Abstract
Objective
The impact of individual antiarrhythmic drugs (AADs) on mortality and hospitalization in atrial fibrillation (AF) was evaluated
Background
Cardiovascular (CV) outcomes in AF patients on pharmacologic rhythm control therapy have not been compared with rate control therapy based on AAD selection.
Methods
We compared CV outcomes in the AFFIRM trial in subgroups defined by the initial AAD selected with propensity score matched subgroups from the rate arm (Rate).
Results
729 amiodarone patients, 606 sotalol patients & 268 class 1C patients were matched. The composite outcome of mortality or CV hospitalizations (CVH) showed better outcomes with Rate compared to amiodarone (Hazard Ratio [HR] 1.18, 95% confidence intervals {CI}:1.03–1.36, p=0.02), sotalol (HR=1.32, CI: 1.13–1.54, p<0.001) and class 1C (HR=1.22, CI: 0.97–1.56, p=0.10). There was a non-significant increase in mortality with amiodarone (HR=1.20, CI: 0.94–1.53, p=0.15) with the risk of non-CV death, being significantly higher with amiodarone versus Rate. (HR=1.11, CI: 1.01–1.24, p=0.04). First CVH event rates at 3 years were 47% for amiodarone, 50% for sotalol and 44% for class 1C versus 40%, 40% and 36% respectively for Rate (amiodarone HR=1.20,CI:1.03–1.40,p=0.02, sotalol HR=1.364, CI:1.16–1.611, p<0.001, class 1C HR=1.24,CI:0.96–1.60,p=0.09). Time to CVH with intensive care unit stay (ICUH) or death was shorter with amiodarone (HR=1.22, CI: 1.02–1.46, p=0.03).
Conclusions
In AFFIRM, composite mortality and CVH outcomes differed for Rate and AADs due to differences in CVH; CVH event rates during follow-up were high for all cohorts, but they were higher for all groups on AADs.
Death, ICUH and non-CV death were more frequent with amiodarone.
Key words for indexing: Atrial Fibrillation, Outcomes Research, Antiarrhythmic Drugs, Clinical Trials, Cardiovascular Outcomes, Cardiovascular Hospitalizations
INTRODUCTION
Atrial fibrillation (AF) is the most prevalent tachyarrhythmia, and is associated with increased mortality, stroke, and recurrent hospitalizations (1, 2). Health care resource consumption due to AF, primarily due to hospitalization, is among the highest for CV diagnoses, but the patterns of these hospitalizations and their relationship to individual therapeutic choices in AF have not been evaluated (3). The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) trial was conducted to examine two treatment strategies for AF, namely rate control or rhythm control (4,5). All-cause mortality, the primary outcome measure, showed a trend toward excess mortality in the rhythm control arm. The antiarrhythmic drugs (AADs) used in the rhythm arm have been cited as a potential cause of the excess mortality (6). Despite concerns regarding their safety, most of the AADs used in AFFIRM remain widely used in clinical practice..
The impact of individual AADs on mortality and hospitalization outcomes in the AFFIRM population in relation to rate control has not been available. In part, this was related to the intent of the AFFIRM investigators to test the treatment strategy hypothesis rather than individual drug therapies. In this report, we examined the impact on outcomes of the selection of amiodarone, sotalol, or a class 1C antiarrhythmic agent (flecainide or propafenone) as the first AAD compared to a rate strategy in the AFFIRM study. AADs selected for this analysis were based on current widespread clinical usage. To address the non-random nature of drug selection in the rhythm arm, we employed propensity score matching derived from 64 baseline patient characteristics deemed to affect antiarrhythmic selection. Propensity score matching has not been employed to assess individual drug outcomes in the AFFIRM trial (7). We compared mortality and hospitalization outcomes in patient subgroups defined by each type of AAD selected as first therapy with propensity score matched subgroups from the rate control arm.
METHODS
Patient selection in AFFIRM
AFFIRM recruited consenting patients who had AF that was likely to be recurrent, warranted therapy and had risk factor(s) for stroke. Patients were candidates for at least two drugs within each strategy and for anticoagulation (4).
Primary objective of analysis
Reassessment of clinical outcomes by initial AAD therapy
The primary objective was to reassess clinical outcomes in the AF population enrolled in the AFFIRM study by initial AAD therapy utilizing a composite principal outcome and its individual components. The principal outcome was a composite of mortality or first cardiovascular (CV) hospitalization. Individual components (all cause mortality and CV hospitalization) were also examined, as were subsets of both CV hospitalizations and all-cause mortality (8). The AAD subgroups were compared to propensity score matched rate subgroups (Rate) and included 1) initial amiodarone therapy (amiodarone cohort), 2) initial sotalol (sotalol cohort), and 3) initial class 1C drug (flecainide or propafenone, class 1C cohort).
Propensity score matched subgroups were selected from the rate control strategy arm (Rate) for each AAD cohort. The score was derived using 62 baseline patient characteristics from the AFFIRM database deemed a priori to potentially affect AAD selection. Two additional characteristics that were determined to be important to achieve balanced cohorts (left ventricular ejection fraction and history of coronary artery disease) were added in a second step. (Table 1)
Table 1.
List of covariates used in propensity score model. Please note that multiple imputation was used for BMI and systolic blood pressure
| Age | Primary Cardiac Diagnosis |
| Sex | Coronary artery Disease |
| Year of Randomization | NYHA Class |
| Site | Current CCS angina class |
| FADS site | Failed Any Antiarrhythmic Drug |
| History of Myocardial Infarction | Number of Antiarrhythmic Drug Failures |
| History of Pulmonary Disease | Failed Amiodarone |
| History of Intracranial Hemorrhage | Failed Disopyramide |
| History of Congestive Heart Failure, Congestive Heart Failure on Enrollment |
Failed Flecainide |
| History of Cardiomyopathy | Failed Moricizine |
| History of Valvular Heart Disease | Failed Procainamide |
| History of Congenital Heart Disease | Failed Propafenone |
| History of Angina | Failed Quinidine |
| History of Diabetes | Failed Sotalol |
| History of Hepatic or Renal Disease | Failed other antiarrhythmic drug |
| History of Symptomatic Brady/Atrioventricular block |
Previous Other CV Procedure |
| History of Resuscitated Cardiac Arrest | Previous Percutaneous Coronary Interventions |
| History of Stroke/Transient Ischemic Attack | Previous Coronary Artery Bypass Grafting |
| History of Peripheral Vascular Disease | Previous Thrombolytic Therapy |
| History of Systemic Embolism | LV ejection fraction |
| History of Hemorrhage or Coagulopathy | Beta stimulant |
| History of Thyroid Disease/ Specific Drugs - Thyroid Replacement |
Theophylline |
| History of Carotid Disease | Diuretic |
Symptoms Constellations Are:
|
Beta Blockers |
| AF Symptoms Frequency | Diltiazem |
| First AF episode | Verapamil |
| Duration of Qualifying AF Episode(s) | BMI |
| Hospitalized for Qualifying Episode | Systolic BP |
| Cardioverted for Qualifying Episode(s) | FADS patient |
| Current Ventricular/Max HR during AF >100 Rate | Other cardiac neurologic interaction |
Abbreviations: AF – atrial fibrillation; BMI – body mass index; BP – blood pressure; CCS – Canadian Cardiovascular Society; FADS – first antiarrhythmic drug substudy; NYHA – New York Heart Association
Secondary objectives
Relating Outcomes to Clinical and Treatment Factors
The severity of CV hospitalizations was characterized by acuity of hospitalization based on concomitant intensive care unit (ICU) stay, CV procedures, CV interventions or emergency room visits. Outcomes in AAD subgroups were related to patient characteristics, underlying disease state, clinical events, and treatment strategy.
Study Outcomes and Definitions
The principal outcome for this analysis was a composite outcome – the first of death from any cause or a CV hospitalization. A CV hospitalization was defined as a hospital admission for CV reasons (per investigator), or for non-CV reasons, but a CV event occurred during the same follow-up interval. Exact dates were available for death but not for hospital admission or discharge. The midpoint of the previous follow-up visit and the follow-up visit when the hospitalization was reported were used to estimate event time for CV hospitalization. Investigators recorded total number of hospital days and total number of ICU days. Visits occurred at 2 months post-randomization, and thereafter every 4 months. Patients who did not experience CV hospitalization or death were censored at the last follow-up visit. For death alone, follow-up information from a vital status sweep (telephone contact with all subjects and national death index scan) at the end of the study was used to determine censoring date.
