–/– mice.90 This observation led to the hypothesis that M5 receptor activity can modulate rewarded behaviors. Consistent with this notion, M5–/– mice showed reduced morphine-conditioned place preference and less severe morphine withdrawal symptoms.91 M5–/– mice also displayed less cocaine-conditioned place preference and decreased rates of cocaine self-administration relative to WT mice (Figures 6 and 7).92,93 These effects appeared to be selective for abuse-related effects of morphine and cocaine, as food-maintained operant behavior and cocaine-induced locomotor activity were not affected by the absence of M5 receptors.93,95,97 In contrast to the findings obtained with cocaine, amphetamine-induced hyperlocomotion, sensitization and evoked dopamine release in the NAcc were increased in M5–/– mice (Figures 8 and 9(95) see, however, Wang et al.,96 who used a different M5–/– mouse line). These discrepant results probably reflect the pharmacological differences between a dopamine reuptake inhibitor (cocaine) and a dopamine-releasing agent (amphetamine).
Figure 6.
Reduced cocaine-conditioned place preference (CPP) in M5–/– mice. Mice were initially habituated to the two-compartment CPP apparatus to determine the side-preference of each individual mouse. Mice were then administered either cocaine (2.5 mg/kg, i.p.) or saline and placed in either the preferred (saline) or nonpreferred (cocaine) compartment for 30 min for 7 days. On the test day, when side-preference was reassessed, cocaine induced significantly less CPP in M5–/– compared to WT mice (‡ p<0.05 vs pretest; ** p < 0.01 vs M5–/– mice). [Reprinted with permission from Journal of Neuroscience Research.]92
Figure 7.

Reduced intravenous cocaine self-administration in M5–/– mice. Intravenous cocaine (0.03, 0.3, 3.2 mg/kg per infusion) self-administration was measured under a progressive ratio schedule of reinforcement in M5–/– (open) and M5± (gray) mutant mice and their WT littermates (black). M5–/– mice reached lower breaking points than WT mice at doses of 0.03 and 0.32 mg/kg per infusion (†p < 0.05, ††p < 0.01 vs WT). [Reprinted with permission from Journal of Neuroscience.]93
Figure 8.
Increased amphetamine-induced hyperlocomotion in M5–/– mice. To induce behavioral sensitization repeated injections of amphetamine (amph; 2 mg/kg, s.c.) were paired with exposure of the mice to an open field arena for 45 min per day. After an initial saline injection at day 0, M5–/– mice and WT controls were divided into two groups that received either saline or amphetamine for 6 days. The repeated amphetamine administration significantly increased locomotor activity in both genotypes, however this effect was significantly greater in M5–/– compared to WT mice (*p < 0.05). After a 6-day, followed by an 8-day drug- and test-free period, all mice were injected with amphetamine on days 12 and 20 and retested. Amphetamine pretreated M5–/– mice showed a significantly increased sensitized locomotor response (*p < 0.05 vs amphetamine-pretreated WT mice). [Reprinted with permission from Psychopharmacology.]95
Figure 9.
