Abstract
Primary angiosarcoma of pulmonary artery is a very rare lesion. We present a case of primary angiosarcoma that was initially misdiagnosed as a subacute massive pulmonary thromboembolism in a 30-year-old man. This rare disease is usually indistinguishable from acute or chronic thromboembolic disease of the pulmonary arteries. The clinical and radiological findings of pulmonary artery angiosarcoma are similar to those of pulmonary thromboembolism. Although the incidence of pulmonary artery angiosarcoma is very low, our case demonstrates that this disease entity should be included in the differential diagnosis of pulmonary thromboembolism. Patients with early identification can have curative potential with aggressive surgical intervention.
Keywords: Pulmonary angiography, Pulmonary artery sarcoma, Pulmonary embolism, Surgical resection, Cardio pulmonary bypass
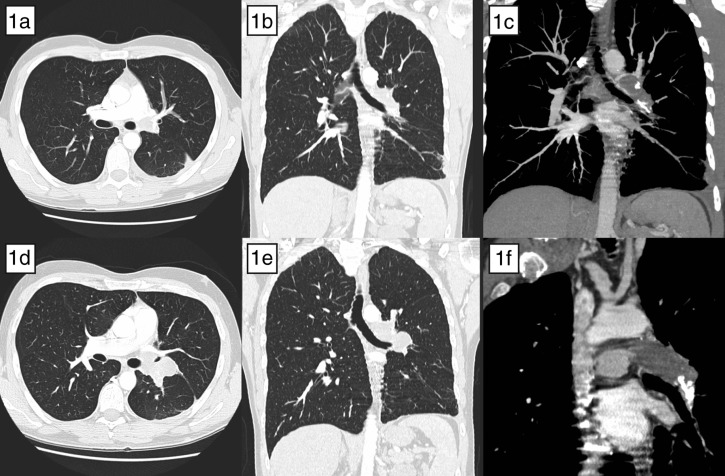
A 30-year-old man initially presented to his general practitioner in July 2009 with persistent chest and back pain. The pain was worse on inspiration and did not allow him to sleep on one side. He went on to develop fever a few days later. Pleural rub was heard on examination. He was treated conservatively with analgesia. In October 2009 due to persistent symptoms, a computed tomography (CT) scan of the chest was performed, which was remarkable for a large filling defect involving the left main pulmonary artery and extending into all sub-branches, most pronounced in the left lower lobe (Fig. 1a–c). He had not been on any long oversea trips or any other condition suggesting a propensity of deep vein thrombosis or pulmonary embolism. He smoked for a short period as youngster and worked in pubs where got exposed to passive smoking. Chest X-ray was normal and laboratory investigation including those for hypercoagualable disorders were within normal limits.
Figure 1:
The CT scan comparing deceptive nature of pulmonary angiosarcoma mimicking pulmonary embolism. Pulmonary embolism has endoluminal thromboemboli extending into the subbranches, where as sarcoma appears to be a mass extending outside the vessel wall. In addition, there is evidence of focal infiltration into the adjacent lung parenchyma. (a–c) CT scan October 2009. (d–f) CT scan March 2010.
A diagnosis of subacute pulmonary thromboembolism was made based on clinical presentation and imaging results, with anticoagulation treatment commenced. Symptoms temporarily subsided but re-occurred in January 2010 with dyspnoea, cough and decreased air entry over the left lung fields. He was treated conservatively with antibiotics. He was seen again in March 2010 for annual check-up where repeat chest X-ray revealed left hilar mass after which he was referred to cardiothoracic unit for intervention. The repeat CT scan showed left hilar neoplasm centred in left main pulmonary artery with occlusion of the lower lobe branches and with new occlusion of left upper lobe branch extending into the left lung parenchyma (Fig. 1d–f). Ventilation scan showed a normal tracer distribution in both lungs and a perfusion scan showed the absence of perfusion of the left lung. Trans-thoracic echocardiography demonstrated normal left and right ventricular chamber size and function. F-18 FDG whole-body positron emission tomography revealed an uptake within the left main pulmonary artery and its proximal branches. There was no evidence of distant metastasis.
Surgery was performed in April 2010. Median sternotomy was performed and cardio pulmonary bypass (CPB) was initiated. The left pulmonary veins were ligated and the left main pulmonary artery was incised. A large tumour completely filled the left main pulmonary artery (Fig. 2). Extension of the incision into the pulmonary trunk revealed that the tumour was confined only to the left side. The portion of tumour located in the left main pulmonary artery and pulmonary trunk, including the left main pulmonary artery and the left lateral wall of the trunk, was excised. The defect was repaired with a bovine pericardial patch and CPB was terminated. The left main bronchus was transected and left pneumonectomy was completed.
Figure 2:
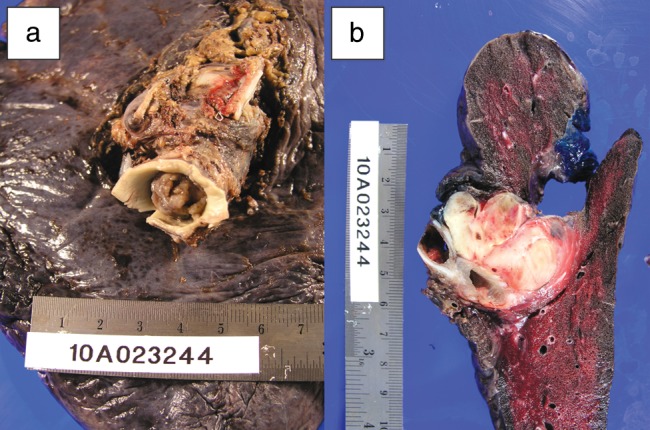
(a and b) Resected left lung and pulmonary artery occluded by the mass.
