Both IFN-γ and TNF-α are integral parts of NK effector machinery and they synergistically enhance the cytolytic function of NK cells.
Keywords: NK cytotoxicity, adhesion molecules, degranulation
Abstract
NK cells control tumor and virus-infected cells through releasing cytotoxic granules and proinflammatory cytokines. IFN-γ and TNF-α secretions and cytotoxicity are regarded as two distinct functions of NK cells with little synergy in between as results of early association of the two functions with distinct subsets of NK populations and of the studies showing target cells developing NK resistance upon IFN-γ treatment. Here, we show that IFN-γ and TNF-α synergistically enhance NK cell cytotoxicity through NF-κB-dependent up-regulation of ICAM-1 expression in target cells, thereby promoting their conjugate formation with NK cells. Neutralizing IFN-γ and TNF-α during cytolysis significantly impaired NK cell lysis of the target cells. Further, tumor cells exhibiting IFN-γ-inducible lysis are generally less-sensitive NK target cells but express inducible levels of ICAM-1. In contrast, sensitive NK targets tend to express higher but less-inducible ICAM-1. Their preferential induction in the lysis of insensitive NK target cells suggests that IFN-γ and TNF-α are functionally linked to and should be regarded as an integral part of NK cytolytic function.
Introduction
NK cells play important roles in controlling viral infections and elimination of tumor cells [1]. They do so by secreting major inflammatory cytokines, such as IFN-γ and TNF-α, and by activating their lytic functions in response to the type I IFNs and other inflammatory cytokines [2–6]. The cytolytic function of NK cells is controlled by a number of activating and inhibitory receptors, as well as adhesion molecules [7–9], and target cell recognition by activating receptors, such as NKG2D, also leads to the production of IFN-γ. IFN-γ and TNF-α have been shown to be essential in viral and tumor clearance [10, 11]. IFN-γ activates the STAT signaling pathway in target cells, leading to the control of infection [12]. Up until now, the main paradigm has been that the cytokine-mediated antiviral and antitumor function of NK cells is separate from their lytic function. In particular, cells treated with IFN-γ became resistant to NK lysis [13–18]. In a sense, NK cells were regarded simply as a major producer of IFN-γ in response to stimulation from other innate cytokines or recognition of target cells. Recently, it was demonstrated that cytolytic CD56dim CD16+ NK cells released abundant IFN-γ at an early stage of their activation [19–21], obscuring the division between IFN-γ production and cytotoxicity functions of NK cells. Although innate cytokines and activating receptor ligands activate the IFN-γ production by NK cells [22], it is not known whether the two major effector functions of NK cells—the killing and cytokine secretion—are coordinated on target cells to further enhance the immune surveillance by NK cells. We show here that the productions of IFN-γ and TNF-α by NK cells are functionally linked to their cytolytic activities. The ability of these inflammatory cytokines to synergistically induce cytolysis in otherwise insensitive target cells suggests that they are an integral part of NK cell effector function. Together, they optimize NK cell-mediated innate immunity.
MATERIALS AND METHODS
Cells, cytokines, and reagents
Polyclonal NK cells were isolated from PBLs and expanded as described previously [23, 24] and then cultured with IMDM, supplemented with 10% human AB serum (Sigma-Aldrich, St. Louis, MO, USA), 10% purified IL-2 (Hemagen Diagnostics, Columbia, MD, USA), and rIL-2 (100 U/ml, National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, MD, USA). All cell lines were from American Type Culture Collection (Manassas, VA, USA). Human rTNF-α and rIFN-γ and their ELISA kits (DuoSet) were from R&D Systems (Minneapolis, MN, USA) and used according to the attached instructions. TPCA-1 and BAY 11-7082 were from Sigma-Aldrich. mAb against human B7-H6 (rat IgM) was a generous gift from Dr. Marco Colonna (Washington University School of Medicine, St. Louis, MO, USA). Polyclonal antibodies against human IFN-γ (ED1210021) and TNF-α (AJ08) were from R&D Systems. mAb against human ICAM-1 (HCD54) was from BioLegend (San Diego, CA, USA). mAb against CD158b (GL183) was from Beckman Coulter (Brea, CA, USA) [25].
Cytotoxicity assays
NK-mediated cytotoxicity was assayed using a Europium-based method (Perkin Elmer, Wellesley, MA, USA). Target cells were washed with a cell culture media (IMDM with 10% FBS), incubated with 1 μl bis(acetoxymethyl) 2,2′:6′,2′′-terpyridine-y,y′′-dicarboxylate-labeling reagent (C136-100; Perkin Elmer) for 45 min at 37°C, and then washed three times with the media containing 1 mM sulfinpyrazone. Approximately 5000 media-resuspended target cells were mixed with varying numbers of effector cells in a 96-well U-bottom microplate, spun down at 300 rpm for 3 min, and incubated for 2–8 h at 37°C in a CO2 incubator. The supernatant (20 μl) from each well was collected and incubated with 200 μl Europium solution (C135-100, Perkin Elmer), and the sample fluorescence was detected using a Wallac microplate reader (Perkin Elmer). For neutralization, an additional 10 μg/ml each of goat anti-human IFN-γ- and TNF-α-neutralizing antibodies (R&D Systems) or 20 μg/ml control goat IgGs (Sigma-Aldrich) was added to the killing assay. The experiments were performed in triplicates, and the data were shown as mean ± sd from one of three experiments. The blocking of the inhibitory KIR with GL183 antibody (1 μg/ml) was carried out using primary polyclonal NK cells, and 38% of them expressed KIR2DL2 against THP-1 cells at an E:T ratio of 8:1.
Flow cytometry and degranulation assays
Cells used for flow cytometric analysis were preincubated with human IgG (10 μg/ml; I4506; Sigma-Aldrich) on ice for 20 min. For flow cytometry staining, the following antibodies were used: APC-conjugated anti-human CD56 (B159; BD PharMingen, San Diego, CA, USA), PE-conjugated anti-human CD107a (H4A3; BD PharMingen), PE-conjugated anti-human HLA-ABC (DX1; BD PharMingen), PE-conjugated anti-human HLA-E (3D12; BioLegend), PE-conjugated anti-human CD48 (BJ40; BioLegend), PE-conjugated anti-human MICA/B (6D4; BD PharMingen), PE-conjugated anti-human ULBP1 (170818; R&D Systems), and PE-conjugated anti-human ULBP2 (165903; R&D Systems). For staining of ICAM-1 (HCD54; BioLegend), ULBP3 (166510; R&D Systems), CD80 (BB1; BD PharMingen), CD86 (2331; BD PharMingen), and FITC-conjugated anti-mouse IgG polyclonal antibody (Dako North America, Inc.) were used as the secondary antibody. For staining of B7-H6, Alexa Fluor 647 goat anti-rat IgM (μ chain; Molecular Probes, Eugene, OR, USA) was used as the secondary antibody. The rat IgM (R4-22; BD PharMingen) was used as an isotype control. After washing cells with PBS (containing 2% FBS), the labeled cells were analyzed by FACSCalibur (BD Biosciences, San Jose, CA, USA). FlowJo software (Tree Star, Ashland, OR, USA) was used for data analysis. For degranulation assays, NK-92MI cells were washed and resuspended in IMDM and then incubated with human IgG for 20 min. Target cells were blocked with human IgG (10 μg/ml) for 20 min on ice. NK-92MI cells (105) were mixed with target cells (2×105) in the presence of 5 μM monensin (Sigma-Aldrich) and PE-conjugated anti-human CD107a mAb, spun down for 3 min at 300 rpm, and then incubated at 37°C for 2 h. Cells were washed and stained with APC-conjugated anti-human CD56 mAb in PBS containing 2% FBS for 30 min on ice. After wash steps, cells were resuspended for FACS analysis.