Increased amphetamine-potentiated nucleus accumbens dopamine release in M5–/– mice. The effect of amphetamine (2 mg/kg, i.p.) on medial forebrain bundle-stimulated dopamine release in the nucleus accumbens of M5–/– and M5+/+ mice was measured by fixed potential amperometry. Amphetamine increased dopamine efflux in both genotypes. This effect was significantly enhanced in M5–/– compared to WT mice (*p < 0.05, **p < 0.01, ***p < 0.001). [reprinted with permission from Psychopharmacology.]95
Muscarinic Receptors and Parkinson’s Disease
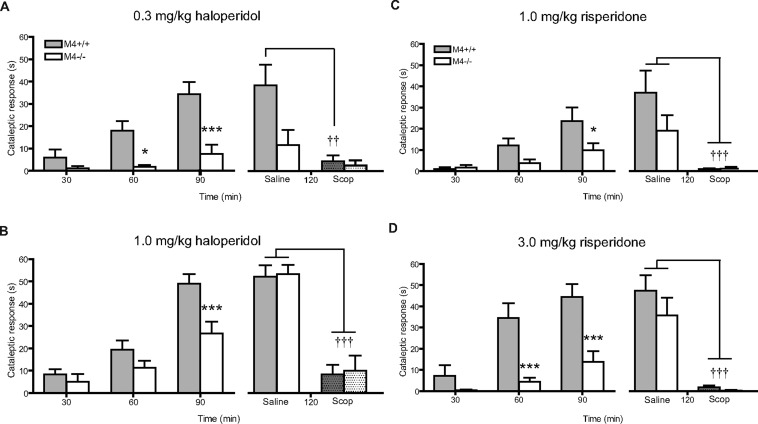
In Parkinson’s disease, the loss of dopamine-containing neurons in the substantia nigra pars compacta disturbs the balance between cholinergic and dopaminergic neurotransmission in the striatum. As discussed above, this balance is essential for proper locomotor control. Initially, Karasawa et al.97 investigated the cataleptic responses induced by haloperidol in M4–/– mice (30 min postinjection). In this study, M4–/– mice displayed a small decrease in cataleptic activity in some tests (not statistically significant). When monitoring cataleptic responses at 60 and 90 min after drug administration (haloperidol and risperidone), we found that the cataleptic response to both drugs was greatly attenuated in M4–/– mice (Figure 10).98 This observation suggested that M4 receptors may play an important role in mediating antipsychotic-induced motor-side effects.98 However, haloperidol and risperidone-induced cataleptic responses were completely abolished by treatment both of WT and M4–/– mice with the nonsubtype-selective muscarinic receptor antagonist scopolamine, indicating that other muscarinic receptor subtypes may also be involved in this activity98 (see however Karasawa et al.97). In agreement with our findings in whole-body M4–/– mice, the cataleptic response to antipsychotics was also attenuated in D1-M4–/– mice,35 suggesting that M4 receptors present on D1 receptor-containing neurons play an important role in mediating drug-induced catalepsy. These data suggest that M4 receptor antagonists may prove useful for the treatment of Parkinson’s disease. However, these findings also raise the possibility that the use of M4 receptor agonists or PAMs for the treatment of schizophrenia may elicit motor side effects. The occurrence of such side effects may be less likely in the case of M4 receptor PAMs, which are only active when M4 receptors are occupied by endogenous acetylcholine.
Figure 10.
Reduced cataleptic effect of antipsychotic drugs in M4–/– mice. Cataleptic responses were measured as the time spent in the placed position (cutoff time: 60 s), at 30, 60, and 90 min after i.p. drug injection. Catalepsy induced by haloperidol (A, B) or risperidone (C, D) was attenuated in M4–/– mice compared to WT mice. (*p < 0.05, ***<0.001). The effect of scopolamine (Scop, 5.0 mg/kg, i.p. after 90 min) was examined 120 min after the initial drug administration. Scopolamine significantly reduced the cataleptic responses in both genotypes (††p < 0.01, †††p < 0.001) [Reprinted with permission from European Journal of Pharmacology.]98
Conclusions
Phenotypic analysis of muscarinic receptor mutant mice has been instrumental in elucidating the physiological roles of the different muscarinic receptor subtypes. In this review, we focused on the M1, M4, and M5 receptors and their potential as drug targets for the treatment of schizophrenia, drug abuse, and Parkinson’s disease. Preclinical data suggest that M1 agonists, M4 agonists and M5 antagonists may prove useful for treating psychostimulant addiction. Moreover, both preclinical and clinical data with xanomeline suggest that M1 and M4 agonists may show clinical efficacy in the treatment of psychosis, including schizophrenia. In addition, animal data indicate that M4 antagonists could be beneficial in the treatment of Parkinson’s disease. Hopefully, muscarinic receptor subtype-selective agonists, PAMs, and antagonists will show efficacy and acceptable side effect profiles in future clinical trials.
The authors declare no competing financial interest.
Author Contributions
⊥ D.D. and M.T. contributed equally to this work.
