Abstract
Background
Live-attenuated influenza vaccine (LAIV) prevents more cases of influenza in immune-competent children than the trivalent inactivated vaccine (TIV). We compared the antibody responses to LAIV or TIV in HIV-infected children.
Methods
Blood and saliva obtained at enrollment, 4 and 24 weeks post-immunization (wpi) from 243 HIV-infected children randomly assigned to TIV or LAIV were analyzed.
Results
Both vaccines increased the anti-influenza neutralizing antibodies at 4 and 24 wpi. At 4 wpi, TIV recipients had 2- to 3-fold higher neutralizing antibody titers than LAIV recipients, but the proportions of subjects with protective titers (≥1:40) were similar between treatment groups (96% to 100% for influenza A and 81% to 88% for influenza B). Both vaccines increased salivary homotypic IgG antibodies, but not IgA antibodies. Both vaccines also increased serum heterosubtypic antibodies. Among HIV-specific characteristics, the baseline viral load correlated best with the antibody responses to either vaccine. We used LAIV-virus shedding as a surrogate of influenza infection. Influenza-specific humoral and mucosal antibody levels were significantly higher in non-shedders than in shedders.
Conclusions
LAIV and TIV generated homotypic and heterosubtypic humoral and mucosal antibody responses in HIV-infected children. High titers of humoral or mucosal antibodies correlated with protection against viral shedding.
Keywords: HIV-infected children, influenza vaccines, antibodies, viral shedding
Introduction
Yearly immunization of HIV-infected individuals with the inactivated trivalent influenza vaccine (TIV) is recommended1, and is efficacious in these patients 2, 3. Because antibody responses to TIV are not always adequate in this population4–6, additional immunization of household contacts is strongly encouraged to further protect HIV-infected patients against influenza. A live attenuated influenza vaccine (LAIV) is licensed in the US for immunization of healthy individuals. This vaccine is more effective than TIV in healthy children7–10; confers protection against infection with mismatched strains of influenza11, 12; and may provide a longer duration of protection than TIV13, 14. Furthermore, >50% of children <5 years of age immunized for the first time with LAIV achieve protective HAI titers ≥1:40 after the first dose of vaccine15.
We demonstrated that LAIV was safe and immunogenic in previously immunized HIV-infected children16. Shedding of the vaccine strain influenza, which occurred in 23% of HIV-infected children, was limited to the first week after vaccination in accordance with the pattern observed in children without HIV infection17. Antibody responses, measured by hemagglutination inhibition (HAI), tended to be lower in LAIV compared with TIV recipients, which also was observed in uninfected children. However, protection conferred against influenza infection by LAIV, which is known not to correlate with HAI antibody titers, is presumed to depend on other immune mechanisms18–24. An immune correlate of protection conferred by LAIV is highly desirable and is being actively pursued.
The goal of this study was to further characterize antibody responses to LAIV and TIV of HIV-infected children, including neutralizing antibodies, mucosal antibodies measured in saliva, and heterosubtypic HAI antibodies, which have not previously been described in this population. We also investigated for the first time the correlation of serum neutralizing antibodies and of mucosal IgG and IgA antibodies with shedding of LAIV viruses.
Methods
Study Population and Design
HIV-infected children and adolescents 5 to 18 years of age were randomly assigned in late September to late November of 2004 to receive LAIV (Arm A) or TIV (Arm B). Inclusion criteria were stable combination antiretroviral therapy for ≥16 weeks prior to immunization; plasma viral load <60,000 copies/ml; CD4 ≥15% within 60 days of enrollment; and immunization with TIV at least once in the 2 years preceding the study. Potential subjects were excluded if they received immunomodulatory therapy within 60 days prior to enrollment, inactivated or live vaccines within 14 and 30 days, respectively, of enrollment, and if they had any of the contraindications listed in the package insert of either vaccine. In each study arm the vaccinees were stratified by the following nadir CD4% (lowest CD4% prior to study entry and/or initiation of HAART) criteria: Group 1 <15%; Group 2 ≥ 15% and < 25%; and Group 3 ≥ 25%.
On study day 0, Arm A (LAIV) received the frozen formulation of Influenza Virus Vaccine Live, Intranasal, (FluMist®; MedImmune) 0.5 mL (0.25 mL per nostril); Arm B (TIV) received Influenza Viral Vaccine, Intramuscular, (Fluzone®; Aventis Pasteur, Inc.) 0.5 mL in the deltoid muscle. The strains comprising the vaccines were those recommended by the U.S. Public Health Service (USPHS) for the 2004/2005 season: A/New Caledonia/20/99 (H1N1); A/Wyoming/3/2003 (H3N2) [an A/Fujian/411/2002-like virus]; and B/Jilin/20/2003 (LAIV) or B/Jiangsu/10/2003 (TIV); both are B/Shanghai/361/2002-like viruses.
Subjects had blood and saliva collected for antibody measurements at study day 0, and weeks 4 and 24. Saliva was collected using OraSure kits as per manufacturer’s instructions. LAIV recipients also had viral shedding from the nares monitored on days 3, 14 and 28 using viral culture and polymerase chain reaction, as previously described16.
Influenza-specific HAI assay (University of Colorado)
Serum samples were treated with receptor-destroying enzyme (RDE) from Vibrio cholerae (Denka-Seiken). These were diluted 1:10 in saline and subsequent serial 2-fold dilutions of the sera were used in a standard HAI assay using 4 hemagglutinating units of the viruses or antigen and 0.75% guinea pig red blood cells. Serum samples with titers ≥10 and ≥40 were considered indicative of immune responses and protection, respectively. The antigens used in the assays were: A/New Caledonia/20/99 (H1N1), A/Wyoming/03/2003 (H3N2), A/Sydney/05/97 (H3N2) and B/Yamanashi/166/98 cold-adapted viruses, and B/Yamanashi/166/98 (Shanghai-like) antigen generously provided by Dr. Alexander Klimov at the Centers for Disease Control and Prevention.