Statistical Methods and Analytical Techniques
Propensity score and establishment of matched cohorts
The goal of development of propensity score matched cohorts was to account for possible confounding variables that may be related to drug selection since the patients were not assigned randomly to specific initial drug therapy in AFFIRM.
Selection of covariates
Propensity score was calculated separately for each AAD subgroup (amiodarone, sotalol, or class 1C). Four patients received more than one AAD, and were excluded. The propensity score model used data from AFFIRM patients randomized to rhythm control. Identical baseline explanatory variables were included in each model, and were prospectively determined by consensus prior to data analysis. (Table 1) This model included explanatory variables that might be considered by clinicians when selecting an AAD, including demographics, clinical characteristics of patients, treating physicians (cardiologists or other), centers, and study design factors. Patients in the First Antiarrhythmic Drug Sub-study (FADS) had their first AAD randomly assigned, so participation in FADS was included as a variable (9). A stepwise model reduction procedure was used to produce a parsimonious model for each propensity score equation. After initial cohort construction, imbalances in two additional variables, coronary artery disease and left ventricular ejection fraction, were identified; these items were added to the model in a second step.
Model building
Proc GLIMMIX in SAS (Version 9.2, SAS Institute, and Cary, NC) was used for building the propensity-matched cohorts. Each model considered all explanatory variables in Table 1. Site was included as a fixed effect for this step. The functional form of response was assessed for continuous variables to determine if transformation was necessary (10). Then, the model was run twice, with site as a fixed and then as a G-sided (generalized) random effect. These models were compared for evidence of extra binomial variability at the investigator site level. Risk score was calculated for each patient in the rate subgroup, and the VMATCH algorithm was used to construct the cohorts (7). Matching was 1-1 between each AAD cohort and the rate cohort.
Descriptive reporting
Once the propensity score matched cohorts were established, baseline demographic and clinical characteristics were tabulated to be consistent with the main AFFIRM publication (5). Tests for differences across matched cohorts were conducted (Fisher’s exact or Chi-square for categorical variables, ANOVA or Wilcoxon for continuous variables).
Principal Outcome
The principal outcome analyzed was a comparison of event time using the log-rank test on an intention-to-treat basis, similar to the primary AFFIRM analysis. Unadjusted Kaplan-Meier survival curves were examined for each propensity-score matched cohort pair. Proportional hazards models were used to obtain hazard ratios and 95% confidence intervals, and to determine the effect in clinically important subgroups.
Sensitivity analyses
To determine the impact of treatment strategy related hospitalizations, e.g. cardioversions, and further define acuity of CV hospitalizations, we repeated the analysis using a composite of death and first hospitalization requiring intensive care unit (ICU) stay. To evaluate the propensity score methodology, a Cox proportional Hazards Model with a frailty term for site was used.
Results
Patient Population
Seven-hundred twenty nine AF patients initially received amiodarone therapy, 606 received initial sotalol therapy and 268 received either initial flecainide or propafenone. The clinical characteristics of these three AAD cohorts based on initial drug therapy selection are shown in Table 2. The AAD cohorts were generally well matched. Patients were usually elderly, with a male predominance, and had recurrent AF associated with cardiac disease. The amiodarone cohort had a slight excess of males (67.4 vs. 61.3% respectively) compared to its matched Rate cohort. More patients in the sotalol cohort had a history of angina compared to Rate (11.1% versus 6.9%).There were no other significant differences. The C statistic for the three propensity models were 0.814 for amiodarone, 0.837 for sotalol, 0.837 for class1c subgroups.
Table 2.
Baseline patient characteristics for entire Rate cohort in AFFIRM (Overall Rate) and the three paired propensity matched cohorts for individual antiarrhythmic drugs and matched rate control groups.
| Overall Rate (original) (N=2027) |
Rate (PS Matched) (N=729) |
Amiodarone (PS Matched) (N=729) |
P-value | Rate (PS Matched) (N=606) |
Sotalol (PS Matched) (N=606) |
P-value | Rate (PS Matched) (N=268) |
Class 1C (PS Matched) (N=268) |
P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age-yr | 69.77 ± 8.91 | 69.7 ± 8.79 | 70.3 ± 9.21 | 0.21 | 70.5 ± 8.4 | 69.6 ± 8.8 | 0.06 | 68.6 ± 9.3 | 68.6 ± 8.9 | 0.94 | |
| Female Sex - no. (%) | 823 (40.6%) | 282 (38.7%) | 238 (32.6%) | 0.02 | 234 (38.6%) | 227 (37.5%) | 0.68 | 144 (53.7%) | 140 (52.2%) | 0.73 | |
| Ethnic Minority Group - no. (%) | 241 (11.9%) | 98(13.4%) | 78 (10.7%) | 0.11 | 70(11.6%) | 57 (9.4%) | 0.22 | 23 (8.6%) | 24 (9.0%) | 0.88 | |
| Predominant Cardiac Diagnosis - no. (%) | 0.92 | 0.74 | 0.66 | ||||||||
| Coronary Artery Disease (MI, angina, etc.) | 497 (24.5%) | 228 (31.3%) | 247 (33.9%) | 165 (27.2%) | 158 (26.1%) | 28 (10.4%) | 31 (11.6%) | ||||
| Dilated Cardiomyopathy | 99 (4.9%) | 53 (7.3%) | 52 (7.1%) | 22 (3.6%) | 20 (3.3%) | 5 (1.9%) | 5 (1.9%) | ||||
| Hypertension | 1045 (51.6%) | 351 (48.1%) | 342 (46.9%) | 291 (48.0%) | 315 (52.0%) | 154 (57.5%) | 156 (58.2%) | ||||
| Valvular Heart Disease | 98 (4.8%) | 30 (4.1%) | 28 (3.8%) | 34 (5.6%) | 34 (5.6%) | 9 (3.4%) | 15 (5.6%) | ||||
| Other | 23 (1.1%) | 9(1.2%) | 7 (1.0%) | 13 (2.1%) | 9 (1.5%) | 3 (1.1%) | 1 (0.4%) | ||||
| No Apparent Heart Disease | 265 (13.1%) | 58 (8.0%) | 53 (7.3%) | 81 (13.4%) | 70 (11.6%) | 69 (25.7%) | 60 (22.4%) | ||||
| History of Congestive Heart Failure - no. (%) | 475 (23.4%) | 223 (30.6%) | 221 (30.3%) | 0.91 | 121 (20.0%) | 112 (18.5%) | 0.51 | 27 (10.1%) | 24 (9.0%) | 0.66 | |
|
Duration of Qualifying Atrial Fibrillation >= 2 days - no. (%) |
1406 (69.4%) | 519 (71.2%) | 525 (72.1%) | 0.7 | 410 (67.7%) | 406 (67.0%) | 0.81 | 175 (65.3%) | 172 (64.2%) | 0.79 | |
|
First Episode of Atrial Fibrillation (vs. Recurrent Episode) - no. (%)2 |
700 (35.8%) | 260 (36.6%) | 254 (34.8%) | 0.48 | 223 (37.9%) | 214 (35.3%) | 0.35 | 78 (29.7%) | 73 (27.2%) | 0.54 | |
|
Any Prerandomization Failure of an Antiarrhythmic Drug - no. (%) |
364(18.0%) | 146 (20.0%) | 139(19.1%) | 0.64 | 83 (13.7%) | 70 (11.6%) | 0.26 | 60 (22.4%) | 69 (25.7%) | 0.36 | |
| Size of Left Atrium Normal - no. (%)3 | 549 (35.3%) | 196 (36.6%) | 184 (33.5%) | 0.28 | 175 (36.1%) | 172 (35.9%) | 0.96 | 94 (43.1%) | 90 (41.9%) | 0.79 | |
| Left Ventricular Ejection Fraction1,3,4 | 54.9±13.1 | 49.4±14.8 | 48.7±16.9 | 0.71 | 57.4±12.1 | 56.7±11.0 | 0.64 | 58.5±11.6 | 61.4± 8.4 | 0.08 | |
| Normal Left Ventricular Ejection Fraction - no. (%)3 | 1131 (74.9%) | 326 (63.8%) | 324 (61.6%) | 0.46 | 374 (80.1%) | 370 (79.4%) | 0.8 | 190 (89.6%) | 195 (89.9%) | 0.94 | |
| Baseline CCS Class | - No Angina | 1835(90.5%) | 637 (87.4%) | 636 (87.2%) | 0.98 | 558(92.1%) | 539 (88.9%) | 0.12 | 256 (95.5%) | 258 (96.3%) | 0.9 |
| - Class I | 135(6.7%) | 60 (8.2%) | 62 (8.5%) | 37(6.1%) | 56 (9.2%) | 8 (3.0%) | 7 (2.6%) | ||||
| - Class II or Greater | 57(2.8%) | 32 (4.4%) | 31 (4.3%) | 11 (1.8%) | 11 (1.8%) | 4 (1.5%) | 3 (1.1%) | ||||
| Baseline NYHA Class - No CHF | 1618(79.8%) | 541(74.2%) | 532 (73.0%) | 0.10 | 517 (85.3%) | 526 (86.8%) | 0.86 | 242 (90.3%) | 240 (89.6%) | 0.85 | |
| - Class I | 215(10.6%) | 81 (11.1%) | 96 (13.2%) | 76 (12.5%) | 70 (11.6%) | 16 (6.0%) | 19(7.1%) | ||||
| - Class II | 158(7.8%) | 89 (12.2%) | 71 (9.7%) | 12 (2.0%) | 9 (1.5%) | 10 (3.7%) | 9 (3.4%) | ||||
| - Class III | 36(1.8%) | 18 (2.5%) | 30 (4.1%) | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | ||||
Abbreviations as in Table 1
Plus-minusvalues are mean ± SD
This information was not collected on the initial version of the data form and therefore was imputed for 143 patients
ECGs were obtained in 3311/4060. The size of the LA was unknown in 185/3311 cases, and left ventricular function (where normal was defined as LVEF = 0.50) was unknown in 279/3311. ECG information was not used in propensity score models.
