Abstract
Mesenchymal stromal cells (MSCs) have been infused in hundreds of patients to date, with minimal reported side effects. However, follow-up is limited and long term side effects are unknown. Because several animal models have raised safety concerns, we sought to develop a system allowing control over the growth and survival of MSCs used therapeutically. We have previously described a suicide system based on an inducible caspase-9 (iCasp9) protein that is activated using a specific chemical inducer of dimerization (CID), analogues of which have been safely tested in a phase I study. Here, we show that MSCs can be easily transduced with this system and selected to high purity (greater than 97%) with clinical grade immunomagnetic procedures. The transduced cells maintain their basic physiology, including expression of surface antigens (such as positivity for CD73, CD90 and CD105, and negativity for hematopoietic markers) and their potential to differentiate into diverse connective tissue lineages (adipocytes, osteoblasts and chondroblasts). Those cells and their differentiated progeny can be selectively eliminated in vitro or in vivo within 24 hours after exposure to pharmacological levels of CID, with evidence of apoptosis in more than 95% of iCasp9-positive cells. In conclusion, we have developed directed MSC killing to provide a necessary safety mechanism for therapies using progenitor cells. We believe that this approach will become of increasing value as clinical applications for MSCs develop further.
Keywords: Mesenchymal stromal cells (MSC), suicide gene, caspase 9
Introduction
Bone marrow derived mesenchymal stromal cells (MSCs) have been defined as a fraction of mononuclear bone marrow cells that adhere to plastic culture dishes in standard culture conditions, are negative for hematopoietic lineage markers and positive for CD73, CD90 and CD105, and able to differentiate in vitro into adipocytes, osteoblasts, and chondroblasts [1, 2]. While their main physiologic role is presumed to be the support of hematopoiesis [3], several reports have also established that MSCs are able to incorporate and possibly proliferate in areas of active growth, such as cicatricial [4] and neoplastic tissues [5, 6], and to home to their native microenvironment and replace the function of diseased cells [7]. Their differentiation potential and homing ability makes MSCs attractive vehicles for cellular therapy, either in their native form for regenerative applications [8, 9], or through their genetic modification for delivery of active biological agents to specific microenvironments such as diseased bone marrow [10] or metastatic deposits [11]. In addition, MSC possess potent intrinsic immunosuppressive activity [12], and to date have found their most frequent application in the experimental treatment of graft-versus-host disease [13] and autoimmune disorders [14].
MSCs have been infused in hundreds of patients with minimal reported side effects [15]. However, follow-up is limited and long term side effects are unknown, and little is known of the consequences that will be associated with future efforts to induce their in vivo differentiation, for example to cartilage or bone, or to genetically modify them to enhance their functionality. Several animal models have raised safety concerns. For instance, spontaneous osteosarcoma formation in culture has been observed in murine derived MSCs [16]. Furthermore, ectopic ossification and calcification foci have been described in mouse and rat models of myocardial infarction after local injection of MSC [17, 18], and their proarrhythmic potential has also been apparent in co-culture experiments with neonatal rat ventricular myocytes [19]. Moreover, bilateral diffuse pulmonary ossification has been observed after bone marrow transplant in a dog, presumably due to the transplanted stromal components [20].
Given the above concerns, we sought to develop a system allowing control over the growth and survival of MSCs used therapeutically. We have described an inducible suicide system based on an inducible caspase-9 (iCasp9) protein that can be activated using a specific chemical inducer of dimerization (CID) [21–23], a functionally identical analogue of which (AP1903) has been safely tested in a phase I study [24]. Here, we show that MSCs are easily transduced with this system, that the transduced cells can be selected with clinical grade procedures and maintain their basic physiology, and that their differentiated progeny can be selectively eliminated by exposure to a CID, suggesting that this approach could effectively augment the safety of transplanted MSCs and their progeny.
Materials and Methods
MSC isolation
MSCs were isolated from healthy donors (protocol H-7122, approved by the Baylor College of Medicine Institutional Review Board). Briefly, post-infusion discarded healthy donor bone marrow collection bags and filters were washed with RPMI 1640 (HyClone, Logan, UT) and plated on tissue culture flasks in DMEM (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS), 2 mM alanyl-glutamine (Glutamax, Invitrogen), 100 units/mL penicillin and 100 μg/mL streptomycin (Invitrogen). After 48 hours, the supernatant was discarded and the cells were cultured in complete culture medium (CCM): α-MEM (Invitrogen) with 16.5% FBS, 2 mM alanyl-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin. Cells were grown to less then 80% confluence and replated at lower densities as appropriate.
Immunophenotyping
Phycoerythrin (PE), fluorescein isothiocyanate (FITC), peridinin chlorophyll protein (PerCP) or allophycocyanin (APC)-conjugated CD14, CD34, CD45, CD73, CD90, CD105 and CD133 monoclonal antibodies were used to stain MSCs. All antibodies were from Becton Dickinson-Pharmingen (San Diego, CA), except where indicated. Control samples labeled with an appropriate isotype-matched antibody were included in each experiment. Cells were analyzed by fluorescence-activated cell sorting FACScan (Becton Dickinson) equipped with a filter set for 4 fluorescence signals.
