Abstract
Muscle weakness and effort intolerance are common in maintenance hemodialysis (MHD) patients. This study characterized morphometric, histochemical, and biochemical properties of limb muscle in MHD patients compared with controls (CTL) with similar age, gender, and ethnicity. Vastus lateralis muscle biopsies were obtained from 60 MHD patients, 1 day after dialysis, and from 21 CTL. Muscle fiber types and capillaries were identified immunohistochemically. Individual muscle fiber cross-sectional areas (CSA) were quantified. Individual fiber oxidative capacities were determined (microdensitometric assay) to measure succinate dehydrogenase (SDH) activity. Mean CSAs of type I, IIA, and IIX fibers were 33, 26, and 28% larger in MHD patients compared with CTL. SDH activities for type I, IIA, and IIX fibers were reduced by 29, 40, and 47%, respectively, in MHD. Capillary to fiber ratio was increased by 11% in MHD. The number of capillaries surrounding individual fiber types were also increased (type I: 9%; IIA: 10%; IIX: 23%) in MHD patients. However, capillary density (capillaries per unit muscle fiber area) was reduced by 34% in MHD patients, compared with CTL. Ultrastuctural analysis revealed swollen mitochondria with dense matrix in MHD patients. These results highlight impaired oxidative capacity and capillarity in MHD patients. This would be expected to impair energy production as well as substrate and oxygen delivery and exchange and contribute to exercise intolerance. The enlarged CSA of muscle fibers may, in part, be accounted for by edema. We speculate that these changes contribute to reduce limb strength in MHD patients by reducing specific force.
Keywords: chronic renal failure, muscle fiber size, muscle fiber oxidative capacity, muscle capillaries, mitochondria
patients with chronic renal failure exhibit skeletal muscle dysfunction, particularly of limb muscles (1). This contributes to impaired muscle strength and endurance capacity and associated effort intolerance (14, 17, 26, 27, 38–40). There have been several excellent reports over the last 30 yr detailing alterations in fiber size and morphometry, ultrastructural abnormalities, altered fiber type proportions, impaired capillarity, and abnormal overall biochemistry (e.g., reduced citrate synthase, an oxidative enzyme; Refs. 4, 5, 11–13, 20, 23, 24, 29, 31–33). There are, however, limitations to several of these studies conducted in patients with chronic renal failure. These include variable results with regard to most of the reported parameters; small numbers of patients studied; suboptimal control subjects (e.g., healthy athletes) or absence of controls; use of older, less precise techniques for assays; nonuniform acquisition and preparation of muscle biopsy specimens for optimal analysis; and different muscles studied, including nonlimb muscles.
We have previously reported low exercise tolerance, endurance capacity, and limb strength in a small cohort of maintenance hemodialysis (MHD) patients (39), as well as impaired limb muscle strength and power in a recent cohort of MHD patients, which are included in this current report (40). The aim of the present study was to evaluate the possible morphometric and biochemical basis for these limb muscle abnormalities in a larger group of MHD patients by examining vastus lateralis muscle biopsies. In addition, we attempted to address some of the limitations of prior studies by including sufficient numbers of MHD patients together with healthy sedentary control subjects of similar age and gender/racial/ethnic distribution. Quantitative histochemical analysis of succinate dehydrogenase (SDH) activity (a mitochondrial oxidative enzyme) within individual fibers, immunohistochemical identification of muscle fiber types, detailed assessment of capillarity, and computer-based image analysis of fiber morphometry were performed. In addition, complementary ultrastructural analyses were conducted with specific attention to muscle mitochondria. We hypothesize that impaired limb muscle oxidative capacity and diffusion reserves account, in part, for reduced muscle endurance capacity in MHD patients.
METHODS
Subjects.
This study was carried out in 60 patients, 37 men and 23 women, who had been undergoing MHD for a mean of 49 ± 57 (SD) mo (range of 6 to 297 mo) and 21 normal control subjects, 16 men and 5 women. The mean age was 44 ± 11 (SD) yr (range of 22 to 64 yr) for MHD patients and 41 ± 12 (SD) yr (range of 22 to 60 yr) for control subjects. Because hemodialysis patients tend to be less active physically, we specifically sought to enroll control subjects who exhibited similar levels of sedentary lifestyle as one of several criteria to control for. Other criteria included controlling for similar age, gender distribution, and racial/ethnic mix. Further, all control subjects were clinically stable without evidence of chronic illnesses or acute inflammatory processes. In particular, control subjects exhibited normal serum creatinine levels with no prior history of renal disease or hypertension. Several criteria were employed to provide optimal control for MHD patients, who had been on dialysis for ≥6 mo before enrollment. These included stable clinical status during the conduct of the study. In particular, no evidence of acute or chronic inflammatory states and absence of severe heart, lung, or liver failure and muscle or joint diseases. No patients had insulin-dependent diabetes, vasculitis, or a functioning renal transplant. None received corticosteroids or abused alcohol or other illicit drugs. As described in our previous study (see Ref. 40 for details) on 51 MHD patients of the current cohort of patients and the same 21 control subjects, a basic inclusion requirement for participation in the study was that each MHD patient and normal control subject gave a history of not engaging in recent manual labor, exercise training, or heavy sports activity. Hemodialysis was performed 3 times per week, with each session lasting 4 h. This study was approved by the Human Subjects Committee of the Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles, Medical Center and by the Institutional Review Board of the Burns and Allen Research Institute at Cedars-Sinai Medical Center, and informed written consent was obtained from all subjects.
