Abstract
Magnetic resonance imaging (MRI) cell tracking has become an important non-invasive technique to interrogate the fate of cells upon transplantation. At least 6 clinical trials have been published at the end of 2010, all of which have shown that real-time monitoring of the injection procedure, initial engraftment, and short-term biodistribution of cells is critical to further advance the field of cellular therapeutics. In MRI cell tracking, cells are loaded with superparamagnetic iron oxide (SPIO) particles that provide an MRI contrast effect through microscopic magnetic field disturbances and dephasing of protons. Magnetic particle imaging (MPI) has recently emerged as a potential cellular imaging technique that promises to have several advantages over MRI, primarily linear quantification of cells, a higher sensitivity, and “hot spot” tracer identification without confounding background signal. Although probably not fully optimized, SPIO particles that are currently used as MRI contrast agent can be employed as MPI tracer. Initial studies have shown that cells loaded with SPIO particles can give a detectable MPI signal, encouraging further development of MPI cell tracking.
Keywords: Magnetic resonance imaging, magnetic particle imaging, superparamagnetic iron oxide, cell tracking
1. WHAT HAS MRI CELL TRACKING TAUGHT US?
MRI cell tracking using superparamagnetic iron oxide (SPIO) particles has now found many applications in understanding the fate of cells following transplantation1, potentially facilitating the translation of promising new cell therapies into the clinic2. SPIO particles endow the cells of interest with hypointense contrast after proper labeling3. One SPIO formulation, Feridex®, was initially approved by the FDA in 1996 and was sold in Europe under the name of Endorem™. It was originally developed as a liver contrast agent (uptake by Kupffer cells) but did not live up to its promise and has, at this time, been taken off the market. It has so far been the only pharmaceutical-grade MR contrast agent used for clinical cell tracking.
The easiest and safest method of SPIO-labeling is spontaneous uptake of particles by phagocytic cells, such as macrophages, microglia, and immature dendritic cells. Neutral, smaller particles, such as ultrasmall SPIO (USPIO) are designed for longer blood half-life applications (e.g., lymph node imaging) and are less efficient for cell labeling. For non-phagocytic cells that do not spontaneously take up SPIO particles, the most widely used labeling strategy is to use cationic (positively charged) transfection agents to shuttle these contrast agents into cells4.
The first pre-clinical studies and the concept of MRI cell tracking were introduced in the early 1990s5-7. Several studies appeared over the years, describing mostly the use of SPIO-labeled immune cells in immunotherapy. It was not until the first serial in vivo studies of cell migration were reported 8, and the use of transfection agents for efficient intracellularlabeling was introduced8-10, that MRI cell tracking saw an explosive growth of pre-clinical studies showing proof-of-concept in many cell migration/homing scenarios1, 11. Because of the emergence of cellular therapeutics, and the need for high-resolution, non-invasive tracking methods that can be used for translational studies, MRI cell tracking entered the clinic in 2005 (Figure 1)12.
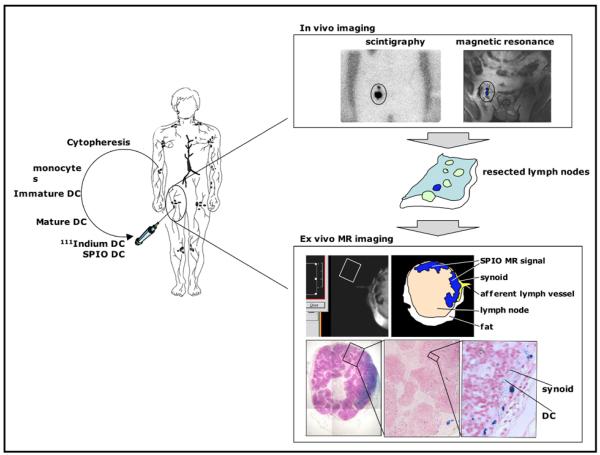
Figure 1.