Outcomes Analysis
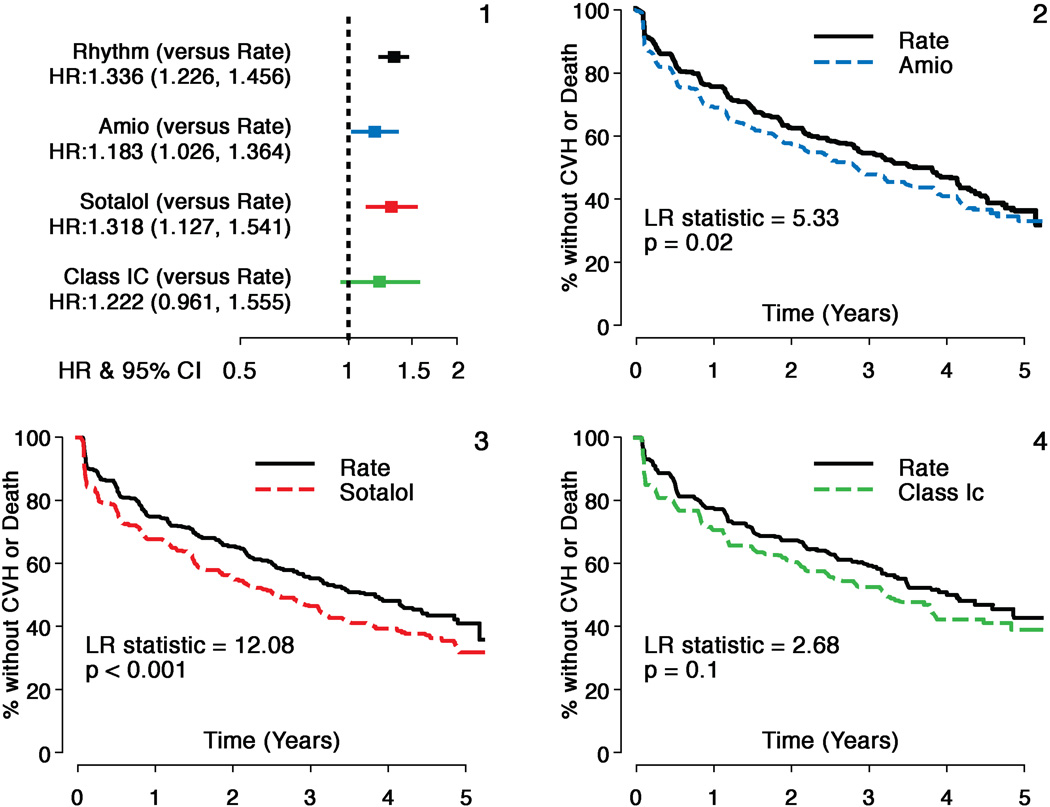
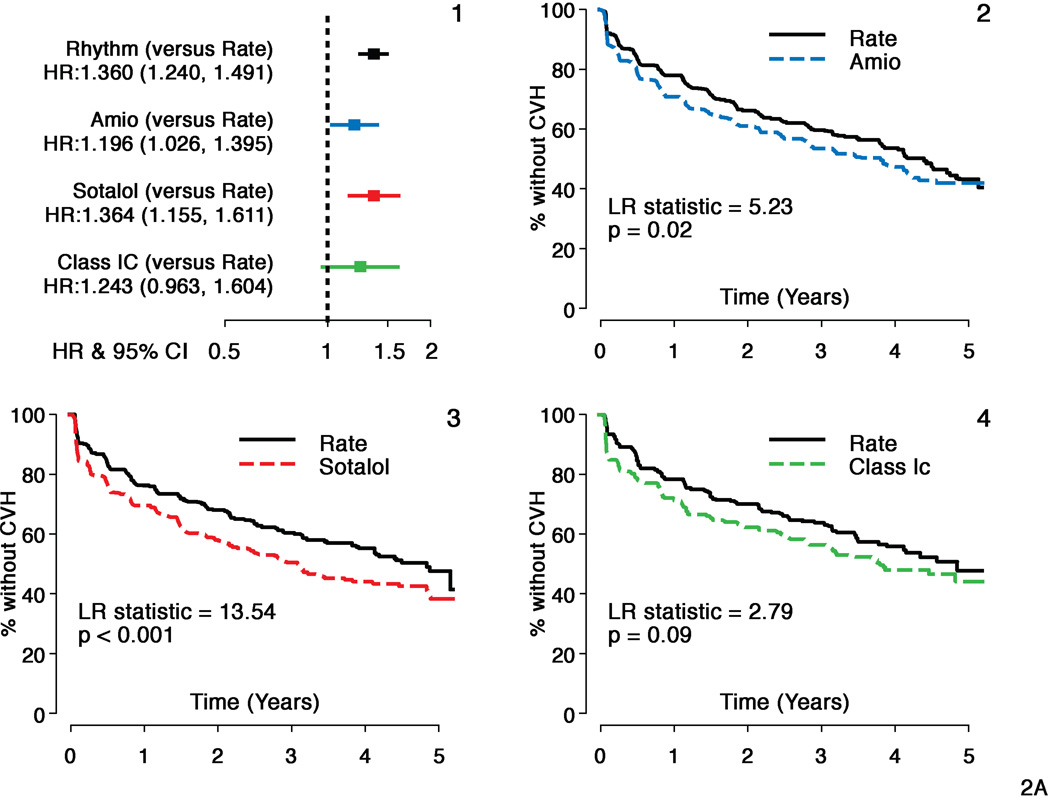
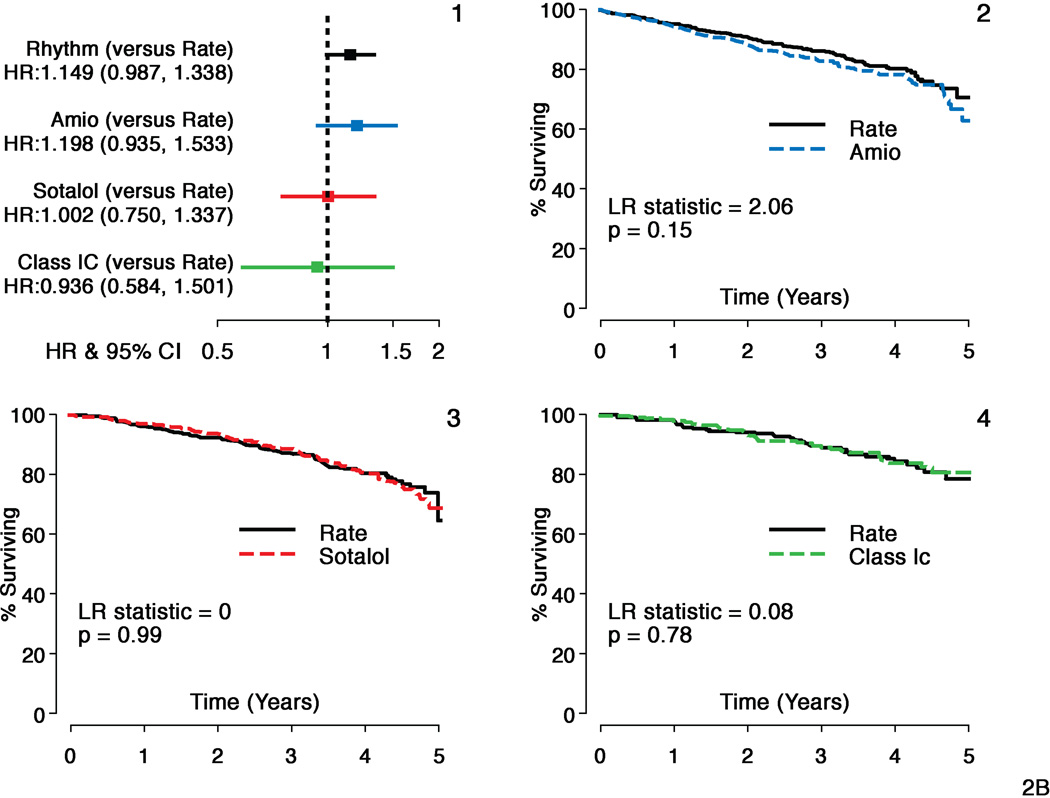
Hazard ratios (HR) and 95% confidence intervals (CI) for the overall comparison (rhythm compared to rate) in AFFIRM and individual AAD subgroups to the matched rate cohort are shown for the composite principal outcome of mortality and first CV hospitalization in Figure 1. All AAD cohorts had inferior principal outcomes compared to Rate (HR for amiodarone=1.18; CI: 1.03–1.36, p=0.02; HR for sotalol =1.32; CI: 1.13–1.54, p<.001 and HR for class 1C =1.22; CI: 0.97–1.56, p=0.10 compared to Rate). In the smaller class 1C cohort, this difference did not reach statistical significance. Figure 2 shows the individual components of the composite endpoint. Risk of CV hospitalization was increased for all 3 AAD cohorts (amiodarone HR=1.20, CI: 1.03–1.40, p=0.05, sotalol HR=1.36, CI: 1.16–1.61, p<0.001 and class 1C HR=1.24, CI: 0.96–1.64, p=0.09 compared to Rate). Ninety one percent of amiodarone patients, 88% of sotalol patients and 78% of class 1C patients were on the initially selected drug at first CV hospitalization. There was no increased mortality risk for sotalol and class 1C cohorts, but an increase in risk was observed for amiodarone (HR= 1.20, CI: 0.94–1.53, p=0.15) compared to Rate, which was not statistically significant. Time to first CV hospitalization was shorter for all AADs compared to Rate. First CVH event rates at 3 yrs were 47% for amiodarone, 50% for sotalol and 44% for class 1C compared to 40%, 40% and 36% respectively for the matched Rate cohorts. CV mortality did not differ between Rate and any of the AAD cohorts (p>0.15 for all comparisons). There was an increased risk of non-cardiovascular mortality with amiodarone (HR=1.11, CI=1.01–1.24, p=0.04) but not with sotalol or class 1C drugs compared to Rate. However, deaths attributable to cancer or pulmonary causes were comparable across each cohort.
