Abstract
Spinal cord hemisection at C2 (C2HS) severs bulbospinal inputs to ipsilateral phrenic motoneurons causing transient hemidiaphragm paralysis. The spontaneous crossed-phrenic phenomenon (sCPP) describes the spontaneous recovery of ipsilateral phrenic bursting following C2HS. We reasoned that the immediate (next breath) changes in tidal volume (VT) induced by ipsilateral phrenicotomy during spontaneous breathing would provide a quantitative measure of the contribution of the sCPP to postinjury VT. Using this approach, we tested the hypothesis that the sCPP makes more substantial contributions to VT when respiratory drive is increased. Pneumotachography was used to measure VT in anesthetized, spontaneously breathing adult male rats at intervals following C2HS. A progressive increase in VT (ml/breath) occurred over an 8 wk period following C2HS during both poikilocapnic baseline breathing and hypercapnic respiratory challenge (7% inspired CO2). The sCPP did not impact baseline breathing at 1–3 days postinjury since VT was unchanged after ipsilateral phrenicotomy. However, by 2 wk post-C2HS, baseline phrenicotomy caused a 16 ± 2% decline in VT; a comparable 16 ± 4% decline occurred at 8 wk. Contrary to our hypothesis, the phrenicotomy-induced declines in VT (%) during hypercapnic respiratory stimulation did not differ from the baseline response at any postinjury time point (all P > 0.11). We conclude that by 2 wk post-C2HS the sCPP makes a meaningful contribution to VT that is similar across different levels of respiratory drive.
Keywords: plasticity, hemisection, phrenicotomy, cervix
severing ipsilateral bulbospinal inputs to phrenic motoneurons via lateral hemisection of the C2 spinal cord (C2HS) transiently paralyzes the hemidiaphragm (22, 23, 27, 48). However, a partial return of ipsilateral phrenic motoneuron inspiratory bursting occurs over a period of weeks to months following C2HS (14, 15, 20, 37). This response has been termed the spontaneous crossed-phrenic phenomenon (sCPP; Refs. 22, 28, 40). The sCPP provides an important experimental model of neuroplasticity and associated functional recovery (i.e., phrenic bursting) after spinal cord injury (22, 28, 40). However, the functional contribution of the sCPP to ventilation (V̇e) has not been definitively established. In other words, it is unclear if the relatively small amount of electrical activity that has been measured in the ipsilateral phrenic nerve after chronic C2HS is sufficient to alter inspiratory tidal volume (VT). Thus it is unknown if C2HS-induced, spontaneous neuroplastic changes associated with the sCPP (22, 23) have a meaningful impact on the respiratory system (20).
The functional impact of the sCPP has been examined to a limited extent (14, 15, 20). Correlations between phrenic nerve activity recorded under anesthesia and V̇e measured in unanesthetized rats suggest that the sCPP makes a small contribution to VT (14, 15, 20). In the most comprehensive study to date (20), Golder et al. (20) evaluated VT 8 wk following C2HS or a “dual lesion” consisting of C2HS and ipsilateral phrenicotomy (i.e., preventing phrenic activity from causing diaphragm contraction). Spontaneously breathing poikilocapnic rats with the dual injury had similar VT levels as those with C2HS alone. However, the volume of augmented breaths (ABs) or “sighs” (19) was reduced after the dual injury. These results lead to the hypothesis that the sCPP makes relatively little contribution to eupneic breathing but becomes functionally relevant when respiratory drive is increased (20, 40). However, a potential confound to this interpretation is that eliminating the sCPP may enhance the compensatory plasticity (28, 40) that occurs in other respiratory motor pools (e.g., contralateral phrenic motoneurons; intercostal motoneurons, etc.) following C2HS. Indeed, even when both phrenic nerves are intact, C2HS injury causes a robust enhancement of contralateral phrenic output (8, 15, 36, 39).
We reasoned, therefore, that measuring inspiratory VT immediately before and after an acute ipsilateral phrenicotomy procedure could enable a more definitive assessment of the functional significance of the sCPP. In other words, the immediate (i.e., “next breath”) drop in VT resulting from disruption of the sCPP should provide a quantitative estimate of its importance. We used this approach to test the hypothesis that the functional significance of the sCPP increases in parallel with respiratory drive. Further, we hypothesized that the functional significance of the sCPP increases in a time-dependent manner over weeks to months post-C2HS. Our final purpose was to establish an experimental method that will enable more direct testing of the functional efficacy of therapeutic interventions aimed at enhancing the sCPP (1, 2, 10, 50).
MATERIALS AND METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Animals.
A total of 37 adult, male Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). Rats receiving C2HS injury were grouped by the following postinjury time frames: “acute” (1–3 days), 2 wk, or 8 wk. Uninjured control rats were age matched to the 2 wk injury group (n = 4) or the 8 wk group (n = 5). A summary of the experimental groups is presented in Table 1.
Table 1.
Age and weight values for all experimental groups
| Baseline Phrenicotomy |
Hypercapnic Phrenicotomy |
|||||
|---|---|---|---|---|---|---|
| n | Age, days | Weight, g | n | Age, days | Weight, g | |
| Uninjured | 5 | 131 ± 13 | 382 ± 17 | 4 | 131 ± 13 | 421 ± 35 |
| 1–3 days | 6 | 91 ± 1 | 302 ± 6* | 4 | 97 ± 1 | 326 ± 18‡ |
| 2 wk | 5 | 109 ± 1 | 333 ± 5† | 4 | 111 ± 1 | 359 ± 9 |
| 8 wk | 4 | 152 ± 1 | 413 ± 8 | 5 | 153 ± 0 | 397 ± 16 |
Values are means ± SE. Spinal cord hemisection at C2 (C2HS) rats were grouped at 1–3 days, 2 wk, and 8 wk postinjury.
P < 0.001,
P < 0.01,
P < 0.05, from uninjured by one-way ANOVA.
Spinal cord injury.
Our anesthesia and injury methods have been previously described (14, 17). Rats were anesthetized by injection of xylazine (10 mg/kg sq) and ketamine (140 mg/kg ip; Fort Dodge Animal Health). The spinal cord was exposed at the C2 level via a dorsal approach, and a left C2HS lesion was induced using a microscalpel followed by aspiration. The dura and overlying muscles were sutured, and the skin was closed with stainless steel wound clips (Stoelting). Rats were given an injection of yohimbine (1.2 mg/kg sq; Lloyd) to reverse the effect of xylazine. Following surgery, animals received an analgesic (buprenorphine; 0.03 mg/kg sq; Hospira) and sterile lactated Ringer solution (5 ml sq). Postsurgical care included administration of buprenorphine (0.03 mg/kg sq) during the initial 48 h postinjury and delivery of lactated Ringer solution (5 ml/day sq) and oral Nutri-cal supplements (1–3 ml; Webster Veterinary) until adequate volitional drinking and eating resumed.
Experimental preparation.