Conjugation assays
NK-92MI cells were labeled with CellTracker Green CMFDA (Molecular Probes), whereas effector cells were labeled with PKH26 dye (Sigma-Aldrich). After labeling, cells were incubated at 37°C for 30 min to allow the excess dye to be bled out. The labeled NK-92MI cells (1×106 cells/mL) were mixed with labeled target cells (2×106). The effector-target cell mixture was centrifuged at 300 rpm for 5 min at 4°C and then incubated at 37°C for the indicated time. After incubation, the cell mixture was resuspended by strong vortexing and fixed by adding ice-cold 0.5% paraformaldehyde. The conjugate formation was analyzed by flow cytometry. The conjugation ratio was calculated as the portion of FL-1 (CellTracker Green CMFDA):FL-2 (PKH26) double-positive events within the FL-1 events.
ICAM-1 transfection and RNAi
Full-length human ICAM-1 cDNA was obtained from OriGene (Rockville, MD, USA) and subcloned into the HindIII and EcoRI sites of a mammalian plasmid pcDNA3 (Invitrogen, Carlsbad, CA, USA) by using a standard molecular biology technique. ICAM-1 expression plasmid or empty vector (2 μg) was delivered to cells by FuGENE HD transfection reagent (Roche Applied Science, Indianapolis, IN, USA), according to the manufacturer′s instructions. About 28 h after ICAM-1 transfection, transfected cells were treated with IFN-γ (100 ng/ml) or TNF-α (50 ng/ml) or left untreated for 20 h, which were then used as the target cell in NK-mediated cytotoxicity assays. For transfecting siRNA against human ICAM-1, three targets were selected with sequences of ACU GUG GUA GCA GCC GCA GUC AUA A (#1, sense), CAG GCC UCA GCA CGU ACC UCU AUA A (#2, sense), and CCA AUU UCU CGU GCC GCA CUG AAC U (#3, sense) and synthesized by Invitrogen. Approximately 2 × 105 cells in six-well plates were transfected with 100 pmol of the indicated siRNAs or the universal negative control (high GC content, Invitrogen) using Lipofectamine RNAiMAX (Invitrogen). Two days after transfection, the siRNA-transfected cells were stimulated with IFN-γ and TNF-α for 20 h and then analyzed by flow cytometry or NK lysis using an E:T ratio of 1:1.
RESULTS
IFN-γ and TNF-α synergistically enhance NK cell lysis of THP-1 cells
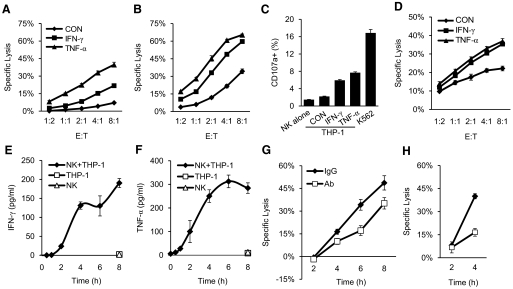
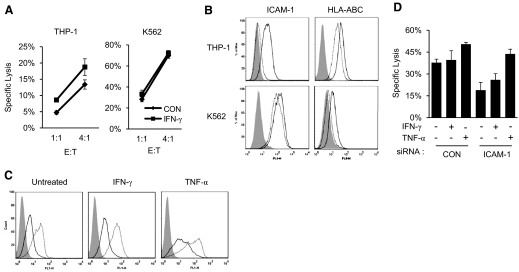
It was shown previously that K562 cells became resistant to NK lysis after IFN-γ treatment [13–15]. However, overnight treatment of THP-1 cells, a myeloid leukemia cell line, with 50 ng/ml IFN-γ resulted in significant enhancement in the target cell lysis by IL-2 expanded, primary NK cells (Fig. 1A) or a NK-like cell line, NK-92MI (Supplemental Fig. 1). Similar enhancement in target cell lysis was also observed in IFN-γ-treated Kasumi-1 cells, also a myeloid leukemia cell line (Fig. 1B and Supplemental Fig. 1). Further, overnight incubation with 25 ng/ml TNF-α also enhanced NK cell lysis of THP-1 and Kasumi-1 cells to a comparable or better level than IFN-γ (Fig. 1). The cytokine-induced NK lysis of THP-1 cells is also evident in a 2-h degranulation assay, in which significantly higher levels of degranulation occurred in NK-92MI cells incubated with THP-1 cells, pretreated with IFN-γ or TNF-α for 20 h versus no treatment (Fig. 1C). A time course and dose-dependent induction with IFN-γ or TNF-α showed that similar enhancements in NK cell lysis of THP-1 were observed in just 6 h treatment, with as low as 10 ng/ml IFN-γ or 80 pg/ml TNF-α (Fig. 1D and Supplemental Fig. 1). As activated NK cells represent a major source of IFN-γ in vivo, we investigated the contribution of endogenous, NK-produced cytokines in lysis of THP-1 target cells. Upon coculturing with THP-1 cells, IL-2 expanded, primary NK cells produced IFN-γ and TNF-α, which reached steady levels of 200–300 pg/ml/105 cells in 4–6 h, as detected by ELISA assays (Fig. 1E and F). Importantly, in the presence of 10 μg/ml each of IFN-γ- and TNF-α-neutralizing antibodies, the NK cell lysis of THP-1 and Kasumi-1 cells was reduced significantly after 4 h incubation (Fig. 1G and H). The lack of antibody neutralization to killings at the 2-h time-point is consistent with the kinetics of IFN-γ and TNF-α production by the coculturing NK cells (Fig. 1E and F). Thus, the cytokines secreted by NK cells directly facilitated their cytolysis of THP-1 and Kasumi-1 cells, suggesting that activated NK cells produce IFN-γ and TNF-α to enhance their lytic function. These results established a direct link between cytokine production and cytolysis of NK cells. As IFN-γ and TNF-α have antiproliferative activities and could induce apoptosis in certain cells [10, 26], we investigated whether the observed enhancement in target cell lysis was a result of an additive effect between cytokine-mediated apoptosis and receptor-mediated cytolysis. However, treatment with IFN-γ or TNF-α for 20 h failed to induce apoptosis in THP-1 cells, as judged by Annexin V/PI staining (Supplemental Fig. 2). This is also consistent with previous reports that THP-1 cells were resistant to TNF-α-induced apoptosis [27]. The failure of cytokines alone to induce the target cell lysis illustrates a synergistic nature between the cytokine production and lytic function of NK cells.