Figure 1. Comparison of Composite Principal Outcome: Individual Antirarrhythmic Drugs versus Rate.
Hazard ratios and Kaplan Meier survival analyses comparing individual antiarrhythmic drugs (AADs) with matched rate cohorts for the composite principal outcome (Time to First CV Hospitalization or Death).
- Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
- Propensity score matched Rate and amiodarone subgroups
- Propensity score matched Rate and sotalol subgroups
- Propensity score matched Rate and class1C subgroups.
All AADs and matched rate cohorts show substantial event rates for the principal outcome during follow-up but all AADs studied had a higher risk of events during follow-up.
Figure 2. First CV Hospitalization: Individual Antirarrhythmic Drugs versus Rate.
Panel A:
Hazard ratios and Kaplan Meier survival analyses comparing individual antiarrhythmic drugs (AADS) with matched rate cohorts for a component of principal outcome - Time to First CV Hospitalization
- Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
- Propensity score matched Rate and amiodarone subgroups
- Propensity score matched Rate and sotalol subgroups
- Propensity score matched Rate and class 1C subgroups.
All AADs and matched rate cohorts show substantial event rates during follow-up but all AADs studied had a significantly higher risk of a first CV hospitalization during follow-up.
Panel B:
Mortality: Individual Antirarrhythmic Drugs versus Rate
Hazard ratios and Kaplan Meier survival analyses comparing individual antiarrhythmic drugs with matched rate cohorts for a component of principal outcome - Time to Death
- Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
- Propensity score matched Rate and amiodarone subgroups
- Propensity score matched Rate and sotalol subgroups
- Propensity score matched Rate and class 1C subgroups.
Sotalol and Class 1C groups and matched rate cohorts show comparable event rates for risk of death during follow-up, but there is a non-significant increase in mortality with amiodarone compared to its matched rate cohort.