These procedures were adapted from our prior publications (9, 14, 17, 29, 41). Isoflurane anesthesia (3–4% in O2) was induced in a closed chamber followed by intraperitoneal injection of urethane (1.6 g/kg; Sigma, St. Louis, MO). The adequacy of urethane anesthesia was confirmed by testing limb withdrawal and palpebral reflexes. Rats were in a supine position throughout the protocol. The trachea was cannulated in the midcervical region and connected in series to a custom-designed, small animal pneumotachograph and volumetric pressure transducer (Grass Instruments, Quincy, MA) for measurement of respiratory air flow. Partial pressure of arterial oxygen (PaO2) was maintained >150 Torr by delivering a hyperoxic gas mixture (FIO2 = 0.50, balance N2; flow rate ∼1.0 l/min) to the tracheostomy tube via a “T-piece” design (13). The femoral vein was catheterized (PE-50) to enable supplemental urethane anesthesia (0.3 g/kg iv; Sigma) if indicated. Another PE-50 catheter was placed in the femoral artery and connected to a pressure transducer (Statham P-10EZ pressure transducer; amplifier CP122 AC/DC strain gauge amplifier; Grass Instruments, West Warwick, RI) for arterial pressure and blood gas measurements. The ipsilateral phrenic nerve was isolated in the cervical region via a ventral approach (32, 40, 41). The exposed nerve was covered in mineral oil but not manipulated at this time. Arterial blood samples (0.2 ml) were drawn during the baseline period (see Experimental protocols) and analyzed for PaO2, carbon dioxide partial pressure (PaCO2), and pH (i-STAT, Waukesha, WI). Blood gas measures were corrected to rectal temperature, which was monitored by rectal thermistor and maintained at 37.5 ± 1 °C by a servo-controlled heating pad (model TC-1000; CWE, Ardmore, PA).
Experimental protocols.
The ipsilateral phrenic nerve was cut during expiration during either the baseline period (n = 20) or during a hypercapnic respiratory challenge (n = 17; Table 1). Baseline breathing was established over a 20-min period during which rats breathed the hyperoxic gas mixture described above. Baseline was followed by a 5-min hypercapnic respiratory challenge (7% CO2, 50% O2, and balance N2; e.g., see Fig. 2). Rats were then returned to baseline conditions, and once breathing had returned to the prehypercapnic values, the ipsilateral phrenic nerve was cut (i.e., baseline phrenicotomy group). After 5 min had elapsed, the hypercapnic challenge was repeated. In a separate group, the ipsilateral phrenic nerve was cut during the third minute of the second hypercapnic challenge (i.e., hypercapnic phrenicotomy group). This time corresponded to a stable period of hypercapnic VT that was similar to the initial hypercapnic response. All rats were returned to baseline conditions following the second hypercapnic challenge.
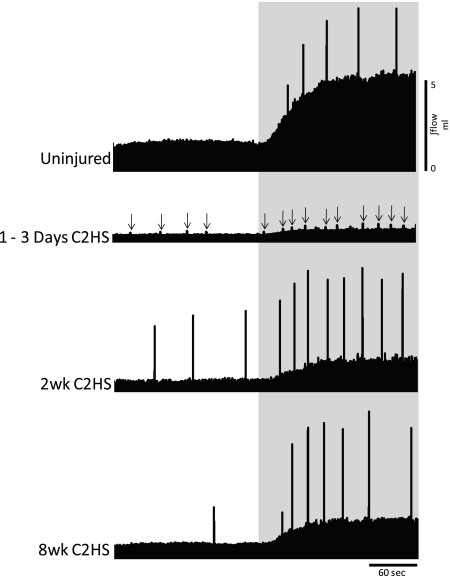
Fig. 2.
Representative examples of tidal volume (VT) during spontaneous breathing at baseline and hypercapnic respiratory challenge. Images show the integrated airflow signals (∫flow) from a control (uninjured) rat and rats studied 1–3 days, 2 wk, and 8 wk following C2HS injury. Shaded area represents the hypercapnic challenge (7% inspired CO2). Spikes in the record are augmented breaths (ABs; see text); the ABs were harder to detect at 1–3 days post-C2HS and are indicated by arrows. C2HS resulted in decreased VT during both baseline and hypercapnic challenge at all postinjury time points. In addition, a reduction in the volume of ABs was observed following C2HS. Scaling is identical in all panels.
A small sample of rats with C2HS (n = 4) was studied to confirm our assumption that reflexive increases in contralateral respiratory muscle activity would not occur during the initial breath following ipsilateral phrenic nerve section. Thus contralateral external intercostal electromyogram (EMG) activity (first intercostal space; n = 2) or contralateral hemidiaphragm EMG activity (medial costal region; n = 2) was assessed during spontaneous breathing in urethane anesthetized rats following C2HS injury. EMG activity was measured using intramuscular fine wire electrodes as previously described (13). Baseline conditions were established as described above. After a stable period of poikilocapnic baseline breathing, the ipsilateral (left) phrenic nerve was sectioned and we examined the immediate impact on the EMG activity of the contralateral respiratory muscles.
Spinal cord histology.
All C2HS lesions were confirmed to extend to the spinal midline as previously described (14, 17, 40). At the conclusion of the phrenicotomy experiment, rats were euthanized by systemic perfusion with saline followed by 4% paraformaldehyde (Sigma). The cervical spinal cord was removed, and 50-μm sections were made in the transverse plane using a vibrotome. Tissue sections were mounted on glass slides (Fisher Scientific, Pittsburgh, PA), stained with Cresyl violet, and evaluated by light microscopy. A histological example of a C2HS lesion is shown in Fig. 1. Consistent with our previous publications (14, 17, 29, 40), the apparent absence of healthy white matter in the ipsilateral C2 spinal cord was taken as confirmation of an anatomically complete C2HS (17).
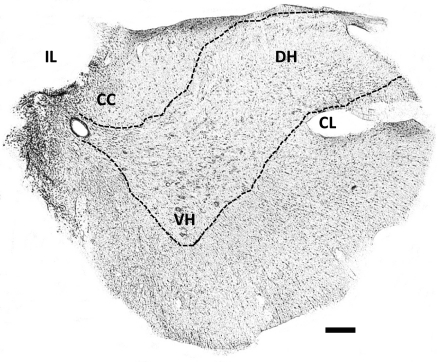
Fig. 1.
Representative histological section illustrating the spinal cord hemisection at C2 (C2HS) lesion. This 40-μm transverse section was taken from the second cervical segment (C2) at 8 wk postinjury and stained with Cresyl violet. The absence of white and grey matter in the ipsilateral (IL) spinal cord suggests an anatomically complete C2HS. CL, contralateral; CC, central canal; VH, ventral horn; DH, dorsal horn. Scale bar = 200 μm.
Data analysis.