Influenza-specific neutralizing antibody assay (MedImmune)
An influenza microneutralization (MN) assay was used to determine specific influenza-neutralizing antibody titers in serum. Serial dilutions of RDE-treated serum were made in duplicate in 96-well plates and influenza virus at pre-established concentration was added to the serum-containing wells. Following incubation for 1 hour at 33°C, the serum-virus mixtures were inoculated onto washed confluent MDCK monolayers containing 2 × 105 cells/well in 96-well plates, and incubated for 6 days at 33°C. Following incubation, culture medium was removed, replaced with Alamar Blue (10% v/v in phosphate buffered saline), and the plates incubated at 33°C for a further 5 hours, following which the absorbance in individual wells was read (531 nm excitatory wavelength and 590 nm emission wavelength) on a Perkin-Elmer Multi Label Counter. Absence of infection and associated cytopathic effect (CPE) was indicated by the ability of intact cells to take up and metabolize Alamar Blue with positive absorbance in the corresponding wells. Conversely, infection of cells and consequent CPE resulted in an inability to metabolize Alamar Blue and absence of absorbance. The neutralizing titer was defined as the reciprocal of the serum dilution in the last well showing evidence of Alamar Blue metabolism. A 4-fold or greater difference in titer between two sera was considered significant.
Influenza-specific salivary antibodies
Influenza strain-specific IgG and IgA were assessed by kinetic ELISA. Microtiter plates (Costar) were coated with Fluzone® at 1.56 ng/mL in carbonate buffer overnight at 4°C. After washing and blocking, serial dilutions of saliva in boric acid solution were added to triplicate wells. Plates were washed, incubated with biotinylated goat anti-human IgG or IgA (Biosource International), followed by streptavidin-peroxidase (Sigma) and TMB substrate. The absorbance of the test wells, measured with a Microplate Reader (Molecular Devices) was interpolated onto a standard curve using a pretitered control saliva sample. Results were divided by the total amount of IgG or secretory IgA in the saliva sample measured by ELISA (ALPCO Diagnostics). Results are expressed as influenza-specific ELISA units/IgG or IgA concentration in saliva.
Statistical Analysis
Paired t-tests or Wilcoxon matched pairs signed ranks tests were used to compare baseline with post-immunization responses. Comparisons across groups that differed by treatment, HIV-specific characteristics or viral shedding, used F tests, Wilcoxon rank sum tests or Fisher’s exact tests. For some of the analyses the data were log10 transformed to normalize their distributions. For data which included values of 0, a very small value (0.001) was added to enable log transformation. Correlation analyses consisted of Spearman or Pearson correlations and linear or logistic regressions with quantitative data for all predictors, except for plasma viral load, which was dichotomized as <400 or ≥400 copies/ml. Statistical significance was defined as an alpha level of p≤0.05.
Results
Demographic Characteristics of the Study Population
The study randomly assigned 243 HIV-infected children to LAIV or TIV treatment arms. The demographic characteristics were similar in the two arms (Table 1). The mean age at enrollment was 12 years. More than 70% of the participants had plasma HIV RNA concentrations (viral load) < 400 copies/ml and CD4% >25 at baseline. However, nadir CD4%, prior to study entry, varied between 0.4 and 41% with a median of 17%. All children were on treatment containing ≥1 nucleoside reverse transcriptase inhibitors. In addition, 22% of the antiretroviral regimens included both non-nucleotide reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI), 60% only PI and 17% only NNRTI.
