Mechanism by which Pseudomonas aeruginosa in infected airways can enhance inflammation by provoking neutrophil production of histamine.
Keywords: mast cell, histidine decarboxylase, Toll-like receptor, endotoxin, Myd88, exotoxin
Abstract
Airway diseases often feature persistent neutrophilic inflammation and infection. In cystic fibrosis bronchitis, for example, Pseudomonas aeruginosa is isolated frequently. Previously, this laboratory revealed that neutrophils become major sources of histamine in mice with tracheobronchitis caused by the wall-less bacterium Mycoplasma pulmonis. To test the hypothesis that more-broadly pathogenic P. aeruginosa (which expresses cell wall-associated LPS and novel toxins) has similar effects, we incubated naïve mouse neutrophils with two strains of P. aeruginosa. Strain PAO1 greatly increased neutrophil histamine content and secretion, whereas strain PA103 depressed histamine production by killing neutrophils. The histamine-stimulating capacity of PAO1, but not PA103-mediated toxicity, persisted in heat-killed organisms. In PAO1-infected mice, lung and neutrophil histamine content increased. However, PAO1 did not alter production by mast cells (classical histamine reservoirs), which also resisted PA103 toxicity. To explore mechanisms of neutrophil-selective induction, we measured changes in mRNA encoding histidine decarboxylase (rate-limiting for histamine synthesis), probed involvement of endotoxin-TLR pathways in Myd88-deficient neutrophils, and examined contributions of pyocyanin and exotoxins. Results revealed that PAO1 increased histamine production by up-regulating histidine decarboxylase mRNA via pathways largely independent of TLR, pyocyanin, and type III secretion system exotoxins. PAO1 also increased histidine decarboxylase mRNA in neutrophils purified from infected lung. Stimulation required direct contact with neutrophils and was blocked by phagocytosis inhibitor cytochalasin D. In summary, Pseudomonas-augmented histamine production by neutrophils is strain-dependent in vitro and likely mediated by up-regulation of histidine decarboxylase. These findings raise the possibility that Pseudomonas-stimulated neutrophils can enhance airway inflammation by producing histamine.
Introduction
Pseudomonas aeruginosa is a widely distributed, opportunistic bacterium causing acute and chronic infections in susceptible individuals. Strains of this organism cause nosocomial infections and are often cultured from respiratory tract secretions of persons with cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease. Structural lung and airway diseases increase risk of colonization and infection with Pseudomonas species [1], which can be difficult to eradicate as a result of an ability of these bacteria to evade innate host defenses and resist antibiotics by multiple mechanisms, including adopting biofilm modes of growth [2–4]. P. aeruginosa pathogenicity is related to expression of a variety of secreted and cell-associated virulence factors, including ExoA, phospholipases, proteases, pyocyanin, and LPS endotoxin. The type III secretion system [5, 6] contributes additional, important virulence determinants—exotoxins ExoS, ExoT, ExoU, and ExoY. The type III secretion phenotype of most clinical isolates of P. aeruginosa is ExoS–/ExoT+/ExoU+/ExoY– or ExoS+/ExoT+/ExoU–/ExoY+. The latter phenotype tends to be more invasive but less cytotoxic and less virulent in the lung [7–9].
Mast cells and neutrophils are among the first lines of defense against bacterial infection. A major function of these cells is to release mediators of inflammation that signal the presence of pathogens and initiate host responses, leading to bacterial killing or containment. Studies conducted previously in this laboratory revealed that tracheobronchitis and pneumonia caused by mycoplasma infection in mice stimulate neutrophils to produce and release histamine, which enhances lung inflammation [10]. Up-regulated production of histamine also has been observed in neutrophils recruited to sites of allergen-induced inflammation in rats [11] and mice [12]. In humans, well-recognized airway inflammatory actions of histamine include bronchoconstriction and vessel leak. Thus, histamine plays a potentially significant role in human airway diseases, which are increasing in prevalence worldwide [13] and tend to feature repeating cycles of infection and inflammation [14, 15]. By a variety of mechanisms, microbes can activate airway neutrophils directly to release inflammatory mediators (including histamine) with potential to augment airflow obstruction. An increased number of airway neutrophils are linked with acute exacerbations of chronic obstructive airway diseases, such as asthma. There is also an increase in productive cough in chronic bronchitis and bronchiectasis and an accelerated decline in lung function [16, 17]. The reasons for persistence in chronic neutrophilic bronchitis remain to be fully explained. Although causes of chronic bronchitis vary, the commonality of symptoms, such as dyspnea, wheeze, and cough [15], suggests that many of the pathological features, including mediators of inflammation, are shared.