Calibration of the pneumotachograph was accomplished using a series of constant volume injections with varying airflow rates. Respiratory airflow signals were amplified (×100 K; CP122 AC/DC strain gage amplifier; Grass Instruments) and recorded on a PC using Spike2 software (Cambridge Electronic Design Limited). The inspiratory phase of the airflow signals were integrated (∫flow) using a customized Spike2 software script (Cambridge Electronic Design Limited), and VT was then calculated offline. Inspiratory and expiratory durations (TI and TE, respectively) were calculated (31) based on the integrated airflow traces as indicated in Fig. 3. Respiratory frequency (f, breaths/min) was calculated as 60/(TI + TE). Rats occasionally showed ABs (Fig. 2). These were identified by characteristic two-phase airflow patterns as previously described (4, 14, 18). The first phase of the AB was indistinguishable from the preceding tidal breath. However, at the peak of inspiration there was a further, distinct increase in the rate of rise of inspiratory flow followed by a prolonged TE.
Fig. 3.
Representative airflow traces showing the impact of phrenicotomy on VT. Examples are provided from a control (uninjured) rat and from separate rats studied 1–3 days or 8 wk following C2HS injury. Phrenicotomy procedure was done to the left phrenic nerve of all rats (C2HS injury was to the left side of the spinal cord). Phrenicotomy occurred between breaths and is denoted by the dashed lines. Immediate (i.e., next breath) change in VT (ΔVT) following phrenicotomy was used to estimate the contribution of signals traveling in the ipsilateral phrenic nerve to inspiratory VT. This example shows that phrenicotomy caused an ∼50% reduction in VT in the control rat. In contrast, phrenicotomy did not change VT at 1–3 days post-C2HS and caused a transient reduction of ∼20% at 8 wk. Scaling is identical in each panel. TI, inspiratory duration; TE, expiratory duration.
One-way ANOVA was used to compare body weight, blood gases, and mean arterial blood pressure cross groups. Prephrenicotomy data including initial baseline and hypercapnic VT and relative increases in VT during hypercapnic challenge were also assessed using one-way ANOVA. Respiratory parameters (e.g., TI, TE, f, and VT) were averaged over the 10 breaths that immediately preceded the phrenicotomy. These values were compared with values measured at the first, third, and fifth breaths following phrenicotomy as well as the breath 1 min following phrenicotomy. These data were compared using two-way repeated measures (RM) ANOVA and the Student-Newman-Keuls post hoc test. For this ANOVA, factor 1 was “treatment” (i.e., control or C2HS groups) and factor 2 was “time” (i.e., time relative to the acute phrenicotomy). Changes in VT (ΔVT) following phrenicotomy were normalized to the prephrenicotomy values as follows: %VT decline = [1 − (postphrenicotomy VT/prephrenicotomy VT)] × 100%.
The final 30 s of the pre- and postphrenicotomy hypercapnic challenges were averaged for comparison using two-way RM ANOVA [factor 1: treatment (lesion group); factor 2: condition (pre or postphrenicotomy)]. Changes in baseline AB f and volume following phrenicotomy were assessed in a subset of C2HS rats (see results) using a one-way ANOVA. Further, since hypercapnia induced ABs in both control and C2HS rats, we analyzed changes in hypercapnic AB f and volume using two-way RM ANOVA [factor 1: treatment (lesion group); factor 2: condition (pre- or postphrenicotomy)]. All data are presented as the means ± SE. A P value of < 0.05 was considered statistically significant.
RESULTS
A time-dependent change in body mass occurred following C2HS as previously reported (9, 14, 15, 17). Thus both the 1- to 3-day and 2 wk post-C2HS groups weighed less than control and 8 wk postinjury rats (Table 1). PaO2 was similar between groups during baseline breathing (Table 2). However, the acutely injured rats (1–3 days postinjury) showed evidence for hypoventilation and arterial acidosis as reflected by increased PaCO2 and decreased pH (P < 0.001 vs. other groups; Table 2). Consistent with prior reports (8, 14), no group differences in mean arterial blood pressure were observed (Table 2).
Table 2.
Blood gas and mean arterial blood pressure values taken during baseline ventilation
| PaO2, Torr | PaCO2, Torr | pH | MAP, mmHg | |
|---|---|---|---|---|
| Uninjured | 190 ± 14 | 45 ± 2 | 7.30 ± 0.01 | 90 ± 5 |
| 1–3 days C2HS | 193 ± 7 | 66 ± 6* | 7.16 ± 0.03* | 80 ± 7 |
| 2 wk post-C2HS | 199 ± 6 | 45 ± 2 | 7.29 ± 0.02 | 81 ± 5 |
| 8 wk post-C2HS | 172 ± 8 | 47 ± 2 | 7.28 ± 0.02 | 91 ± 6 |
Values are means ± SE. PaO2, arterial oxygen partial pressure; PaCO2, carbon dioxide partial pressure; MAP, mean arterial blood pressure.
P < 0.001, from all other groups by one-way ANOVA.
Effect of C2HS on ventilation.
Representative examples of airflow signals in control and C2HS rats are provided in Figs. 2 and 3. Before describing the impact of phrenicotomy, we first provide an overview of how C2HS influenced breathing during the initial (i.e., prephrenicotomy) measurements. C2HS did not significantly influence f, TI, or TE during the initial baseline period (Table 3). A tendency for increased f was noted during baseline breathing in the 8 wk C2HS groups, but this did not reach statistical significance (P = 0.16, Table 3). Some between-group differences in f were noted during hypercapnia (Table 3). Control (101 ± 2% baseline) and 1- to 3-day post-C2HS rats (99 ± 5% baseline) did not alter their breathing frequency during hypercapnic challenge. In contrast, both 2 wk (91 ± 3% baseline) and 8 wk rats (90 ± 4% baseline) showed a decline in f during hypercapnia reflecting an elongation of TI (P < 0.001; Table 3).
Table 3.
Respiratory frequency, inspiratory duration, and expiratory duration during prephrenicotomy baseline and hypercapnic respiratory challenge
|
f, breaths/min |
TI, s |
TE, s |
||||
|---|---|---|---|---|---|---|
| BL | CO2 | BL | CO2 | BL | CO2 | |
| Uninjured | 97 ± 3 | 98 ± 5 | 0.24 ± 0.01 | 0.23 ± 0.01* | 0.38 ± 0.03 | 0.38 ± 0.04 |
| 1–3 days-C2HS | 77 ± 10 | 75 ± 8 | 0.34 ± 0.05 | 0.35 ± 0.05‡ | 0.47 ± 0.12 | 0.46 ± 0.10 |
| 2 wk post-C2HS | 97 ± 9 | 89 ± 9* | 0.28 ± 0.04 | 0.30 ± 0.04‡ | 0.36 ± 0.09 | 0.41 ± 0.11 |
| 8 wk post-C2HS | 110 ± 11 | 100 ± 15* | 0.26 ± 0.03 | 0.28 ± 0.03‡ | 0.29 ± 0.08 | 0.35 ± 0.11 |
Values are means ± SE. f, Respiratory frequency; TI, inspiratory duration; TE, expiratory duration; BL, prephrenicotomy baseline; CO2, hypercapnic respiratory challenge.