Differentiation studies in vitro
Adipocytic differentiation
MSCs (7.5×104 cells) were plated in wells of 6-well plates in NH AdipoDiff Medium (Miltenyi Biotech, Auburn, CA). Medium was changed every third day for 21 days. Cells were stained with Oil Red O solution (obtained by diluting 0.5% w/v Oil Red O in isopropanol with water at a 3:2 ratio), after fixation with 4% formaldehyde in phosphate buffered saline (PBS).
Osteogenic differentiation
MSCs (4.5×104 cells) were plated in 6-well plates in NH OsteoDiff Medium (Miltenyi Biotech). Medium was changed every third day for 10 days. Cells were stained for alkaline phosphatase activity using Sigma Fast BCIP/NBT substrate (Sigma-Aldrich, St. Louis, MO) as per manufacturer instructions, after fixation with cold methanol.
Chondroblastic differentiation
MSC pellets containing 2.5×105 to 5×105 cells were obtained by centrifugation in 15 mL or 1.5 mL polypropylene conical tubes and cultured in NH ChondroDiff Medium (Miltenyi Biotech). Medium was changed every third day for a total of 24 days. Cell pellets were fixed in 4% formalin in PBS and processed for routine paraffin sectioning. Sections were stained with alcian blue or using indirect immunofluorescence for type II collagen (mouse anti-collagen type II monoclonal antibody MAB8887, Millipore, Billerica, MA) after antigen retrieval with pepsin (Thermo Scientific, Fremont, CA).
iCasp9-ΔCD19 retrovirus production and transduction of MSCs
The SFG.iCasp9.2A.ΔCD19 (iCasp-ΔCD19) retrovirus [23] consists of iCasp9 linked, via a cleavable 2A-like sequence, to truncated human CD19 (ΔCD19). iCasp9 is a human FK506-binding protein (FKBP12) with an F36V mutation, which increases the binding affinity of the protein to a synthetic homodimerizer (AP20187 or AP1903), connected via a Ser-Gly-Gly-Gly-Ser linker to human caspase 9, whose recruitment domain (CARD) has been deleted, its function replaced by FKBP12. The 2A-like sequence encodes a 20 amino acid peptide from Thosea Asigna insect virus, which mediates more than 99% cleavage between a glycine and terminal proline residue, to ensure separation of iCasp9 and ΔCD19 upon translation. ΔCD19 consists of human CD19 truncated at amino acid 333, which removes all conserved intracytoplasmic tyrosine residues that are potential sites for phosphorylation. A stable PG13 clone producing Gibbon ape leukemia virus (Gal-V) pseudotyped retrovirus was made by transiently transfecting Phoenix Eco cell line (ATCC product #SD3444; ATCC, Manassas, VA) with SFG.iCasp9.2A.ΔCD19, which yielded Eco-pseudotyped retrovirus. The PG13 packaging cell line (ATCC) was transduced 3 times with Eco-pseudotyped retrovirus to generate a producer line that contained multiple SFG.iCasp9.2A.ΔCD19 proviral integrants per cell. Single-cell cloning was performed, and the PG13 clone that produced the highest titer was expanded and used for vector production. Retroviral supernatant was obtained via culture of the producer cell lines in IMDM (Invitrogen) with 10% FBS, 2 mM alanyl-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin. Supernatant containing the retrovirus was collected 48 and 72 hours after initial culture. For transduction, approximately 2×104 MSCs/cm2 were plated in CM in 6-well plates, T75 or T175 flasks. After 24 hours, medium was replaced by viral supernatant diluted 10-fold together with polybrene (final concentration 5 μg/mL) and the cells were incubated at 37°C in 5% CO2 for 48 hours, after which cells were maintained in complete medium.
Cell enrichment
For inducible iCasp9-ΔCD19-positive MSC selection for in vitro experiments, retrovirally transduced MSC were enriched for CD19-positive cells using magnetic beads (Miltenyi Biotec) conjugated with anti-CD19 (clone 4G7), per manufacturer instructions. Cell samples were stained with PE- or APC- conjugated CD19 (clone SJ25C1) antibody to assess the purity of the cellular fractions.
Apoptosis studies in vitro
Undifferentiated MSCs
The chemical inducer of dimerization (CID) (AP20187; ARIAD Pharmaceuticals, Cambridge, MA) was kindly provided by Dr. David Spencer (Baylor College of Medicine) and added at 50 nM to iCasp9-transduced MSCs cultures in complete medium. Apoptosis was evaluated 24 hours later by FACS analysis, after cell harvest and staining with annexin V-PE and 7-AAD in annexin V binding buffer (BD Biosciences, San Diego, CA). Control iCasp9-transduced MSCs were maintained in culture without exposure to CID.
Differentiated MSCs
Transduced MSCs were differentiated as described above. At the end of the differentiation period, CID was added to the differentiation media at 50 nM. Cells were stained appropriately for the tissue being studied, as described above, and a contrast stain (methylene azur or methylene blue) was used to evaluate the nuclear and cytoplasmic morphology. In parallel, tissues were processed for terminal deoxynucleotidyl-transferase dUTP nick end labeling (TUNEL) assay as per manufacturer instructions (In Situ Cell Death Detection Kit, Roche Diagnostics, Mannheim, Germany). For each time point, four random fields were photographed at a final magnification of 40× and the images were analyzed with ImageJ software version 1.43o (NIH, Bethesda, MD). Cell density was calculated as the number of nuclei (DAPI positivity) per unit of surface area (in mm2). The percentage of apoptotic cells was determined as the ratio of the number of nuclei with positive TUNEL signal (FITC positivity) to the total number of nuclei. Controls were maintained in culture without CID.