Figure 1. IFN-γ- and TNF-α-induced lysis of THP-1 and Kasumi-1 cells by NK cells.
(A and B) Lysis of THP-1 (A) or Kasumi-1 (B) cells, with or without prior treatment of IFN-γ or TNF-α. IL-2 expanded, primary NK cells were used at the indicated E:T ratios. (C) Degranulation of NK-92MI cells alone and in the presence of untreated [control (CON)] and IFN-γ- or TNF-α-treated THP-1 cells. K562 cells were used as a positive control. (D) NK cell lysis of THP-1 cells treated with IFN-γ or TNF-α for 6 h. (E and F) Target cells mediated production of IFN-γ and TNF-α. Primary NK cells (105) were incubated with an equal number of THP-1 cells for the indicated time, and then the supernatants were collected for ELISA assays. (G and H) Lysis of THP-1 (G) and Kasumi-1 (H) cells by primary NK cells at an E:T ratio of 2:1 for the indicated time in the presence of neutralizing antibodies against IFN-γ and TNF-α or control IgG. The experiments were performed in triplicates, and the data were shown as mean ± sd from one of three experiments.
Changes in class I MHC and NKG2D ligand expressions are insufficient for IFN-γ- and TNF-α-induced THP-1 lysis
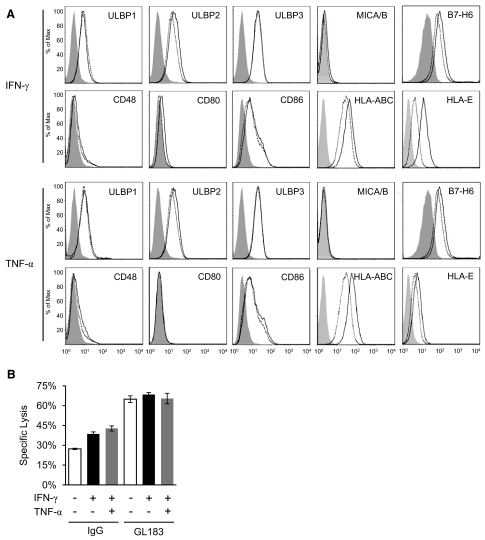
Previously, IFN-γ was shown to up-regulate class I MHC and down-regulate NKG2D ligand expressions in certain tumor cells, thereby rendering them less susceptible to NK lysis [28]. To investigate the mechanism of the IFN-γ- and TNF-α-induced NK lysis of THP-1 cells, we examined the cytokine effects on the expressions of class I MHC and activating NKR ligands on the target cells. It is possible that the cytokines up-regulate the expression of activating receptor ligands or down-regulate the expression of class I MHC on THP-1 cells, thus rendering them more sensitive to NK lysis. THP-1 cells express several NKG2D ligands (ULBP1–3) and a NKp30 ligand (B7-H6; Fig. 2A). Overnight treatment of THP-1 with 50 ng/ml IFN-γ or 25 ng/ml TNF-α did not significantly affect the expressions of these known ligands (Fig. 2A). THP-1 cells express abundant class I MHC molecules on their surface, and IFN-γ or TNF-α treatment did not decrease but rather resulted in a slight increase in HLA expression on THP-1 cells (Fig. 2A), indicating that MHC expression may not contribute to the cytokine-induced THP-1 lysis by NK cells. Although the overall HLA expressions on THP-1 are increased in response to the cytokine treatment, it is possible that the inhibitory KIRs on the primary NK cells are in mismatch with HLA alleles on THP-1 cells. The KIR-HLA mismatch would result in the absence of KIR inhibition and could account for the IFN-γ-induced NK lysis through up-regulation of HLA expressions. THP-1 cells express C1 allotype HLA-Cw3 (Cw*0303/0303) [29, 30]. To distinguish the presence of specific NK and THP-1 KIR-HLA mismatch as the mechanism for the IFN-γ-induced THP-1 lysis through induction of HLA expressions, we typed primary NK cells for the expression of KIR2DL2 and blocked the HLA-Cw3-specific, inhibitory KIRs with antibody GL183 during the cytokine-induced THP-1 lysis. Blocking KIR with GL183 resulted in significant increases in NK lysis of THP-1 cells in the presence and absence of the cytokines (Fig. 2B), demonstrating, as expected, the presence of a functional KIR-HLA inhibition. Thus, the cytokine-induced target cell lysis not related to HLA mediated target cell lysis as a result of KIR mismatch.
Figure 2. Change of NK ligands upon IFN-γ or TNF treatment.
(A) The expression of known ligands of activating NKRs and class I MHC molecules on THP-1 cells. Untreated (dotted lines), IFN-γ (50 ng/ml), or TNF-α (25 ng/ml) overnight-treated (black lines) THP-1 cells were stained with indicated antibodies and analyzed by flow cytometry. Isotype controls are shown in gray-shaded histograms. (B) Effect of inhibitory KIR on IFN-γ-induced lysis of THP-1. The cytotoxicity assays were carried out in the presence of a KIR-blocking antibody, GL183, or its isotype control using an E:T ratio of 8:1. The experiments were repeated for at least two times, and one representative result was shown.