P < 0.05, **P < 0.01,
P < 0.001, from baseline by two-way repeated-measures ANOVA.
The C2HS injury caused a substantial reduction in VT (ml/breath) during both baseline and hypercapnia in all groups (see “prephrenicotomy” data point in Fig. 4). Injured rats in the 1- to 3-day group produced the smallest baseline VT (P < 0.05 vs. all other groups). A progressive increase in baseline VT was measured over the 8 wk period following C2HS. In particular, 2 wk C2HS rats had increased VT compared with the 1- to 3-day postinjury group, and 8 wk rats had greater VT than both 2 wk and 1- to 3-day groups (Fig. 4A). However, baseline VT remained below control values in the 8 wk C2HS rats (P < 0.05; Fig. 4A). All C2HS rats also had lower VT during hypercapnia compared with controls (P < 0.05; Fig. 4B). As with the baseline condition, the smallest VT values were observed in the 1- to 3-day group. However, since PaCO2 in these rats was already elevated (Table 2), a reduced hypercapnic VT response was anticipated. Partial recovery of hypercapnic VT was seen in 2- and 8 wk C2HS rats (Fig. 4B). The relative increase in hypercapnic VT (%baseline) was similar between control (266 ± 23%) and chronic C2HS rats (2 wk: 222 ± 17%; 8 wk: 218 ± 20%; data not shown), but this response was substantially blunted in the 1- to 3-day post-C2HS group (166 ± 14%; P < 0.05).
Fig. 4.
Effect of phrenicotomy on VT (ml/breath) during baseline breathing (A) and hypercapnic respiratory challenge (B). Phrenicotomy caused an immediate (next breath) and robust decline in VT in uninjured rats. In contrast, phrenicotomy caused modest declines in VT in C2HS rats. *P < 0.05, ***P < 0.001, compared with prephrenicotomy values; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with uninjured; †P < 0.01, compared with 8 wk C2HS; ‡P < 0.05, ‡‡‡P < 0.001, compared with 1–3 days post-C2HS.
Immediate impact of ipsilateral phrenicotomy.
Representative examples of airflow and VT before and after phrenicotomy in control and C2HS rats are provided in Fig. 3. Reductions in VT following acute phrenicotomy in the two control groups (i.e., age-matched to the 2- and 8 wk C2HS groups, respectively) were similar during baseline (P = 0.27) and hypercapnia (P = 0.95). These data were therefore combined to form a single control group (Table 1) for each condition. Likewise, similar reductions in VT following ipsilateral phrenicotomy occurred at 1- and 3-day post-C2HS during baseline (P = 0.64) and hypercapnia (P = 0.40). Therefore, these data were combined into a single 1- to 3-day group for each condition.
In control rats, baseline phrenicotomy caused an immediate reduction in f that reflected an elongation of TE (Table 4). In contrast, baseline phrenicotomy did not significantly affect breathing pattern in any of the C2HS groups (Table 4). Phrenicotomy during the hypercapnic challenge did not influence f in control or C2HS rats (Table 5).
Table 4.
Respiratory frequency, inspiratory duration, and expiratory duration pre- and postipsilateral phrenicotomy during baseline ventilation
| Pre-PhrX | Breath 1 | Breath 3 | Breath 5 | 60 s | |
|---|---|---|---|---|---|
| f, breaths/min | |||||
| Uninjured | 98 ± 3 | 90 ± 2‡ | 88 ± 2‡ | 87 ± 2‡ | 93 ± 2† |
| 1–3 days C2HS | 84 ± 8 | 84 ± 8 | 82 ± 8 | 83 ± 7 | 85 ± 8 |
| 2 wk post-C2HS | 94 ± 6 | 96 ± 5 | 94 ± 5 | 93 ± 6 | 92 ± 6 |
| 8 wk post-C2HS | 109 ± 10 | 112 ± 9 | 110 ± 10 | 112 ± 10 | 111 ± 9 |
| TI, s | |||||
| Uninjured | 0.23 ± 0.01 | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.00 | 0.28 ± 0.04* |
| 1–3 days C2HS | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.22 ± 0.01 | 0.27 ± 0.04† |
| 2 wk post-C2HS | 0.29 ± 0.04 | 0.28 ± 0.04 | 0.29 ± 0.04 | 0.29 ± 0.04 | 0.31 ± 0.05 |
| 8 wk post-C2HS | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.24 ± 0.01 |
| TE, s | |||||
| Uninjured | 0.38 ± 0.02 | 0.47 ± 0.01‡ | 0.46 ± 0.02† | 0.45 ± 0.02† | 0.37 ± 0.05 |
| 1–3 days C2HS | 0.53 ± 0.06 | 0.53 ± 0.06 | 0.54 ± 0.06 | 0.53 ± 0.06 | 0.47 ± 0.06† |
| 2 wk post-C2HS | 0.36 ± 0.07 | 0.36 ± 0.07 | 0.36 ± 0.07 | 0.36 ± 0.08 | 0.35 ± 0.08 |
| 8 wk post-C2HS | 0.34 ± 0.04 | 0.33 ± 0.03 | 0.34 ± 0.04 | 0.32 ± 0.04 | 0.31 ± 0.03 |
Values are mean ± SE. PhrX, ipsilateral phrenicotomy.
P < 0.05,
P < 0.01,
P < 0.001, from prephrenicotomy by two-way repeated-measures ANOVA.
Table 5.
Respiratory frequency, inspiratory duration, and expiratory duration pre- and postipsilateral phrenicotomy during hypercapnic respiratory challenge
| Pre-PhrX | Breath 1 | Breath 3 | Breath 5 | 60 s | |
|---|---|---|---|---|---|
| f, breaths/min | |||||
| Uninjured | 90 ± 8 | 86 ± 9 | 86 ± 8 | 83 ± 8a | 83 ± 7a |
| 1–3 days C2HS | 70 ± 4 | 71 ± 4 | 69 ± 4 | 71 ± 6 | 70 ± 5 |
| 2 wk post-C2HS | 102 ± 8 | 105 ± 9 | 103 ± 10 | 103 ± 8 | 105 ± 9 |
| 8 wk post-C2HS | 91 ± 6 | 92 ± 6 | 90 ± 6 | 82 ± 7c | 88 ± 4 |
| TI, s | |||||
| Uninjured | 0.25 ± 0.01 | 0.22 ± 0.01c | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.01b |
| 1–3 days C2HS | 0.41 ± 0.02e | 0.40 ± 0.02 | 0.40 ± 0.02 | 0.40 ± 0.02 | 0.44 ± 0.01c |
| 2 wk post-C2HS | 0.45 ± 0.01e | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.45 ± 0.01 | 0.46 ± 0.01 |
| 8 wk post-C2HS | 0.36 ± 0.05d | 0.36 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.04 | 0.37 ± 0.05 |
| TE, s | |||||
| Uninjured | 0.44 ± 0.07 | 0.51 ± 0.09a | 0.48 ± 0.07 | 0.50 ± 0.08 | 0.48 ± 0.08 |
| 1–3 days C2HS | 0.46 ± 0.04 | 0.46 ± 0.04 | 0.47 ± 0.04 | 0.46 ± 0.06 | 0.42 ± 0.05 |
| 2 wk post-C2HS | 0.15 ± 0.03d | 0.14 ± 0.03 | 0.15 ± 0.04 | 0.14 ± 0.03 | 0.13 ± 0.04 |
| 8 wk post-C2HS | 0.30 ± 0.08 | 0.30 ± 0.08 | 0.31 ± 0.08 | 0.38 ± 0.10b | 0.31 ± 0.08 |
Values are means ± SE.