Funding Statement
National Institutes of Health, United States
References
- Smythies J. (2005) Section I. The cholinergic system. Int. Rev. Neurobiol. 64, 1–122. [DOI] [PubMed] [Google Scholar]
- Wess J.; Eglen R. M.; Gautam D. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discovery 6, 721–733. [DOI] [PubMed] [Google Scholar]
- Langmead C. J.; Watson J.; Reavill C. (2008a) Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 117, 232–243. [DOI] [PubMed] [Google Scholar]
- Davis A. A.; Fritz J. J.; Wess J.; Lah J. J.; Levey A. I. (2010) Deletion of M1 Muscarinic Acetylcholine Receptors Increases Amyloid Pathology In Vitro and In Vivo. J. Neurosci. 30(12), 4190–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G.; Morelli M.; Consolo S. (1994) Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 17, 228–233. [DOI] [PubMed] [Google Scholar]
- Lester D. B.; Rogers T. D.; Blaha C. D. (2010) Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 16, 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T.; Miura M.; Suzuki T.; Nishimura K.; Masuda M. (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr. Gerontol Int. 10(Suppl 1), S148–S157. [DOI] [PubMed] [Google Scholar]
- Fahn S.; Burke R.; Stern Y. (1990) Antimuscarinic drugs in the treatment of movement disorders. Prog. Brain Res. 84, 389–397. [DOI] [PubMed] [Google Scholar]
- Tandon R.; Shipley J. E.; Greden J. F.; Mann N. A.; Eisner W. H.; Goodson J. A. (1991) Muscarinic cholinergic hyperactivity in schizophrenia. Relationship to positive and negative symptoms. Schizophr. Res. 4, 23–30. [DOI] [PubMed] [Google Scholar]
- Perry E. K.; Perry R. H. (1995) Acetylcholine and hallucinations: disease-related compared to drug-induced alterations in human consciousness. Brain Cognit. 28, 240–258. [DOI] [PubMed] [Google Scholar]
- Halpern J. H. (2004) Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol Ther. 102, 131–138. [DOI] [PubMed] [Google Scholar]
- Terry A. V. (2008) Role of central cholinergic system in the therapeutics of schizophrenia. Curr. Neuropharmacol. 6, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. (2004) Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 44, 423–450. [DOI] [PubMed] [Google Scholar]
- Wess J. (1996) Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 10, 69–99. [DOI] [PubMed] [Google Scholar]
- Caulfield M. P.; Birdsall N. J. M. (1998) Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 50, 279–290. [PubMed] [Google Scholar]
- Caulfield M. D. (1993) Muscarinic receptors–characterization, coupling and function. Pharmacol. Ther. 58, 319–379. [DOI] [PubMed] [Google Scholar]
- Levey A. I. (1993) Immunological localization of M1-M5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 52, 441–448. [DOI] [PubMed] [Google Scholar]
- Vilaro M. T.; Mengod G.; Palacios J. M. (1993) Advances and limitations of the molecular neuroanatomy of cholinergic receptors: the example of multiple muscarinic receptors. Prog. Brain Res. 98, 95–101. [DOI] [PubMed] [Google Scholar]
- Wei J.; Walton A. E.; Milici A.; Buccafusco J. J. (1994) M1-M5 muscarinic receptor distribution in rats CNS by RT-PCR and HPLC. J. Neurochem. 63, 815–821. [DOI] [PubMed] [Google Scholar]
- Weiner D. M.; Levey A. I.; Brann M. R. (1990) Expression of muscarinic acetylcholine and dopamine receptor messenger-RNAs in rat basal ganglia. Proc. Natl. Acad. Sci. U.S.A. 87, 7050–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V.; Normand E.; Bloch B. (1992) Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J. Neurosci. 12, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch S. M.; Gutekunst C. A.; Rees H. D.; Heilman C. J.; Levey A. I. (1994) Distribution of M1-M4 muscarinic receptor proteins in the rat striatum - light and electron-microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 14, 3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch S. M.; Levey A. I. (1995) Diverse pre- and post-synaptic expression of m1-m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 56, 931–938. [DOI] [PubMed] [Google Scholar]