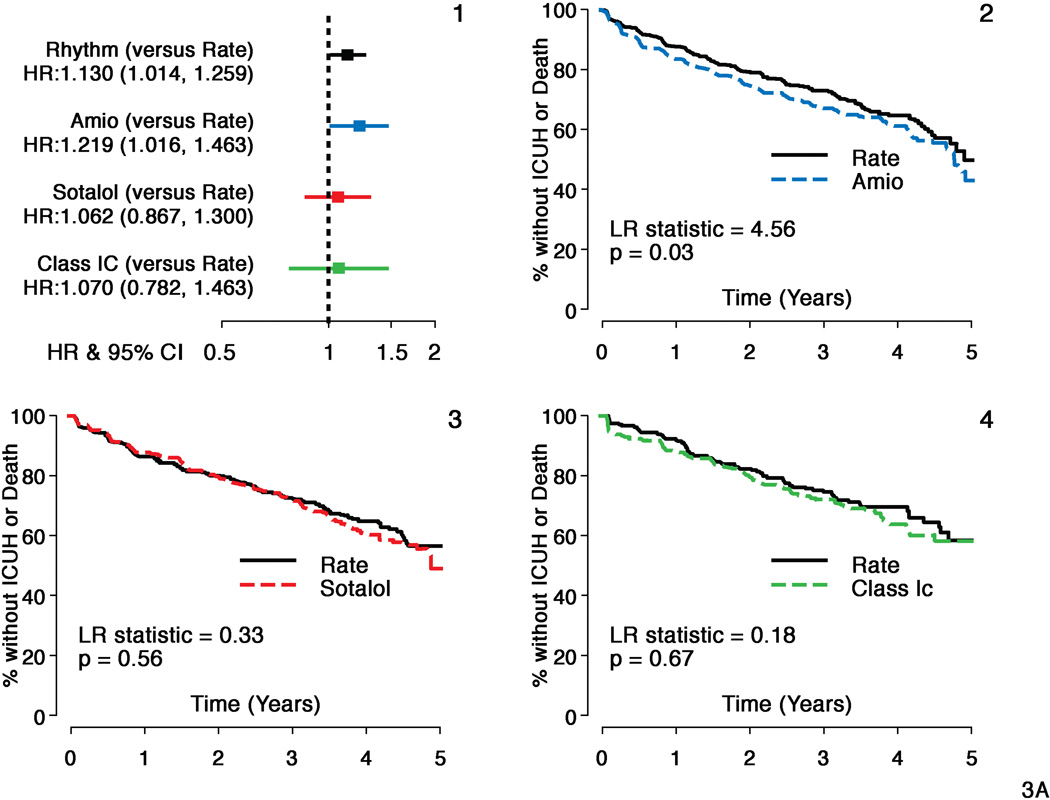
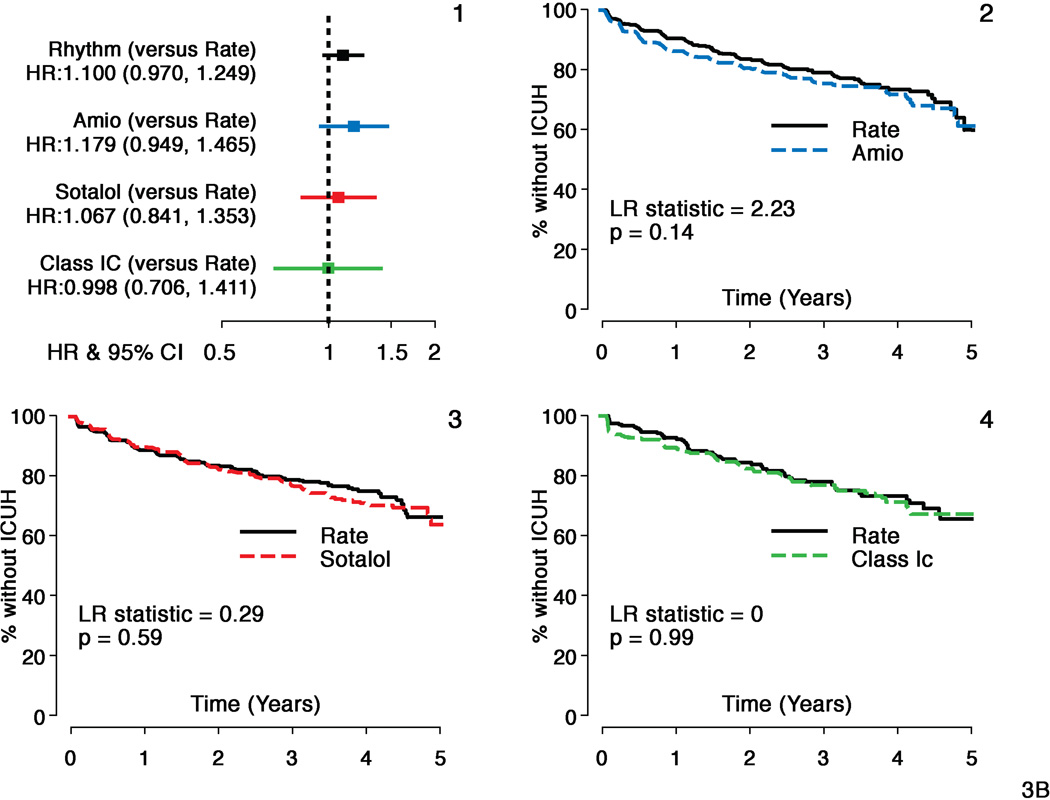
A composite of death or ICU hospitalizations showed moderately increased risk with amiodarone (HR = 1.22, CI: 1.02–1.46, p=0.03) but not with sotalol or class 1C agents (HR 1.06, CI: 0.87–1.30, p=0.56 and HR=1.07, CI: 0.78–1.46, p=0.67 respectively) compared to Rate (Figure 3, panel A). There was no difference in time to ICU hospitalizations for sotalol and class 1C compared to Rate, but a non-significant increased risk was noted for amiodarone (HR= 1.18, CI: 0.95–1.47, p=0.14) (Figure 3, panel B). All-cause hospitalizations were increased in amiodarone compared to Rate (HR=1.19, CI: 1.05–1.35, p=0.008) and in sotalol compared to Rate (HR=1.22, CI: 1.06–1.41, p=0.005). There was no increased risk of all-because hospitalization with class 1C compared to Rate.
Figure 3. Secondary Composite Outcome (ICU Hospitalizations or Death): Individual Antirarrhythmic Drugs versus Rate.
Panel A:
Hazard ratios and Kaplan Meier survival analyses comparing individual antiarrhythmic drugs (AADs) with matched rate cohorts for secondary composite outcome - Time to First Hospitalization with intensive care unit stay (ICUH) or Death
- Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
- Propensity score matched Rate and amiodarone subgroups
- Propensity score matched Rate and sotalol subgroups
- Propensity score matched Rate and c 1C subgroups.
Composite outcome shows time to ICUH or death was shorter with amiodarone but not with sotalol or class 1C versus Rate during follow-up.
Panel B:
Comparison of ICU Hospitalizations: Individual Antirarrhythmic Drugs versus Rate
Hazard ratios and Kaplan Meier survival analyses comparing individual antiarrhythmic drugs with matched rate cohorts for secondary outcome - Time to First ICU Hospitalization (ICUH)
- Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
- Propensity score matched Rate and amiodarone subgroups
- Propensity score matched Rate and sotalol subgroups
- Propensity score matched Rate and class1C subgroups.
Time to ICUH was comparable for sotalol and Class 1C groups compared to matched rate cohorts but a non-significant increased risk was seen with amiodarone compared to rate during follow-up.
Concomitant beta-blocker therapy did not alter outcomes for either sotalol or class 1C cohorts for either mortality or CV hospitalization risk (CVH for sotalol HR=1.09, CI: 0.89–1.34, for death HR=1.15, CI: 0.81–1.63; CVH for class 1C HR=0.75, CI: 0.60–1.03), for death HR=0.65, CI: 0.40–1.07). Amiodarone-Rate cohort patients who were taking beta blockers had increased mortality risk (CVH risk for amiodarone HR=1.06, CI: 0.90–1.25, for death HR=1.53, CI: 1.16–2.02). There was no evidence of a treatment-digoxin interaction for the principal outcome. Time-dependent digoxin use was significantly associated with CVH in the amiodarone-Rate cohorts (HR: 1.43, CI: 1.21–1.68) and in the class1C-Rate cohorts (HR: 1.36, CI: 1.04–1.77), but not in the sotalol-Rate cohorts (HR: 1.15, CI: 0.96–1.37). After adjusting for time-dependent digoxin use, AADs still increased the risk of CVH (HR for amiodarone = 1.34, CI: 1.13–1.57, HR for sotalol= 1.40, CI: 1.17–1.67 compared to matched rate patients, HR for class1C =1.34, CI: 1.03–1.75 compared to the respective AAD rate-matched patients). The increased risk of CV hospitalization or death was consistent across clinically important subgroups including coronary disease, female gender and age for amiodarone and sotalol patients, presence of thyroid disease only in amiodarone patients but in none of the subgroups examined for the class 1C patients. These results are detailed in the next section.
CV hospitalizations categorized by intensity, duration and associated procedures are tabulated in Table 3. There were substantially more hospitalizations of <3 days duration associated with cardioversion in the amiodarone and sotalol cohorts than matched rate cohorts. Cardioversion occurred at similar rates in the matched class 1C and Rate cohorts (7.2%). CV hospitalizations with a length of stay of <3 days with a cardioversion procedure alone (without another CV procedure, emergency room visits or ICU stay, i.e. events that may reflect adherence to AF rhythm control treatment strategy only) constituted 6.1 %, 6.1% and 4.0% of first CV hospitalizations for amiodarone, sotalol, and class 1C, respectively. The corresponding rates in the matched Rate cohorts were 1.9%, 1.6%, and 0.9% respectively. Stroke, embolism and major bleeds accounted for only a minority of first CVH in both AAD and rate cohorts (Table 3). Warfarin use at first CVH or death was slightly but not significantly higher in the rate cohorts.
Table 3.