In vivo killing studies in murine model
All mouse experiments were performed in accordance with the Baylor College of Medicine animal husbandry guidelines. To assess the persistence of modified MSCs in vivo, we used a SCID mouse model and an in vivo imaging system. MSCs were transduced with retroviruses coding for the enhanced green fluorescent protein-firefly luciferase (eGFP-FFLuc) gene alone or together with the iCasp9-ΔCD19 gene. Cells were sorted for eGFP positivity by fluorescence activated cell sorting using a MoFlo flow cytometer (Beckman Coulter, Fullerton, CA). Doubly transduced cells were also stained with PE-conjugated anti-CD19 and sorted for PE-positivity. SCID mice (8–10 weeks old) were injected subcutaneously with 5×105 MSCs with and without iCasp9-ΔCD19 in opposite flanks. Mice received two intraperitoneal injections of 50 μg of CID 24 h apart starting a week later. For in vivo imaging of MSCs expressing eGFP-FFLuc, mice were injected intraperitoneally with D-luciferin (150 mg/kg) and analyzed using the Xenogen-IVIS Imaging System. Total luminescence (a measurement proportional to the total labeled MSCs deposited) at each time point was calculated by automatically defining regions-of-interest (ROIs) over the MSC implantation sites. These ROIs included all areas with luminescence signals at least 5% above background. Total photon counts were integrated for each ROI and an average value calculated. Results were normalized so that time zero would correspond to 100% signal.
In a second set of experiments, a mixture of 2.5×106 eGFP-FFLuc-labeled MSCs and 2.5×106 eGFP-FFLuc-labeled, iCasp9-ΔCD19-transduced MSCs was injected subcutaneously in the right flank, and the mice received two intraperitoneal injections of 50 μg of CID 24 h apart starting 7 days later. At several time points after CID injection, the subcutaneous pellet of MSCs was harvested using tissue luminescence to identify and collect the whole human specimen and to minimize mouse tissue contamination. Genomic DNA was then isolated using QIAmp® DNA Mini (Qiagen, Valencia, CA). Aliquots of 100 ng of DNA were used in a quantitative PCR (qPCR) to determine the number of copies of each transgene using specific primers and probes (for the eGFP-FFLuc construct: forward primer 5'–TCCGCCCTGAGCAAAGAC–3', reverse 5'–ACGAACTCCAGCAGGACCAT–3', probe 5' FAM, 6-carboxyfluorescein–ACGAGAAGCGCGATC–3' MGBNFQ, minor groove binding non-fluorescent quencher; iCasp9-ΔCD19: forward 5'–CTGGAATCTGGCGGTGGAT–3', reverse 5'–CAAACTCTCAAGAGCACCGACAT–3', probe 5' FAM–CGGAGTCGACGGATT–3' MGBNFQ). Known numbers of plasmids containing single copies of each transgene were used to establish standard curves. We determined that 100 ng of DNA isolated from “pure” populations of singly eGFP-FFLuc- or doubly eGFP-FFLuc- and iCasp9-transduced MSCs had similar numbers of eGFP-FFLuc gene copies (~3.0×104), as well as zero and 1.7×103 of iCasp9-ΔCD19 gene copies, respectively. Untransduced human cells and mouse tissues had zero copies of either gene in 100 ng of genomic DNA. Because the copy number of the eGFP gene is the same on identical amounts of DNA isolated from either population of MSCs (iCasp9-negative or positive), the copy number of this gene in DNA isolated from any mixture of cells will be proportional to the total number of eGFP-FFLuc-positive cells (iCasp9-positive plus negative MSCs). Moreover, because iCasp9-negative tissues do not contribute to the iCasp9 copy number, the copy number of the iCasp9 gene in any DNA sample will be proportional to the total number of iCasp9-positive cells. Hence, if G is the total number of GFP-positive and iCasp9-negative cells and C the total number of GFP-positive and iCasp9-positive cells, for any DNA sample then NeGFP = g·(C+G) and NiCasp9 = k·C, where N represents gene copy number and g and k are constants relating copy number and cell number for the eGFP and iCasp9 genes, respectively. Thus NiCasp9/NeGFP = (k/g)·[C/(C+G)], i.e., the ratio between iCasp9 copy number and eGFP copy number is proportional to the fraction of doubly transduced (iCasp9-positive) cells among all eGFP positive cells. Although the absolute values of NiCasp9 and NeGFP will decrease with increasing contamination by murine cells in each MSC explant, for each time point the ratio will be constant regardless of the amount of murine tissue included, since both types of human cells are physically mixed. Assuming similar rates of spontaneous apoptosis in both populations (as documented by in vitro culture) the quotient between NiCasp9/NeGFP at any time point and that at time zero will represent the percentage of surviving iCasp9-positive cells after exposure to CID. All copy number determinations were done in triplicate.
Statistical Analysis
Paired 2-tailed Student's t-test was used to determine the statistical significance of differences between samples. All numerical data are represented as mean ± 1 standard deviation.