- Smiley J. F.; Levey A. I.; Mesulam M. M. (1999) M2 muscarinic receptor immunolocalization in cholinergic cells of the monkey basal forebrain and striatum. Neuroscience 90, 803–814. [DOI] [PubMed] [Google Scholar]
- Yasuda R. P.; Ciesla W.; Flores L. R.; Wall S. J.; Li M.; Satkus S. A.; Weisstein J. S.; Spagnola B. V.; Wolfe B. B. (1993) Development of antisera selective for M4 and M5 muscarinic cholinergic receptors -distribution of M4 and M5 receptors in rat-brain. Mol. Pharmacol. 43, 149–157. [PubMed] [Google Scholar]
- Levey A. I.; Edmunds S. M.; Koliatsos V.; Wiley R. G.; Heilman C. J. (1995) Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J. Neurosci. 15, 4077–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L.; Platt B.; Riedel G. (2011) Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 221, 443–465. [DOI] [PubMed] [Google Scholar]
- Yan Z.; Flores-Hernandez J.; Surmeier D. J. (2001) Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 103, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Basile A. S.; Gomeza J.; Volpicelli L. A.; Levey A. I.; Wess J. (2002) Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J. Neurosci. 22, 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E.; Ciliax B. J.; Levey A. I. (1997) Differential expression of D1 and D2 dopamine and M4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synaps.e 27, 357–366. [DOI] [PubMed] [Google Scholar]
- Onali P.; Olianas M. C. (2002) Muscarinic M4 receptor inhibition of dopamine D1-like receptor signaling in rat nucleus accumbens. Eur. J. Pharmacol. 448, 105–111. [DOI] [PubMed] [Google Scholar]
- Vilaro M. T.; Palacios J. M.; Mengod G. (1990) Localization of M5 muscarinic receptor mRNA in rat brain examined by in situ hybridisation histochemistry. Neurosci. Lett. 114, 154–159. [DOI] [PubMed] [Google Scholar]
- Yamada M.; Lamping K. G.; Duttaroy A.; Zhang W.; Cui Y.; Bymaster F. P.; McKinzie D. L.; Felder C. C.; Deng C. X.; Faraci F. M.; Wess J. (2001) Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 98, 14096–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R.; Noquchi T.; Yuhki M.; Kitamura N.; Hiquchi M.; Saido T. C.; Seki K.; Itohara S.; Kawano M.; Tanemura K.; Takashima A.; Yamada K.; Kondoh Y.; Kanno I.; Wess J.; Yamada M. (2006) Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol. Dis. 24, 334–344. [DOI] [PubMed] [Google Scholar]
- Jeon J.; Dencker D.; Wörtwein G.; Woldbye D. P. D.; Cui Y.; Davis A. A.; Levey A. I.; Schuütz G.; Sager T. N.; Mørk A.; Li C.; Deng C.; Fink-Jensen A.; Wess J. (2010) A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulation dopamine-dependent behaviors. J. Neurosci. 30, 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P. J.; Christopoulos A.; Lindsley C. W. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discovery 8, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubik J.; Bacakova L.; El-Fakahany E. E.; Tucek S. (1997) Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol. Pharmacol. 52, 172–179. [DOI] [PubMed] [Google Scholar]
- Lanzafame A.; Christopoulos A. (2004) A investigation of the interaction if a putative allosteric modulator, N-(2,3-diphenyl-1,2,4-thiadiazole-5-(2H)-ylidene) methanamine hydrobromide (SCH-202676), with M1 muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 308, 830–837. [DOI] [PubMed] [Google Scholar]
- Lazareno S.; Dolezal V.; Popham A.; Birdsall N. J. (2004) Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol. Pharmacol. 65, 257–266. [DOI] [PubMed] [Google Scholar]