Cardiovascular hospitalization profiles in the propensity score matched patient cohorts for individual antiarrhythmic drugs in the AFFIRM trial
| Amiodarone | Rate | Sotalol | Rate | Class 1C | Rate | |
|---|---|---|---|---|---|---|
| CV Hosp. | 342 | 309 | 310 | 252 | 126 | 111 |
| # Fatal First CVH - no. (%) | 13 (3.8%) | 14 (4.5%) | 11(3.5%) | 5 (2.0%) | 3(2.4%) | 8(7.2%) |
| CVH < 3 d - no. (%) | 96(28.1%) | 95(30.7%) | 82(26.5%) | 70(27.8%) | 41(32.5%) | 35(31.5%) |
| CVH < 3d + CV- no. (%) | 40(11.7%) | 11(3.6%) | 35(11.3%) | 9(3.6%) | 12(9.5%) | 8(7.2%) |
| CVH<3d, CV, no ER/ICU - no. (%) |
21(6.1%) | 6(1.9%) | 19(6.1%) | 4 (1.6%) | 5(4.0%) | 1 (0.9%) |
| CVH > 3 d - no. (%) | 246(71.9%) | 214(69.3%) | 228(73.5%) | 182 (72.2%) | 85(67.5%) | 76(68.5%) |
| ICU Days First CVH- no. (%) | 95(27.8%) | 84(27.2%) | 66(21.3%) | 72 (28.6%) | 32(25.4%) | 34(30.6%) |
| Warfarin use at First CVH (% of CVH) |
279 (81.6%) |
275 (89.0%) |
273 (88.1%) |
227 (90.1%) |
108 (85.7%) |
101 (91.0%) |
| Bleeds/stroke/embolic events(% of CVH) |
42 (12.3%) |
54 (17.5%) |
28 (9.1%) |
40 (15.9%) |
13 (14.1%) |
18 (16.2%) |
| Warfarin use at above Event (% of event) |
31 (73.8%) |
47 (87.0%) |
17 (60.7%) |
31 (77.5%) |
12 (67.7%) |
7 (50.0%) |
Abbreviations: CV – cardiovascular; CVH – Cardiovascular hospitalization; ER – emergency room visit; ICU – intensive care unit stay;
Potential Risk Factors for CV Hospitalization (Tables 4 and 5)
Table 4.
Relationship between Baseline Characteristics and Risk of Cardiovascular Hospitalization
| Amiodarone | Rate | Sotalol | Rate | Class 1C | Rate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (PSM) (N=729) |
(PSM) (N=729) |
(PSM) (N=606) |
(PSM) (N=606) |
(PSM) (N=268) |
(PSM) (N=268) |
|||||||||||
| Age - yr | 70.3 ± 9.21 | 70.26 ± 9.21 | 69.7 ± 8.79 | 69.67 ± 8.79 | 0.212 | 69.6 ± 8.8+ | 69.56 ± 8.83 | 70.5 ± 8.4 | 70.48 ± 8.35 | 0.061* | 68.6 ± 8.9 | 68.64 ± 8.93 | 68.6 ± 9.3 | 68.58 ± 9.33 | 0.94 | |
| Female Sex - no. (%) | 238 (32.6%) | 238 (32.6%) | 282 (38.7%)* | 282 (38.7%) | 0.016* | 227 (37.5%) | 227 (37.5%) | 234 (38.6%) | 234 (38.6%) | 0.679 | 140 (52.2%) | 140 (52.2%) | 144 (53.7%) | 144 (53.7%) | 0.729 | |
| Minority Group - no. (%) | 78 (10.7%) | 78 (10.7%) | 98 (13.4%) | 98 (13.4%) | 0.108 | 57 (9.4%) | 57 (9.4%) | 70 (11.6%) | 70 (11.6%) | 0.223 | 24 (9.0%) | 24 (9.0%) | 23 (8.6%) | 23 (8.6%) | 0.879 | |
| CAD (MI, angina etc.) | 247 (33.9%) | 247 (33.9%) | 228 (31.3%) | 228 (31.3%) | 0.921 | 158 (26.1%) | 158 (26.1%) | 165 (27.2%) | 165 (27.2%) | 0.742 | 31 (11.6%) | 31 (11.6%) | 28 (10.4%) | 28 (10.4%) | 0.655 | |
| Dilated Cardiomyopathy | 52 (7.1%) | 52 (7.1%) | 53 (7.3%) | 53 (7.3%) | 20 (3.3%) | 20 (3.3%) | 22 (3.6%) | 22 (3.6%) | 5 (1.9%) | 5 (1.9%) | 5 (1.9%) | 5 (1.9%) | ||||
| Hypertension | 342 (46.9%) | 342 (46.9%) | 351 (48.1%) | 351 (48.1%) | 315 (52.0%) | 315 (52.0%) | 291 (48.0%) | 291 (48.0%) | 156 (58.2%) | 156 (58.2%) | 154 (57.5%) | 154 (57.5%) | ||||
| Valvular Heart Disease | 28 (3.8%) | 28 (3.8%) | 30 (4.1%) | 30 (4.1%) | 34 (5.6%) | 34 (5.6%) | 34 (5.6%) | 34 (5.6%) | 15 (5.6%) | 15 (5.6%) | 9 (3.4%) | 9 (3.4%) | ||||
| No Apparent Heart Disease | 53 (7.3%) | 53 (7.3%) | 58 (8.0%) | 58 (8.0%) | 70 (11.6%) | 70 (11.6%) | 81 (13.4%) | 81 (13.4%) | 60 (22.4%) | 60 (22.4%) | 69 (25.7%) | 69 (25.7%) | ||||
| History of CHF - no. (%) | 221 (30.3%) | 221 (30.3%) | 223 (30.6%) | 223 (30.6%) | 0.909 | 112 (18.5%) | 112 (18.5%) | 121 (20.0%) | 121 (20.0%) | 0.512 | 24 (9.0%) | 24 (9.0%) | 27 (10.1%) | 27 (10.1%) | 0.659 | |
| Qualifying AF >2d - no. (%) | 525 (72.1%) | 525 (72.1%) | 519 (71.2%) | 519 (71.2%) | 0.696 | 406 (67.0%) | 406 (67.0%) | 410 (67.7%) | 410 (67.7%) | 0.806 | 172 (64.2%) | 172 (64.2%) | 175 (65.3%) | 175 (65.3%) | 0.786 | |
| First Episode of AF -no.(%) | 254 (34.8%) | 254 (34.8%) | 260 (36.6%) | 260 (36.6%) | 0.482 | 214 (35.3%) | 214 (35.3%) | 223 (37.9%) | 223 (37.9%) | 0.349 | 73 (27.2%) | 73 (27.2%) | 78 (29.7%) | 78 (29.7%) | 0.537 | |
| Prior Failure of AAD - no. (%) | 139 (19.1%) | 139 (19.1%) | 146 (20.0%) | 146 (20.0%) | 0.644 | 70 (11.6%) | 70 (11.6%) | 83 (13.7%) | 83 (13.7%) | 0.261 | 69 (25.7%) | 69 (25.7%) | 60 (22.4%) | 60 (22.4%) | 0.363 | |
| Normal LA Size - no. (%) | 184 (33.5%) | 184 (33.5%) | 196 (36.6%) | 196 (36.6%) | 0.282 | 172 (35.9%) | 172 (35.9%) | 175 (36.1%) | 175 (36.1%) | 0.955 | 90 (41.9%) | 90 (41.9%) | 94 (43.1%) | 94 (43.1%) | 0.791 | |
| Mean LVEF (%) | 48.7 ±16.9 | 48.66 ±16.91 | 49.4 ±14.8 | 49.40 ±14.84 | 0.709 | 56.7 ±11.0 | 56.74 ±11.03 | 57.4 ±12.1 | 57.39 ±12.06 | 0.64 | 61.4 ± 8.4 | 61.39 ± 8.36 | 58.5 ±11.6 | 58.51 ±11.58 | 0.081* | |
| CCS Class | - No Angina | 636 (87.2%) | 636 (87.2%) | 656 (90.0%) | 637 (87.4%) | 0.976 | 539 (88.9%)* | 539 (88.9%) | 564 (93.1%) | 558 (92.1%) | 0.122 | 258 (96.3%) | 258 (96.3%) | 259 (96.6%) | 256 (95.5%) | 0.897 |
| 62 (8.5%) | 60 (8.2%) | 56 (9.2%) | 37 (6.1%) | 7 (2.6%) | 8 (3.0%) | |||||||||||
| - Class I | 62 (8.5%) | 49 (6.7%) | 56 (9.2%) | 31 (5.1%) | 7 (2.6%) | 4 (1.5%) | ||||||||||
| - Class ≥ II | 31 (4.3%) | 31 (4.3%) | 24 (3.3% | 32 (4.4%) | 11 (1.8%) | 11 (1.8%) | 11 (1.8%) | 11 (1.8%) | 3 (1.1%) | 3 (1.1%) | 5 (1.9%) | 4 (1.5%) | ||||
| NYHA Class | - No CHF | 532 (73.0%) | 532 (73.0%) | 539 (73.9%)+ | 541 (74.2%) | 0.095* | 526 (86.8%) | 526 (86.8%) | 527 (87.0%) | 517 (85.3%) | 0.861 | 240 (89.6%)+ | 240 (89.6%) | 248 (92.5%) | 242 (90.3%) | 0.853 |
| 96 (13.2%) | 81 (11.1%) | 70 (11.6%) | 76 (12.5%) | 19 (7.1%) | 16 (6.0%) | |||||||||||
| - Class I | 96 (13.2%) | 81 (11.1%) | 70 (11.6%) | 58 (9.6%) | 19 (7.1%) | 13 (4.9%) | ||||||||||
| - Class II | 71 (9.7%) | 71 (9.7%) | 91 (12.5%) | 89 (12.2%) | 9 (1.5%) | 9 (1.5%) | 19 (3.1%) | 12 (2.0%) | 9 (3.4%) | 9 (3.4%) | 4 (1.5%) | 10 (3.7%) | ||||
| - Class III | 30 (4.1%) | 30 (4.1%) | 18 (2.5%) | 18 (2.5%) | 1 (0.2%) | 1 (0.2%) | 2 (0.3%) | 1 (0.2%) | 0 (0.0%) | 3 (1.1%) | ||||||
Abbreviations: Amio - amiodarone
Table 5.