Muscle biopsies.
Biopsies were obtained from the right vastus lateralis muscle as previously described (40). One muscle sample was prepared for histochemical analyses. Of note, all muscle samples were processed by a single experienced investigator (M. Fournier) in an identical fashion for all cases. The sample was observed under a dissecting scope, rapidly blotted of extra fluid, and cleaned of fat and blood if present, and the fiber orientation was verified. The muscle specimen was mounted on cork with optimal cutting temperature compound (Tissue-Tek, Sakura Finetek, Torrance, CA), oriented for transverse sectioning (i.e., with fibers perpendicular to cork surface), and then rapidly frozen in isopentane, which had been cooled to its melting point by liquid nitrogen. The fresh frozen muscle samples were stored at −80°C until analysis. Of note, all histochemical, quantitative histochemical and immunohistochemical studies, including initial sectioning of muscle specimens were performed by a single experienced investigator to minimize variation in sample processing and analysis.
Ultrastructural analysis.
As ultrastructural analyses are extremely labor intensive, such studies were performed in only 25% of the entire cohort, i.e., in 15 MHD patients. In these patients, another small sample of the muscle biopsy was fixed in 2.5% glutaraldehyde, postfixed in osmium tetroxide, and, following standard procedures for dehydration, embedded in plastic. One-micrometer “semi-thin” sections stained with methylene blue were assessed by light microscopy. Thin sections stained with lead citrate and uranyl acetate were examined by electron microscopy. With the use of our previously reported study protocol (19), mitochondria were evaluated for swelling, disintegration, presence of inclusions, and myocytes for arrangement, preservation of Z-bands, lysis of myocytes, glycogen distribution, and increase in quantity. Each of these parameters were semiquantitatively graded from 0 (normal) to 3+ (most abnormal).
Immunohistochemistry: muscle fiber types and capillaries.
For the assessment of muscle fiber classification and capillarity, serial cross-sections of the muscle sample were cut (10 μm thickness) using a cryostat (model 2800E; Reichert-Jung, Nussloch, Germany) kept at −20°C. Muscle cryosections were dried at room temperature, fixed in cold acetone for 5 min, washed with PBS for 5 min, and incubated in 5% goat serum for 15 min at room temperature. For the identification of capillaries, muscle sections were exposed to a monoclonal mouse antibody (MAb; IgG1) against human endothelial cells, specifically platelet/endothelial adhesion molecule (PECAM-1) or CD31 (clone JC/70A; DAKO North America, Carpinteria, CA) for 1 h at room temperature. Various anti-myosin heavy chains (MyHC) MAbs were used for the indirect immunoperoxidase identification of MyHC isoforms within single fibers as previously described (18). Our aim in this study was to classify muscle fibers into the major fiber types only, i.e., based on the MyHC isoform mainly expressed. We did not specifically attempt to explore coexpressing/hybrids fibers. Serial muscle sections were incubated for 1 h at room temperature in one the following MAbs: A4.951, reacting with human MyHC I (β/slow); N2.261, reacting with human MyHCs I + IIA; and A4.74, reacting with human fast MyHCs (i.e., IIA + IIX). These mouse MAbs (IgG1) were raised from hybridoma cell lines obtained from the American Type Culture Collection. Sections were processed as previously described (18).
Muscle morphometry.
Muscle fiber proportions (into types I, IIA, and IIX), fiber cross-sectional areas (CSAs), and capillaries were determined from microscopic images of digitized muscle sections, using a computer-based imaging-processing system as we previously described (18). A microscope stage micrometer was used to calibrate the imaging system for morphometry. Using a ×20 microscope objective, we determined that each pixel had an area of 0.15 μm2. Muscle fiber proportions, CSAs, and capillaries were determined from samples of 150–200 fibers for each subject. Group means for each fiber type were computed from the average values obtained from each individual subject (i.e., no pooling of fibers). Individual fiber CSAs were determined from the number of pixels within manually outlined fiber boundaries. Several indexes of capillarity were determined as follows: 1) the capillary-to-fiber ratio, i.e., the total number of capillaries divided by the total number of fibers within the muscle section; 2) the capillary density, defined as the number of capillaries per square millimeter of muscle area; and 3) the number of capillaries per fiber (and per fiber type), i.e., capillary contacts per fiber, or the number of capillaries surrounding each fiber.