- Shirey J. K.; Xiang Z.; Orton D.; Brady A. E.; Johnson K. A.; Williams R.; Ayala J. E.; Rodriguez A. L.; Wess J.; Weaver D.; Niswender C. M.; Conn J. P. (2008) An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat. Chem. Biol. 4, 42–50. [DOI] [PubMed] [Google Scholar]
- Bridges T. M.; LeBois E. P.; Hopkins C. R.; Wood M. R.; Jones C. K.; Conn J. P.; Lindsley C. W. (2010) The antipsychotic potential of muscarinic allosteric modulation. Drug News Perspect. 23, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. Y.; McKinzie D. L.; Bose S.; Mitchell S. N.; Witkin J. M.; Thompson R. C.; Christopoulos A.; Lazareno S.; Birdsall N. J.; Bymaster F. P.; Felder C. C. (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 105, 10978–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. K.; Brady A. E.; Davis A. A.; Xiang Z.; Bubser M.; Tantawy M. N.; Kane A. S.; Bridges T. M.; Kennedy J. P.; Bradley S. R.; Peterson T. E.; Ansari M. S.; Baldwin R. M.; Kessler R. M.; Deutch A. Y.; Lah J. J.; Levey A. I.; Lindsley C. W.; Conn P. J. (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 28, 10422–10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois E. P.; Bridges T. M.; Lewis L. M.; Dawson E. S.; Kane A. S.; Xiang Z.; Jadhav S. B.; Yin H.; Kennedy J. P.; Meiler J.; Niswender C. M.; Jones C. K.; Conn P. J.; Weaver C. D.; Lindsley C. W. (2010) Discovery and charaterization of novel subtype-selective allosteric agonists for the investigation of M1 receptor function in the central nervous system. ACS Chem. Neurosci. 1, 104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.; Seager M. A.; Wittmann M.; Jacobson M.; Bickel D.; Burno M.; Jones K.; Graufelds V. K.; Xu G.; Pearson M.; McCampbell A.; Gaspar R.; Shughrue P.; Danziger A.; Regan C.; Flick R.; Pascarella D.; Garson S.; Doran S.; Kreatsoulas C.; Veng L.; Lindsley C. W.; Shipe W.; Kuduk S.; Sur C.; Kinney G.; Seabrook G. R.; Ray W. J. (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl. Acad. Sci. U.S.A. 106, 15950–15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlo J. E.; Niswender C. M.; Days E. L.; Bridges T. M.; Xiang Y.; Rodriguez A. L.; Shirey J. K.; Brady A. E.; Nalywajko T.; Luo Q.; Austin C. A.; Williams M. B.; Kim K.; Williams R.; Orton D.; Brown H. A.; Lindsley C. W.; Weaver C. D.; Conn P. J. (2009) Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol. 75, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. R.; Bridges T. M.; Sheffler D. J.; Cho H. P.; Lewis L. M.; Days E.; Daniels J. S.; Jones C. K.; Niswender C. M.; Weaver C. D.; Conn P. J.; Lindsley C. W.; Wood M. R. (2011) Discovery and optimization of a novel, selective and brain penetrant M1 positive allosteric modulator (PAM): the development of ML169, an MLPCN probe. Bioorg. Med. Chem. Lett. 21, 2697–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. V.; Shipe W. D.; Bunda J. L.; Nolt M. B.; Wisnoski D. D.; Zhao Z.; Barrow J. C.; Ray W. J.; Ma L.; Wittmann M.; Seager M. A.; Koeplinger K. A.; Hartman G. D.; Lindsley C. W. (2010) Parallel synthesis of N-biaryl quinolone carboxylic acids as selective M1 positive allosteric modulators. Bioorg. Med. Chem. Lett. 20(2), 531–536. [DOI] [PubMed] [Google Scholar]