P < 0.05,
P < 0.01,
P < 0.001, from prephrenicotomy values;
P < 0.05,
P < 0.01 fP < 0.001, from uninjured by two-way repeated-measures ANOVA.
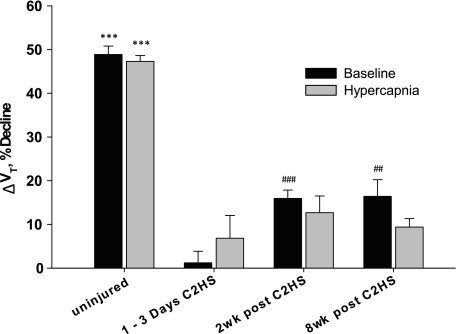
The immediate impact of phrenicotomy on VT was assessed in both absolute values (i.e., Δml/breath; Fig. 4) and relative to the preceding breaths (i.e., %decline; Fig. 5). Our primary outcome measure was the %decline in VT immediately (next breath) following phrenicotomy. Baseline phrenicotomy in control rats caused an immediate reduction in VT by 49 ± 2% (Figs. 3–5). Control rats were able to compensate for the loss of hemidiaphragm activity as evidenced by the progressive increase in VT over the subsequent breaths (Fig. 4A). However, VT did not return to prephrenicotomy values within 60 s postphrenicotomy (Fig. 4A). The decline in VT following phrenicotomy in C2HS rats was considerably less than in controls. Indeed, phrenicotomy caused no measurable changes in baseline VT at 1–3 days post-C2HS (ΔVT = 1 ± 3%; Figs. 4 and 5). However, by 2 wk post-C2HS ipsilateral phrenicotomy caused an immediate 16 ± 2% reduction in VT (Figs. 4 and 5). Phrenicotomy triggered a similar decline of VT in the 8 wk post-C2HS group (16 ± 4%; Fig. 5). Rats with chronic C2HS were able to rapidly compensate for the loss of ipsilateral diaphragm activity. In both groups, VT had returned to baseline (prephrenicotomy) values within three breaths (Fig. 4).
Fig. 5.
Immediate (next breath) decline in VT following phrenicotomy. Change in VT was expressed relative to the prephrenicotomy baseline (ΔVT, %decline). Note that performing the phrenicotomy procedure during the hypercapnic challenge caused declines in VT similar to what occurred during the baseline condition. ***P < 0.001, compared with all C2HS; ##P < 0.01, compared with 1–3 days post-C2HS; ###P < 0.001, from 1–3 days post-C2HS.
Phrenicotomy during the hypercapnic challenge produced qualitatively similar responses to what was observed during baseline (Figs. 3–5). Control rats showed an immediate decline in VT of 47 ± 1% (Fig. 5). As with the baseline response, C2HS rats showed a comparatively smaller change in VT after hypercapnic phrenicotomy. Surprisingly, the relative drop in VT during hypercapnia was similar at each postinjury time point (1–3 days: 7 ± 5, 2 wk: 13 ± 4; 8 wk: 9 ± 2%; Fig. 5). In other words, a time-dependent and progressive increase in sCPP contribution to hypercapnic VT was not apparent. The relative magnitude of the change in VT following phrenicotomy and hypercapnia in 1- to 3-day injured rats merits a further comment. Because the 1 to 3-day C2HS rats had much lower absolute VT compared with the other groups, the ∼7% reduction in VT following phrenicotomy (Fig. 5) corresponded to a change of just 0.1 ± 0.1 ml/breath. As such, the contribution of the sCPP to hypercapnic VT at 1 to 3 days postinjury should be interpreted cautiously. Finally, the compensatory responses to phrenicotomy were similar to those observed during the baseline condition. Specifically, hypercapnic VT returned to prephrenicotomy levels within three breaths in all injured groups, while in control rats the reduced VT persisted for 60 s following phrenicotomy (Fig. 4B).
Effect of phrenicotomy on subsequent hypercapnic ventilatory responses.
Hypercapnic challenge was performed both before and after phrenicotomy in all control and C2HS rats in the baseline phrenicotomy group. Phrenicotomy in control rats caused a blunting of the subsequent hypercapnic ventilatory response that reflected reductions in both f and VT (Fig. 6). These blunted ventilatory responses, however, remained significantly greater than the response of phrenic-intact C2HS rats (Fig. 6). All C2HS rats maintained VT, f, and V̇e at prephrenicotomy values when assessed during the postphrenicotomy hypercapnic challenge (Fig. 6). Thus, in contrast to the control rats, C2HS animals were able to fully compensate for the acute loss of ipsilateral hemidiaphragm activity. The normalized hypercapnic ventilation data (i.e., %prephrenicotomy) were qualitatively similar to the absolute values (not shown).
Fig. 6.
Impact of phrenicotomy on the hypercapnic ventilatory response. VT, respiratory frequency (f), and ventilation (V̇e) were measured during hypercapnic respiratory challenge before and after (> 60 s) phrenicotomy in control uninjured rats and rats with C2HS injury. Phrenicotomy procedure blunted the hypercapnic ventilatory response in uninjured rats but did not impact the response in C2HS rats. A progressive recovery in the hypercapnic ventilatory response was observed whereby V̇e was highest in rats at 8 wk following injury. ***P < 0.001, **P < 0.01, *P < 0.05, compared with uninjured; #P < 0.05, compared with 1–3 days post-C2HS.
Effect of phrenicotomy on spontaneous ABs.