Single fiber SDH activity.
Fiber oxidative capacity was determined by quantifying the activity of SDH (a key mitochondrial enzyme in the Krebs cycle) in individual muscle fibers. The methodology employed to quantitate SDH activity has been described in detail in previous reports (e.g., Refs. 7, 8, 18, 37). The mean SDH activity of each fiber was expressed as millimoles of fumarate per liter of tissue per minute. The same fibers sampled for measurement of CSA were analyzed for SDH from each specimen. The SDH activity of each individual fiber was used to determine the mean SDH activity for each fiber type. Group means for each fiber type were computed from the average values obtained from each individual subject (i.e., no pooling of fibers).
Statistical analysis.
The distribution of data was tested for normality. Statistical analysis was performed using ANOVA (SigmaStat v. 2.0; Jandel, Richmond, CA). If a significant interaction was found, post hoc analysis (Newman-Keuls test) was used to compare differences in independent groups. An α-level of 0.05 was used to determine significance. Values are presented as means ± SE in results except in Tables 1 and 2.
Table 1.
Characteristics of subjects
| MHD Patients1 | Control Subjects2 | |
|---|---|---|
| Number, M/F | 37/23 | 16/5 |
| Racial/ethnic background | ||
| African American, M/F | 14/13 | 3/2 |
| Hispanic, M/F | 16/8 | 10/2 |
| Caucasian, M/F | 2/1 | 1/1 |
| Philipino, M/F | 4/0 | 2/0 |
| Asian, M/F | 0/1 | 0/0 |
| Other, M/F | 1/0 | 0/0 |
| Age, yr | 43.8 ± 11.0* | 40.9 ± 12.4 |
| Duration of hemodialysis, mo | 49.0 ± 57.1 | – |
| Diabetes mellitus, M/F | 4/3 | 0/0 |
Table 2.
Body composition of subjects
| MHD Patients1 |
Control Subjects2 |
|||||
|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |
| Number | 37 | 23 | 60 | 16 | 5 | 21 |
| Height, cm | 170.1 ± 7.0* | 161.7 ± 7.8 | 166.9 ± 8.3 | 171.4 ± 8.8 | 161.4 ± 8.9 | 169.0 ± 9.7 |
| Weight, kg | 72.2 ± 13.8 | 75.2 ± 26.7 | 73.3 ± 19.5 | 73.2 ± 10.4 | 61.6 ± 8.5 | 70.4 ± 11.0 |
| Body mass index, kg/m2 | 25.6 ± 4.8 | 28.2 ± 8.3 | 26.6 ± 6.4 | 24.9 ± 2.9 | 23.6 ± 2.2 | 24.0 ± 3.0 |
RESULTS
Subject demographics and clinical data.
Characteristics of MHD and control subjects are provided in Table 1. Body composition of patients and controls are depicted in Table 2.
Muscle fiber proportions and morphology.
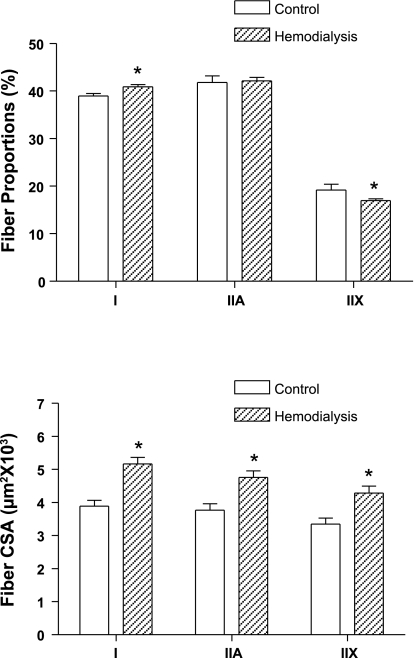
The muscle fibers of the vastus lateralis muscle were classified based on their immunoreactions to antibodies against specific MyHCs, and the proportions of types I, IIA, and IIX muscle fibers were determined for both control subjects and MHD patients. There was a small, but significantly greater proportion of type I fibers and a similar lower proportion of type IIX fibers in MHD patients compared with control subjects (P < 0.05; Fig. 1, top). The mean CSA from individual type I, IIA, and IIX vastus lateralis muscle fibers in MHD patients were larger by 33% (P < 0.001), 26% (P < 0.01), and 28% (P < 0.05), respectively, compared with those of control subjects (Fig. 1, bottom).