- Thomsen M.; Conn P. J.; Lindsley C.; Wess J.; Boon J. Y.; Fulton B. S.; Fink-Jensen A.; Caine S. B. (2010) Attenuation of cocaine’s reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J. Pharmacol. Exp. Ther. 332, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M., Lindsley C. W., Conn P. J., Wessell J. E., Fulton B. S., Wess J., and Caine S. B. (2011) Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology published online Oct 5, 2011. DOI : 10.1007/s00213-011-2516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. E.; Jones C. K.; Bridges T. M.; Kennedy J. P.; Thompson A. D.; Heiman J. U.; Breininger M. L.; Gentry P. R.; Yin H.; Jadhav S. B.; Shirey J. K.; Conn P. J. (2008) Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine induced hyperlocomotor activity in rats. J. Pharmacol. Exp. Ther. 327, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. P.; Bridges T. M.; Gentry P. R.; Brogan J. T.; Kane A. S.; Jones C. K.; Brady A. E.; Shirey J. K.; Conn P. J.; Lindsley C. W. (2009) Synthesis and structure-activity relationships of allosteric potentiators of the m(4) muscarinic acetylcholine receptor. ChemMedChem 4, 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.; Roth B. L. (2010) Allosteric antipsychotics: M4 muscarinic potentiators as novel treatments for schizophrenia. Neuropsychopharmacology 35, 851–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; Marlo J. E.; Niswender C. M.; Jones C. K.; Jadhav S. B.; Gentry P. R.; Plumley H. C.; Weaver C. D.; Conn P. J.; Lindsley C. W. (2009) Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J. Med. Chem. 52(11), 3445–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. M.; Kennedy J. P.; Cho H. P.; Breininger M. L.; Gentry P. R.; Hopkins C. R.; Conn P. J.; Lindsley C. W. (2010) Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M5 PAM. Bioorg. Med. Chem. Lett. 15;20(2), 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler T. J.; Bymaster F. P.; Tandon R.; Copolov D.; Dean B. (2007) Towards a muscarinic hypothesis of schizophrenia. Mol. Psychiatry 12, 232–246. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K.; Katsifis A.; Mattner F.; Huang X. F. (2004) Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology 29(3), 619–625. [DOI] [PubMed] [Google Scholar]
- Deng C.; Huang X. F. (2005) Decreased density of muscarinic receptors in the superior temporal gyrusin schizophrenia. J. Neurosci. Res. 81(6), 883–890. [DOI] [PubMed] [Google Scholar]
- Scarr E.; Sundram S.; Keriakous D.; Dean B. (2007) Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biol. Psychiatry 61(10), 1161–1170. [DOI] [PubMed] [Google Scholar]
- Dean B.; Crook J. M.; Opeskin K.; Hill C.; Keks N.; Copolov D. L. (1996) The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol. Psychiatry 1(1), 54–58. [PubMed] [Google Scholar]
- Dean B.; McLeod M.; Keriakous D.; McKenzie J.; Scarr E. (2002) Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 7(10), 1083–1091. [DOI] [PubMed] [Google Scholar]
- Crook J. M.; Dean B.; Pavey G.; Copolov D. (1999) The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci. 64(19), 1761–1771. [DOI] [PubMed] [Google Scholar]
- Crook J. M.; Tomaskovic-Crook E.; Copolov D. L.; Dean B. (2000) Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol. Psychiatry 48(5), 381–388. [DOI] [PubMed] [Google Scholar]
- Crook J. M.; Tomaskovic-Crook E.; Copolov D. L.; Dean B. (2001) Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am. J. Psychiatry 158(6), 918–925. [DOI] [PubMed] [Google Scholar]
- Salah-Uddin H.; Scarr E.; Pavey G.; Harris K.; Hagan J. J.; Dean B.; Challiss R. A.; Watson J. M. (2009) Altered M1 muscarinic acetylcholine receptor (CHRM1)-Galphaq/11 coupling in a schizophrenia endophenotype. Neuropsychopharmacology 34(9), 2156–2166. [DOI] [PubMed] [Google Scholar]
- Borda T.; Perez Rivera R.; Joensen L.; Gomez R. M.; Sterin-Borda L. (2002) Antibodies against cerebral M1 cholinergic muscarinic receptor from schizophrenic patients: molecular interaction. J. Immunol. 168(7), 3667–3674. [DOI] [PubMed] [Google Scholar]
- Ganzinelli S.; Borda T.; Sterin-Borda L. (2006) Regulation of m1 muscarinic receptors and nNOS mRNA levels by autoantibodies from schizophrenic patients. Neuropharmacology 50(3), 362–371. [DOI] [PubMed] [Google Scholar]