First clinical MRI cell tracking study. Monocytes are obtained by cytopheresis from stage-III melanoma patients. They are cultured and labeled with SPIO particles and 111In-oxine. Cells are then injected intranodally into a (either cervical, inguinal or axillary) lymph node basin that is to be resected and their biodistribution is monitored in vivo by scintigraphy and MRI at 3 Tesla. The resected lymph nodes can be visualized with high resolution MRI at 7 Tesla and histology. Adapted from Ref.12
What have we learned from MRI cell tracking? First, it is feasible, using a clinical routine setup, to detect SPIO-labeled cells, not only in the injected lymph node, but also in the nearby lymph nodes they migrated to (Figure 1). This occurred with cells containing approximately 30 pg iron per cell13, with MRI performed at 3 Tesla and using conventional pulse sequences. Using labeling with 111In-oxine in parallel, it was estimated that the sensitivity at the coil set-up at a resolution of 0.5 × 0.5 × 3.5 mm was approximately 15,000 cells12. It also became evident that, due to its flexible 3D multi-planar nature, MRI was superior to radionuclide imaging with regard to the detection of the accurate number of nodes that contained injected DCs.
Second, in eight patients, cells were found to be accidentally misinjected in half the cases, This poor successful injection rate for procedures performed by experienced radiologists was not known until the results of the MRI cell tracking were available. On the radionuclide scans, only a “cloud” of radioactivity was visible, in the area of the draining lymph node bed, but, when this was cross-referenced with the MRI scans containing anatomical information, it was clear that the cells were either injected in the surrounding muscle or subcutaneous fat. The results of this first clinical MRI cell tracking study are a testament to the absolute need for a non-invasive technique that can assess the accuracy of successful cell injections, and which can preferably guide the actual injection itself as well, in real-time.
2. WHAT ARE THE LIMITATIONS OF MRI CELL TRACKING?
Due to its indirect detection of cells through the SPIO effect on proton relaxation, there are several limitations inherent to MRI cell tracking. These include 1) the difficulty to absolutely quantify cell concentration and iron content - part of the difficulty relies in the existence of different relaxation regimes (dependent on the agglomeration state and size of SPIO cluster); 2) the difficulty of discriminating SPIO-labeled cells in areas of hemorrhage and traumatic injury (which are often present in targets of cell therapy), as caused by the proton dephasing effects of methemoglobin, ferritin, and hemosiderin (especially at higher fields); 3) the occasional misinterpretation of isolated “black spots” due to differences in magnetic susceptibility effects around blood vessels and air-tissue interfaces (i.e. stomach and GI tract); and 4) the inability to track cells in areas devoid of proton signal (i.e., the lungs).
In addition, MRI cell tracking using SPIO labels cannot discriminate live from dead cells, as the label persists upon cell death. Also, when cells divide rapidly, parental cells dilute the label among daughter cells and at some point the label will become undetectable. These two limitations will also exist in MPI. In order to only image live cells, and with a marker that does not dilute, MRI reporter genes are required. While several approaches have been pursued (see review by Gilad et al.)14, no widely used, robust MRI reporter gene-based cell tracking system yet exists.
3. WHAT IS THE PROMISE OF MPI CELL TRACKING?
MPI promises to take away some of these limitations. First described in 200515, it relies on the non-linear response of magnetic material as a direct manner for detecting the presence of a SPIO nanoparticle agent in an oscillating magnetic field. Spatial encoding can be realized by a static, inhomogeneous magnetic field, saturating the magnetic material almost everywhere except in the vicinity of a special point, the field free point.
As the detection is through direct effects, it is a true “hot spot” or tracer technique, without any confounding other sources of signal. Therefore, SPIO-labeled cells should be detectable in areas of hemorrhage or traumatic injury, as neither methemoglobin, ferritin, or hemosiderin are superparamagnetic. There should also be no MPI signal present originating from magnetic susceptibility effects around blood vessels or air-tissue interfaces, and cells should be detectable in the lungs. Experimental studies need to be performed in order to determine whether or not it is possible to absolutely quantify cell concentration and iron content, that is, if the MPI signal is the same for single isolated SPIO particles as it is for clustered, larger agglomerates often encountered in biological samples.
At the present time, preliminary studies have shown that stem cells can be readily detected with an MPI spectrometer at biologically relevant concentrations, and that MPI enables a linear quantification of both cell number and iron content over a wide range of concentrations, regardless of the state of SPIO as free or intracellular entity. It was also evident that SPIO particles having equivalent efficacy (“molar relaxivity”) on MRI displayed significant differences in generating MPI signal. The underlying physicochemical phenomena are, at present, poorly understood, opening up up a new avenue of research in synthesizing and testing of novel SPIO formulations that can be used as MPI tracers.