Fig. 1.
Proportions of types I, IIA, and IIX muscle fibers (top) in the vastus lateralis of control subjects (open bars) and maintenance hemodialysis (MHD) patients (hatched bars). There was a small (2%), but significantly greater, proportion of type I fibers and lower proportion of type IIX fibers in MHD patients (P < 0.05). Mean cross-sectional areas (CSA) from individual type I, IIA, and IIX muscle fibers (bottom) were significantly larger by 33% (P < 0.001), 26% (P < 0.01), and 28% (P < 0.05), respectively, in MHD patients. Values are means ± SE. *Significantly different from control group.
SDH activity.
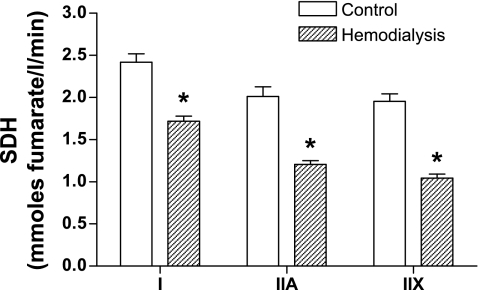
Vastus lateralis muscle oxidative capacity was assessed by measuring the rate of SDH activity within individual muscle fibers. The mean SDH activities from individual type I, IIA, and IIX muscle fibers in the vastus lateralis of MHD patients were found to be lower by 29% (P < 0.0001), 40% (P < 0.0001), and 47% (P < 0.0001), respectively, compared with those of control subjects (Fig. 2).
Fig. 2.
Mean succinate dehydrogenase (SDH) activity from individual type I, IIA, and IIX muscle fibers in the vastus lateralis of MHD patients (hatched bars) were lower by 29% (P < 0.0001), 40% (P < 0.0001), and 47% (P < 0.0001), respectively, compared with control subjects (open bars). Values are means ± SE. *Significantly different from control group.
Muscle fiber capillarity.
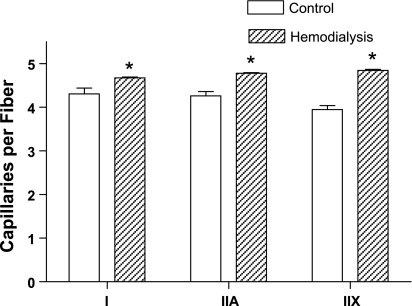
Muscle fiber capillarization was measured in both control subjects and MHD patients, and several indexes of capillarity are shown in Table 3. There were a significantly greater number of capillary contacts per fiber in MHD patients, compared with control subjects; this was observed in relation to all fiber types. As shown in Table 3, the mean number of capillaries contacting individual muscle fibers was 12% greater in MHD patients (P < 0.001), compared with control subjects. When observed in each individual fiber type, the mean number of capillaries around type I, IIA, and IIX fibers in the vastus lateralis of MHD patients were significantly greater by 9% (P < 0.001), 10% (P < 0.001), and 23% (P < 0.001), respectively, compared with control subjects (Fig. 3). The capillary-to-fiber ratio (i.e., the total number of capillaries divided by the total number of fibers within a muscle section) in the vastus lateralis of MHD patients was significantly greater by 11% (P < 0.0001), compared with control subjects (Table 3). However, the capillary density (the number of capillaries per square millimeter of muscle area) in the vastus lateralis of MHD patients was found to be lower by 34% (P < 0.0001), compared with control subjects (Table 3).
Table 3.
Indexes of capillarity in control and chronic hemodialysis patients
| Capillaries per Fiber | Capillary-to-Fiber Ratio | Capillary Density, number/mm2 | |
|---|---|---|---|
| Control | 4.22 ± 0.45 | 1.66 ± 0.16 | 544 ± 162 |
| Hemodialysis | 4.74 ± 0.11* | 1.84 ± 0.08§ | 362 ± 113§ |
Values are means ± SE.
P < 0.001, significantly different from control.
P < 0.0001, significantly different from control.
Fig. 3.
Mean number of capillaries per individual type I, IIA, and IIX muscle fibers in the vastus lateralis of MHD patients (hatched bars) were significantly greater by 9% (P < 0.001), 10% (P < 0.001), and 23% (P < 0.001), respectively, compared with control subjects (open bars). Values are means ± SE. *Significantly different from control group.
Ultrastructural analysis.
One-third of MHD patients in whom ultrastructural analyses were performed revealed normal organelles. Mitochondrial changes were, however, appreciated in two-thirds of MHD patients. These were often quite subtle and consisted of dilatation (grade 1–2+) of isolated and widely scattered mitochondria. Of those patients with mitochondrial abnormalities, myocyte and Z-band disruption were evident in 9% and depleted glycogen in 18%. These abnormalities were graded 1–2+. No abnormal inclusions were present, and there was no evidence of cell lysis. Figure 4 depicts an example of the muscle ultrastructure of a control subject and MHD patient.