- Bymaster F. P.; Carter P. A.; Peters S. C.; Zhang W.; Ward J. S.; Mitch C. H.; Calligaro D. O.; Whitesitt C. A.; DeLapp N.; Shannon H. E.; Rimvall K.; Jeppesen L.; Sheardown M. J.; Fink-Jensen A.; Sauerberg P. (1998) Xanomeline compared to other muscarinic agents on stimulation of phosphoinositide hydrolysis in vivo and other cholinomimetic effects. Brain Res. 795(1–2), 179–190. [DOI] [PubMed] [Google Scholar]
- Bymaster F. P.; Shannon H. E.; Rasmussen K.; Delapp N. W.; Mitch C. H.; Ward J. S.; Calligaro D. O.; Ludvigsen T. S.; Sheardown M. J.; Olesen P. H.; Swedberg M. D.; Sauerberg P.; Fink-Jensen A. (1998) Unexpected antipsychotic-like activity with the muscarinic receptor ligand (5R,6R)6-(3-propylthio-1,2,5-thiadiazol- 4-yl)-1-azabicyclo[3.2.1]octane. Eur. J. Pharmacol. 356, 109–119. [DOI] [PubMed] [Google Scholar]
- Shannon H. E.; Rasmussen K.; Bymaster F. P.; Hart J. C.; Peters S. C.; Swedberg M. D. B.; Jeppesen L.; Sheardown M. J.; Sauerberg P.; Fink-Jensen A. (2000) Xanomeline, an M1/M4 preferring muscarinic cholinergic receptor agonist, produces antipsychotic-like activity in rats and mice. Schizophr. Res. 42, 249–259. [DOI] [PubMed] [Google Scholar]
- Stanhope K. J.; Mirza N. R.; Bickerdike M. J.; Bright J. L.; Harrington N. R.; Hesselink M. B.; Kennett G. A.; Lightowler S.; Sheardown M. J.; Syed R.; Upton R. L.; Wadsworth G.; Weiss S. M.; Wyatt A. (2001) The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J. Pharmacol. Exp. Ther. 299, 782–789. [PubMed] [Google Scholar]
- Andersen M. B.; Fink-Jensen A.; Peacock L.; Gerlach J.; Bymaster F.; Lundbaek J. A.; Werge T. (2003) The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in Cebus apella monkeys. Neuropsychopharmacology 28(6), 1168–1175. [DOI] [PubMed] [Google Scholar]
- Jones C. K.; Eberle E. L.; Shaw D. B.; McKinzie D. L.; Shannon H. E. (2005) Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. J. Pharmacol. Exp. Ther. 312(3), 1055–1063. [DOI] [PubMed] [Google Scholar]
- Bymaster F. P.; Felder C.; Ahmed S.; McKinzie D. (2002) Muscarinic receptors as a target for drugs treating schizophrenia. Curr. Drug Targets 1, 163–181. [DOI] [PubMed] [Google Scholar]
- Bodick N. C.; Offen W. W.; Levey A. I.; Cutler N. R.; Gauthier S. G.; Satlin A.; Shannon H. E.; Tollefson G. D.; Rasmussen K.; Bymaster F. P.; Hurley D. J.; Potter W. Z.; Paul S. M. (1997) Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in alzheimer disease. Arch Neurol. 54, 465–473. [DOI] [PubMed] [Google Scholar]
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann J.; Dubé S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 165, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Woolley M. L.; Carter H. J.; Gartlon J. E.; Watson J. M.; Dawson L. A. (2009) Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur. J. Pharmacol. 603, 147–149. [DOI] [PubMed] [Google Scholar]
- Gerber D. J.; Sotnikova T. D.; Gainetdinov R. R.; Huang S. Y.; Caron M. G.; Tonegawa S. (2001) Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 98(26), 15312–15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T.; Yamada M.; Duttaroy A.; Wess J. (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 21(14), 5239–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras S. G.; Murphy G. G.; Hamilton S. E.; Mitchell S. L.; Rahnama N. P.; Nathanson N. M.; Silva A. J. (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 6(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Felder C. C.; Porter A. C.; Skillman T. L.; Zhang L.; Bymaster F. P.; Nathanson N. M.; Hamilton S. E.; Gomeza. J.; Wess J.; McKinzie D. L. (2001) Elucidating the role of muscarinic receptors in psychosis. Life Sci. 68, 2605–2613. [DOI] [PubMed] [Google Scholar]
