Abstract
OBJECTIVE
Diabetic patients with foot ulcers usually manifest with high amputation and mortality rates. Preliminary evidence supports the effectiveness of stem cell (St) therapy on diabetic foot ulcers. The objective of this study was to evaluate the efficacy of stem cells in the healing of wounds among streptozotocin-induced diabetic albino rats.
METHODS
Thirty male albino rats were divided into three groups each of 10 rats: control group, diabetic control (DC) group and St group. Diabetes was induced by intra-peritoneal injection of streptozotocin. A full thickness circular wound of ∼10 mm in diameter was performed on the front of right legs of all rats. In the diabetic St group, the wounds were treated by injection of umbilical cord blood-derived CD34+ stem cells into the wound bed. Half of each group rats were sacrificed after 1 week and the rest after 2 weeks. The wound areas were used for histopathology, immunohistochemistry and transmission electron microscope studies. Assessment of wound surface area, epidermal thickness, blood vessel proliferation and collagen deposition were performed.
RESULTS
There was a significant decrease in mean wound surface area, increase in mean epidermal thickness, blood vessel proliferation and collagen deposition in the St group compared with the DC group.
CONCLUSION
Treatment with CD34+-enriched cells decreased wound size, accelerated epidermal healing and dramatically accelerated revascularization of the wounds compared with the DC group.
Keywords: CD34+ stem cells, Diabetic foot ulcers, Angiogenesis
INTRODUCTION
Diabetic foot is a major end-stage complication of diabetes mellitus (DM) [1]. It is estimated that 15% of all patients with diabetes develop lower extremity ulcers, and 15–20% of those patients require amputation [2]. The prevalence of diabetic foot ulcers in the Arab world in general is high, compared with Western countries [3]. Recently, few studies demonstrated the efficacy and associated healing mechanisms of local therapy of progenitor cells in a preclinical model of diabetic ischaemic foot ulcer [4–6]. Previous studies have also shown that CD34+ cells have the capacity to differentiate into progenitor cells that are further able to incorporate into newly forming blood vessels in pathological and non-pathological conditions [7]. Multipotent non-embryonic stem cells are available in large numbers in umbilical cord blood (UCB), and they are capable of giving rise to different types of cells that can be used in regenerative medicine applications [8].
There are only a few studies that used UCB CD34+ cells in healing of diabetic foot ulcers; therefore, the present study was carried out with an objective to evaluate the efficacy of UCB-derived stem cells in the wound healing and its angiogenesis effect on diabetic animals. If such stem cells were proved to be effective in animals, then it should be studied in clinical use.
METHODS
The study was carried out at the Surgery, Histology, and Physiology Departments of the Suez Canal University's Faculty of Medicine, in Ismailia, Egypt. The experimental study was performed in accordance with national laws on animal experiments and with the permission of the local university ethics commission.
Thirty apparently healthy male albino rats were used in this study. They were randomly divided into three groups each of 10 rats: control group, diabetic control (DC) group and stem cell (St) group. Diabetes was induced in DC and St groups by intra-peritoneal injection of streptozotocin in 0.05 M Na citrate, pH 4.5, at a dose of 50 mg/kg body weight for two consecutive days. In both the DC and St groups, blood sugar was monitored daily by glucometer. After 1 week of having blood sugar >250 mg/dl, a full thickness circular wound of ∼10 mm in diameter was created on the front right legs of all the rats using a sterile biopsy punch. The St group was treated by injection of UCB-derived CD34+ stem cells into the wound bed. No dressings were applied on the wounds throughout the duration of the experiment. All animals were provided with food and water, maintained at 25–28°C and housed in large cages with free mobility.
Preparation and transplantation of stem cells
Obtaining the UCB: after obtaining informed consent, the UCB (cells source) was collected from normal healthy volunteer pregnant donors undergoing full-term normal vaginal deliveries. Females with known history of hepatitis, infectious diseases, DM, severe hypertension, abortions or bad obstetric history were excluded. The UCB was collected while the placenta was still in utero. Using strict aseptic techniques, 50 ml of UCB were withdrawn from the umbilical vein and collected in sterile tubes containing 5 ml of citrate phosphate dextrose adenine-l (CPDA-l) anticoagulant. Samples were collected separately, stored at 4°C and processed within 24 h [9].