IFN-γ or TNF-α enhances NK lysis through up-regulation of ICAM-1 expression in THP-1
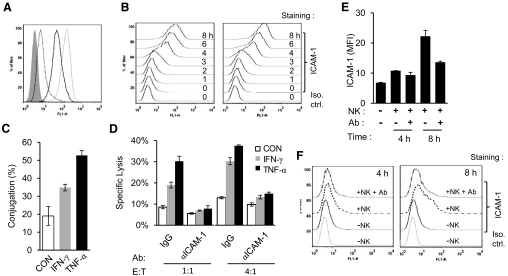
As IFN-γ and TNF-α did not significantly influence the expression of known, activating NK ligands nor did the cytokines decrease the class I MHC expression in THP-1, we then investigated the possible role of the cytokines in regulating the adhesion molecules involved in NK lysis. In particular, IFN-γ and TNF-α are known to up-regulate ICAM-1 expression in tumor cell lines [31–34] and increase the killing of a neuroblastoma cell line by LAK cells [35]. Indeed, IFN-γ and TNF-α induced ICAM-1 expression in THP-1 cells upon overnight treatment (Fig. 3A). Interestingly, TNF-α-treated THP-1 cells expressed more ICAM-1 and were more sensitive to NK lysis than the IFN-γ-treated cells (Figs. 1A and 3A). In fact, the cytokine-induced ICAM-1 expression was evident after 3-4 h of stimulation (Fig. 3B). To examine if the increased ICAM-1 expression leads to enhanced target cell adhesion with NK cells, we carried out a conjugation assay between NK-92MI and THP-1 cells using FACS analysis [36]. When THP-1 cells pretreated with 50 ng/ml IFN-γ or 25 ng/ml TNF-α for 20 h were coincubated with NK-92MI cells for 20 min, the level of conjugation was significantly higher in IFN-γ- and TNF-α-treated THP-1 cells than the control (Fig. 3C), suggesting IFN-γ and TNF-α induce ICAM-1 expression in THP-1 cells to promote the target cell adhesion to NK cells. This cytokine-induced NK lysis of THP-1 cells was abolished in the presence of 5 μg/ml anti-ICAM-1 antibody (Fig. 3D). As ICAM-1 was up-regulated within 4 h of cytokine stimulation, and neutralizing IFN-γ and TNF-α significantly reduced NK lysis of THP-1 cells (Figs. 1G and 3B), we investigated whether the NK cell produced cytokine-stimulated target cell ICAM-1 expression during NK cytolysis. IL-2-activated NK cells were coincubated with THP-1 cells at a 2:1 ratio of NK:THP-1 cells for 4 and 8 h in the presence or absence of IFN-γ- and TNF-α-neutralizing antibodies. With the use of CD56 staining to exclude NK cells, the ICAM-1 expression on THP-1 cells, resulting from the coincubation with NK cells, was determined by FACS analyses. The results showed that coincubation of THP-1 cells with NK cells for 4 and 8 h significantly up-regulated ICAM-1 expression on the target cells, whereas this NK cell-induced ICAM-1 expression is diminished in the presence of 10 μg/ml IFN-γ- and TNF-α-neutralizing antibodies (Fig. 3E and F). The lower ICAM-1 expressions induced by NK cells compared with those by exogenous cytokines may reflect their concentration differences (Fig. 3B and F). Thus, NK cell-secreted cytokines directly induced target cell ICAM-1 expression. The kinetics of ICAM-1 up-regulation is also consistent with the lack of the significant blocking effect in the first 4 h of NK cytolysis of THP-1 cells by anti-IFN-γ and anti-TNF-α antibodies (Fig. 1).
Figure 3. ICAM-1 is required for IFN-γ- and TNF-α-induced target cell lysis.
(A) ICAM-1 expression on THP-1 cells (dotted line), THP-1 cells treated with IFN-γ (black line), or TNF-α (light, gray line) for 20 h. Isotype control (Iso. ctrl.) is shown in gray-shaded histograms. (B) The ICAM-1 expression on THP-1 cells was detected by FACS analysis at 0-, 1-, 2-, 3-, 4-, 6-, and 8-h time-points following the treatment with IFN-γ (left panel) or TNF-α (right panel). (C) IFN-γ and TNF-α enhanced the conjugation between NK and THP-1 cells. (D) Lysis of THP-1 cells by NK-92MI cells at an E:T ratio of 1:1 and 4:1 for 2 h in the presence of ICAM-1 blocking antibody or control IgG. (E and F) Coincubation of THP-1 cells with primary NK cells for 4 and 8 h increased ICAM-1 expression on THP-1 cells, and the ICAM-1 induction is reduced significantly in the presence of IFN-γ- and TNF-α-neutralizing antibodies. The same data were presented as a bar diagram (E) and histogram (F). The experiments were repeated for at least two times, and one representative result was shown. FL1-H, FL-1 height; MFI, mean fluorescence intensity.
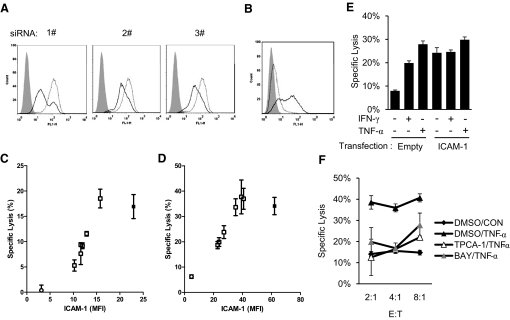
As ICAM-1 also contributes to basal target cell lysis, blocking ICAM-1 is inadequate to demonstrate its specific contribution to IFN-γ- and TNF-α-induced target cell sensitization. To further address the role of ICAM-1 in the cytokine-induced THP-1 sensitization, we attempted to knock down the TNF-α-induced ICAM-1 expression in THP-1 using three ICAM-1-specific siRNAs. Compared with THP-1 cells transfected with control siRNA, the cells transfected with individual ICAM-1-targeted siRNA displayed 40–70% knocked-down in ICAM-1 expression upon overnight induction with 1 ng/ml TNF-α (Fig. 4A). By combining the three different ICAM-1-specific siRNAs, we were able to achieve various levels of IFN-γ- and TNF-α-induced ICAM-1 expressions on the knocked-down THP-1 cells. More importantly, when these siRNA-transfected THP-1 cells were used as target cells to assess IFN-γ-induced NK lysis, the results showed that the extent of NK lysis of the siRNA-transfected THP-1 cells correlated well with their surface ICAM-1 expression with a two-tailed Spearman correlation P value of 0.002 (Fig. 4C). Similarly, TNF-α-induced NK lysis of THP-1 also correlated with their surface ICAM-1 expression (Fig. 4D; P=0.005). These data demonstrated that ICAM-1 expression is required for target cell sensitization to NK-mediated cytotoxicity. To address if the expression of ICAM-1 is sufficient to sensitize THP-1 cells to NK lysis, we transfected THP-1 cells with human ICAM-1, cloned into a mammalian expression plasmid pcDNA3 (Fig. 4B). The NK lysis of ICAM-1-transfected THP-1 cells was increased greatly compared with untransfected THP-1 cells without cytokine stimulation (Fig. 4E). The NK lysis of ICAM-1-transfected THP-1 cells is comparable with that of TNF-α-treated, untransfected THP-1 cells, suggesting that ICAM-1 expression is sufficient to sensitize THP-1 for NK cytolysis. Further, the cytolysis of these ICAM-1-transfected THP-1 cells displayed a reduced response to IFN-γ or TNF-α stimulation compared with untransfected THP-1 cells. Thus, up-regulation of ICAM-1 is necessary and sufficient for IFN-γ- and TNF-α-mediated target cell sensitization to NK lysis.
Figure 4. The expression of ICAM-1 correlates with susceptibility to NK lysis.