Table 1.
Demographic characteristics of the study population
| Parameters | LAIV1 | TIV1 |
|---|---|---|
| Number | 122 | 121 |
| Male | 65 (53%) | 64 (53%) |
| Female | 57 (47%) | 57 (47%) |
| White | 20 (16%) | 11 (9%) |
| Black | 72 (59%) | 82 (68%) |
| Hispanic | 27 (22%) | 26 (21%) |
| Age mean (min; 25%; 75%; max)2 | 11.4 (5; 9; 14; 18) | 11.9 (6; 10; 14; 18) |
| Entry CD4% Median (range) | 34.0 (15.0–51.0) | 34.0 (16.0–52.0) |
| Nadir CD4% Median (range) | 18.0 (1.0–41.0) | 17.0 (0.4–41.0) |
| Log10 Viral Load Mean (STD) | 2.7 (0.7) | 2.7 (0.7) |
| N (%) with Viral Load<400copies/mL | 87 (71) | 86 (71) |
| CD8% Median (range) | 32.0 (12.0–65.0) | 34.0 (15.0–58.0) |
| CD19% Median (range) | 19.0 (4.0–43.0) | 18.0 (5.0–47.0) |
LAIV = live attenuated influenza vaccine; TIV = trivalent influenza vaccine (inactivated)
min = minimum; max=maximum
MN Responses to LAIV and TIV in HIV-Infected Children on HAART
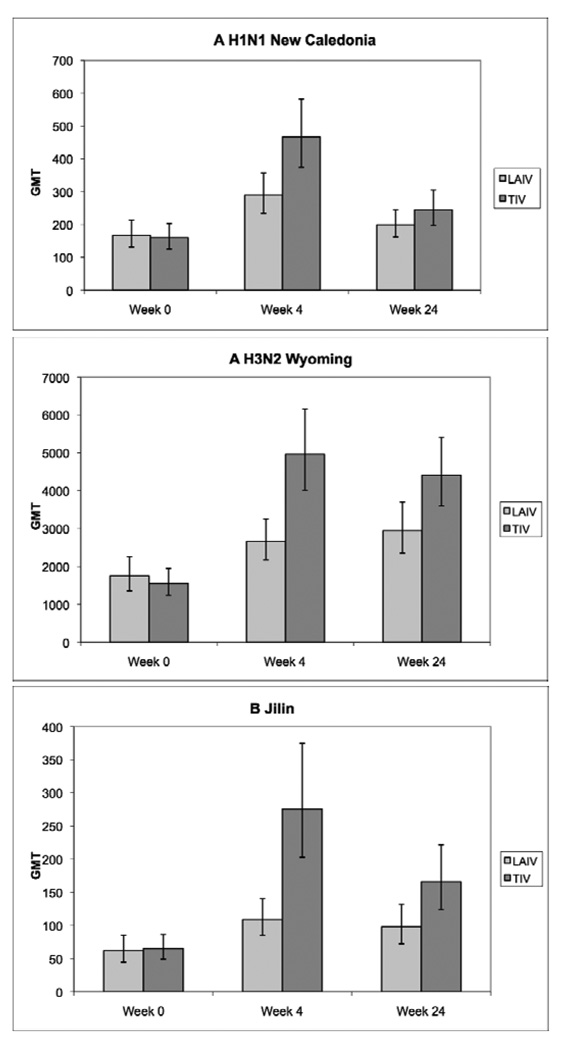
The baseline MN titers for A H1N1 New Caledonia, A H3N2 Wyoming and B Jilin were similar in the two treatment arms (Fig 1). At 4 and 24 weeks after immunization, both TIV and LAIV recipients showed significant increases in MN titers against all strains in the vaccines (p≤0.02), but the week 4 MN titers were higher among TIV compared with LAIV recipients (p≤0.002).
Figure 1.
Neutralizing antibody (MN) responses to LAIV and TIV in HIV-infected children on HAART. Data were derived from 234 HIV-infected children randomly assigned to receive LAIV or TIV during the 2004–2005 influenza season. Bars represent geometric mean titers (GMT) and 95% CI. Baseline values were similar in both treatment groups. MN titers were significantly higher in TIV compared with LAIV recipients at weeks 4 and 24 for all strains except for A H1N1 New Caledonia at week 24 (F test).
An MN titer ≥1:40 has been associated with ≥50% reduction in influenza infection and morbidity in children25. Table 2 shows that both treatment groups had similar proportions of subjects with MN titers ≥1:40 at baseline, week 4 and week 24 for each of the 3 strains in the vaccines.
Table 2.
HIV-infected children on HAART with protective anti-influenza MN titers (≥40) before and after vaccination with TIV or LAIV.
| Viral strains | Week | LAIV | TIV | p-value* |
|---|---|---|---|---|
| A H1N1 New Caledonia | 0 | 88% (103/117) | 86% (100/116) | 0.70 |
| 4 | 96% (109/114) | 100% (109/109) | 0.06 | |
| 24 | 96% (106/110) | 97% (111/114) | 0.72 | |
| A H3N2 Wyoming | 0 | 100% (114/114) | 99% (113/114) | 1.00 |
| 4 | 100% (104/104) | 100% (104/104) | N.A. | |
| 24 | 100% (100/100) | 100% (107/107) | N.A. | |
| B Jilin | 0 | 66% (75/113) | 72% (84/117) | 0.39 |
| 4 | 81% (86/106) | 88% (95/108) | 0.19 | |
| 24 | 80% (82/103) | 84% (97/115) | 0.38 |
Fisher’s exact test
Analyses to identify correlates of the magnitude of the MN response to TIV and LAIV in HIV-infected children on HAART (Table 3) showed the strongest correlation with baseline MN titers (p<0.0001). The multivariate analysis including baseline MN titers, plasma viral load, CD4%, CD8% and CD19%, showed that among HIV-specific immunologic and virologic parameters, only the plasma viral load at baseline was independently associated with responses to all 3 viruses in TIV recipients and to A H1N1 New Caledonia in LAIV recipients.
Table 3.
Factors associated with the magnitude of influenza neutralizing antibody responses to vaccination in HIV-infected children on HAART.