Separation and purification of CD34+ cells: separation of UCB CD34+ stem cells was carried out according to the method described by immuno-magnetic separation technique [10]. By mixing and incubation, CD34+ cells were bound to Dynabeads M-450 CD34. The formed rosettes were isolated from the suspension using a DYNAL Magnetic Particle Concentrator (DYNAL MPC). With subsequent incubation with DETACHaBEAD, CD34 gently detached isolated cells from the beads. A DYNAL MPC was then used to separate the purified, positively selected CD34+ cells from the released Dynabeads M-450 CD34.
Assessment of the quantity and quality of the separated cells: the quantity of the isolated CD34+ cells was assessed by putting the sample in an automated cell counter. The quality of the isolated CD34+ cells was determined by using a tyrpan blue dye exclusion test where the viable cells were not stained [11].
Transplantation of human UCB stem cells: after preparation of UCB stem cells, a dose of 0.5 × 106 UCB stem cells/rat was injected locally in the wound bed.
The surface area of wounds was measured immediately before sacrificing the animals using wound tracing in which a pen was used to trace the outline of the wound directly onto a transparent film. To detect early signs of healing, half of each group of rats was sacrificed after 1 week and the rest after 2 weeks. The wound areas were excised with a rim of 5 mm of normal surrounding skin and were used for light (histopathological and immunohistochemical) and transmission electron microscope studies.
Light microscopy
Skin specimens were fixed in 10% neutral buffered formalin solution. Wounds were serially sectioned (5 µm thick) perpendicular to the wound surface. They were then processed for histological (H&E & Masson's trichrome) and immunohistochemical staining using CD31 anti-human (Ventana Medical Systems Inc., Strasburg). Quantitative measurements were carried out using the image analyser (Leica Imaging System) to measure: (i) epidermal thickness in H&E-stained sections, (ii) colour area percentage of collagen fibres in Masson's trichrome-stained sections, (iii) vascular area density [7]: mean percent of vascular lumina per wound area and (iv) vessel index [9] (vessels/mm2): number of blood vessels per square millimetre. Vascular area density and vessel index were measured in CD31-immunostained sections. For morphometric analysis, two different fields were imaged per section and five sections (each 10th from the series, distance 50 µm from each other) were chosen per wound. Therefore, 10 captures of every stain were used per animal. The image analyser was calibrated for colour and distance measurements before use.
Electron microscopy
Specimens for electron microscopy were immediately immersed in 2.5% glutaraldehyde solution. They were trimmed into small pieces, then were put in the same fixative at 4°C for 24 h. The specimens were then washed overnight in several changes of 0.1 M sodium phosphate buffer, pH 7.4, at 4°C. They were later post-fixed in 2% osmium tetroxide in 0.1 M sodium phosphate buffer, pH 7.4, at room temperature. After dehydration in ascending grades of cold ethanol and propylene oxide, the skin specimens were embedded in Spurr's resin. Semithin sections stained with toluidine blue were obtained for observation. Ultrathin sections stained with uranyl acetate and lead citrate were examined at 80 kV under the transmission electron microscope (Jeol, JEM 1010, Japan).
Statistical analysis
Results were summarized using descriptive statistics. These were presented as mean ± SD and compared using a Student's t-test. Significance was set at P < 0.05 for all comparisons. All statistical analyses were performed with the aid of SPSS 15 (SPSS Inc., Chicago, IL, USA) software.
RESULTS
Diabetic control group
DC 1 week: the wound surface area was significantly bigger than the control group (Table 1). Examination of H&E-stained sections showed that the mean epidermal thickness was significantly decreased, compared with the control group (Table 1). Masson's trichrome-stained sections revealed that the mean colour area percentage of collagen was significantly decreased, compared with the control group (Table 1). Immunostained sections showed that vascular area density and the vessel index were significantly decreased, compared with the control group (Table 1).
DC 2 weeks: the wound surface area was significantly bigger than the control group (Table 1). Examination of H&E-stained sections revealed healing of the ulcers in some animals (Fig. 1); however, the epidermis was thin, where most of its cells were flat, and lie on area of the dermis deficient in collagen fibres. The mean epidermal thickness in this group was significantly decreased, compared with the control group (Table 1). Masson's trichrome-stained sections revealed a decrease in collagen fibres in papillary dermis of the wound area (Fig. 2). The mean colour area percentage of collagen was significantly decreased, compared with the control group (Table 1). Immunostained section showed that the vascular area density and the vessel index were significantly decreased, compared with the control group (Fig. 3, Table 1). Electron microscopic examination of skin sections showed an abnormal appearance of cells in the stratum basale and stratum spinosum (Fig. 4). Their nuclei appeared to be irregular with condensed chromatin and wide perinuclear space. The cytoplasm showed vacuoles, swollen mitochondria with disrupted crestae and dilatation of the rough endoplasmic reticulum; however, the appearance of desmosomes and hemidesmosomes was normal. There was also widening of some parts of the intercellular space.