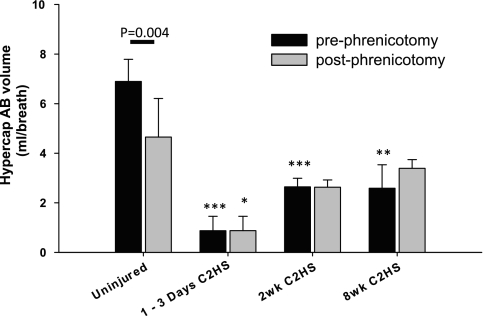
Representative examples of AB behavior during spontaneous breathing are shown in Fig. 2. While ABs were not observed in control rats during baseline breathing, they were observed in a subset of C2HS rats. Of those rats exhibiting ABs, no apparent differences could be detected across the three C2HS groups. Specifically, AB frequency (P = 0.38) and volume (P = 0.34) were similar across C2HS groups and thus these data were pooled. Overall, 40% of C2HS rats showed ABs during baseline conditions at a rate of 30 ± 9 per hour. Phrenicotomy significantly reduced the rate of AB occurrence to just 4 ± 3 per hour (P = 0.02). However, the phrenicotomy procedure did not diminish baseline AB volume in C2HS rats (117 ± 92%). Hypercapnic challenge induced the appearance of ABs in control rats and increased the frequency of ABs in C2HS groups (e.g., Fig. 2). The average frequency of ABs was similar in control (88 ± 17 per hour) and C2HS rats (100 ± 22 per hour) during hypercapnia (P = 0.66). Phrenicotomy did not influence hypercapnic AB f either within or between groups (not shown). As expected, phrenicotomy diminished hypercapnic AB volume in control rats (62 ± 21%; P = 0.004; Fig. 7). However, similar to the baseline response hypercapnic AB volume was not altered following phrenicotomy in C2HS rats (102 ± 3%; P = 0.63; Fig. 7).
Fig. 7.
Impact of phrenicotomy on the volume of spontaneous ABs. Data presented here were collected during a hypercapnic respiratory challenge. Two-way ANOVA was not possible with the poikilocapnic baseline data since ABs were not observed in control uninjured rats at baseline. Only the uninjured animals showed a reduction in AB volume following phrenicotomy. ***P < 0.001, **P < 0.01, *P < 0.05, compared with uninjured.
Effect of phrenicotomy on contralateral respiratory muscle EMG activity.
Anecdotally, we noted in supplemental experiments that acute phrenicotomy caused no discernable immediate (next breath) change in the contralateral EMG activity of the hemidiaphragm or external intercostals muscles in C2HS rats (data not shown). Thus changes in VT following ipsilateral phrenicotomy result from primarily, if not exclusively, the immediate paralysis of the ipsilateral hemidiaphragm. Compensatory EMG responses in accessory muscles and the contralateral diaphragm are not rapid enough to “mask” the contribution of the ipsilateral hemidiaphragm after phrenicotomy.
DISCUSSION
There are two particularly novel aspects of our results. First, contraction of the ipsilateral hemidiaphragm following C2HS makes a meaningful contribution to VT during quiet breathing (i.e., baseline conditions) as early as 2 wk following C2HS. However, the impact of the sCPP on ventilation was similar during both baseline and respiratory challenge. Therefore, the functional significance of the sCPP does not appear to increase relative to respiratory demand. This observation contrasts with previous assertions that the sCPP is relevant primarily during periods of increased respiratory drive (20). Second, progressive increases in the functional impact of the sCPP occurred only during the initial 2 wk following C2HS. In other words, after chronic C2HS injury, there was not an increased (or preferential) reliance on the sCPP to generate tidal volume. This observation does not necessarily preclude a time-dependent increase in the output of the sCPP (14, 15, 37); rather, it indicates that any increases in ipsilateral phrenic motor output are occurring in parallel with compensatory increases in other motor outputs (e.g., intercostal muscles).
Commentary on methods and diaphragm biomechanics.
Here we provide a brief discussion regarding the phrenicotomy method and the interpretation of subsequent changes in VT. First, the phrenicotomy procedure necessitated that the rats be deeply anesthetized. Anesthesia has the potential to suppress sCPP activity and accordingly could lead to an underestimation of the potential contribution of ipsilateral phrenic activity to VT in unanesthetized animals. Second, we recognize that the immediate decrease in VT following ipsilateral phrenicotomy does not necessarily represent decreases in transdiaphragmatic pressure (Pdi) that directly resulted from diaphragm paralysis. Rather, alterations in the biomechanical relationships between the diaphragm, rib cage, abdominal cavity, and accessory respiratory muscles may alter Pdi secondary to diaphragm paralysis. It should be emphasized that the biomechanical relationship between diaphragm contraction and generation of Pdi is complex (6). Contraction of the diaphragm produces forces with an “insertional” component (i.e., force developed through shortening of myofibers that insert into the ribs) and an “appositional component” (i.e., force exerted through the diaphragm “zone of apposition” due to increases in abdominal pressure; Ref. 47). The net action of the diaphragm (and resultant Pdi) will depend on the balance of these two forces. In our experiments, we did not attempt to characterize how C2HS or the phrenicotomy-induced cessation of diaphragm activity altered diaphragm and chest wall biomechanics. Regardless of the underlying biomechanical changes, however, the immediate change in VT following phrenicotomy provides a quantitative evaluation of the importance of hemidiaphragm contraction. In this regard, it is striking that VT decreased dramatically immediately following phrenicotomy in spinal intact rats, as might be predicted if diaphragm contraction is a primary contributor to Pdi under these conditions. A final comment is warranted regarding postural impacts on diaphragm function. Similar to prior reports (9, 14, 15, 17, 41), rats were studied in the supine position to provide suitable access to the trachea and phrenic nerves. Studies in humans have clearly demonstrated that posture can impact diaphragm function. For example, the diaphragm expands the rib cage less when contraction takes place in the supine compared with the upright position (6). However, the impact of supine vs. prone breathing on pulmonary biomechanics in quadrupedal rodents is less clear.
Contribution of the sCPP to VT following C2HS.
Even months after C2HS, the ipsilateral phrenic nerve and/or hemidiaphragm shows comparatively little inspiratory activity during “quiet breathing” (11, 14–17, 37). It is difficult to say precisely how much inspiratory activity is present since the actual number of active ipsilateral phrenic motor units during spontaneous breathing after C2HS is unknown. Recruitment of even a relatively low number of motor units, as has been suggested by prior studies (14–17, 37), could make a meaningful contribution to VT. Our data are consistent with this possibility since ipsilateral phrenicotomy during baseline conditions significantly reduced VT as early as 2 wk post-C2HS. Thus the current results indicate a functional role for the sCPP in the recovery of quiet breathing during the initial weeks following chronic spinal cord injury (11).
The relative importance of the sCPP to baseline VT was similar across 2 to 8 wk postinjury. There are several potential explanations for this plateau in the functional contribution of the sCPP. First, the sCPP may primarily act to “stabilize” the ipsilateral hemidiaphragm and thereby minimize paradoxical movements and enhance the effectiveness of contralateral hemidiaphragm contractions. This action might require only a relatively small number of active ipsilateral phrenic motor units as would be expected to occur by 2 wk postinjury (14, 15). Secondly, in the spontaneously breathing rat, the output of the sCPP may not increase significantly after the first few weeks post-C2HS injury. In this scenario, the spontaneously occurring spinal cord plasticity associated with increased ipsilateral phrenic output (see Refs. 22, 23, 52) may be essentially complete within 2 wk. In addition, vagal inhibition of ipsilateral phrenic activity is much more pronounced at 8 vs. 2 wk following C2HS (32). Therefore, potential increases in sCPP output in spontaneously breathing, vagally intact animals could be constrained by activation of vagal afferent neurons. It follows that acute phrenicotomy in vagotomized rats may have revealed a time-dependent increase in the contribution of the sCPP to VT.