Fig. 4.
Representative ultrastructure of the vastus lateralis muscle from a healthy control subject (top) and from a MHD patient (bottom). Note a cluster of swollen mitochondria (arrows) and an area of Z-band disruption (*) with hemodialysis. Note difference in scale bars between control and MHD. Mitochondria (arrows) in controls are clearly seen at ×3 the magnification of MHD, and their average size is still smaller than or comparable with MHD.
DISCUSSION
We (39) have previously reported low exercise tolerance, endurance capacity and limb muscle strength in a small cohort of MHD patients compared with healthy control subjects with similar age, gender and ethnicity. Indeed, constant work rate was significantly lower in MHD assigned to an exercise training cohort at baseline, compared with control subjects (32% of control values; Ref. 39). Similarly, with incremental cycle ergometry studies, peak work rate and peak V̇o2 were significantly lower in the same cohort of patients, compared with healthy control subjects (i.e., 43 and 57% of control values; Ref. 39). Further, quadriceps strength measured with the five-repetitive maximum technique was reduced in the MHD patients (strength 78% that of controls; Ref. 39). Thus our results are consistent with the impaired exercise capacity and exercise endurance previously reported in MHD patients (17, 39). Of interest, the decrement in leg muscle strength appeared less than the substantial curtailment in exercise capacity. Our present study was thus designed to evaluate possible morphometric and biochemical abnormalities that may underlie these observations in a large cohort of MHD patients compared with healthy control subjects.
Muscle fiber oxidative capacity.
Despite robust data demonstrating a 31% decrease in citrate synthase activity in locomotor muscles of rats with chronic renal failure (2), the data in patients are conflicting. For example, citrate synthase activity was not reduced in quadriceps muscle biopsies from young adult patients with chronic renal failure (23). Further, Pastoris et al. (28) reported increased activities of several oxidative enzymes (citrate synthase, SDH) in limb muscle biopsies from chronic renal failure patients. By contrast, reduced fractional synthesis rate of mitochondrial proteins and reduced activity of muscle oxidative enzymes (citrate synthase and muscle cytochrome C oxidase) were reported in vastus lateralis muscle biopsies of patients with chronic renal failure compared with healthy controls (3). Our findings are in keeping with reduced activity of mitochondrial oxidative enzymes. In addition, we add to the literature that SDH activity in specific muscle fiber types (i.e., I, IIA, and IIX fibers) of the vastus lateralis in MHD patients is reduced. Furthermore, our study was performed on substantially greater numbers of patients (3 to 10 times) than has been previously reported regarding muscle oxidative capacity.
In this regard, the present study has several strengths. These include a large cohort of patients with controls (similar age, gender distribution, and racial/ethnic backgrounds) as well as validated quantitative histochemical techniques (8), which enabled us to assess SDH activity within individual fibers and fiber types. This methodological approach provides more specific data than those obtained from the use of muscle homogenates for biochemical analyses. Our study is the first to demonstrate reduced SDH activity in type I, IIA, and IIX fibers of the vastus lateralis muscle in MHD patients. This has important implications for muscle fatigability and endurance, as type I and IIA fibers are more oxidative, are recruited first for low level activities and exhibit greater fatigue resistance (e.g., Refs. 16, 36). Thus significantly reduced oxidative capacity of limb muscle fibers likely explains, at least in part, impaired exercise endurance in MHD patients.
Muscle fiber capillarity.
In the present study, despite modest increments in number of capillaries per fiber, significant decrement in capillary density (i.e., a decrease in capillaries per unit area of contractile muscle tissue) was noted in the MHD patients compared with controls. Thus, despite an attempt to increase capillarity, the increment in fiber size was greater so that the number of capillaries per square millimeter of muscle area (i.e., capillary density) was less in hemodialysis patients. Of interest, our absolute values for our three indexes of capillarity were almost identical to those reported by Sakkas et al. (32) in limb muscles of MHD patients. Thus, in the present study, we hypothesize that adaptations to augment capillary supply, while positive, would likely be insufficient to support and maintain adequate diffusion capabilities and reserve for oxygen and nutrient exchange, particularly under conditions of the increased demand of exercise. This concept is supported by Sala et al. (34) who reported low oxygen muscle conductance in limb muscles of patients with chronic renal failure. Of interest, impaired capillarity in rats with chronic kidney disease was evident even in the early stages of disease and more profound in locomotor skeletal muscles (15).
Muscle fiber proportions and morphometry.