Relationship between Time Dependent Changes in Clinical Status and Risk of Cardiovascular Hospitalizations
| Amio versus Rate | Sotalol versus Rate | Class 1C versus Rate | |||||
|---|---|---|---|---|---|---|---|
| HR and 95% CI | p-value | HR and 95% CI | p-value | HR and 95% CI | p-value | ||
|
SR to AF NYHA Class |
1.87(1.40, 2.50) | <.0001 | 1.76(1.29, 2.41) | <.001 | 1.11(0.64, 1.94) | 0.7139 | |
| I | 1.82(1.45, 2.29) | <.0001 | 1.35(1.00, 1.82) | <.0001 | 2.17(1.30, 3.63) | <.0001 | |
| II | 2.28(1.78, 2.93) | 1.81(1.19, 2.77) | 1.95(1.00, 3.82) | ||||
| III | 3.51(2.42, 5.09) | 3.72(2.19, 6.33) | 4.23(1.49, 12.04) | ||||
| IV | 7.44(3.42, 16.20) | 15.61(4.65, 52.47) | 22.45(6.01, 83.82) | ||||
|
Increase in NYHA class CHC Class |
1.72(1.35, 2.20) | <.0001 | 1.98(1.39, 2.83) | <.001 | 1.25(0.67, 2.34) | 0.4772 | |
| I | 2.20(1.68, 2.89) | <.0001 | 1.26(0.82, 1.92) | 0.4877 | 2.58(1.28, 5.19) | <.001 | |
| II | 3.57(2.40, 5.30) | 1.62(0.87, 3.01) | 5.42(2.09, 14.01) | ||||
| III | 3.73(1.61, 8.64) | 2.20(0.64, 7.48) | 6.37(1.19, 34.01) | ||||
| IV | 4.08(1.29, 12.88) | 1.96(0.45, 8.61) | 28.74(3.10, 266.46) | ||||
| Increase in CHC Class | 1.25(0.87, 1.80) | 0.2218 | 2.35(1.40, 3.92) | <.01 | 0.90(0.36, 2.22) | 0.8144 | |
| VR | 1.13(1.04, 1.24) | 0.0067 | 1.10(1.00, 1.21) | 0.0518 | 0.99(0.84, 1.16) | 0.8984 | |
| Increase in VR by ≥ 15 bmp | 1.25(0.96, 1.64) | 0.1017 | 1.58(1.20, 2.07) | <.01 | 1.62(1.04, 2.51) | 0.0328 |
Abbreviations: SR – sinus rhythm; AF – atrial fibrillation; VR – ventricular rate; NYHA – New York Heart Association heart failure class; CHC – Canadian Heart Association Classification for angina pectoris. Hazard ratio for VR is the increase in risk associated with a 15 bpm increase in VR
Baseline historical characteristics that increased risk of CV hospitalization with AAD compared to matched rate cohorts are shown in Table 4. Female gender was associated with increased risk in sotalol and class 1C cohorts compared to matched Rate cohorts, but this was not observed in the amiodarone-Rate cohort comparison. A history of heart failure, coronary disease and diabetes at enrollment were associated with increased risk for CV hospitalization in all antiarrhythmic drug cohorts. Pulmonary disease at baseline was associated increased risk of CV hospitalization with amiodarone, and age >75 years with sotalol. There was evidence of significant AAD - comorbidity interactions only in the amiodarone cohort; age >75 years and thyroid disease were associated with increased risk for amiodarone patients but not for their matched Rate counterparts. A significant increased risk for CV hospitalization was maintained for amiodarone and sotalol compared to Rate despite adjustments for age, gender or any of these comorbidities.
Time- dependent changes in clinical status that increased risk of CV hospitalization are shown in Table 5. In the amiodarone patient cohort, relapse from sinus rhythm to AF and increase in NYHA class by 1 or more were associated with a 1.9 and 1.7-fold increase in CVH risk, respectively. For sotalol, relapse from sinus rhythm to AF, increase ≥ 1 in NYHA class, increase in angina class by 1 or more and ventricular rate increase ≥15 bpm were all associated with increased risk for CV hospitalization. For class 1C, ventricular rate increase ≥15 bpm was associated with increased risk. Higher absolute ventricular rate (in steps of 15 bpm) was associated with increased risk for sotalol and class 1C patients. Overall, a higher NYHA class was associated with increased risk for all cohorts and higher angina class for amiodarone and class 1C patients.
Discussion
Analyses of overall and secondary outcomes for the AF population in the AFFIRM study have suggested no overarching benefit of a particular strategy (5,11–13). There was, however, a non-significant increase in mortality in the rhythm arm with an excess in pulmonary and cancer deaths (5, 14). This finding raised the specter of AAD therapy related mortality risk. The impact of individual AAD selection on both mortality and hospitalization compared to rate has not been available due to the investigator determined process for AAD selection, which makes unbiased comparisons challenging. However, such an analysis is still relevant and potentially informative since most of these agents are currently in widespread clinical use and still employed in clinical trials (15, 16).
To evaluate these agents individually, we employed propensity score matching to permit comparative analysis with the rate control patients. (17) In this report, it produced highly comparable Rate and AAD cohorts for demographics, disease status and severity, prior interventions, and therapy. (Table 1)
Major findings of Study
1. Clinical outcomes, especially CV hospitalization, are affected by initial anti-arrhythmic drug selection
The present analysis demonstrates inferior performance in the principal clinical outcome for the individual AADs studied versus rate control for the AFFIRM population. This difference in composite outcome was largely due to excess and earlier CV hospitalizations for each AAD. Sotalol and class 1C cohorts were comparable to Rate for all cause mortality. The hazard ratio comparing amiodarone to Rate was very similar to the overall AFFIRM study result for mortality risk with rhythm control, but in this small matched cohort the power to see a significant difference was low (< 30%). Initial amiodarone therapy was associated with significantly increased risk of non-CV death, and mortality plus ICU hospitalization. The sotalol and class 1C cohorts were similar to Rate with respect to these outcomes, suggesting that the excess CV hospitalizations seen with these drugs were less serious events than those seen with amiodarone.
2. Cardiovascular hospitalization was extremely common with AF therapies in AFFIRM
From our data, we can estimate overall CV hospitalization risk for AF populations and its relation to therapy selection during the period 1995–2001. CV hospitalization incidence ranged from 36–50% at three years for rate and rhythm therapies. CV hospitalization rates in the AFFIRM Rate subgroups were similar to those seen in the placebo (rate control therapies only) arm of ATHENA (36.3% at 2.5 years) (15).