We emphasize that the current data do not necessarily preclude a progressive increase in ipsilateral phrenic motor output after chronic C2HS as previously reported (15, 37). Nantwi et al. (37) were the first to report that spontaneous inspiratory activity recorded from the ipsilateral phrenic nerve increases over weeks to months postinjury. In the study of Nantwi's et al. (37), anesthetized and spontaneously breathing rats were studied at intervals following C2HS. Spontaneous CPP activity could not be detected at 4 wk post-C2HS but was present in ∼50% of rats at 6–8 wk post-C2HS and occurred in 100% of rats at 12–16 wk post-C2HS. In the current experiments, ipsilateral phrenic nerve activity was not measured, and accordingly we draw no conclusions regarding the “strength” of the sCPP; rather we used this preparation to examine the relative contribution of the ipsilateral phrenic nerve in C2HS rats to VT. The apparent discrepancy between the data of Nantwi's et al. (i.e., no sCPP inspiratory bursting at 4 wk postinjury) and the current data (i.e., a functional contribution of the ipsilateral phrenic nerve at 2 wk post-C2HS) may indicate that tonic bursting in the ipsilateral phrenic nerve is functionally important in the first few weeks following C2HS. In any case, if neural activity associated with the sCPP becomes more robust over time postinjury (15, 37), then our data indicate that this is occurring in parallel with compensatory increases in other respiratory motor outputs (e.g., intercostal muscles). In this scenario, the relative contribution of the sCPP would remain static as shown by the current data.
Numerous studies (14–17) have shown that ipsilateral phrenic bursting after chronic C2HS increases substantially during chemical respiratory challenge. Similarly, progressive improvements in hypercapnic V̇e (whole body plethysmography) occur over weeks to months following C2HS (14). These observations are consistent with the hypothesis that the primary role of the sCPP is to enable respiratory behaviors requiring large VT (20). Why then did ipsilateral phrenic activity make only modest contributions to VT when respiratory drive was substantially increased with hypercapnia? We hypothesize that parallel increases in the contribution of the contralateral hemidiaphragm and/or intercostal muscles during respiratory stimulation ensures that the relative contribution of the sCPP to VT remains stable. In this regard, we again emphasize that the number of active motor units in the ipsilateral hemidiaphragm after chronic C2HS is unknown. For example, if the number of active phrenic motor units is relatively low under baseline conditions, the robust increase in ipsilateral phrenic nerve activity seen during chemical challenge in C2HS rats (15–17) could reflect a very modest degree of motor unit recruitment. The apparently large increase in relative phrenic activity (%baseline) may therefore make only a small contribution to pulmonary biomechanics (and VT), particularly during ongoing recruitment of accessory respiratory muscles.
Compensation following phrenicotomy.
Changes in VT following diaphragm paralysis or paresis are mitigated by compensatory increases in the activity of other respiratory muscles (3, 28, 40, 43, 51). For example, both rostral (1st space) and caudal (6–10th space) intercostal muscle EMG activity is substantially increased following bilateral phrenicotomy in rats (43). Indeed, respiratory compensatory responses are so robust that rats maintain V̇e after bilateral phrenicotomy with virtually no disturbance to their sleep-wake cycles (43). Spontaneously breathing, awake dogs also have only minor reductions (26) or even no change in VT (45) following bilateral diaphragm paralysis. Unilateral diaphragm paralysis also evokes compensatory increases in accessory respiratory muscle activity including the contralateral parasternal muscles (26, 46) and transverse abdominus (26). These compensatory responses remain robust after vagotomy, suggesting that vagally mediated lung afferents are not essential to this process (46). Rather, the increased output of other respiratory muscles after diaphragm paralysis appears to reflect a diminished influence of inhibitory phrenic afferent neurons with additional contribution from arterial hypercapnia (3, 46). It is difficult, however, to compare the time course (e.g., onset time) of the compensatory responses across studies since prior work has induced diaphragm paralysis with selective anesthesia (e.g., lidocaine, bupivacaine, and vecuronium; Refs. 3, 26, 46). Accordingly, the onset of diaphragm paralysis will have a slower time course compared with phrenicotomy, and to our knowledge prior studies have not attempted to examine immediate (i.e., next breath) changes in VT. Nevertheless, these previously described compensatory responses almost certainly contributed to the time-dependent recovery of VT that occurred over the initial breaths following phrenicotomy (e.g., Fig. 4).
Axotomy and phrenic afferent neurons.
Axotomy can trigger neuronal depolarization (33) and increases in phrenic nerve activity (42). However, axotomy effects should be limited to ipsilateral phrenic motoneurons with limited, if any, impact on contralateral motor pools. In addition to axotomy-induced changes in membrane potential, the acute removal of ipsilateral phrenic afferent activity could have a rapid impact on contralateral phrenic output. Approximately 45% of phrenic nerve axons are afferent fibers (30), and a few studies (24, 25) have shown that phrenic afferents can inhibit phrenic motor activity on the contralateral side of the spinal cord. Removal of inhibitory phrenic afferents could therefore disinhibit contralateral phrenic motoneurons (and possibly intercostal motoneurons; Ref. 7) and thus facilitate the short-term recovery of VT seen in C2HS rats. However, in a few supplemental experiments, we could not detect any rapid (i.e., within 1–3 breaths) changes in the EMG activity of the contralateral hemidiaphragm or external intercostal muscles following phrenicotomy. Phrenic afferents are also capable of exerting excitatory effects on supraspinal respiratory neurons (44). Consistent with this notion, we noted that phrenicotomy induced immediate changes in respiratory frequency in uninjured rats. However, phrenicotomy did not alter respiratory frequency in C2HS rats, a finding that is consistent with plasticity in supraspinal respiratory neurons following C2HS (21).
Our data also indicate that phrenic afferent neurons are involved in triggering ABs following C2HS. Two prior studies (14, 20) indicate that ABs occur with much greater frequency following C2HS but with substantially reduced volumes compared with uninjured controls. In the current study, we noted a sharp decline in AB frequency observed following phrenicotomy in C2HS animals. Accordingly, afferent information from the ipsilateral hemidiaphragm may be responsible for the increase in AB frequency that occurs after chronic C2HS injury (14, 20). Regarding the contribution of the sCPP to the volume of ABs, a prior study (20) that utilized a dual injury method (simultaneous C2HS and ipsilateral phrenicotomy) indicated that the sCPP made a significant contribution. However, in our study AB volumes in C2HS rats were similar both before and after the acute ipsilateral phrenicotomy. We suggest that determination of the direct contribution of the sCPP to AB volume would require phrenicotomy during an AB cycle, a technical challenge to say the least.