Table 1:
Histological analysis of DC and control groups
| Control | DC | P value | |
|---|---|---|---|
| Wound surface area (mm2) | |||
| 1 week | 4.63 ± 3.34 | 12.86 ± 9.58 | 0.045 |
| 2 weeks | 1.4 ± 0.89 | 3.8 ± 2.07 | 0.047 |
| Epidermal thickness (µm) | |||
| 1 week | 9.15 ± 1.23 | 1.12 ± 0.1 | <0.001 |
| 2 weeks | 11.79 ± 2.51 | 7.15 ± 1.45 | 0.004 |
| Colour area % of collagen | |||
| 1 week | 21.34 ± 4.78 | 19.03 ± 2.83 | 0.005 |
| 2 weeks | 25.45 ± 4.52 | 20.89 ± 3.81 | 0.018 |
| Vascular area density (%) | |||
| 1 week | 26.19 ± 9.63 | 10.85 ± 2.88 | 0.022 |
| 2 weeks | 27.66 ± 7.57 | 13.49 ± 6.92 | 0.024 |
| Vessel index (vessels/mm2) | |||
| 1 week | 54.7 ± 17.4 | 33 ± 13 | 0.007 |
| 2 weeks | 79.1 ± 31.6 | 36 ± 11.6 | <0.001 |
Figure 1:
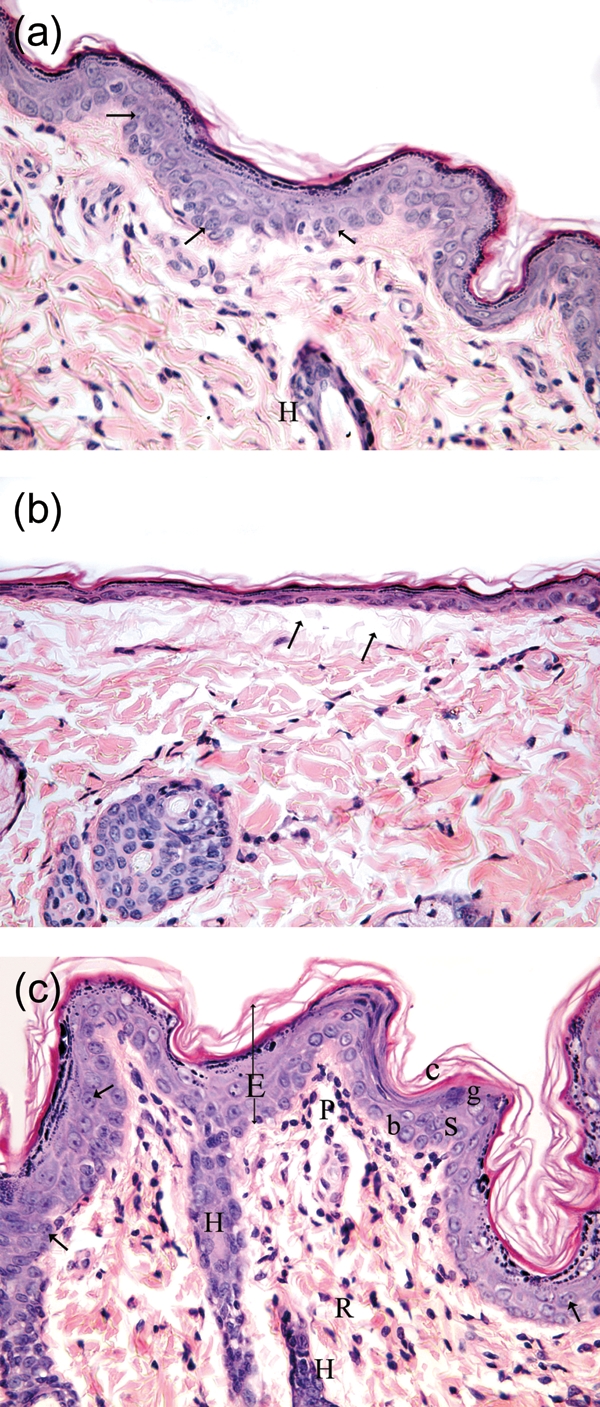
(a) Photomicrograph of a skin section from the St 2 week group showing complete healing of the ulcer and normal appearance of the epidermis and dermis. The epidermis appears of normal thickness showing all the strata (similar to the control group) and some of its cells show mitotic figures (arrow). There is also a part of hair follicle (H) in the dermis. (H&E ×400.) The epidermis shows the stratum basale (b), stratum spinosum (s), where both layers show mitotic figures (arrow), stratum granulosum (g) and stratum corneum (c). There are also parts of hair follicles (H) in the dermis. (b) Photomicrograph of a skin section from the DC 2 week group showing that the ulcer is covered with very thin epidermis where the cells are flat and lie on an area of the dermis deficient in collagen fibres (arrow). (c) Photomicrograph of a skin section from the control group 2 weeks, showing complete healing of the ulcer and normal histological appearance of the epidermis (E) and dermis (papillary layer, P; reticular layer, R).