| Parameter | A H1N1 New Caledonia |
A H3N2 Wyoming |
B Jilin |
|||
|---|---|---|---|---|---|---|
| Rho$ | P value | Rho | P value | Rho | P value | |
| Baseline Titers (N= 175 to 231) | ||||||
| Age | 0.043 | 0.51 | −0.106 | 0.11 | 0.043 | 0.33 |
| Baseline plasma HIV RNA#$ | −0.093 | 0.16 | −0.222 | <0.001 | −0.232 | <0.001 |
| Baseline CD4%#$ | 0.063 | 0.34 | 0.131 | 0.05 | 0.237 | <0.001 |
| Nadir CD4% | −0.025 | 0.70 | 0.128 | 0.05 | 0.107 | 0.10 |
| Baseline CD8%#$ | −0.209 | <0.01 | −0.237 | <0.001 | −0.250 | <0.001 |
| Baseline CD19% | 0.196 | 0.01 | 0.101 | 0.19 | 0.087 | 0.26 |
| Week 4 Titers of LAIV Recipients (N=76 to 114) | ||||||
| Age | −0.008 | 0.94 | −0.211 | 0.04 | −0.084 | 0.39 |
| Baseline plasma HIV RNA* | −0.074 | 0.43 | −0.287 | <0.01 | −0.365 | <0.001 |
| Baseline CD4% | 0.100 | 0.29 | −0.038 | 0.70 | 0.210 | 0.03 |
| Nadir CD4% | −0.033 | 0.73 | 0.060 | 0.55 | 0.176 | 0.07 |
| Baseline CD8%# | −0.212 | 0.02 | −0.170 | 0.08 | −0.287 | <0.01 |
| Baseline CD19% | 0.063 | 0.57 | 0.092 | 0.43 | 0.192 | 0.09 |
| Baseline Neutralizing Titers#* | 0.547 | <0.0001 | 0.686 | <0.0001 | 0.756 | <0.0001 |
| Week 4 Titers of TIV Recipients (N=77 to 109) | ||||||
| Age | −0.069 | 0.48 | −0.237 | 0.02 | −0.068 | 0.49 |
| Baseline plasma HIV RNA#* | −0.318 | <0.001 | −0.217 | 0.03 | −0.377 | <0.0001 |
| Baseline CD4% | 0.073 | 0.45 | 0.157 | 0.11 | 0.246 | 0.01 |
| Nadir CD4% | −0.077 | 0.42 | 0.068 | 0.49 | 0.069 | 0.48 |
| Baseline CD8%# | −0.289 | <0.01 | −0.166 | 0.09 | −0.337 | <0.001 |
| Baseline CD19%# | 0.336 | <0.01 | 0.006 | 0.96 | 0.224 | 0.05 |
| Baseline Neutralizing Titers#* | 0.639 | <0.0001 | 0.642 | <0.0001 | 0.685 | <0.0001 |
Significant in the univariate Pearson correlation analysis for ≥2 influenza strains.
Significant in the multivariate linear regression analysis.
Coefficient of correlation.
Mucosal Antibody Responses to LAIV and TIV in HIV-Infected Children on HAART
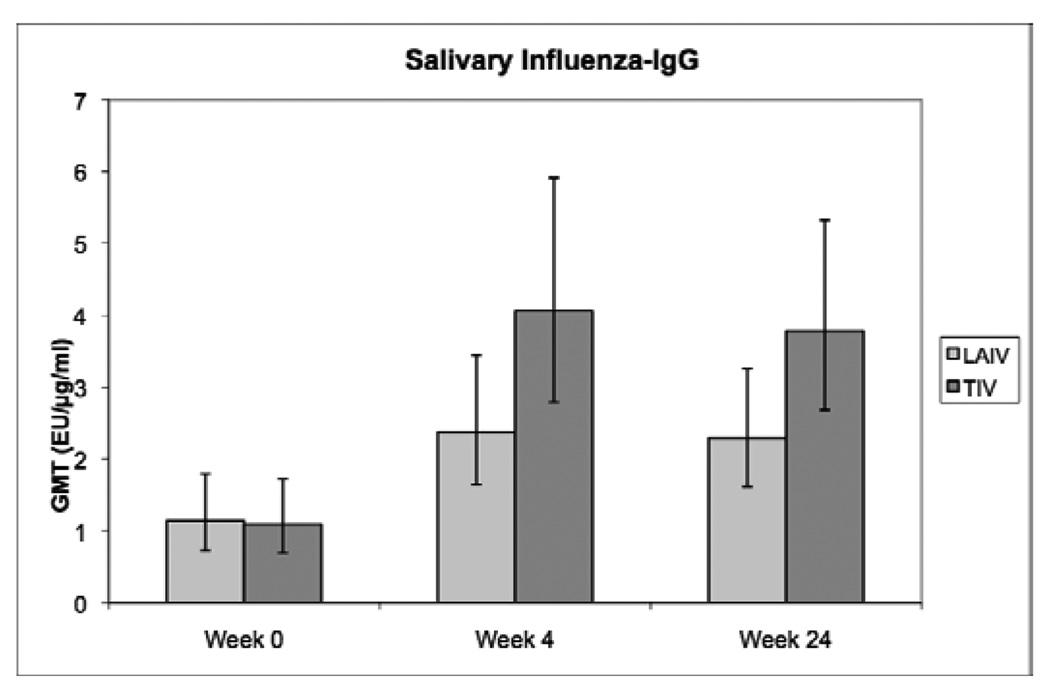
Figure 2 shows the changes in influenza-specific salivary IgG after vaccination. The salivary responses to the vaccines followed the same pattern as the MN responses. In addition, salivary influenza-IgG concentrations were significantly associated with serum antibody titers against influenza A H3N2 Wyoming at all time points (p<0.0001). The multivariate analysis showed that week 4 salivary anti-influenza IgG responses to either vaccine were independently associated with baseline concentrations and with baseline plasma HIV viral load (table 4).
Figure 2.
Influenza-specific salivary IgG antibody responses to LAIV and TIV in HIV-infected children on HAART. Data were derived from 234 HIV-infected children randomly assigned to receive LAIV or TIV during the 2004–2005 influenza season. Bars represent geometric mean titers (GMT) and 95% CI. For the subjects who did not have detectable influenza-IgG in the saliva (N of 19, 7 and 6 at weeks 0, 4 and 24, respectively), an arbitrary value of 0.001 was used for the analysis. Baseline values were similar in both treatment groups. The increase from baseline in the influenza-IgG concentrations was significantly higher in TIV compared with LAIV recipients at week 4 (p=0.05; F test), but not at week 24 (p=0.12; F test).
Table 4.