Results
MSCs are readily transduced with iCasp9-ΔCD19 and maintain their basic phenotype
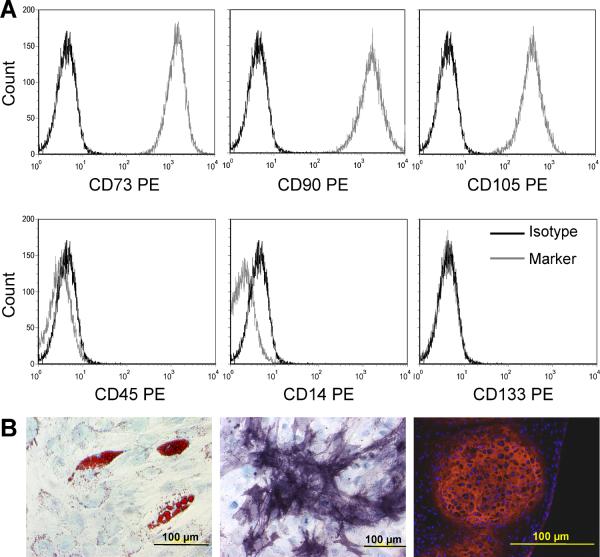
Flow cytometric analysis of MSCs from 3 healthy donors showed they were uniformly positive for CD73, CD90 and CD105 and negative for the hematopoietic markers CD45, CD14, CD133 (Fig. 1 A) and CD34 (not shown). Their differentiation potential into adipocytes, osteoblasts and chondroblasts was confirmed in specific assays (Fig. 1 B), demonstrating that these cells are bona fide MSCs.
Figure 1. MSCs isolated from healthy individuals.
A. The mononuclear adherent fraction isolated from bone marrow was homogenously positive for CD73, CD90 and CD105 and negative for hematopoietic markers. B. These cells were able to differentiate into adipocytes (left, oil red and methylene blue), osteoblasts (center, alkaline phosphatase-bromochloroindolyl phosphate/nitroblue tetrazolium and methylene blue) and chondroblasts (right, anti-type II collagen antibody-Texas red and DAPI) when cultured in specific media.
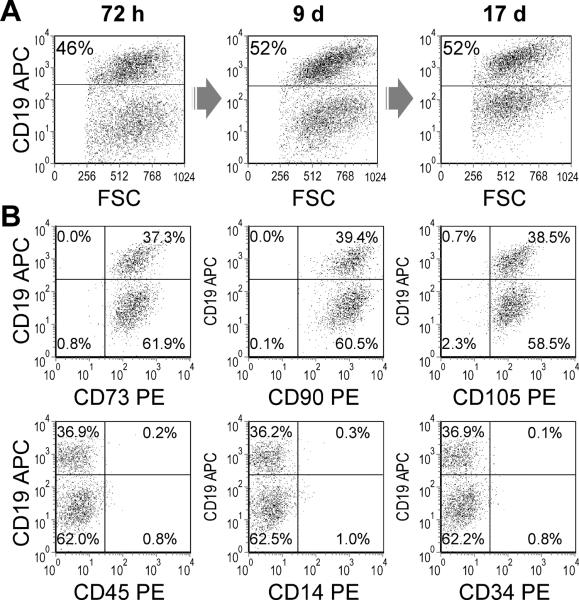
These early passage MSCs were transduced with an iCasp9-ΔCD19 retroviral vector, encoding an inducible form of caspase 9. Under optimal single transduction conditions, 47 ± 6% of the cells expressed CD19, a truncated form of which is transcribed in cis with iCasp9, serving as a surrogate for successful transduction and allowing selection of transduced cells. This percentage of CD19 positivity was stable over more than two weeks in culture suggesting no deleterious or growth advantageous effects of the construct on MSCs (Fig. 2 A). To further address the stability of the construct, a population of iCasp9-positive cells purified by a fluorescence activated cell sorter (FACS) was maintained in culture: no significant difference in CD19-positivity was observed over six weeks (96.5 ± 1.1% at baseline versus 97.4 ± 0.8% after 43 days, P = 0.46). The phenotype of the iCasp9-CD19-positive cells was otherwise identical to that of untransduced cells, with virtually all cells positive for CD73, CD90 and CD105 and negative for hematopoietic markers (Fig. 2 B), confirming that the genetic manipulation of MSCs did not modify their basic characteristics.
Figure 2. Human MSCs are readily transduced with iCasp9-ΔCD19 and maintain their phenotype.
A. MSCs underwent a single round of transduction with iCasp9-ΔCD19 retrovirus. The percentage of CD19-positive cells, a surrogate for successful transduction with iCasp9, remains constant for more than 2 weeks. B. Successfully transduced and non-transduced cells retain the characteristic MSC surface phenotype; positive for CD73, CD90 and CD105 and negative for hematopoietic markers.