- Gomeza J.; Zhang L.; Kostenis E.; Felder C.; Bymaster F.; Brodkin J.; Shannon H.; Xia B.; Deng C. X.; Wess J. (1999) Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 10483–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara E. T.; Bymaster F. P.; Davis R. J.; Wade M. R.; Perry K. W.; Wess J.; McKinzie D. L.; Felder C.; Nomikos G. G. (2004) M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 18, 1410–1412. [DOI] [PubMed] [Google Scholar]
- Schmidt L. S.; Thomsen M.; Weikop P.; Dencker D.; Wess J.; Woldbye D. P. D.; Wörtwein G.; Fink-Jensen A. (2011) Increased self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology 216, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D.; Wörtwein G.; Weikop P.; Jeon J.; Thomsen M.; Sager T. N.; Mørk A.; Woldbye D. P. D.; Wess J.; Fink-Jensen A. (2011) Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J. Neurosci. 31(16), 5905–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan K. A.; Dykstra L. A. (2007) Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berlin). 191(4), 985–993. [DOI] [PubMed] [Google Scholar]
- Koob G. F.; Sanna P. P.; Bloom F. E. (1998) Neuroscience of addiction. Neuron 21, 467–476. [DOI] [PubMed] [Google Scholar]
- Forster G. L.; Blaha C. D. (2000) Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 12(10), 3596–3604. [DOI] [PubMed] [Google Scholar]
- Forster G. L.; Blaha C. D. (2003) Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur. J. Neurosci. 17(4), 751–762. [DOI] [PubMed] [Google Scholar]
- Forster G. L.; Yeomans J. S.; Takeuchi J.; Blaha C. D. (2002) M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J. Neurosci. 22(1), RC190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A. S.; Fedorova I.; Zapata A.; Liu X.; Shippenberg T.; Duttaroy A.; Yamada M.; Wess J. (2002) Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc. Natl. Acad. Sci. U.S.A. 99(17), 11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink-Jensen A; Fedorova I; Wörtwein G; Woldbye D. P.; Rasmussen T; Thomsen M; Bolwig T. G.; Knitowski K. M.; McKinzie D. L.; Yamada M; Wess J; Basile A (2003) Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J. Neurosci. Res. 74(1), 91–96. [DOI] [PubMed] [Google Scholar]
- Thomsen M.; Woldbye D. P.; Wörtwein G.; Fink-Jensen A.; Wess J.; Caine S. B. (2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J. Neurosci. 25, 8141–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M.; Wörtwein G.; Fink-Jensen A.; Woldbye D. P.; Wess J.; Caine S. B. (2007) Decreased prepulse inhibition and increased sensitivity to muscarinic, but not dopaminergic drugs in M5 muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berlin) 192(1), 97–110. [DOI] [PubMed] [Google Scholar]
- Schmidt L. S.; Miller A. D.; Lester D. B.; Bay-Richter C.; Schülein C.; Frikke-Schmidt H.; Wess J.; Blaha C. D.; Woldbye D. P.; Fink-Jensen A.; Wortwein G. (2010) Increased amphetamine-induced locomotor activity, sensitization, and accumbal dopamine release in M5 muscarinic receptor knockout mice. Psychopharmacology (Berl) 207(4), 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Ng K.; Hayes D.; Gao X.; Forster G.; Blaha C.; Yeomans J. (2004) Decreased amphetamine-induced locomotion and improved latent inhibition in mice mutant for the M5 muscarinic receptor gene found in the human 15q schizophrenia region. Neuropsychopharmacology 29(12), 2126–2139. [DOI] [PubMed] [Google Scholar]
- Karasawa H.; Taketo M. M.; Matsui M. (2003) Loss of anti-cataleptic effect of scopolamine in mice lacking muscarinic acetylcholine receptor subtype 4. Eur. J. Pharmacol. 468, 15–19. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A.; Schmidt L. S.; Dencker D.; Schülein C.; Wess J.; Wörtwein G.; Woldbye D. P. D. (2011) Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic receptor. Eur. J. Pharmacol. 656, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]