Factors associated with the magnitude of influenza-specific salivary IgG in HIV-infected children on HAART.
| Parameter | Rho | P value |
|---|---|---|
| Baseline Concentrations (N= 171 to 232) | ||
| Age | −0.038 | 0.56 |
| Baseline plasma HIV RNA#$ | −0.154 | 0.02 |
| Baseline CD4%#$ | 0.229 | <0.001 |
| Nadir CD4%#$ | 0.136 | 0.04 |
| Baseline CD8%#$ | −0.253 | <0.0001 |
| Baseline CD19%#$ | 0.169 | 0.03 |
| Week 4 Concentrations of LAIV Recipients (N=86 to 113) | ||
| Age | −0.075 | 0.43 |
| Baseline plasma HIV RNA#* | −0.420 | <0.0001 |
| Baseline CD4%# | 0.298 | <0.01 |
| Nadir CD4% | −0.017 | 0.85 |
| Baseline CD8%# | −0.414 | <0.0001 |
| Baseline CD19% | 0.192 | 0.08 |
| Baseline Salivary Influenza-IgG Concentrations#* | 0.470 | <0.0001 |
| Week 4 Concentrations of TIV Recipients (N=85 to 111) | ||
| Age | −0.147 | 0.12 |
| Baseline plasma HIV RNA#* | −0.322 | <0.001 |
| Baseline CD4%# | 0.277 | <0.01 |
| Nadir CD4% | 0.039 | 0.69 |
| Baseline CD8%# | −0.343 | <0.001 |
| Baseline CD19% | 0.146 | 0.19 |
| Baseline Salivary Influenza-IgG Concentrations#* | 0.462 | <0.0001 |
Significant in the univariate Pearson correlation analysis.
Significant in the multivariate linear regression analysis.
Influenza-specific salivary IgA were detected only in a minority of study participants. There were no significant increases in salivary influenza-IgA at 4 weeks after LAIV or TIV administration. At 24 weeks after vaccination, salivary influenza-IgA concentrations were significantly higher compared to baseline in TIV recipients (p<0.01), but not in LAIV recipients (p=0.17). Salivary influenza-IgA concentrations were weakly correlated with influenza A H3N2 serum antibody titers and with salivary influenza-IgG concentrations at baseline (rho of 0.184 and 0.146, respectively; p≤0.03 for both). Week 4 salivary influenza-IgA responses to either vaccine were significantly associated with baseline salivary influenza-IgA concentrations. In TIV recipients, week 4 salivary influenza-IgA concentrations also were associated with week 4 serum antibody titers (rho=0.242, p=0.01) and weakly associated with salivary influenza-IgG concentrations (rho=0.166, p=0.09). In either treatment arm, there were no significant associations of salivary influenza-IgA concentrations with baseline plasma viral load, CD4%, CD8% or CD19%.
Immunecorrelates of Protection against Shedding of LAIV-contained viruses
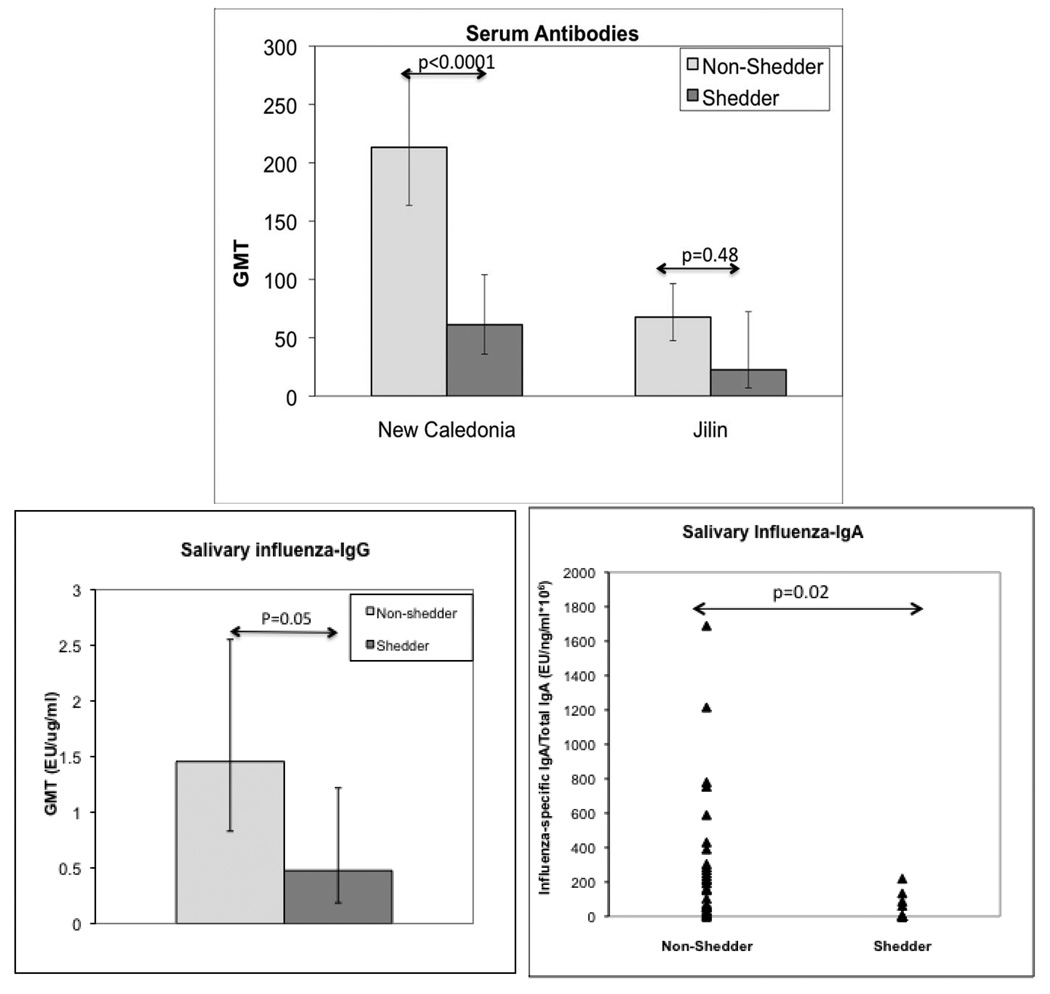
To identify immune defenses associated with protection against influenza infection in HIV-infected children on HAART, we used shedding of the vaccine virus as a surrogate of infection (Fig 4). There were 113 children with baseline antibody and vaccine virus excretion data. Of these, 22 children shed A H1N1 New Caledonia, 9 B Jilin and 3 A H3N2 Wyoming vaccine viruses during the first week after immunization. Children who shed A H1N1 New Caledonia had significantly lower HAI and MN titers (p<0.001 for both) than children without shedding. The comparison of subjects who shed B Jilin showed similar trends, but due to the smaller number of subjects who shed B Jilin, the MN or HAI titer differences between shedders and non-shedders did not reach statistical significance. An analysis of the factors associated with protection against shedding of A H3N2 Wyoming vaccine virus was not performed due to the small number of shedders. The comparison of influenza-IgG and IgA between shedders of any influenza vaccine strain and non-shedders showed increased concentrations of both types of antibodies in non-shedders (p=0.05 and 0.02, respectively).