Histamine is a major mediator of allergic inflammation and may contribute to airway obstruction in humans. Its effects on airway include smooth muscle contraction, vagal afferent nerve and mucous gland stimulation, and altered vascular and epithelial permeability [18–22]. Potentially, histamine predisposes to microbial colonization by impairing epithelial integrity, leading to vicious cycles of bacterial invasion and inflammation. Sputum from subjects with acute exacerbations of asthma, chronic bronchitis, pneumonia, and cystic fibrosis can contain high levels of histamine [23–25]. Previously, histamine was thought to be produced mainly by cells associated with allergy, especially mast cells and basophils; however, neutrophils are the major cell type in airways infected with P. aeruginosa and other bacteria. In a model of tracheobronchitis and pneumonia produced by Mycoplasma pulmonis, which is a minimally invasive bacterium without a cell wall that remains confined to the respiratory tract in immunocompetent mice, we found that neutrophils become a major source of histamine [10]. The present studies investigate whether the more-invasive, Gram-negative bacterium P. aeruginosa, which is a common and serious opportunistic pathogen, stimulates neutrophils and mast cells to produce and release histamine. Although M. pulmonis and P. aeruginosa differ remarkably in structure, growth requirements, and virulence factors, they share an ability to cause long-lasting airway infections. The studies presented here show that P. aeruginosa can induce neutrophils (but not mast cells) to produce histamine in vitro and can elevate airway and lung histamine in vivo. These results suggest the possibility that asthma-like symptoms, such as wheezing, in exacerbations of obstructive airway diseases triggered by infection, may be caused, in part, by neutrophil-derived histamine.
MATERIALS AND METHODS
Mice
C57BL/6 WT mice were purchased from Charles River (Hollister, CA, USA). C57BL/6 Myd88−/− mice were obtained from the Anthony DeFranco laboratory at University of California at San Francisco (San Francisco, CA, USA). Mice were housed under specific pathogen-free barrier conditions as described [26, 27] and in some experiments, were infected at 8–10 weeks of age with P. aeruginosa. This study was conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of NIH (Bethesda, MD, USA), and the protocol was approved by the Institutional Animal Care and Use Committee of the University of California at San Francisco (Permit Number AN079696).
Purification of naïve neutrophils
Naïve neutrophils were isolated and purified as described [28] from femoral BM of uninfected WT C57BL/6 and Myd88−/− mice. Purity was established by flow cytometric assessment of FITC-conjugated anti-Gr-1 binding and by direct visualization after cytostaining with Diff-Quik (American Scientific Products, McGaw Park, IL, USA).
Measurement of histamine production and release by neutrophils incubated with P. aeruginosa
Neutrophil histamine production and release were assessed in a 96-well plate assay. Cells were incubated with PBS or with P. aeruginosa strain PAO1, PA103, isogenic PAO1 strain ΔExoS with genetic deletion of the gene encoding ExoS {kindly provided by Dr. Susanne Fleiszig (University of California at Berkeley, Berkeley, CA, USA) and originating in the laboratory of Dr. Arne Rietsch at Case Western Reserve University (Cleveland, OH, USA) [29]}, or LPS O-saccharide-defective isogenic PAO1 strain gmd (obtained as described [30]) at 37°C for 200 min at a density of 107 cells/ml. Experiments were performed using neutrophil:P. aeruginosa ratios ranging from 1:0 to 1:100 with live bacteria or with organisms killed by heating to 65°C for 30 min. In some experiments, WT neutrophils were precultured for 30 min with 5–15 μg/ml anti-TLR4 (eBioscience, San Diego, CA, USA) or rat IgG2a isotype control (eBioscience). Neutrophils were then incubated with bacteria to explore effects of TLR4 on histamine production. Supernatants were collected from cell suspensions centrifuged after incubation with bacteria. Pellets were washed three times and sonicated. Samples were stored at –80°C until use. Cytotoxicity was assessed using a CytoTox 96 kit (Promega, Madison, WI, USA) to measure release of LDH. Additional experiments probed potential involvement of phagocytosis by preincubating neutrophils for 30 min with 1–10 μg/ml cytochalasin D (Sigma-Aldrich, St. Louis, MO, USA), followed by incubation for 3 h with PAO1 bacteria.