iCasp9-ΔCD19 transduced MSCs undergo selective apoptosis after exposure to CID in vitro
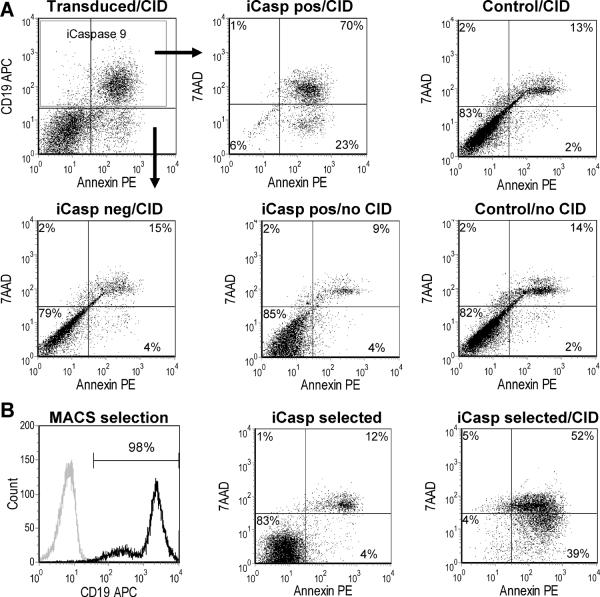
The proapoptotic gene product iCasp9 is activated by a small chemical inducer of dimerization (CID), AP20187, an analogue of tacrolimus that binds the FK506-binding domain present in the iCasp9 product. Non-transduced MSCs have a spontaneous rate of apoptosis in culture of approximately 18% (± 7%) as do iCasp9-positive cells at baseline (15 ± 6%, P = 0.47). Addition of CID (50 nM) to MSC cultures after transduction with iCasp9-ΔCD19 results in the apoptotic death of more than 90% of iCasp9-positive cells within 24 hrs (93 ± 1%, P < 0.0001), while iCasp9-negative cells retain an apoptosis index similar to that of non-transduced controls (20 ± 7%, P = 0.99 and P = 0.69 vs. non-transduced controls with or without CID respectively) (Fig. 3). Analysis of a highly purified iCasp9-positive population at later time points after a single exposure to CID shows that the small fraction of iCasp9-negative cells expands and that a population of iCasp9-positive cells remains, but that the latter can be killed by re-exposure to CID. Hence, we detected no iCasp9-positive population resistant to further killing by CID (Fig. S1).
Figure 3. Human MSCs expressing iCasp9 are selectively driven to apoptosis in vitro after exposure to a small molecule chemical inducer of dimerization.
A. After transduction of MSCs with iCasp9, the chemical inducer of dimerization (CID) was added at 50 nM to cultures in complete medium. Apoptosis was evaluated 24 hours later by FACS analysis, after cell harvest and staining with annexin V-PE and 7-AAD. Ninety-three percent of the iCasp9-CD19-positive cells (iCasp pos/CID) became annexin positive versus only 19% of the negative population (iCasp neg/CID), a proportion comparable to non-transduced control MSC exposed to the same compound (Control/CID, 15%) and to iCasp9-CD19-positive cells unexposed to CID (iCasp pos/no CID, 13%), and similar to the baseline apoptotic rate of non-transduced MSCs (Control/no CID, 16%). B. Magnetic immunoselection of iCap9-CD19-positive cells can be achieved to high degree of purity. More than 95% of the selected cells become apoptotic after exposure to CID.
iCasp9-ΔCD19 transduced MSCs maintain the differentiation potential of unmodified MSCs and their progeny is killed by exposure to CID
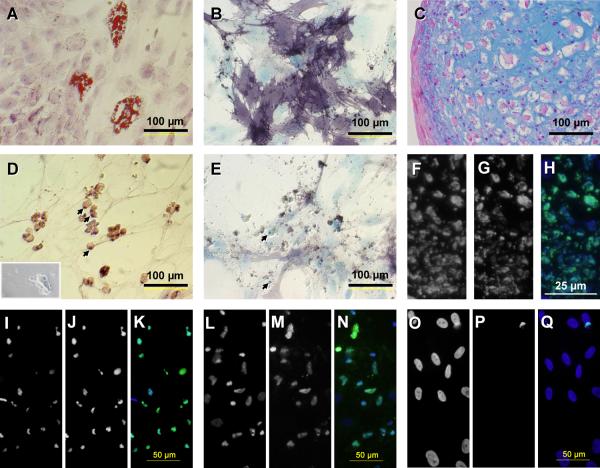
To discover if the CID can selectively kill the differentiated progeny of iCasp9-positive MSCs, we used immunomagnetic selection for CD19 to increase the purity of the modified population (>90% after one round of selection, Fig. 2B). The iCasp9-positive cells thus selected were able to differentiate in vivo into all connective tissue lineages studied (Fig. 4). After 24 hours of exposure to 50 nM of CID, we observed microscopic evidence of apoptosis with membrane blebbing, cell shrinkage and detachment, and presence of apoptotic bodies throughout the adipogenic and osteogenic cultures. A TUNEL assay showed widespread positivity in adipogenic and osteogenic cultures and the chondrocytic nodules (Fig. 4), which increased over time (Fig. S2). Hence, iCasp9 remains functional even after MSC differentiation, and its activation results in the death of the differentiated progeny.
Figure 4. Human MSCs expressing iCasp9 retain the differentiation potential of unmodified MSCs and their progeny is killed by exposure to CID in vitro.