(A) Knockdown of ICAM-1 on THP-1 cells with siRNA. TNF-α induced ICAM-1 expression on THP-1 cells transfected with control siRNA (dotted lines) or ICAM-1-specific siRNAs (black lines). Isotype control staining is shown in gray-shaded histograms. (B) ICAM-1 expression on empty vector-transfected (dotted line) or ICAM-1-transfected (black line) THP-1 cells. (C and D) Correlation between IFN-γ (C)- or TNF-α (D)-induced ICAM-1 expression on various siRNA-transfected THP-1 cells and their lysis by NK-92MI cells. (E) Overexpression of ICAM-1 is sufficient for THP-1 cells to sensitize to NK-92MI-mediated lysis. (F) TPCA-1 and BAY 11-7082 (BAY) inhibit TNF-α-induced sensitization of THP-1 cells to NK-92MI-mediated cytotoxicity. The experiments were repeated for at least two times, and one representative result was shown.
IFN-γ- and TNF-α-induced target cell lysis depends on NF-κB pathway
IFN-γ and TNF-α have been shown to induce ICAM-1 expression through the activating NF-κB signaling pathway [37, 38]. To further evaluate the involvement of the NF-κB pathway in IFN-γ- and TNF-α-induced target cell sensitization to NK cytolysis, we carried out the cytokine-induced NK cytolysis of THP-1 cells in the presence of NF-κB pathway-specific inhibitors—TPCA-1 (2 μM) and BAY 11-7082 (10 μM)—to block the function of IKK-β and IκBα [39, 40], respectively. The results showed that TPCA-1 and BAY 11-7082 strongly blocked TNF-α-induced killing of THP-1 cells (Fig. 4F), confirming the involvement of the NF-κB pathway in the cytokine-induced target cell sensitization.
IFN-γ and TNF-α induce tumor cell ICAM-1 expression to promote NK cytolysis
Previously, prolonged IFN-γ treatment resulted in resistance to NK cytolysis in certain sensitive target cell lines, such as K562, a human erythrokeukemia cell line [13–15]. Despite the known role of class I MHC in protection against NK lysis and the ability of IFN-γ to up-regulate MHC expression [41, 42], the contribution of MHC to IFN-γ-induced NK resistance was less clear, with evidence indicating that class I MHC expression may not be the only factor affecting the cytokine-mediated NK cytolysis [16–18]. In mouse tumors expressing high levels of H60, the effect of IFN-γ-induced NK resistance was shown to be partly a result of IFN-γ down-regulation of NKG2D ligand expression [28]. To investigate the contrasting effects of IFN-γ in NK-mediated lysis of THP-1 and K562 cells (Fig. 5A), we examined ICAM-1 expression in response to IFN-γ in K562 cells. Unlike THP-1 cells, whose basal ICAM-1 expression is low but increased by approximately fivefold in response to IFN-γ, K562 cells expressed a high level of ICAM-1 but failed to increase further upon IFN-γ treatment (Fig. 5B). In contrast to ICAM-1 expression, THP-1 cells express abundant HLA, whereas K562 expressed little. However, both cell lines exhibited a similar increase in HLA expression upon IFN-γ stimulation (Fig. 5B). To test whether the higher endogenous ICAM-1 expression rendered K562 cells nonresponsive to IFN-γ stimulation and thus, insensitive to the cytokine-induced NK cytolysis, we transfected K562 cells with an ICAM-1-specific siRNA (1#) or its control siRNA. The siRNA transfection significantly reduced the expression of ICAM-1 compared with the control transfection (Fig. 5C). However, more importantly, ICAM-1 siRNA transfection significantly reduced NK lysis of K562 cells compared with the control siRNA transfection, and the ICAM-1 knock-down K562 cells exhibited an increased response in NK cytolysis to IFN-γ and TNF-α stimulation (Fig. 5D). These results showed that the difference in ICAM-1 expression and response to IFN-γ stimulation between THP-1 and K562 cells contributed to their sensitivity to the cytokine-induced NK cytolysis.
Figure 5. Basal levels of ICAM-1 determine target response to inducible NK lysis.
(A) Effects of IFN-γ on NK-92MI-mediated killing against THP-1 and K562 cells. (B) Expression of ICAM-1 and HLA-ABC on IFN-γ-treated (thick lines) and untreated (dotted lines) cells. The isotype controls are shown as gray-shaded histograms. ICAM-1 expression (C) on and NK lysis (D) of K562 cells transfected with (1#) siRNA against human ICAM-1 or universal high GC content control. The transfected cells were treated with or without IFN-γ (100 ng/ml) or TNF-α (50 ng/ml) for 20 h before FACS analysis and cytotoxicity assays using NK-92MI as effector cells at an E:T ratio of 1:1.
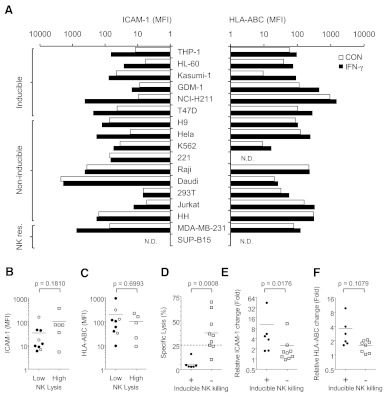
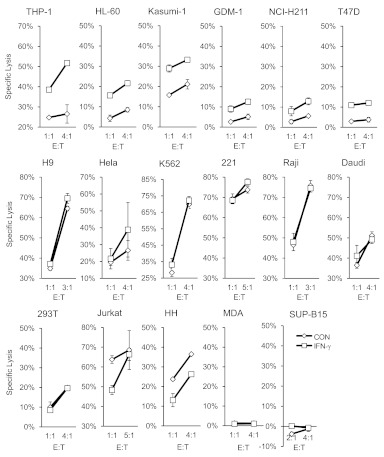
To further examine whether ICAM-1 expression and its response to IFN-γ stimulation correlate with the ability of the cytokine to sensitize tumor cells for NK killings, we measured ICAM-1 expressions on a number of tumor cell lines and their response to NK cytolysis after IFN-γ treatment (see Fig. 7A). The majority of tumor cells tested here is susceptible to NK cytolysis (Fig. 6), and their lysis levels are proportional to the E:T ratios, indicating that they express ligands for activating NKRs. In contrast, MDA-MB-231 and SUP-B15, which exhibited minimum lysis by NK-92MI, independent of the E:T ratio, are NK-resistant target cells, suggesting that these cells lack ligands for activating NKRs. To examine the effect of IFN-γ on NK cytolysis of susceptible target tumor cells, we further divided the susceptible tumor cells into sensitive (high) and insensitive (low sensitivity) cytolytic targets. NK-sensitive tumor cells include K562, 721.221, H9, Raji, Daudi, and Jurkat cells. The insensitive targets include THP-1, HL-60, T47D, Kasumi-1, GDM-1, NCI-H211, Hela, 293T, and HH cells. Although NK-sensitive target cells appear to express higher constitutive levels of ICAM-1, the correlation is not statistical significant (Fig. 7A and B). Similarly, HLA expressions on tumor cells failed to reverse-correlate with their sensitivity to NK cytolysis, despite that the known, HLA-negative target cells, such as 