4. WHAT IS NEEDED TO DEVELOP MPI CELL TRACKING?
MPI cell tracking is still in its infancy. Pre-clinical prototype animal scanners have been built, but are not yet commercially available. An alliance of Philips Healthcare and Bruker Biospin has been announced, and it is expected that machines will become available during the next year. This will allow investigators to test novel SPIO particles, determine MPI cell tracking sensitivity, and its spatial resolution, which are both inherent to the physical properties of SPIO particles. There appear to be no physical constraints towards developing a whole body human scanner16, but a clinical SPIO agent will have to be developed now that the clinical MRI agents Feridex® (Endorem) and Resovist® are no longer available. At the 2011 SPIE conference, some new SPIO formulations were presented with promising results17. To exploit its full potential, an anatomical imaging modality combined with MPI is highly desirable, and whether this will be MRI or computed tomography (CT) remains to be seen.
5. ACKNOWLEDGMENTS
J.W.M.B. is supported by NIH EUREKA RO1 DA026299. JB, BG, and MK are supported by the German Federal Ministry of Education and Research under grant numbers FKZ 13N9079 and FKZ 13N11086.
REFERENCES
- [1].Long CM, Bulte JWM. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin. Biol. Ther. 2009;9:293–306. doi: 10.1517/14712590802715723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bulte JWM. In vivo MRI cell tracking: clinical studies. Am. J. Roentgenol. 2009;193:314–325. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bulte JWM, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- [4].Frank JA, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- [5].Bulte JWM, et al. Selective MR imaging of labeled human peripheral blood mononuclear cells by liposome mediated incorporation of dextran-magnetite particles. Magn. Reson. Med. 1993;29:32–37. doi: 10.1002/mrm.1910290108. [DOI] [PubMed] [Google Scholar]
- [6].Hawrylak N, et al. Nuclear magnetic resonance (NMR) imaging of iron oxide-labeled neural transplants. Exp. Neurol. 1993;121:181–192. doi: 10.1006/exnr.1993.1085. [DOI] [PubMed] [Google Scholar]
- [7].Yeh TC, Zhang W, Ildstad ST, Ho C. Intracellular labeling of T-cells with superparamagnetic contrast agents. Magn. Reson. Med. 1993;30:617–625. doi: 10.1002/mrm.1910300513. [DOI] [PubMed] [Google Scholar]
- [8].Bulte JWM, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- [9].Hoehn M, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frank JA, et al. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad. Radiol. 2002;9(Suppl 2):S484–487. doi: 10.1016/s1076-6332(03)80271-4. [DOI] [PubMed] [Google Scholar]
- [11].Muja N, Bulte JWM. Magnetic resonance imaging of cells in experimental disease models. Progr. NMR Spectr. 2009;55:61–77. doi: 10.1016/j.pnmrs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de Vries IJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- [13].Verdijk P, et al. Sensitivity of magnetic resonance imaging of dendritic cells for in vivo tracking of cellular cancer vaccines. Int. J. Cancer. 2007;120:978–984. doi: 10.1002/ijc.22385. [DOI] [PubMed] [Google Scholar]
- [14].Gilad AA, Winnard PT, Jr., van Zijl PCM, Bulte JWM. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- [15].Gleich B, Weizenecker J. Tomographic imaging using the nonlinear response of magnetic particles. Nature. 2005;435:1214–1217. doi: 10.1038/nature03808. [DOI] [PubMed] [Google Scholar]
- [16].Weizenecker J, Borgert J, Gleich B. A simulation study on the resolution and sensitivity of magnetic particle imaging. Phys. Med. Biol. 2007;52:6363–6374. doi: 10.1088/0031-9155/52/21/001. [DOI] [PubMed] [Google Scholar]
- [17].Ferguson RM, Khandhar AP, Krishnan KM. Biomedical Applications in Molecular, Structural, and Functional Imaging. Disney Coronado Springs eEsort; SPIE Conference; Lake Buena Vista, FL, USA. 2011.p. 31. [Google Scholar]