Human MSCs were immunomagnetically selected for CD19 (thus iCasp9) expression, with a purity greater than 91%. After culture in specific differentiation media, iCasp9-positive cells were able to give rise to adipocytic (A, oil red and methylene azur), osteoblastic (B, alkaline phosphatase-BCIP/NBT and methylene blue) and chondroblastic lineages (C, alcian blue and nuclear red) lineages. These differentiated tissues are driven to apoptosis by exposure to 50 nM CID (D–H). Note numerous apoptotic bodies (arrows), cytoplasmic membrane blebbing (inset) and loss of cellular architecture (D and E); widespread TUNEL positivity in chondrocytic nodules (F–H), and adipogenic (I–K) and osteogenic (L–N) cultures, in contrast to that seen in untreated iCasp9-transduced controls (adipogenic condition shown, O–Q) (F, I, L, O, DAPI; G, J, M, P,TUNELFITC; H, K, N, Q, overlay).
iCasp9-ΔCD19 transduced MSCs undergo selective apoptosis after in vivo exposure to CID
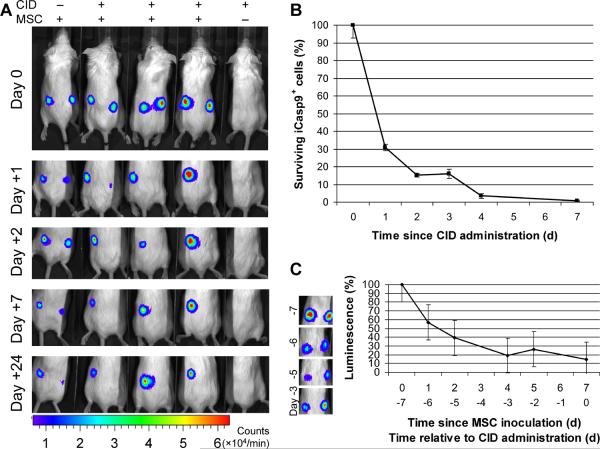
Although intravenously injected MSC already appear to have a short in vivo survival time [25], cells injected locally may survive longer and produce correspondingly more profound adverse effects. To assess the in vivo functionality of the iCasp9 suicide system in such a setting, we injected SCID mice subcutaneously with MSCs. We doubly transduced MSCs with the eGFP-FFLuc (previously described) and iCasp9-ΔCD19 genes and singly transduced MSCs with eGFP-FFLuc. The eGFP-positive (and CD19-positive, where applicable) fractions were isolated by fluorescence activated cell sorting, with a purity > 95%. Each animal was injected subcutaneously with iCasp9-positive and control MSCs (both eGFP-FFLuc-positive) in opposite flanks. Localization of the MSCs was evaluated using the Xenogen-IVIS Imaging System. As anticipated from earlier studies [25], the luminescence data showed a substantial loss of human MSCs over the first 96 h (Fig. 5 C) after local delivery of MSCs, even before administration of CID, with only approximately 20% cells surviving after one week. From that time point onward, however, there were significant differences between the survival of icasp9-positive MSCs with and without dimerizer drug. Seven days after MSC implantation, animals were given two injections of 50 μg of CID, 24 hours apart. As illustrated in Fig. 5 A, the MSCs transduced with iCasp9 were quickly killed by the drug, as demonstrated by the disappearance of their luminescence signal. Cells negative for iCasp9 were not affected by the drug. Animals not injected with the drug showed persistence of signal in both populations up to a month after MSC implantation. To further quantify cell killing, we developed qPCR assays to measure copy numbers of the eGFP-FFLuc and iCasp9-ΔCD19 genes. We injected mice subcutaneously with a 1:1 mixture of doubly and singly transduced MSCs and administered CID as above, one week after MSC implantation. We collected MSCs explants at several time points, isolated genomic DNA from these samples and performed the qPCR assays on identical amounts of DNA. Under these conditions (see Methods), at any time point, the ratio of iCasp9-ΔCD19 to eGFP-FFLuc copy numbers is proportional to the fraction of viable iCasp9-positive cells. We saw progressive killing of iCasp9-positive cells (>99%) so that the proportion of surviving iCasp9-positive cells was reduced to 0.7% of the original population after one week (Fig. 5 B). Therefore, MSCs transduced with iCasp9 can be selectively killed in vivo after exposure to CID, but otherwise persist.
Figure 5. Human MSCs expressing iCasp9 are selectively killed in vivo after exposure to CID.
A. Human MSCs were singly transduced with the eGFP-firefly luciferase (FFLuc) gene or doubly transduced with eGFP-FFLuc and iCasp9-ΔCD19 genes. The eGFP-positive or eGFP and CD19 double-positive fractions were isolated by fluorescence activated cell sorting, with a purity > 95%. NOD-SCID animals were injected subcutaneously on day −7 with eGFP-FFLuc-positive iCasp9-positive MSCs in the right flank and control eGFP-positive MSCs in the left flank. Localization of the MSCs was tracked by the Xenogen-IVIS Imaging System. Seven days (day 0) after MSC implantation, animals were given two intraperitoneal injections of 50 μg of CID, 24 hours apart. iCasp-positive MSCs were quickly killed by the drug, as demonstrated by the disappearance of their luminescence signal. Negative control cells were unaffected by the drug, while iCasp9 positive cells persisted in the absence of drug. B. In another set of experiments, a 1:1 mixture of singly and doubly transduced MSCs was injected subcutaneously in the right flank and the mice received CID as above. The subcutaneous pellet of MSCs was harvested at different time points, genomic DNA was isolated and qPCR was used to determine copy numbers of the eGFP-FFLuc and iCasp9-ΔCD19 genes. Under these conditions, the ratio of the iCasp9 to eGFP gene copy numbers is proportional to the fraction of iCasp9-positive cells among total human cells (see Methods for details). The ratios were normalized so that time zero corresponds to 100% of iCasp9-positive cells. We observed a progressive decrease in the percentage of iCasp9-positive cells down to ~0.7% of the original population after one week. C. Serial examination of animals after subcutaneous inoculation of MSCs (prior to CID injection) shows evidence of spontaneous apoptosis in both cell populations (as demonstrated by a fall in the overall luminescence signal to ~20% of the baseline). This has been previously observed after systemic and local delivery of MSCs in xenogeneic models.