Conclusion.
The sCPP makes a significant contribution to postinjury breathing following C2HS during eupneic conditions. However, taken as a whole, our results indicate that increased output of the contralateral diaphragm and other accessory respiratory muscles (i.e., compensation) is probably more important than the sCPP in promoting respiratory recovery after C2HS. Specifically, a time-dependent increase in VT occurred over a 2-mo period postinjury despite a plateau in the contribution of the sCPP (Fig. 5). Accordingly, we hypothesize that spontaneous improvements in VT beyond the initial weeks post-C2HS primarily reflect plasticity taking place in inspiratory motor circuits other than the sCPP. Plasticity in the neurons and/or networks controlling the accessory muscles has received considerably less attention than the phrenic circuitry (34, 35). However, at least one prior report (12) indicates that intercostal motoneurons have a greater capacity for plasticity than do phrenic motoneurons. Functionally, the sCPP may serve as a “stop-gap” support mechanism until neuroplastic processes enable more robust compensation in other motor pathways.
Our results also validate the C2HS model for use in rehabilitation studies. The limited contribution of the sCPP to recovery (current data; Ref. 14) provides a template for testing strategies intended to increase spinal synaptic efficacy after spinal cord injury (1, 2, 5, 16, 49). Moreover, the methods described herein may prove useful to evaluate the impact of treatments intended to strengthen the sCPP such as intermittent hypoxia (16, 49), activation of spinal neurons via channel rhodopsin (1, 2), and neuronal replacement strategies (10, 38, 50).
GRANTS
B. J. Dougherty was supported by National Institutes of Health National Research Service Award Predoctoral Fellowship F31NS063659. Additional support was provided by National Institutes of Health Grants 1R21-HL104294-01 (to D. D. Fuller) and 1R01-NS-054025 (to P. J. Reier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.J.D., K.-Z.L., and D.D.F. conception and design of research; B.J.D. and K.-Z.L. performed experiments; B.J.D. analyzed data; B.J.D., K.-Z.L., M.A.L., P.J.R., and D.D.F. interpreted results of experiments; B.J.D. prepared figures; B.J.D. drafted manuscript; B.J.D., K.-Z.L., M.A.L., P.J.R., and D.D.F. edited and revised manuscript; B.J.D., K.-Z.L., M.A.L., P.J.R., and D.D.F. approved final version of manuscript.
REFERENCES
- 1. Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci 28: 11862–11870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alilain WJ, Silver J. Shedding light on restoring respiratory function after spinal cord injury. Front Mol Neurosci 2: 18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brichant JF, De Troyer A. On the intercostal muscle compensation for diaphragmatic paralysis in the dog. J Physiol 500: 245–253, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cherniack NS, von Euler C, Glogowska M, Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol Scand 111: 349–360, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann NY Acad Sci 1198: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Troyer A, Loring SH. Action of the respiratory muscles. In: Handbook of Physiology. The Respiratory System. Bethesda, MD: Am Physiol Soc, 1986, vol III, sect. 3, p. 443–462 [Google Scholar]
- 7. De Troyer AD. The canine phrenic-to-intercostal reflex. J Physiol 508: 919–927, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol 200: 74–81, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir Physiol Neurobiol 162: 160–167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dougherty BJLK, Ross HH, Reier PJ, Fuller DD. Recovery of inspiratory tidal volume following lateral cervical spinal cord injury: the role of ipsilateral phrenic activity and the impact of spinal serotonergic cell transplants. In: 2010 Neuroscience Meeting Planner, Program No 6617 San Diego, CA: Society for Neuroscience, 2010 [Google Scholar]
- 11. Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf Proc IEEE Eng Med Biol Soc 2009: 404–407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211: 97–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuller DD, Golder FJ, Olson EB, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol 100: 800–806, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol 165: 245–253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci Lett 373: 89–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci Lett 373: 89–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494–2501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci 21: 8680–8689, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol 169: 85–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol 94: 795–810, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol 72: 211–225, 1981 [DOI] [PubMed] [Google Scholar]
- 25. Jammes Y, Buchler B, Delpierre S, Rasidakis A, Grimaud C, Roussos C. Phrenic afferents and their role in inspiratory control. J Appl Physiol 60: 854–860, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Katagiri M, Young RN, Platt RS, Kieser TM, Easton PA. Respiratory muscle compensation for unilateral or bilateral hemidiaphragm paralysis in awake canines. J Appl Physiol 77: 1972–1982, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci 31: 538–547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169: 123–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langford LA, Schmidt RF. An electron microscopic analysis of the left phrenic nerve in the rat. Anat Rec 205: 207–213, 1983 [DOI] [PubMed] [Google Scholar]
- 31. Lee KZ, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. J Neurophysiol 102: 2184–2193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats. J Appl Physiol 109: 377–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J 18: 1934–1936, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79: 1640–1649, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair 13: 225–234, 1999 [Google Scholar]
- 38. Polentes J, Stamegna JC, Nieto-Sampedro M, Gauthier P. Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol Dis 16: 638–653, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respir Physiol Neurobiol 147: 235–251, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol 169: 94–101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandhu MS, Lee KZ, Fregosi RF, Fuller DD. Phrenicotomy alters phrenic long-term facilitation following intermittent hypoxia in anesthetized rats. J Appl Physiol 109: 279–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandhu MS, Lee KZ, Fregosi RF, Fuller DD. Phrenicotomy alters phrenic long-term facilitation following intermittent hypoxia in anesthetized rats. J Appl Physiol 109: 279–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sherrey JH, Megirian D. After phrenicotomy the rat alters the output of the remaining respiratory muscles without changing its sleep-waking pattern. Respir Physiol 81: 213–225, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Speck DF, Revelette WR. Excitation of dorsal and ventral respiratory group neurons by phrenic nerve afferents. J Appl Physiol 62: 946–951, 1987 [DOI] [PubMed] [Google Scholar]
- 45. Stradling JR, Kozar LF, Dark J, Kirby T, Andrey SM, Phillipson EA. Effect of acute diaphragm paralysis on ventilation in awake and sleeping dogs. Am Rev Respir Dis 136: 633–637, 1987 [DOI] [PubMed] [Google Scholar]
- 46. Teitelbaum J, Borel CO, Magder S, Traystman RJ, Hussain SN. Effect of selective diaphragmatic paralysis on the inspiratory motor drive. J Appl Physiol 74: 2261–2268, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Urmey WF, De Troyer A, Kelly KB, Loring SH. Pleural pressure increases during inspiration in the zone of apposition of diaphragm to rib cage. J Appl Physiol 65: 2207–2212, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Vinit S, Kastner A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir Physiol Neurobiol 169: 115–122, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169: 210–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. White TE, Lane MA, Sandhu MS, O'Steen BE, Fuller DD, Reier PJ. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol 225: 231–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82: 803–814, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol 209: 399–406, 2008 [DOI] [PubMed] [Google Scholar]