3. Clinical characteristics and initial antiarrhythmic drug selection rather than treatment strategy influenced cardiovascular hospitalization risk
Potential mechanisms proposed for increased CV hospitalizations include hospitalizations related to change in AAD therapy with associated cardioversion or possible higher warfarin discontinuation rates with potential complications (5,12). Our analysis of CV hospitalizations related solely to cardioversions for the rhythm control strategy, while higher than in matched Rate cohorts, demonstrated a fairly low incidence in all AAD cohorts. Stoke, embolism and major bleeds also had a low incidence that was comparable in the matched Rate cohorts. Longer hospitalizations, ICU stays and other CV procedures constituted the bulk of CV hospitalizations, suggesting more serious clinical conditions. Differences in CV hospitalization rates persisted across clinically important subgroups such as the elderly, women, and coronary disease patients.
CV Hospitalizations in Atrial Fibrillation: Insights from the AFFIRM trial
CV hospitalization has become a major endpoint for clinical trials. It can impact treatment strategy recommendations, and regulatory approval of new therapies but is rarely used in AF trials (15, 18–20). CV hospitalizations in AF are costly, with average costs estimated to exceed $12,000 per AF admission in the USA, and 3 billion US dollars in annual costs (21). AF hospitalizations are widely assumed to be related to AF recurrences, but such an assumption has neither been critically verified and quantified, nor has the uniformity of this risk been assessed across AF subpopulations or treatments (22).
To date, small trials of non-pharmacologic therapies and one large pharmacologic therapy trial have provided some information about CV hospitalization in AF (15, 18–20). Analysis of the AFFIRM database provides important additional data from a large randomized controlled trial over a long follow-up. CV hospitalizations presaged mortality but it was unclear how these events related to treatment strategy and clinical condition (12). Given the observations with respect to ICU hospitalizations, CV hospitalizations are usually related to serious morbidity, with treatment strategy related hospitalizations such as for a change of drug therapy or for cardioversion being a relatively small component. Excess CV hospitalization events observed with the AADs evaluated are associated with age, gender and co-morbidity status. There is a residual excess CV hospitalization risk even after adjustment for these historical factors which is related to AAD use. Additionally, CV hospitalization risk can be related to changes in cardiovascular disease status longitudinally. Time-dependent changes that impact risk can include either AF relapses or worsening of major cardiovascular symptoms of the underlying disease. Based on our analysis, we propose that both baseline patient characteristics and time dependent changes in clinical status contribute to CV hospitalization risk. Any heart failure or coronary disease was associated with increased risk in all three matched cohorts but was more common in the amiodarone and matched Rate cohorts. An increase in heart failure or angina class by one or more increased risk of CV hospitalization. These findings make a strong case for baseline disease state variables and change in clinical status leading to CV hospitalization.
Relapse from sinus rhythm to AF was also related to CV hospitalization, suggesting failure of rhythm control as a potential mechanism. Finally, specific interactions of antiarrhythmic agents such as amiodarone with comorbidities such as thyroid disease suggest additional mechanisms leading to hospitalization. The reasons for CV hospitalizations are multiple and multifactorial. AF patients have varying risk for the principal outcome in this analysis based on these factors.
Limitations
Propensity score matching cannot correct for erroneous omission or inclusion of variables that might have affected AAD selection but it is a significant improvement over naïve subgroup analyses. Some of the hospitalizations may be the result of routine patient care for rhythm control rather than for medical necessity, but these still occur in current clinical practice. The AFFIRM study did not capture detailed reasons for hospitalization or drug doses. Exact dates of hospitalization were not collected, which results in decreased precision in estimates of time to hospitalization, but probably not for the comparison of matched cohorts.
Acknowledgments
Dr. Saksena is or has been a consultant, investigator and research grant recipient for the National Heart Lung and Blood Institute, Medtronic Inc., St. Jude Medical Inc., sanofi-aventis, Sorin group, Aryx Pharmaceuticals and has been a speaker bureau member for sanofi aventis. Dr. Wyse is a consultant to Boehringer Ingelheim, Medtronic, Bristol Myers Squibb/Pfizer, sanofi Aventis, Biotronik, Boston Scientific/Guidant, NHLBI, Duke Clinical Research Institute, European Commission, Merck, Medtronic, and Bayer and speaker bureau member for sanofiaventis. Dr. Waldo is a consultant to Biotronik, St. Jude Medical, Daiichi, Sankyo Pharmaceuticals, Medtronic, Inc., Astellas, Biosense Webster, Inc., Bristol Meyers Squibb, Portola, Boehringer Ingelheim, CardioInsight Technologies, Merck, AtriCure, Inc.sanofi aventis and is a speaker for sanofi aventis.
Abbreviations
- AF
atrial fibrillation
- AAD
antiarrhythmic drug
- CV
cardiovascular
- ICU
intensive care unit
- AFFIRM
Atrial Fibrillation Follow-Up Investigation of Rhythm Management
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs Reynolds and Freemantle are consultants for sanofi Aventis. Ms. Slee, Dr. Y. Rosenberg, Ms. S. Grant, Ms. E. Thomas, and Mr. S. Rathod have no conflicts of interest.
Dr. Saksena and Ms. Slee had full access to the entire data and the entire investigator group had full access to the analyses.
References
- 1.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998 Sep 8;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.AF Hospitalisations in the USA. 2009 June; www.nhlbi.nih.gov/resources/docs/cht-book.htm.
- 3.Miyasaka Y, Barnes ME, Gersh BJ, et al. Changing trends of hospital utilization in patients after their first episode of atrial fibrillation. Am J Cardiol. 2008;102:568–572. doi: 10.1016/j.amjcard.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Planning and Steering Committees of the AFFIRM Study for the NHLBI AFFIRM Investigators. Atrial Fibrillation Follow-up Investigation of Rhythm Management -- the AFFIRM study design. Am J Cardiol. 1997;79:1198–1202. [PubMed] [Google Scholar]
- 5.The AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 6.Corley SD, Epstein AE, DiMarco JP, et al. AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983:41–55. [Google Scholar]
- 8.Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials- greater precision with greater uncertainty. Journal of the American Medical Association. 2003;289:2554–2559. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]
- 9.AFFIRM First Antiarrhythmic Drug Substudy Investigators Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM sub study of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20–29. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 10.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 11.Sherman DG, Kim SG, Boop BS, et al. National Heart, Lung, and Blood Institute AFFIRM Investigators. Occurrence and characteristics of stroke events in the Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) study. Arch Intern Med. 2005;165(10):1185–1191. doi: 10.1001/archinte.165.10.1185. [DOI] [PubMed] [Google Scholar]
- 12.Wyse DG, Slee A, Epstein AE, et al. Alternative endpoints for mortality in studies of patients with atrial fibrillation: the AFFIRM study experience. Heart Rhythm. 2004;1:531–537. doi: 10.1016/j.hrthm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins LS, Brodsky M, Schron E, et al. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg JS, Sadaniantz A, Kron J, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109:1973–1980. doi: 10.1161/01.CIR.0000118472.77237.FA. [DOI] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Crijns HJ, van Eickels M, et al. ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360 doi: 10.1056/NEJMoa0803778. 668-78.10. [DOI] [PubMed] [Google Scholar]
- 16.Wilber DJ, Pappone C, Neuzil P, et al. ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 17.Bosco JLF, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 19.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study) Eur Heart J. 2006;27:216–221. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 20.Rao BH, Saksena S. Impact of "hybrid therapy" on long-term rhythm control and arrhythmia related hospitalizations in patients with drug-refractory persistent and permanent atrial fibrillation. J Interv Card Electrophysiol. 2007;18:127–136. doi: 10.1007/s10840-007-9091-3. [DOI] [PubMed] [Google Scholar]
- 21.Naccarelli G, Johnston S, Lin J, Patel P, Schulman K. Cost Burden of Cardiovascular Hospitalization and Mortality in ATHENA-Like Patients with Atrial Fibrillation/Atrial Flutter in the United States. Clinical Cardiology. 2010;33:270–279. doi: 10.1002/clc.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–504. doi: 10.1016/j.amjcard.2004.04.068. [DOI] [PubMed] [Google Scholar]