Discussion
We have demonstrated the feasibility of engineering human MSCs to express a safety mechanism using an inducible suicide protein. Our data show that MSC can be readily transduced with the suicide gene iCasp9 coupled to the selectable surface maker CD19. Expression is stable both in MSC and their differentiated progeny, and does not evidently alter their phenotype or potential for differentiation. These transduced cells can be killed in vitro and in vivo if they are exposed to the small molecule chemical induced of dimerization designed to bind to the iCasp9.
Any successful cell based therapy requires transplanted cells to survive the period between their harvest and their ultimate in vivo clinical application. Conversely, every safe cell based therapy will require the ability to control the unwanted growth and activity of successfully transplanted cells. Although MSCs have been administered to many patients without notable side effects [15], recent reports highlight the need for protection [16–19, 26], and this need will likely become ever greater as we exploit the potential of transplanted MSC to differentiate into lineages including bone and cartilage, and as we modify them genetically and epigenetically to enhance their functionality. Subjects receiving MSCs that have been genetically modified to release biologically active proteins might particularly benefit from the added safety provided by a suicide gene [5, 6].
The suicide system we use offers several potential advantages over others in existence. First, strategies involving nucleoside analogues, such as those combining Herpes Simplex Virus thymidine kinase [27] (HSV-tk) with ganciclovir (GCV) and bacterial or yeast cytosine deaminase [28, 29] (CD) with 5-fluoro-cytosine (5-FC), are cell-cycle dependent and are unlikely to be effective in the post-mitotic tissues [30] that may be formed during the application of MSCs to regenerative medicine. Moreover, even in proliferating tissues the mitotic fraction does not comprise all cells, and a significant portion of the graft may survive and remain dysfunctional. Secondly, the prodrugs required for suicide may themselves have therapeutic uses that are therefore excluded (e.g. GCV), or may be toxic (e.g. 5-FC), either as a result of their metabolism by non-target organs (like most cytochrome P450 substrates) [31], or due to diffusion to neighboring tissues after activation by target cells (e.g. CB1954, a substrate for bacterial nitroreductase) [32]. In contrast, the CID used has no known therapeutic value and has shown no evidence of toxicities even at doses ten fold higher than those required to activate the iCasp9 [24]. Thirdly, nonhuman enzymatic systems, such as HSV-tk and DC, carry a high risk of destructive immune responses against transduced cells [33, 34]. Both the iCasp9 suicide gene as well as our selection marker CD19, are of human origin, and thus should be less likely to induce unwanted immune responses. Although linkage of expression of the selectable marker to the suicide gene by a 2A-like cleavable peptide of nonhuman origin could pose problems, this product is 20 amino acids long, and is likely less immunogenic than a nonhuman protein. Finally, the effectiveness of suicide gene activation in iCasp9-positive cells compares favorably to killing of cells expressing other suicide systems, with 90% or more of iCasp9-modified T cells eliminated after a single dose of dimerizer, a level that should be clinically efficacious [34, 35].
There are, nonetheless, potential problems with the iCasp9 system. Loss of expression due to silencing of the construct is frequently observed after retroviral transduction of mammalian cells [36]. Our system, however, showed no evidence for such an effect, with no decrease in expression or induced death even after one month in culture. A second potential problem is the development of resistance in cells that have upregulated anti-apoptotic genes, an effect already observed in other suicide systems involving different elements of the programmed cell death pathways such as Fas [37]. We chose iCasp9 as our suicide gene because it was less likely to have this limitation. Compared to other members of the apoptotic cascade, activation of caspase 9 occurs late in the apoptotic pathway and it should therefore bypass the effects of anti-apoptotic regulators, such as c-FLIP and bcl-2 family members [21]. Perhaps the greatest potential limitation of the system is spontaneous dimerization of iCasp9 causing unwanted cell death and poor persistence. This effect has certainly been observed in inducible systems using Fas [38, 39], but our observations of low spontaneous death rate in transduced cells and long term persistence of transgenic cells in vivo argue against this possibility as a significant problem. Finally, integration events deriving from retroviral transduction of MSCs may potentially drive deleterious mutagenesis, especially when there are multiple insertions of the retroviral vector. This could offset the benefit of our retrovirally transduced suicide system. However, in our experience using clinical grade retroviral supernatant obtained from stable producer cell lines and similar culture conditions to transduce T lymphocytes [23], our T cells contain 1 to 3 integrants at most (the supernatant containing circa 1×106 viral particles/mL). The substitution of lentiviral for retroviral vectors could further reduce the risk of genotoxicity, especially in cells with high self-renewal and differentiation potential [40].