HAI and MN titers ≥1:40 are considered predictors of protection against influenza infection and morbidity in children. To test the ability of these thresholds to predict protection against viruses contained in LAIV, we compared the proportions of shedders and non-shedders with antibody titers higher than the above listed thresholds (Table 5). Neither HAI nor MN titers ≥1:40 were completely protective against vaccine virus shedding. Influenza A H1N1 shedders had significantly lower proportions of subjects with protective HAI or MN titers compared with non-shedders (p≤0.01). There was a trend towards lower prevalence of MN titers ≥1:40 among B Jilin shedders compared with non-shedders (p=0.06), but no difference in the proportions of subjects with HAI titers ≥1:40. There were no significant differences in MN or HAI titers between shedders and non-shedders of A H3N2 Wyoming.
Table 5.
HAI titers against mismatched influenza strains in HIV-infected children immunized with LAIV compared with those in children immunized with TIV.
| Influenza Strains |
Week | LAIV | TIV | P value* | ||
|---|---|---|---|---|---|---|
| N | GMT (95% CI) | N | GMT (95% CI) | |||
| A H3N2 Sydney |
0 | 116 | 65.2 (52.6, 80.8) | 119 | 78.3 (63.4, 96.8) | 0.23 |
| 4 | 114 | 88.2 (72.7, 106.9) | 112 | 191.1 (157.3, 232.0) | <.0001 | |
| 24 | 110 | 100.2 (82.0, 122.4) | 116 | 169.5 (139.5, 206.0) | 0.0003 | |
| B Yamanashi | 0 | 116 | 13.4 (10.3, 17.6) | 119 | 14.4 (11.1, 18.8) | 0.71 |
| 4 | 114 | 17.3 (13.4, 22.2) | 112 | 44.1 (34.2, 56.8) | <.0001 | |
| 24 | 110 | 16.7 (12.7, 21.9) | 116 | 36.6 (28.1, 47.7) | <.0001 | |
F test
Heterosubtypic HAI responses to LAIV and TIV in HIV-infected children
At 4 weeks after vaccination, HAI antibody titers were measured against A H3N2 Sydney and B Yamanashi, which were not included in the 2004/05 influenza vaccines. Both LAIV and TIV recipients showed increases in antibody titers to these mismatched influenza strains (Table 5). Although baseline HAI titers against the mismatched strains were similar in the two treatment groups, TIV recipients had significantly higher HAI titers compared with LAIV recipients against either mismatched influenza virus at 4 and 24 weeks after vaccination.
Discussion
Both LAIV and TIV administration increased influenza-specific MN titers in HIV-infected children on HAART. The elevated MN titers persisted for at least 6 months, suggesting that the influenza-specific sero-protection conferred by each vaccine to HIV-infected children on HAART lasts through an entire influenza season. Although compared with LAIV, TIV administration resulted in significantly higher MN titers against two of the three influenza serotypes in the vaccine, the proportions of subjects with post-immunization influenza MN titers ≥1:40, the threshold associated with protection against infection and morbidity in HIV-uninfected children, was similar among LAIV and TIV recipients for all the viruses in the vaccines. In addition, we used shedding of the LAIV strains to validate the protective value of MN titers >1:40 in HIV-infected children.
The MN responses to the influenza vaccines of HIV-infected children on HAART were strongly associated with baseline MN titers, which negatively correlated with baseline plasma viral load and CD8% and positively correlated with baseline CD4%. Furthermore, a multivariate analysis showed that the week 4 response to all three viruses in TIV and to one of the viruses in LAIV was also independently associated with baseline plasma viral load. Taken together, these findings underscore the importance of controlling viral replication and preserving CD4 cells for the development of protective immune responses to influenza vaccines and wild type infection.
Since influenza infection is acquired through colonization of the respiratory tract, mucosal immunity is deemed to play an important role in protection against influenza infection. Using saliva as a surrogate sample for mucosal immunity, we showed that both TIV and LAIV increased the salivary influenza-specific IgG titers. Influenza-specific IgA antibody was present only in a minority of vaccinees at baseline and did not increase significantly at 4 weeks after immunization. At 24 weeks after immunization, the influenza-specific IgA levels in saliva were significantly higher compared to the baseline levels in LAIV recipients, but not in TIV recipients. The limited advantage of LAIV over TIV with respect to mucosal IgA antibody responses was surprising, because LAIV is administrated intranasally and we expected it to stimulate higher local antibody production compared with TIV. The extent to which the antibody titers in the saliva reflect the antibody production in the respiratory tract has been debated. In this study, we showed a significant association between the influenza-specific IgA and IgG concentrations in saliva and protection against shedding of LAIV viruses. Since LAIV is administered intranasally, we interpreted these results as an indication that salivary influenzas-specific antibody correlates with functional nasal influenza-specific antibody.