In the present study, we observed in the MHD patients compared with the controls a modestly but significantly higher proportion of type I fibers coupled with a modestly lower proportion of type IIX fibers. These results could be interpreted to be compensatory for the overall decrease in oxidative capacity in that the proportion of fibers associated with the greatest endurance capacity is augmented. It should be noted, however, that variable findings, often in small cohorts of patients, have been reported in the literature with regard to the relative proportions of the different muscle fibers in patients with chronic renal failure. For example, Fahal et al. (14) reported no change in fiber proportions in the quadriceps muscle of dialysis patients. By contrast, in the vastus lateralis muscle of MHD patients, Clyne et al. (11) reported an increased proportion of type IIA fibers and decrease in type IIX fibers (as in our data), while Molsted et al. (24) described an increase in IIX fibers and decrement in type I fibers.
In the present study, significantly greater CSAs of individual fibers types were observed compared with control subjects. We interpret this as likely reflecting the influence of fiber edema, despite our efforts to limit fluid overload. (Patients were dialyzed 24 h before all biopsies.) This is in keeping with the study of Montanari et al. (25), who demonstrated increased muscle intra- and extracellular total water fractions in patients on regular long-term dialysis. Our findings thus likely represent “real world” reality, which may have functional implications (see below). It is also of interest that fiber splitting has been reported in dialysis patients, a situation in which large fibers split as they outstrip their nuclear domain size to adequately support muscle protein turnover (13, 22). Data in the literature are also highly variable and derived mostly from small numbers of patients. Further, the measurement of muscle fiber CSAs from biopsy material is subject to major technical challenges. These include the quality of the biopsy sample processing (single highly experienced investigator for all specimens) and analysis (single highly experienced investigator for all sectioning and assays) to ensure, for example, optimal orientation of fibers before freezing, optimization of sectioning in the cross-sectional plane, staining procedures, and methods to calculate CSA (computer-based image analysis to measure the number of pixels within outlined fibers is optimal). We utilized methods designed to control for all these factors except for precise control of muscle fiber length, which was not possible utilizing small biopsy samples. The biopsies were rapidly processed immediately following acquisition in a relatively uniform time frame, which likely reduced variances related to the resting length at which fiber bundles were frozen.
Skeletal muscle atrophy is generally cited in reviews on the influences of chronic renal failure on muscle morphometry/dysfunction (e.g., Ref. 1). However, reports in the literature provide controversial findings. Four studies, in which control patients were included, reported no significant atrophy of muscle fiber types (11, 13, 14, 24, 28). In the study by Fahal et al. (14), a tendency (not significant) to reduced CSA of type IIA fibers was reported. Most of the studies in which muscle fiber atrophy was reported were flawed, as they lacked control subjects, and conclusions were drawn with reference to data published by other authors or, in one instance, by assessing variance in terms of SDs (4, 10, 20, 33, 35). In one of these studies, hypertrophy of type I fibers with atrophy of type II fibers was reported (4).
Ultrastructural changes.
Several abnormalities on electron microscopy have been reported in patients with chronic renal failure. These include mitochondrial changes, myofibrillar degeneration, disruption of Z-bands, myofilament loss, and glycogen deposits (5, 13, 35). In the present study, the major abnormalities observed were mitochondrial in nature. This would tend to support the reduction in muscle oxidative capacity as reflected by the significant reduction of the mitochondrial enzyme SDH in muscle fibers of MHD patients.
Clinical implications.
Our results highlight impaired oxidative capacity and altered capillarity in all fiber types in the vastus lateralis muscle of MHD patients. These alterations would be expected to impair energy production as well as substrate and oxygen delivery and exchange, contributing to effort intolerance and reduced endurance capacity. The mitochondrial abnormalities evident on electron microscopy support these possibilities. The slight increase in the fiber proportions of type I fibers may well be an adaptive response to preserve endurance capacity by increasing the proportion of those fibers that are most fatigue resistant. The larger fiber CSAs that we observed do not necessarily imply increased muscle bulk. Indeed we hypothesize that muscle fiber edema likely account for our observations. This being the case, we speculate that the relative hypertrophy contributes to reduced limb strength in MHD patients by reducing specific force (i.e., reduced functional contractile muscle protein per unit CSA of muscle), likely due to the excessive water content/edema of the muscle. Since in chronic renal failure muscle proteolysis may be enhanced via activation of the ubiquitin-proteasome pathways (6), likely associated with caspase-3 influences (9, 41). Thus the amount of contractile muscle mass may be even more reduced, further contributing to muscle weakness. Reduced contractility and/or muscle bulk could also limit endurance, particularly under conditions of increased demand, as the critical ratio of force production to maximal force generation is exceeded (30). Lastly, undernutrition or sepsis, which often occurs in MHD patients, would be expected to further reduce metabolic energy reserves and the ability to produce force (21, 37).