Figure 2:
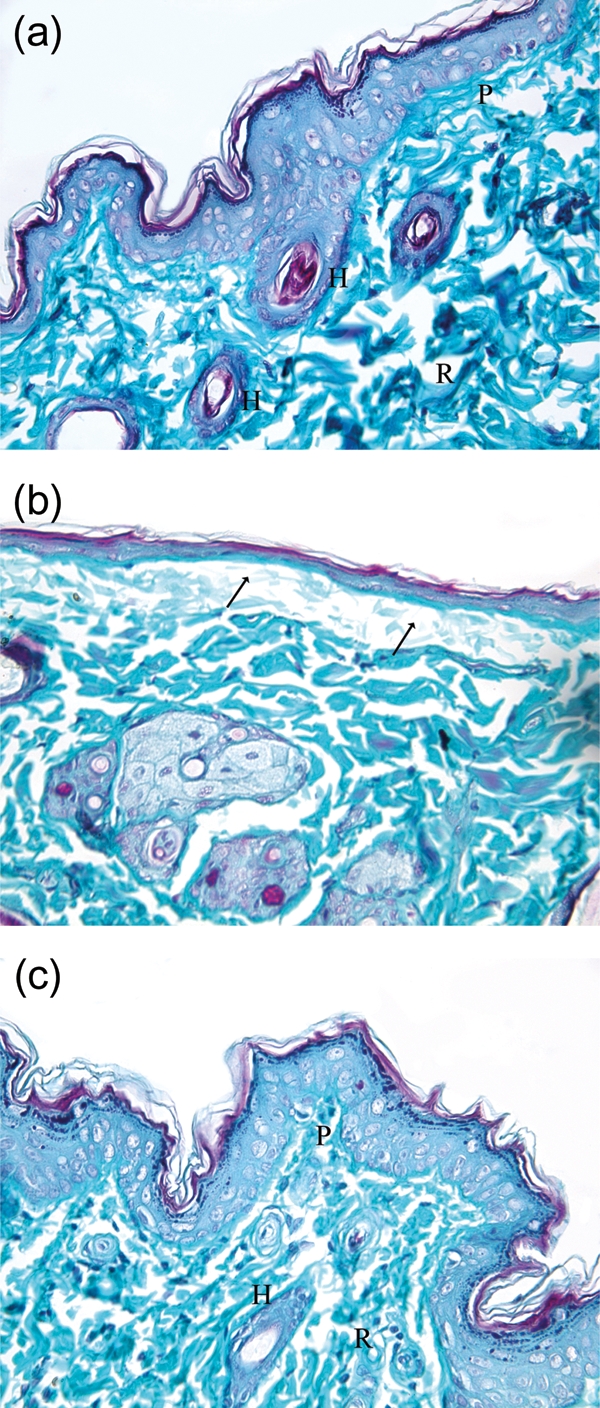
(a) Photomicrograph of a skin section from the control group showing normal appearance of dermal collagen fibres. The fibres are fine in the papillary layer (P) and coarse in the reticular layer (R) and around the hair follicles (H). (b) Photomicrograph of a skin section from the DC 2 week group showing marked decrease in collagen fibres under the ulcer area (arrow). (c) Photomicrograph of a skin section from the St 2 week group showing normal appearance of dermal collagen fibres. The fibres are fine in the papillary layer (P) and coarse in the reticular layer (R) and around the hair follicles (H). (Masson's trichrome ×400.)