721.221, Daudi, and K562, are very sensitive to NK lysis (Fig. 7C). More interestingly, these tumor cells exhibited variable response to IFN-γ-induced NK lysis (Fig. 6), and they can be divided into IFN-γ-inducible and noninducible groups. In addition to THP-1 and Kasumi-1, NK cell killing of GDM-1, HL60, NCI-H211, and T47D was enhanced upon IFN-γ stimulation and thus, is IFN-γ-inducible (Fig. 6). The IFN-γ-noninducible cells include H9, Hela, K562, 721.221, Daudi, Raji, 293T, Jurkat, and HH, whose lysis by NK cells exhibited no change or slight reduction in response to IFN-γ treatment. Overall, IFN-γ-inducible tumor cells were less sensitive to NK lysis than the noninducible cells (Fig. 7D). Further, the IFN-γ-inducible tumor cells also exhibited significantly larger changes in ICAM-1 expression than the noninducible group target cells (Fig. 7E). Although the class I MHC expressions also appeared to be up-regulated by IFN-γ among the inducible tumor cells (Fig. 7F), the increased sensitivity to NK lysis of these cells suggests that their ICAM-1-mediated tumor sensitization is a primary effect over the MHC-mediated tumor resistance to NK lysis. This correlation between IFN-γ-induced ICAM-1 expressions and their increased sensitivity to NK lysis suggests that a general function of IFN-γ is to induce target cell lysis through ICAM-1 expression. It is worth noting that tumor cells in the IFN-γ-inducible group are less sensitive to NK killings than the noninducible target cells (Fig. 7D). Alternatively, tumor cells that failed to respond positively to IFN-γ-induced NK cytolysis, such as K562 cells, are generally more susceptible to NK lysis. This inverse correlation between target cell susceptibility to NK lysis and their response to IFN-γ stimulation is consistent with activated NK cells to produce cytokines for the sensitization of otherwise resistant tumor cells for NK cytolysis.
Figure 7. Distinct effects of IFN-γ on different tumor cell lines.
(A) Expression profiles of ICAM-1 and HLA-ABC on multiple tumor cell lines with or without IFN-γ treatment. NK res., NK resistance. (B) Comparison of basal levels of ICAM-1 on sensitive (high) and insensitive (low sensitivity) cytolytic target. (U=15.0; n1=9; n2=6; P=0.1810 two-tailed). (C) Comparison of basal levels of HLA-ABC on sensitive and insensitive cytolytic targets (U=19.0; n1=9; n2=5; P=0.6993 two-tailed). (D) Comparison of NK-mediated lysis of untreated-inducible and noninducible cells. The specific lysis of each target cell, based on the data from Fig. 6, at an E:T ratio of 1:1. The cutoff of sensitivity of NK lysis was shown as a dashed line. The distributions in the two groups differed significantly (U=1.0; n1=6; n2=9; P=0.0008 two-tailed). (E) Comparison of relative induction of ICAM-1 on inducible and noninducible cells. The distributions in the two groups differed significantly (U=7.0; n1=6; n2=9; P=0.0176 two-tailed). (F) Comparison of relative induction of HLA-ABC on inducible and noninducible cells (U=11.0; n1=6; n2=8; P=0.1079 two-tailed). The statistical analysis was done by Mann-Whitney U test using GraphPad Prism software. Inducible and noninducible target cells were shown as closed and open symbols, respectively.
Figure 6. Effects of IFN-γ on NK-92MI lysis of multiple tumor cell lines using the indicated E:T ratio.
The experiments were performed in triplicates, and the data were shown as mean ± sd from one of at least two experiments. 221, 721.221; MDA, MDA-MB-231.
DISCUSSION
Cytolytic activity and cytokine production are two main functions of NK cells, which represent a major source of IFN-γ in vivo. IFN-γ and TNF-α are known as major proinflammatory cytokines, important for host protection against viral infection and tumor; however, the direct role of the cytokines in NK cell effector function has not been well defined. IFN-γ-producing NK cells were found to have low cytotoxicity, whereas conversely, cytotoxic NK cells expressed a low level of IFN-γ [43]. In addition, IFN-γ was known to induce target cell resistance to NK cytolysis [28, 44–46]. Recently, it was demonstrated that cytolytic CD56dimCD16+ NK cells can produce IFN-γ over the duration of their cytolytic activity [19, 21]. In the present study, we show that IFN-γ and TNF-α sensitize target cells to NK cytotoxicity, and intrinsic cytokines produced by activated NK cells promote target cell lysis, as neutralization of these cytokines significantly impaired the lytic function. Using THP-1 cells, we showed that the cytokine-induced ICAM-1 expression is necessary and sufficient to sensitize target cells for NK lysis. Tumor cells exhibit varying sensitivity to NK cytolysis, although a few exceptions exist, such as the NK-resistance tumor cells, MDA-MB-231 and SUP-B15, which exhibited minimum cytolysis, independent of NK:target ratios. The majority of tumor cells that we examined exhibited target killings dependent on the E:T ratios and thus, susceptible to NK lysis. The lack of direct correlation between constitutive ICAM-1 expression and tumor sensitivity to NK lysis is most likely a result of variable expressions of other activating and inhibitory NKR ligands on different tumor cells. The correlation between ICAM-1 up-regulation and their increased sensitivity to NK cytolysis suggests that in general, IFN-γ modulates NK lysis through the expression of the adhesion receptor. In general, tumor cells that are sensitized by IFN-γ and TNF-α express lower but inducible levels of ICAM-1 than tumor cells refractory to cytokine treatment. As many IFN-γ- and TNF-α-noninducible tumor cells are indeed sensitive NK target cells, they may not need further sensitization by the cytokines. It is worth noting that IFN-γ and TNF-α antibodies only partially blocked the NK cell-mediated ICAM-1 up-regulation in THP-1 cells (Fig. 3F), suggesting that the cytokine effect is more profound than that shown in Fig. 1G and H at later time-points.