The immediate postoperative course was uncomplicated, and he was discharged home on post-operative day 7. The tumour was diagnosed histologically with the aid of immunohistochemical staining as a leiomyosarcoma completely occluding the left pulmonary artery and secondary branches. There was no evidence of metastasis to lymphnodes and resection margins were clear.
At his last follow-up appointment in March 2011, the patient was doing well with no recurrence.
DISCUSSION
Primary malignant tumours of the pulmonary arteries are very rare with low incidence. Therefore, most of the cases reported in the literature have been identified at autopsy. They are nearly always a highly malignant sarcoma that typically obtain their origin from the intima of the pulmonary trunk or the bulbous cordis. The first published case was reported in 1923 from an autopsy by Mandelstamm, and since then, a couple of hundred patients with primary pulmonary artery (PA) sarcoma have been described [1–6].
We present a case of primary angiosarcoma of the pulmonary trunk that was initially misdiagnosed as a subacute massive pulmonary thromboembolism in a 30-year-old man. This is an extremely rare disease that is usually indistinguishable from acute or chronic thromboembolic disease of the pulmonary arteries because the clinical and radiologic findings of pulmonary artery angiosarcoma are similar to those of pulmonary thromboembolism. Although the incidence of pulmonary artery angiosarcoma is very low, literature demonstrates that this disease entity should be included in the differential diagnosis of pulmonary thromboembolism, especially in patients who do not respond to anticoagulant therapy or present with no identifiable source of thromboembolic events. The diagnosis of chronic thromboembolic pulmonary hypertension is often missed or delayed for months or years. It can be speculated that in the majority of patients with fast-growing PA tumours and progressive cardiopulmonary dysfunction, the diagnosis will not be established before death.
Presentation usually is with chest pain, cough, haemoptysis and syncope are characteristic symptoms of significant pulmonary vascular obstruction. Several reports suggest that CT and magnetic resonance imaging (MRI) may be the most useful investigation for differentiation between tumour and thrombosis [4]. After application of gadolinium–diethylene–triamine–pentaacetic acid, the heterogenous enhancement demonstrated by MRI is characteristic of a vascularized tumour. This can be added with preoperative pathological diagnosis for better planning of surgical approach with endovascular catheter biopsy.
Most report suggests that radical resection as the only effective therapy and might benefit from neoadjuvant or adjuvant radiotherapy, chemotherapy or both [1, 4].
Blackmon et al. reviewed the published literature which showed the median survival was 36.5 ± 20.2 months for patients undergoing an attempt at curative resection compared with 11 ± 3 months for those undergoing incomplete resection. The median survival was 24.7 ± 8.5 months for patients undergoing multimodality treatment compared with 8.0 ± 1.7 months for patients having single-modality therapy [1]. After resection surveillance should be done with regular follow-up by history, physical examination and contrast-enhanced CT scan to detect early recurrence.
In summary, a presumptive diagnosis of primary artery sarcoma should always be considered before deciding on a final diagnosis of pulmonary embolism. Unless less radical operations are considered, complete resection with CPB offers a good prognosis in the treatment of primary leiomyosarcoma.
Conflict of interest: none declared.
REFERENCES
- 1.Blackmon SH, Rice DC, Correa AM, Mehran R, Putnam JB, Smythe WR. Management of primary pulmonary artery sarcomas. Ann Thorac Surg. 2009;87:977–84. doi: 10.1016/j.athoracsur.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Kim JB, Kim SH, Lim SY, Roh SY, Cho GY, Song HJ. Primary angiosarcoma of the pulmonary trunk mimicking pulmonary thromboembolism. Echocardiography. 2010;27:E23–6. doi: 10.1111/j.1540-8175.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 3.Mattoo A, Fedullo PF, Kapelanski D, Ilowite JS. Pulmonary artery sarcoma: a case report of surgical cure and 5-year follow-up. Chest. 2002;122:745–7. doi: 10.1378/chest.122.2.745. [DOI] [PubMed] [Google Scholar]
- 4.Mayer E, Kriegsmann J, Gaumann A, Kauczor HU, Dahm M, Hake U. Surgical treatment of pulmonary artery sarcoma. J Thorac Cardiovasc Surg. 2001;121:77–82. doi: 10.1067/mtc.2001.111423. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmeier A, Semik M, Fallenberg EM, Scheld HH. Leiomyosarcoma of the pulmonary artery—a diagnostic chameleon. Eur J Cardiothorac Surg. 2001;20:1049–51. doi: 10.1016/s1010-7940(01)00939-3. [DOI] [PubMed] [Google Scholar]
- 6.Shimono T, Yuasa H, Yuasa U, Yasuda F, Adachi K, Tokui T. Pulmonary leiomyosarcoma extending into left atrium or pulmonarytrunk: complete resection with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;115:460–1. doi: 10.1016/S0022-5223(98)70290-9. [DOI] [PubMed] [Google Scholar]