Figure 3:
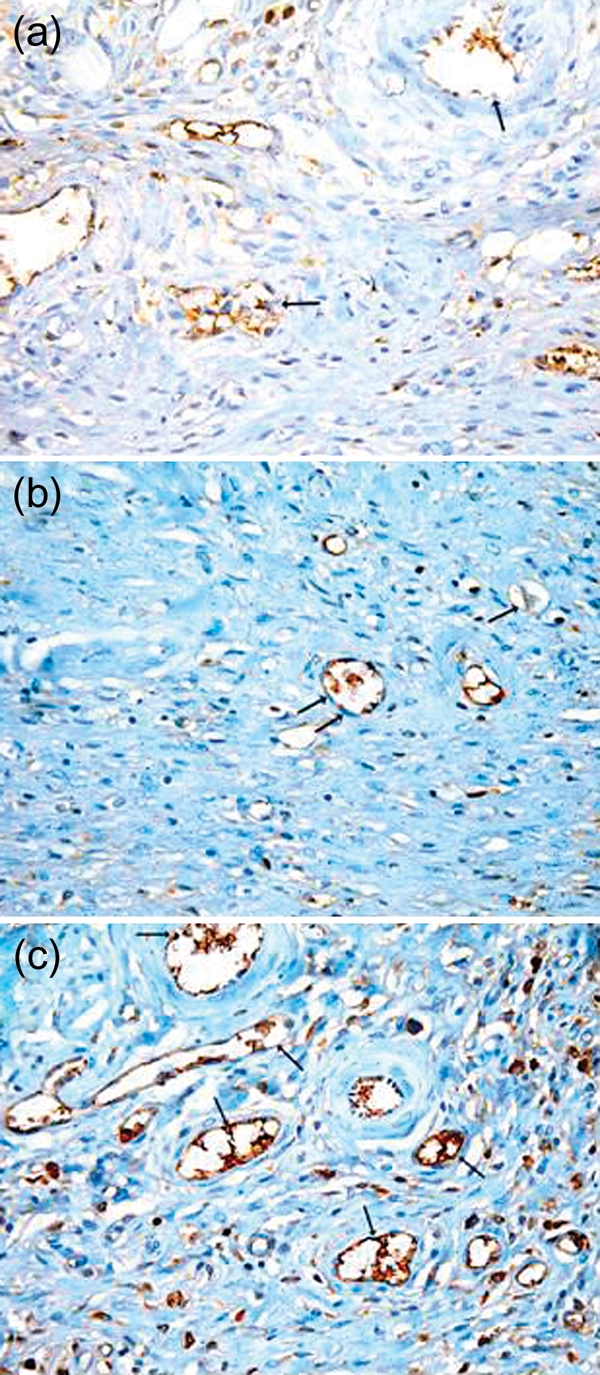
(a) Photomicrograph of a skin section from the control group showing normal vasculature in the dermis of the wound area; however, the reaction is mostly negative in the endothelial cells (arrow). (b) Photomicrograph of a skin section from the DC 2 week group showing an area of the dermis with decreased number of blood vessels. The reaction in the endothelial cells is mostly negative (arrow). (c) Photomicrograph of a skin section from the St 2 week group showing an area of the dermis with increased number of blood vessels which are mostly functioning and contains RBCs (arrow). There is a strong positive reaction in the endothelial cells lining the vessels. (Immunostaining for CD31 anti-body ×400.)