HLA molecules serve as ligands for inhibitory NKRs. Several reports have shown that IFN-γ inhibited NK-mediated killing by up-regulation of HLA molecules and down-regulation of NKG2D ligands on target cells [28, 44–46]. Indeed, HLA class I molecules were up-regulated in response to IFN-γ treatment in most tumor cells (Fig. 7A and C). However, the IFN-γ-induced HLA expressions do not correlate inversely with the target cell sensitization by IFN-γ and TNF-α (Fig. 7C). Nonetheless, for tumor cells expressing low levels of HLA, the increase in HLA expression upon IFN-γ treatment is consistent with their reduced killing by NK cells. Thus, for target cells expressing low levels of ICAMs, the effect of cytokine-induced ICAM-1 expression overcomes inhibitory HLA expression, resulting in target cell sensitization. Conversely, for cells expressing high levels of ICAMs, the effect of up-regulation of ICAM-1 by IFN-γ in target lysis may be less compared with that of HLA, resulting in reduced target cell lysis upon IFN-γ treatment.
Up-regulation of ICAM-1 could not promote NK-mediated cytotoxicity without ligands of activating NKRs. In humans, NKG2D ligands, including MICA, MICB, and ULBP1–6, could be modulated during virus infection or tumorigenesis [47]. Upon treatment with differentiation-promoting myeloid growth factors, together with IFN-γ, cell-surface levels of ULBP1 were up-regulated on AML blasts [48]. THP-1 cells express ULBP1, ULBP2, and ULBP3 but not ULBP4 and ULBP5 [49] and B7-H6 (Fig. 2A) [50]. However, IFN-γ or TNF-α stimulation only marginally increased the expression of ULBP2 and B7-H6 in THP-1 cells (Fig. 2A). The expressions of CD48, CD80, and CD86, which are ligands of 2B4 and CD28, also remain unchanged after the cytokine treatment (Fig. 2A). Activation and inflammation induce ICAM-1 expression in many cells. Perforin deficiency in familial hemophagocytic lymphohistiocytosis disorder results in accumulation of activated macrophages and lymphocytes [51], suggesting a role for the NK cell in maintaining a homeostatic lymphocyte population. It is conceivable that increased expression of ICAM-1 contributes in part to the preferential killing of activated macrophages and lymphocytes by NK cells. In conclusion, IFN-γ and TNF-α are an integral part of NK effector machinery, and they synergistically enhance the cytolytic function of NK cells.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of NIAID, NIH. We thank Marco Colonna (Washington University School of Medicine) for the antibody against human B7-H6, and we thank Eric O. Long and Sumati Rajagopalan (NIAID, NIH) for the supply of primary human NK cells and helpful suggestions.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- APC
- allophycocyanin
- CMFDA
- 5-chloromethylfluorescein diacetate
- FL-1/2
- fluorescence 1/2
- KIR
- killer cell Ig-like receptor
- MICA/B
- MHC class I chain-related genes A/B
- NIAID
- National Institute of Allergy and Infectious Diseases
- RNAi
- RNA interference
- siRNA
- small interfering RNA
- TPCA-1
- 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide
- ULBP1–3
- UL16-binding protein-1–3
AUTHORSHIP
R.W., J.J.J., N.C.S., and Z.Z. conducted the experiments. P.D.S. and R.W. designed the study, analyzed the data, and wrote the manuscript.
REFERENCES
- 1. Biron C. A., Nguyen K. B., Pien G. C., Cousens L. P., Salazar-Mather T. P. (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 [DOI] [PubMed] [Google Scholar]
- 2. Welsh R. M., Jr. (1978) Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J. Exp. Med. 148, 163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grundy J. E., Trapman J., Allan J. E., Shellam G. R., Melief C. J. (1982) Evidence for a protective role of interferon in resistance to murine cytomegalovirus and its control by non-H-2-linked genes. Infect. Immun. 37, 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. (1978) Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature 273, 759–761 [DOI] [PubMed] [Google Scholar]
- 5. Trinchieri G., Santoli D., Koprowski H. (1978) Spontaneous cell-mediated cytotoxicity in humans: role of interferon and immunoglobulins. J. Immunol. 120, 1849–1855 [PubMed] [Google Scholar]
- 6. Santoli D., Trinchieri G., Lief F. S. (1978) Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J. Immunol. 121, 526–531 [PubMed] [Google Scholar]
- 7. Long E. O. (2008) Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol. Rev. 224, 70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanier L. L. (2005) NK cell recognition. Annu. Rev. Immunol. 23, 225–274 [DOI] [PubMed] [Google Scholar]
- 9. Moretta L., Biassoni R., Bottino C., Cantoni C., Pende D., Mingari M. C., Moretta A. (2002) Human NK cells and their receptors. Microbes Infect. 4, 1539–1544 [DOI] [PubMed] [Google Scholar]
- 10. Ikeda H., Old L. J., Schreiber R. D. (2002) The roles of IFN γ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 13, 95–109 [DOI] [PubMed] [Google Scholar]
- 11. Balkwill F. (2009) Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 [DOI] [PubMed] [Google Scholar]
- 12. Miyagi T., Gil M. P., Wang X., Louten J., Chu W. M., Biron C. A. (2007) High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 204, 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grönberg A., Ferm M. T., Ng J., Reynolds C. W., Ortaldo J. R. (1988) IFN-γ treatment of K562 cells inhibits natural killer cell triggering and decreases the susceptibility to lysis by cytoplasmic granules from large granular lymphocytes. J. Immunol. 140, 4397–4402 [PubMed] [Google Scholar]
- 14. De Fries R. U., Golub S. H. (1988) Characteristics and mechanism of IFN-γ-induced protection of human tumor cells from lysis by lymphokine-activated killer cells. J. Immunol. 140, 3686–3693 [PubMed] [Google Scholar]
- 15. Maio M., Altomonte M., Tatake R., Zeff R. A., Ferrone S. (1991) Reduction in susceptibility to natural killer cell-mediated lysis of human FO-1 melanoma cells after induction of HLA class I antigen expression by transfection with B2m gene. J. Clin. Invest. 88, 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maziarz R. T., Mentzer S. J., Burakoff S. J., Faller D. V. (1990) Distinct effects of interferon-γ and MHC class I surface antigen levels on resistance of the K562 tumor cell line to natural killer-mediated lysis. Cell. Immunol. 130, 329–338 [DOI] [PubMed] [Google Scholar]
- 17. Ramirez R., Solana R., Carracedo J., Alonso M. C., Pena J. (1992) Mechanisms involved in NK resistance induced by interferon-γ. Cell. Immunol. 140, 248–256 [DOI] [PubMed] [Google Scholar]
- 18. Nishimura M., Mitsunaga S., Akaza T., Mitomi Y., Tadokoro K., Juji T. (1994) Protection against natural killer cells by interferon-γ treatment of K562 cells cannot be explained by augmented major histocompatibility complex class I expression. Immunology 83, 75–80 [PMC free article] [PubMed] [Google Scholar]