Influenza-specific salivary IgG antibodies were highly correlated with MN titers both before and after immunization with either vaccine. Furthermore, the same immunologic and virologic parameters that affected the MN titers also determined the influenza-specific salivary concentrations. Taken together, these findings suggested that the salivary influenza-specific IgG may represent a transudate, although local influenza-specific IgG production could not be ruled out. In contrast to IgG, neither baseline nor week 4 influenza-specific salivary IgA correlated with serum HAI or MN (not shown) titers, suggesting that the IgA was the result of local production. Influenza-specific salivary IgA responses to vaccination correlated with baseline influenza-specific IgA concentrations, but did not correlate with any of the HIV-specific immunologic or virologic parameters. The latter findings have to be interpreted with caution, because most subjects did not have detectable levels of influenza-specific IgA in the saliva, which may have weakened the ability to detect any associations.
The influenza serotypes in the seasonal vaccines are not always well matched to the serotypes that circulate during the influenza season. Compared with TIV, LAIV administration confers increased protection against mismatched influenza serotypes in children8, 11, but the opposite phenomenon was suggested in adults26, 27. We measured the heterosubtypic HAI antibody responses to LAIV and TIV in HIV-infected children against influenza viruses that circulated several years before this study was performed. Administration of either vaccine was followed by an increase of the HAI antibody titers to heterosubtypic viruses, which were in fact highly correlated with the HAI titers to the viruses in the vaccines, suggesting cross reactivity at the level of antibodies or memory B cells.
This study showed that HIV-infected children on HAART respond to both LAIV and TIV with MN, mucosal and heterosubtypic antibody production. As previously demonstrated in healthy children19, 28, 29, antibody titers tended to be higher after TIV compared with LAIV administration. However the proportion of HIV-infected children that achieved MN antibody titers considered protective against influenza were similar among LAIV and TIV recipients. Both humoral and mucosal antibody titers were associated with protection against shedding of LAIV viruses, suggesting that antibodies in both compartments may contribute to the protection against infections caused by influenza viruses in general.
Figure 3.
Comparison of serum and salivary influenza-specific antibodies in HIV-infected LAIV recipients who excreted ≥1 vaccine viruses (shedders) or did not (non-shedders). Data were derived from 109 HIV-infected children on HAART who received LAIV 2004/2005 and had baseline antibody measurements. There were 22 influenza A H1N1 New Caledonia, 9 B Jilin and 3 A H3N2 Wyoming shedders. For the salivary influenza-IgG and IgA analyses, all shedders were analyzed together irrespective of the type of virus they shed. The F test was used for the statistical analysis.
Acknowledgments
We thank Nancy Tustin, Cristina Gonzaga and Howard Gutzman for technical and data management support.
This research was supported by grant number 5 MO1-RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH; N01-HD-3-3162 and by Grant Number U01AI068632 and 1 U01 AI068616 from the National Institute of Allergy and Infectious Diseases; and N01-HD-3-3345 and contract N01-HD-33162 (AW) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; and by MedImmune LLC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
Appendix
IMPAACT/PACTG P1057 sites and contributors:
Site 3701 Johns Hopkins University Hospital (Beth Griffin, RN; Nancy Hutton, MD; Mary Joyner, NP; Andrea Ruff, MD); 4701 Duke University – Pediatric (Joan Wilson, RN; Mary Jo Hassett, RN; Carole Mathison; John Swetnam); 5006 Harlem Hospital (Elaine Abrams, MD; Maxine Frere, RN; LisaGaye Robinson, MD); 5012 NYU Medical Center/Bellevue (William Borkowsky, MD; Sandra Deygoo, BS; Siham Akleh, RN; Aditya Kaul, MD); 5018 University of South Florida Physicians Group (Jorge Lujan-Zilberman, MD; Patricia Emmanuel, MD; Carolyn Graisbery, RN; Carina Rodriguez, MD); 5024 Children's Hospital Kings and Daughters (Randall G. Fisher, MD; Kenji M. Cunnion, MD, MPH; Laura Sass, MD; Donna Sandifer, RN); 5026 Mount Sinai (Mary S. Dolan, RN; Roberto Posada, MD); 5038 Yale University School of Medicine (Warren A. Andiman, MD; Leslie Hurst, BS; Sostena Romano, APRN, MBA); 5040 SUNY Health Science Center (Denise Ferraro, RN; Michele Kelly, PNP; Margaret Oliver, LPN); 5051 University of Florida Health Science Center (Mobeen Rathore, MD; Nizar Maraqa, MD; Kathy Thomas, MA; Angela Lala, LPN); 5052 The Children's Hospital- U. of Colorado; (Mark Abzug MD; Emily Barr; Megan Canon; Josephine Greenquist); 5057 University of Rochester - Pediatric Component (Geoffrey A. Weinberg, MD; Francis Gigliotti, MD; Barbra Murante, RNC, PNP; Susan Laverty, RN); 5095 Tulane University (Margarita Silio, MD; Thomas Alchediak, MD; Cheryl Borne, RN; Sheila Bradford, RN); 6701 The Children's Hospital of Philadelphia IMPAACT CTU (Steven D. Douglas, MD; Richard M. Rutstein, MD; Carol A. Vincent, CRNP, MSN; Patricia C. Coburn, RN, BSN); 7301 University of Massachusetts Medical School (Katherine Luzuriaga, MD; Richard Moriarty, MD; William (Jerry) Durbin, MD; Donna Picard, RN); 60336 Baylor College of Medicine (Chivon D. McMullen-Jackson, RN, ADN; Theresa Aldape, LMSW; Mary E. Paul, MD; Heidi L. Schwarzwald, MD, MPH); 60422 St. Jude/Memphis (Gregory Storch, MD; Laura Pickering, RN; Katherine Knapp, MD; Jill Utech, RN); 60444 Family Clinical Trials Center (Mavis Dummitt, RN; Caroline Nubel; Stefan Hagmann, MD; Murli Purswani, MD); 2901 Boston Children's Hospital; 3601 UCLA Medical Center; 4001 Children's Hospital of Chicago; 4501 UCSF Medical Center; 4601 UCSD Medical Center; 5008 Children's Hospital at SUNY Downstate; 5013 Jacobi Medical Center; 5031 City Hospital at San Juan; 5041 Children's Hospital of Michigan; 5055 Children's Diagnostic & Treatment Center of South Florida; 5056 University of Florida at Gainesville; 6501 St. Jude Children's Research Hospital; 7701 University of Alabama, Birmingham; 60341 Columbia Collaborative - HIV/AIDS; 60349 University of Miami Ped. Perinatal HIV/AIDS; 60358 NJ Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009 Jul 31;58(RR-8):1–52. [PubMed] [Google Scholar]