While a small proportion of iCasp9-positive MSCs persists after a single exposure to CID, these surviving cells can be subsequently be killed following re-exposure to CID. In vivo, there is >99% depletion with two doses, but it is likely that repeated doses of CID will be needed for maximal depletion in the clinical setting. In any case, potential ways of providing extra safety include additional rounds of cell sorting to further increase the purity of the cell populations administered and the use of more than one suicide gene system to enhance the efficiency of killing.
We used the CD19 molecule, which is physiologically expressed by B lymphocytes, as our selectable marker for transduced cells, because we believe it has advantages over other available selection systems, such as neomycin phosphotransferase (neo) and truncated low affinity nerve growth factor receptor (ΔLNGFR). neo encodes a potentially immunogenic foreign protein and requires a 7-day culture in selection medium, increasing the complexity of the system and potentially damaging the selected cells [41]. ΔLNGFR expression should allow for isolation strategies similar to other surface markers, but these are not widely available for clinical use [42] and a lingering concern remains about the oncogenic potential of ΔLNGFR [43]. In contrast, magnetic selection of iCasp9-positive cells by CD19 expression using a clinical grade device is readily available and without notable effects on subsequent cell growth or differentiation.
Conclusion
We have developed directed MSC killing to provide a necessary safety mechanism for therapies using progenitor cells. We believe that this approach will become of increasing value as clinical applications for MSCs develop further.
Supplementary Material
Acknowledgments
This work was supported by a Specialized Centers for Cell-based Therapy Grant NIH-NHLBI 1 U54 HL081007.
Footnotes
Disclosure of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
References
- 1.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Lee RH, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 6.Studeny M, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 9.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 13.Le Blanc K, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 14.Tyndall A, Uccelli A. Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.63. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz EM, Michael A, Francesco F. Mesenchymal Stromal Cells. Biol Blood Marrow Transplant. 2007;13:53–57. [Google Scholar]
- 16.Tolar J, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 17.Yoon Y-S, Park J-S, Tkebuchava T, Luedeman C, Losordo DW. Unexpected Severe Calcification After Transplantation of Bone Marrow Cells in Acute Myocardial Infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 18.Breitbach M, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 19.Chang MG, et al. Proarrhythmic Potential of Mesenchymal Stem Cell Transplantation Revealed in an In Vitro Coculture Model. Circulation. 2006;113:1832–1841. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 20.Sale GE, Storb R. Bilateral diffuse pulmonary ectopic ossification after marrow allograft in a dog. Evidence for allotransplantation of hemopoietic and mesenchymal stem cells. Exp Hematol. 1983;11:961–966. [PubMed] [Google Scholar]
- 21.Straathof KC, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintarelli C, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iuliucci JD, et al. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J Clin Pharmacol. 2001;41:870–879. doi: 10.1177/00912700122010771. [DOI] [PubMed] [Google Scholar]
- 25.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 27.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 28.Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci U S A. 1992;89:33–37. doi: 10.1073/pnas.89.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kievit E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417–1421. [PubMed] [Google Scholar]
- 30.Chester JD. Cancer gene therapy. In: Knowles MA, Selby P, editors. Introduction to the cellular and molecular biology of cancer. Oxford University Press; New York: 2005. pp. 458–479. [Google Scholar]
- 31.Wei MX, et al. Experimental tumor therapy in mice using the cyclophosphamide-activating cytochrome P450 2B1 gene. Hum Gene Ther. 1994;5:969–978. doi: 10.1089/hum.1994.5.8-969. [DOI] [PubMed] [Google Scholar]
- 32.Denny WA. Nitroreductase-based GDEPT. Curr Pharm Des. 2002;8:1349–1361. doi: 10.2174/1381612023394584. [DOI] [PubMed] [Google Scholar]
- 33.Riddell SR, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 34.Bonini C, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 35.Ciceri F, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 36.Lange C, Blankenstein T. Loss of retroviral gene expression in bone marrow reconstituted mice correlates with down-regulation of gene expression in long-term culture initiating cells. Gene Ther. 1997;4:303–308. doi: 10.1038/sj.gt.3300395. [DOI] [PubMed] [Google Scholar]
- 37.Thomis DC, et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001;97:1249–1257. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 38.Spencer DM, et al. Functional analysis of Fas signaling in vivo using synthetic inducers of dimerization. Curr Biol. 1996;6:839–847. doi: 10.1016/s0960-9822(02)00607-3. [DOI] [PubMed] [Google Scholar]
- 39.MacCorkle RA, Freeman KW, Spencer DM. Synthetic activation of caspases: artificial death switches. Proc Natl Acad Sci U S A. 1998;95:3655–3660. doi: 10.1073/pnas.95.7.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montini E, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 41.Sauce D, et al. Retrovirus-mediated gene transfer in primary T lymphocytes impairs their anti-Epstein-Barr virus potential through both culture-dependent and selection process-dependent mechanisms. Blood. 2002;99:1165–1173. doi: 10.1182/blood.v99.4.1165. [DOI] [PubMed] [Google Scholar]
- 42.Bonini C, et al. Safety of retroviral gene marking with a truncated NGF receptor. Nat Med. 2003;9:367–369. doi: 10.1038/nm0403-367. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.