Figure 4:
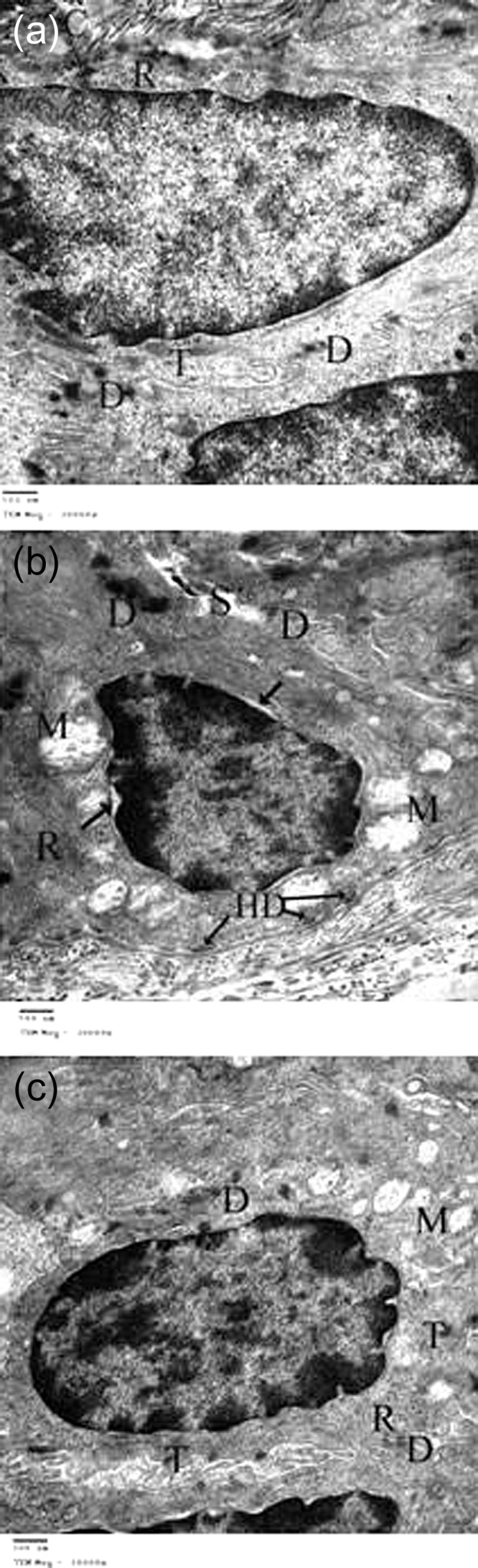
(a) TEM photomicrograph of a skin section from the control group, showing one of the cells of stratum basale lying on the basement membrane (arrow) to which it is attached by haemidesmosomes (arrow heads). Underneath the basement membrane, there are collagen fibres (C). The nucleus is oval with fine chromatin and the cytoplasm contains lots of free ribosomes (R) and tonofilaments (T). There are also desmosomes (D) between the cell and adjacent cells. (b) TEM photomicrograph of a skin section from the DC 2 week group showing a basal cell. The nucleus shows condensed chromatin, widening of the perinuclear space (arrow) and the cytoplasm shows swollen mitochondria with disrupted crestae (M) and ribosomes (R). There is also widening of the intercellular space (S); however, the appearance of desmosomes (D) and hemidesmosomes (HD) is normal. (c) TEM photomicrograph of a skin section from the St 2 week group showing one of the cells of stratum basale which appears to be similar to the control group. The nucleus is oval with fine chromatin and the cytoplasm contains lots of free ribosomes (R), tonofilaments (T) and some mitochondria (M). Note the presence of desmosomes (D) between the cell and adjacent cells. (TEM ×20 000.)
Stem cell group
St 1 week: the wound surface area was significantly smaller compared with DC 1 week group (Table 2). H&E-stained sections revealed that the mean epidermal thickness was significantly increased, compared with the DC 1 week group (Table 2). Masson's trichrome-stained sections revealed that the mean colour area percentage of collagen was significantly increased, compared with the DC 1 week group (Table 2). Immunostained sections showed that the vascular area density and the vessel index were significantly increased, compared with the DC 1 week group (Table 2).
St 2 weeks: the wound surface area was significantly smaller compared with the DC 2 week group (Table 2). H&E-stained sections revealed that the skin of most of the animals showed normal histological structure of the epidermis and dermis (Fig. 1). The epidermis appeared of normal thickness, showing all the strata and some of its cells showed mitotic figures. The mean epidermal thickness was significantly increased compared with the DC 2 week group (Table 2). The dermis also appeared to be normal with the presence of hair follicles. Masson's trichrome-stained sections revealed normal appearance of dermal collagen fibres in the wound area (Fig. 2). The mean colour area percentage of collagen was significantly increased compared with the DC 2 week group (Table 2). Immunostained sections using CD31 anti-human revealed an increased number of dermal blood vessels that were mostly functioning and contained RBCs. There was a strong positive reaction in the endothelial cells lining these vessels (Fig. 3). The vascular area density was significantly increased compared with the DC 2 week group (Table 2). Electron microscopic examination of skin sections showed normal appearance of cells in the stratum basale and spinosum (Fig. 4).