- 19. Fauriat C., Long E. O., Ljunggren H. G., Bryceson Y. T. (2010) Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115, 2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juelke K., Killig M., Luetke-Eversloh M., Parente E., Gruen J., Morandi B., Ferlazzo G., Thiel A., Schmitt-Knosalla I., Romagnani C. (2010) CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood 116, 1299–1307 [DOI] [PubMed] [Google Scholar]
- 21. De Maria A., Bozzano F., Cantoni C., Moretta L. (2011) Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-{γ} on activation. Proc. Natl. Acad. Sci. USA 108, 728–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryceson Y. T., March M. E., Ljunggren H. G., Long E. O. (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214, 73–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rajagopalan S., Moyle M. W., Joosten I., Long E. O. (2010) DNA-PKCs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells. Sci. Signal. 3, ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. March M. E., Long E. O. (2011) β2 Integrin induces TCRζ-Syk-phospholipase C-γ phosphorylation and paxillin-dependent granule polarization in human NK cells. J. Immunol. 186, 2998–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vitale M., Sivori S., Pende D., Moretta L., Moretta A. (1995) Coexpression of two functionally independent p58 inhibitory receptors in human natural killer cell clones results in the inability to kill all normal allogeneic target cells. Proc. Natl. Acad. Sci. USA 92, 3536–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen G., Goeddel D. V. (2002) TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 [DOI] [PubMed] [Google Scholar]
- 27. Rushworth S. A., MacEwan D. J. (2008) HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood 111, 3793–3801 [DOI] [PubMed] [Google Scholar]
- 28. Bui J. D., Carayannopoulos L. N., Lanier L. L., Yokoyama W. M., Schreiber R. D. (2006) IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J. Immunol. 176, 905–913 [DOI] [PubMed] [Google Scholar]
- 29. Radaev S., Sun P. D. (2003) Structure and function of natural killer cell surface receptors. Annu. Rev. Biophys. Biomol. Struct. 32, 93–114 [DOI] [PubMed] [Google Scholar]
- 30. Wu L., Martin T. D., Carrington M., KewalRamani V. N. (2004) Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318, 17–23 [DOI] [PubMed] [Google Scholar]
- 31. Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. (1986) Induction by IL 1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J. Immunol. 137, 245–254 [PubMed] [Google Scholar]
- 32. Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. (1988) Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J. Immunol. 141, 1665–1669 [PubMed] [Google Scholar]
- 33. Möst J., Schwaeble W., Drach J., Sommerauer A., Dierich M. P. (1992) Regulation of the expression of ICAM-1 on human monocytes and monocytic tumor cell lines. J. Immunol. 148, 1635–1642 [PubMed] [Google Scholar]
- 34. Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. (1989) Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59, 1203–1211 [DOI] [PubMed] [Google Scholar]
- 35. Naganuma H., Kiessling R., Patarroyo M., Hansson M., Handgretinger R., Gronberg A. (1991) Increased susceptibility of IFN-γ-treated neuroblastoma cells to lysis by lymphokine-activated killer cells: participation of ICAM-1 induction on target cells. Int. J. Cancer 47, 527–532 [DOI] [PubMed] [Google Scholar]
- 36. Barber D. F., Faure M., Long E. O. (2004) LFA-1 contributes an early signal for NK cell cytotoxicity. J. Immunol. 173, 3653–3659 [DOI] [PubMed] [Google Scholar]
- 37. Jahnke A., Johnson J. P. (1994) Synergistic activation of intercellular adhesion molecule 1 (ICAM-1) by TNF-α and IFN-γ is mediated by p65/p50 and p65/c-Rel and interferon-responsive factor Stat1 α (p91) that can be activated by both IFN-γ and IFN-α. FEBS Lett. 354, 220–226 [DOI] [PubMed] [Google Scholar]
- 38. Roebuck K. A., Finnegan A. (1999) Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 66, 876–888 [DOI] [PubMed] [Google Scholar]
- 39. Podolin P. L., Callahan J. F., Bolognese B. J., Li Y. H., Carlson K., Davis T. G., Mellor G. W., Evans C., Roshak A. K. (2005) Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IκB kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell proliferation. J. Pharmacol. Exp. Ther. 312, 373–381 [DOI] [PubMed] [Google Scholar]
- 40. Pierce J. W., Schoenleber R., Jesmok G., Best J., Moore S. A., Collins T., Gerritsen M. E. (1997) Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103 [DOI] [PubMed] [Google Scholar]
- 41. Kärre K., Ljunggren H. G., Piontek G., Kiessling R. (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature 319, 675–678 [DOI] [PubMed] [Google Scholar]
- 42. Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. (1984) Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell 38, 745–755 [DOI] [PubMed] [Google Scholar]
- 43. Caligiuri M. A. (2008) Human natural killer cells. Blood 112, 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwinn N., Vokhminova D., Sucker A., Textor S., Striegel S., Moll I., Nausch N., Tuettenberg J., Steinle A., Cerwenka A., Schadendorf D., Paschen A. (2009) Interferon-γ down-regulates NKG2D ligand expression and impairs the NKG2D-mediated cytolysis of MHC class I-deficient melanoma by natural killer cells. Int. J. Cancer 124, 1594–1604 [DOI] [PubMed] [Google Scholar]
- 45. Grönberg A., Kiessling R., Masucci G., Guevara L. A., Eriksson E., Klein G. (1983) γ-Interferon (IFN-γ) produced during effector and target interactions renders target cells less susceptible to NK-cell-mediated lysis. Int. J. Cancer 32, 609–616 [DOI] [PubMed] [Google Scholar]
- 46. Derré L., Corvaisier M., Charreau B., Moreau A., Godefroy E., Moreau-Aubry A., Jotereau F., Gervois N. (2006) Expression and release of HLA-E by melanoma cells and melanocytes: potential impact on the response of cytotoxic effector cells. J. Immunol. 177, 3100–3107 [DOI] [PubMed] [Google Scholar]
- 47. Champsaur M., Lanier L. L. (2010) Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 235, 267–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nowbakht P., Ionescu M. C., Rohner A., Kalberer C. P., Rossy E., Mori L., Cosman D., De Libero G., Wodnar-Filipowicz A. (2005) Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood 105, 3615–3622 [DOI] [PubMed] [Google Scholar]
- 49. Bacon L., Eagle R. A., Meyer M., Easom N., Young N. T., Trowsdale J. (2004) Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. J. Immunol. 173, 1078–1084 [DOI] [PubMed] [Google Scholar]
- 50. Brandt C. S., Baratin M., Yi E. C., Kennedy J., Gao Z., Fox B., Haldeman B., Ostrander C. D., Kaifu T., Chabannon C., Moretta A., West R., Xu W., Vivier E., Levin S. D. (2009) The B7 family member B7–H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 206, 1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stepp S. E., Dufourcq-Lagelouse R., Le Deist F., Bhawan S., Certain S., Mathew P. A., Henter J. I., Bennett M., Fischer A., de Saint Basile G., Kumar V. (1999) Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286, 1957–1959 [DOI] [PubMed] [Google Scholar]