Conclusion.
We conclude that impaired muscle strength and endurance capacity as previously reported by us and others in MHD patients may in part be explicable on the basis of biochemical and morphometric aberrations in limb muscle fibers. We speculate that impaired muscle fiber oxidative capacity and capillarity of all major muscle fiber types would impair energy production together with reduced substrate and oxygen delivery and contribute to reduce endurance capacity and exercise tolerance. We further speculate that reduced limb muscle strength is due to reduced specific muscle force (i.e., force per unit CSA of the muscle). Loss of effective contractile muscle mass via proteolytic and other pathways, together with muscle fiber edema and fiber size enlargement, is likely an important factor accounting for reduced specific force in MHD patients.
GRANTS
This research was supported by funds from the National Institutes of Health Grants DK-054457 and HL-071227 and the resources of the General Clinical Research Center Grant M01-RR00425 of the National Center for Research Resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.I.L., M.F., T.W.S., R.C., and J.D.K. conception and design of research; M.I.L., M.F., T.W.S., R.C., A.H.C., and J.D.K. interpreted results of experiments; M.I.L. and M.F. drafted manuscript; M.I.L., M.F., H.W., T.W.S., R.C., A.H.C., and J.D.K. edited and revised manuscript; M.I.L. and J.D.K. approved final version of manuscript; M.F., H.W., T.W.S., R.C., A.H.C., and J.D.K. performed experiments; M.F., H.W., and A.H.C. analyzed data; M.F. and A.H.C. prepared figures.
REFERENCES
- 1. Adams GR, Vaziri ND. Skeletal muscle dysfunction in chronic renal failure: effects of exercise. Am J Physiol Renal Physiol 290: F753–F761, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Adams GR, Zhan CD, Haddad FA, Vaziri NA. Voluntary exercise during chronic renal failure in rats. Med Sci Sports Exerc 37: 557–562, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab 278: E219–E225, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Ahonen RE. Light microscopic study of striated muscle in uremia. Acta Neuropathol (Berl) 50: 163–166, 1980 [DOI] [PubMed] [Google Scholar]
- 5. Ahonen RE. Striated muscle ultrastructure in uremic patients and in renal transplant recipients. Acta Neuropathol (Berl) 49: 51–55, 1980 [DOI] [PubMed] [Google Scholar]
- 6. Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol 17: 1388–1394, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Blanco CE, Fournier M, Sieck GC. Metabolic variability within individual fibres of the cat tibialis posterior and diaphragm muscles. Histochem J 23: 366–374, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Blanco CE, Sieck GC, Edgerton VR. Quantitative histochemical determination of succinate dehydrogenase activity in skeletal muscle fibers. Histochem J 20: 230–243, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Boivin MA, Battah SI, Dominic EA, Kalantar-Zadeh K, Ferrando A, Tzamaloukas AH, Dwivedi R, Ma TA, Moseley P, Raj DSC. Activation of caspase-3 in the skeletal muscle during haemodialysis. Eur J Clin Invest 40: 903–910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castaneda C, Gordon PL, Uhlin KL, Levey AS, Kehayias JJ, Dwyer JT, Fielding RA, Roubenoff R, Singh MF. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. Ann Intern Med 135: 965–976, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Clyne N, Esbjörnsson M, Jansson E, Jogestrand T, Lins LE, Pehrsson SK. Effects of renal failure on skeletal muscle. Nephron 63: 395–399, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Conjard A, Ferrier B, Martin M, Caillette A, Carrier H, Baverel G. Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol 6: 68–74, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Diesel W, Emms M, Knight BK, Noakes TD, Swanepoel CR, vanZyl-Smit R, Kaschula RO, Sinclair-Smith CC. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Dis 22: 677–684, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Fahal IH, Bell GM, Bone JM, Edwards RHT. Physiologic abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant 12: 119–127, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Flisinski M, Brymora A, Elminowska-Wenda G, Bogucka J, Walasik K, Stefanska A, Odrowaz-Sypniewska G, Maitius J. Influence of different stages of experimental chronic kidney disease on rats locomotor and postural skeletal muscles microcirculation. Ren Fail 30: 443–451, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Johansen KL. Physical functioning and exercise capacity in patients on dialysis. Adv Ren Replace Ther 6: 141–148, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kern PA, Simsolo RB, Fournier M. Effects of weight loss on muscle fiber type, fiber size, capillarity and succinate dehydrogenase activity in humans. J Clin Endocrinol Metab 84: 4185–4190, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Kopple JD, Cohen AH, Wang H, Qing D, Tang Z, Fournier M, Lewis M, Casaburi R, Storer T. Effect of exercise on mRNA levels for growth factors in skeletal muscle of hemodialysis patients. J Ren Nutr 16: 312–324, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kouidi E, Albani M, Natsis K, Megalopoulos A, Gigis P, Guiba-Tziampiri O, Tourkantonis A, Deligiannis A. The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol Dial Transplant 13: 685–699, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Lin MC, Ebihara S, El Dwairi Q, Hussain SN, Yang L, Gottfried SB, Comtois A, Petrof BJ. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med 158: 1656–1663, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Mantilla CB, Sill RV, Aravamudan B, Zhan WZ, Sieck GC. Developmental effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol 104: 787–794, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Miro O, Marrades RM, Roca J, Sala E, Masanes F, Campistol JM, Torregrosa JV, Casademont J, Wagner PD, Cardellach F. Skeletal muscle mitochondrial function is preserved in young patients with chronic renal failure. Am J Kidney Dis 39: 1025–1031, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Molsted S, Eidemak I, Sorensen HT, Kristensen JH, Harrison A, Andersen JL. Myosin heavy-chain isoform distribution, fibre-type composition and fibre size in skeletal muscle of patients on haemodialysis. Scand J Urol Nephrol 41: 539–545, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Montanari A, Graziani G, Borghi L, Cantaluppi A, Simoni I, Lorenzano E, Ponticelli C, Novarini A. Skeletal muscle water and electrolytes in chronic renal failure. Effects of long-term regular dialysis. Nephron 39: 316–320, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Moore GE, Bertocci LA, Painter PL. 31P-magnetic resonance spectroscopy assessment of subnormal oxidative metabolism in skeletal muscle of renal failure patients. J Clin Invest 91: 420–424, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore GE, Parsons DB, Stray-Gundersen J, Painter PL, Brinker KR, Mitchell JH. Uremic myopathy limits aerobic capacity in hemodialysis patients. Am J Kidney Dis 22: 277–287, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Pastoris O, Aqualani R, Foppa P, Bovio G, Segagni S, Baiardi P, Catapano M, Maccario M, Salvadeo A, Dossena M. Altered muscle energy metabolismin post-absorptive patients with chronic renal failure. Scand J Urol Nephrol 31: 281–287, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Putman CT, Xu X, Gillies E, MacLean IM, Bell GJ. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol 92: 376–384, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Roussos C, Macklem PT. Diaphragm fatigue in man. J Appl Physiol 43: 189–197, 1977 [DOI] [PubMed] [Google Scholar]
- 31. Sakkas GK, Ball D, Mercer TH, Sargeant AJ, Tolfrey K, Naish PF. Atrophy of non-locomotor muscle in patients with end-stage renal failure. Nephrol Dial Transplant 18: 2074–2081, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Sakkas GK, Ball D, Sargeant AJ, Mercer TH, Koufaki P, Naish PF. Skeletal muscle morphology and capillarization of renal failure patients receiving different dialysis therapies. Clin Sci (Lond) 107: 617–623, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Sakkas GK, Sargeant AJ, Mercer TH, Ball D, Koufaki P, Karatzaferi C, Naish PF. Changes in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol Dial Transplant 18: 1854–1861, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Sala E, Noyszewski EA, Campitol JM, Marrades RM, Dreha S, Torregrossa JV, Beers JS, Wagner PD, Roca J. Impaired muscle oxygen transfer in patients with chronic renal failure. Am J Physiol Regul Integr Comp Physiol 280: R1240–R1248, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Shah AJ, Sahgal V, Quintanilla AP, Subramani V, Singh H, Hughes R. Muscle in chronic uremia–a histochemical and morphometric study of human quadriceps muscle biopsies. Clin Neuropathol 2: 83–89, 1983 [PubMed] [Google Scholar]
- 36. Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and non-ventilatory behaviors. J Appl Physiol 66: 2539–2545, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity and fatigue resistance. J Appl Physiol 66: 2196–2205, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 65: 719–724, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Storer TW, Casaburi R, Sawelson S, Kopple JD. Endurance exercise training during hemodialysis improves strength, power, fatigability and physical performance in maintenance hemodialysis patients. Nephrol Dial Transplant 20: 1429–1437, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Wang H, Casaburi R, Taylor WE, Aboellail H, Storer TW, Kopple JD. Skeletal muscle mRNA for IGF-IEa, IGF-II, and IGF-I receptor is decreased in sedentary chronic hemodialysis patients. Kidney Int 68: 352–361, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Workeneh BT, Rondon-Berrios Zhang L, Hu Z, Ayehu G, Ferrando A, Kopple JD, Wang H, Storer T, Fournier M, Lee SW, Du J, Mitch WE. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J Am Soc Nephrol 17: 3233–3239, 2006 [DOI] [PubMed] [Google Scholar]