- 2.Yamanaka H, Teruya K, Tanaka M, et al. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005 Jun 1;39(2):167–173. [PubMed] [Google Scholar]
- 3.Atashili J, Kalilani L, Adimora AA. Efficacy and clinical effectiveness of influenza vaccines in HIV-infected individuals: a meta-analysis. BMC Infect Dis. 2006;6:138. doi: 10.1186/1471-2334-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amendola A, Boschini A, Colzani D, et al. Influenza vaccination of HIV-1-positive and HIV-1-negative former intravenous drug users. J Med Virol. 2001 Dec;65(4):644–648. doi: 10.1002/jmv.2085. [DOI] [PubMed] [Google Scholar]
- 5.Iorio AM, Alatri A, Francisci D, et al. Immunogenicity of influenza vaccine (1993–94 winter season) in HIV-seropositive and -seronegative ex-intravenous drug users. Vaccine. 1997 Jan;15(1):97–102. doi: 10.1016/s0264-410x(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 6.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine. 2000 Jul 1;18(26):3040–3049. doi: 10.1016/s0264-410x(00)00079-7. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N Engl J Med. 1998 May 14;338(20):1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 8.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007 Feb 15;356(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi S, Vertruyen A, Aristegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006 Oct;25(10):870–879. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 10.Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006 Oct;25(10):860–869. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 11.Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000 Feb;136(2):168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 12.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008 Feb;82(3):1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam JS, Capeding MR, Lum LC, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J. 2007 Jul;26(7):619–628. doi: 10.1097/INF.0b013e31806166f8. [DOI] [PubMed] [Google Scholar]
- 14.Rhorer J, Ambrose CS, Dickinson S, et al. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine. 2009 Feb 11;27(7):1101–1110. doi: 10.1016/j.vaccine.2008.11.093. [DOI] [PubMed] [Google Scholar]
- 15.Bracco Neto H, Farhat CK, Tregnaghi MW, et al. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine-naive children. Pediatr Infect Dis J. 2009 May;28(5):365–371. doi: 10.1097/INF.0b013e31819219b8. [DOI] [PubMed] [Google Scholar]
- 16.Levin MJ, Song LY, Fenton T, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008 Aug 5;26(33):4210–4217. doi: 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block SL, Yogev R, Hayden FG, Ambrose CS, Zeng W, Walker RE. Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age. Vaccine. 2008 Sep 8;26(38):4940–4946. doi: 10.1016/j.vaccine.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol (Basel) 2003;115:97–104. [PubMed] [Google Scholar]
- 19.Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999 Dec 10;18(9–10):899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 20.Forrest BD, Pride MW, Dunning AJ, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008 Jul;15(7):1042–1053. doi: 10.1128/CVI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammitt LL, Li S, Patterson-Bartlett J, et al. Kinetics of viral shedding and immune responses to cold-adapted influenza vaccine. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.09.041. in press. [DOI] [PubMed] [Google Scholar]
- 22.Boyce TG, Gruber WC, Coleman-Dockery SD, et al. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine. 1999 Aug 20;18(1–2):82–88. doi: 10.1016/s0264-410x(99)00183-8. [DOI] [PubMed] [Google Scholar]
- 23.Gorse GJ, Otto EE, Powers DC, Chambers GW, Eickhoff CS, Newman FK. Induction of mucosal antibodies by live attenuated and inactivated influenza virus vaccines in the chronically ill elderly. J Infect Dis. 1996 Feb;173(2):285–290. doi: 10.1093/infdis/173.2.285. [DOI] [PubMed] [Google Scholar]
- 24.Tomoda T, Morita H, Kurashige T, Maassab HF. Prevention of influenza by the intranasal administration of cold-recombinant, live-attenuated influenza virus vaccine: importance of interferon-gamma production and local IgA response. Vaccine. 1995 Feb;13(2):185–190. doi: 10.1016/0264-410x(95)93134-u. [DOI] [PubMed] [Google Scholar]
- 25.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979 Jan;35(1):69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 26.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006 Dec 14;355(24):2513–2522. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009 Sep 24;361(13):1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 28.Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr., Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994 Jan;169(1):68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 29.Piedra PA, Glezen PW. Influenza in children: epidemiology, immunity and vaccines. Semin Pediatr Infect Dis. 1991;2:140–146. [Google Scholar]