Table 2:
Histological analysis of DC and St groups
| DC | St | P value | |
|---|---|---|---|
| Wound surface area (mm2) | |||
| 1 week | 12.86 ± 9.58 | 5.88 ± 5.19 | 0.048 |
| 2 weeks | 3.8 ± 2.07 | 1 ± 1.4 | 0.029 |
| Epidermal thickness (µm) | |||
| 1 week | 1.12 ± 0.1 | 8.55 ± 1.78 | <0.001 |
| 2 weeks | 7.15 ± 1.45 | 10.73 ± 2.47 | 0.015 |
| Colour area % of collagen | |||
| 1 week | 19.03 ± 2.83 | 22.12 ± 5.99 | <0.001 |
| 2 weeks | 20.89 ± 3.81 | 24.13 ± 6.46 | 0.023 |
| Vascular area density (%) | |||
| 1 week | 10.85 ± 2.88 | 29.29 ± 11.71 | 0.043 |
| 2 weeks | 13.49 ± 6.92 | 32.13 ± 5.03 | <0.001 |
| Vessel index (vessels/mm2) | |||
| 1 week | 33 ± 13 | 51.6 ± 21 | 0.001 |
| 2 weeks | 36 ± 11.6 | 73.7 ± 23.1 | <0.001 |
DISCUSSION
Skin ulcers are a severe and frequent complication of diabetes and constitute a significant burden to healthcare systems all over the world [12]. Numerous studies demonstrated that a subset of haematopoietic cells can function as adult stem cells, and it appears that leucocytes expressing the cell surface antigen CD34+ are enriched for these stem cells [13]. Blood-derived stem cells are capable of differentiating into a variety of cell types, including endothelial cells [14]. Impaired wound healing in diabetic patients results from multi-factorial deficits, including inefficient reparative angiogenesis as well as aberrant control of cell survival; thus it may be clinically relevant that transplantation of stem cells restored reparative angiogenesis in diabetic ulcers through stimulation of endothelial cells survival, proliferation and migration [15, 16].
Diabetes affects the three phases of wound healing; the inflammatory phase through compromising the immune system, the proliferative phase through suppression of collagen deposition and formation of new vessels (angiogenesis) [4] and the remodelling phase in which re-organization of collagen occurs to restore the tissue structural integrity [17]. In the present study, diabetes caused delayed healing in most of the animals. Furthermore, the skin of healed ulcers appeared to be abnormal as was seen with light and electron microscopic examination. There was also a significant increase in wound surface area and significant decrease in each of the epidermal thickness, colour area percentage of collagen, vascular area density and vessel index, compared with the control groups. These data were in agreement with other studies [18]. Previous studies have reported diminished proliferative capacity, abnormal morphology of fibroblasts derived from diabetic ulcers and impaired fibroblast migration to wound in diabetic patients [19]. Similar findings were also documented in the present study with the use of electron microscope. Another important impairment to healing of diabetic wound is lack of migration of keratinocytes and fibroblasts [20]; this would result in reduction of collagen deposition and delay in wound healing. Impaired angiogenesis is a clinically significant problem in diabetic patients [21], and clinical trials indicate that therapies designed to improve vascularization can improve the outcomes in patients with severe skin wounds and diabetic ulcers [22].
In the present study, the use of CD34+ stem cells revealed marked improvement and healing of wounds in most of the animals. Light and electron microscopic examination revealed that the newly formed skin was of normal appearance. There was also a significant decrease in wound surface area in addition to the significant increase in all other parameters (epidermal thickness, colour area percentage of collagen, vascular area density and vessel index) compared with the DC groups after 1 week from the study. These significant differences were maintained up to the second week. The same finding was shown in other studies [13].
Previous studies demonstrated that injection of CD34+-enriched peripheral blood mononuclear cells into the ischaemic limbs of diabetic mice could rapidly and significantly improve the blood flow to the limbs [23]. In this study, we tested the potential of these same cells to improve vascularization of skin wounds in diabetic rats. Our data indicated that treatment with UCB enriched for CD34+ cells dramatically enhanced revascularization of the wound by 7 days after wounding and injection of stem cells. This was different from other studies which showed that the vessel index was similar in DC and treated wounds [13]. Nevertheless, there were dramatic differences in vascular area density in both studies between the DC and St groups. At 2 weeks, both studies showed that the vascular area density and vessel index were significantly higher in the St group compared with the DC group.
Different mechanisms were suggested for the action by which CD34+ stem cells transplantation leads to tissue repair. One of these mechanisms is differentiation into mature endothelial cells leading to angiogenesis, or expression of angiogenic growth factors in a paracrine way to stimulate neovascularization at the site of the cell graft [24]. Immunohistochemical staining with CD31 anti-human, in the present study, confirmed this mechanism. Beside the increase in vasculature, the immunostained sections from the St groups showed a strong positive reaction for the CD31 anti-human in the endothelial cells of the newly formed vessels. Another mechanism suggested by other authors [25] was that CD34+ stem cells have transdifferentiation potential and can contribute to the formation of the newly formed keratinocytes as well as dermal fibroblasts that deposit collagen and share in the healing process. Our study revealed that stem cells improved keratinocytes formation as well as collagen deposition and hence promoted diabetic wound healing.
In conclusion, CD34+ stem cells are simple and effective therapy for diabetic wounds. They stimulate blood vessel proliferation and collagen deposition. Further studies are required to evaluate the efficacy of this therapy in clinical trials.
Conflict of interest: none declared.
REFERENCES
- 1.Boulton AJ, Meneses P, Ennis WJ. Diabetic foot ulcers: a framework for prevention and care. Wound Repair Regen. 1999;7:7–16. doi: 10.1046/j.1524-475x.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey S, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 3.Al-Wahbi AM. The diabetic foot in the Arab world. Saudi Med J. 2006;27:147–53. [PubMed] [Google Scholar]
- 4.Gu Y, Zhang J, Qi L. Comparative study on autologous implantation between bone marrow stem cells and peripheral blood stem cells for treatment of lower limb ischemia. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21:675–8. [PubMed] [Google Scholar]
- 5.Kirana S, Stratmann B, Lammers D, Negrean M, Stirban A, Minartz P, et al. Wound therapy with autologous bone marrow stem cells in diabetic patients with ischaemia-induced tissue ulcers affecting the lower limbs. Int J Clin Pract. 2007;61:690–2. doi: 10.1111/j.1742-1241.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang XF, Wu YX, Wang HM, Xu YF, Lü X, Zhang YB, et al. Autologous peripheral blood stem cells transplantation in treatment of 62 cases of lower extremity ischemic disorder. Zhonghua Nei Ke Za Zhi. 2005;44:95–8. [PubMed] [Google Scholar]
- 7.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate neovascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–77. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 8.Harris DT. Non-hematological uses of cord blood stem cells. Br J Hematol. 2009;147:177–84. doi: 10.1111/j.1365-2141.2009.07767.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JE. Umbilical cord blood stem cell transplantation. Cancer Treat Res. 1995;76:195–213. doi: 10.1007/978-1-4615-2013-9_8. [DOI] [PubMed] [Google Scholar]
- 10.Miltenyi S, Müller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–8. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 11.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3:Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 12.Harrington C, Zagari MJ, Corea J, Klitenic J. A cost analysis of diabetic lower-extremity ulcers. Diabetes Care. 2000;23:1333–8. doi: 10.2337/diacare.23.9.1333. [DOI] [PubMed] [Google Scholar]
- 13.Sivan-Loukianovaa OA, Awada V, Stepanovica J, Bickenbachb GC, Schattemana GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–77. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 14.Shi Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 15.Procházka V, Gumulec J, Chmelová J, Klement P, Klement GL, Jonszta T, et al. Autologous bone marrow stem cell transplantation in patients with end-stage chronical critical limb ischemia and diabetic foot. Vnitr Lek. 2009;55:173–8. [PubMed] [Google Scholar]
- 16.Zhang M, Yu G. Research progress of stem cells transplantation for treating diabetic foot. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:358–61. [PubMed] [Google Scholar]
- 17.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Omar SM. Effect of chromium picolinate on histological skin alterations of streptozocin-diabetic rats. A light and electron microscopic study Egypt. J Histol. 2010;33:178–91. [Google Scholar]
- 19.Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res. 1999;291:93–9. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- 20.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30–9. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–45. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 22.Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of Becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335–46. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- 23.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–8. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Zhou B, Han ZC. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. A comparison between CD34+ and CD34− mononuclear cells. Thromb Haemost. 2006;95:301–11. doi: 10.1160/TH05-06-0442. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413–37. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]