Incubation of neutrophils with PAO1 strain P. aeruginosa-conditioned medium and with PAO1 cultured as biofilms
PAO1 bacteria, cultured planktonically overnight, were sedimented at 13,000 g, followed by collection of PAO1-conditioned supernatant and quantification of bacteria in the resuspended pellet by measuring absorbance at 600 nm. For biofilm experiments, autoclaved, 7 mm-diameter, 0.45-μm pore nylon membranes (Millipore, Billerica, MA, USA) placed on a LB agar plate were spotted with 25 μl of a PAO1 suspension containing 2.5 × 106 CFU and cultured in air at 37°C for 48 h to allow formation of a biofilm. Some biofilm-containing membranes were immersed in PBS and vortexed to release bacteria into solution. The numbers of biofilm-derived PAO1 in suspension were estimated spectrophotometrically as above. Each membrane contained ∼109 CFU. Individual membranes containing PAO1 biofilms were incubated by immersion in 150 μl culture medium containing 1.5 × 106 BM-derived naïve neutrophils and cocultured for 200 min at 37°C (5% CO2). Neutrophils incubated at a ratio of 1:10 with PAO1 bacteria, which had been cultured planktonically in medium, were positive controls. PAO1, which had been cultured as biofilms and resuspended, was incubated with neutrophils at the same ratios.
Incubation of neutrophils with ExoS, pyocyanin, and LPS
Recombinant P. aeruginosa ExoS was expressed in Escherichia coli and purified as described [31].
Pyocyanin was purified by chloroform extraction of PAO1 strain P. aeruginosa as described [32]. LPS was purchased from Sigma-Aldrich. Neutrophil histamine production and release were assessed as described above after incubation of cells with PBS or purified ExoS (1–500 nM), pyocyanin (10–250 ng/ml), or LPS (10–1000 ng/ml) for 200 min.
Mast cell culture and degranulation assays
The LAD-2 human mast cell line [33], kindly provided by Dr. Dean Metcalfe (NIAID, NIH, Bethesda, MD, USA) and colleagues, was cultured in StemPro-34 medium (Invitrogen, Carlsbad, CA, USA), supplemented with recombinant human stem cell factor (PeproTech, Rocky Hill, NJ, USA; 100 ng/ml). Mouse mast cells were differentiated in vitro from BM cells. Briefly, cells harvested from femoral BM of 5- to 7-week-old C57BL/6 WT mice were cultured in IL-3-containing medium as described [26] for 4–8 weeks to generate BMCMC of >95% purity, as assessed by the presence of metachromatic granules in toluidine blue-stained cells. Mast cells (105/well) and P. aeruginosa PAO1 or PA103 were coincubated at 37°C for 1 h in Tyrode's buffer containing Ca++ and Mg++ with mast cell:P. aeruginosa ratios ranging from 1:0 to 1:100, using live or heat-killed bacteria. Cell supernatants and pellets were collected. Pellets were resuspended, washed three times, and sonicated. Samples were stored at –80°C prior to conducting assays for histamine, LTB4, LTC4, and PGD2. Cytotoxicity was assessed as above by measuring release of LDH.
P. aeruginosa lung infection, BAL, and purification of neutrophils from mice with pneumonia
For most experiments, WT mice were anesthetized intramuscularly with ketamine and xylazine, infected intratracheally with 5 × 106 P. aeruginosa PAO1 in 50 μl PBS, and killed 4 h, 2 days, and 1 week after infection. To obtain BALF, a 22-gauge catheter was inserted into surgically exposed tracheal lumens. Fluid was collected from one, 0.8-ml PBS BAL/mouse. Supernatants were stored at –80°C until use. Cell pellets were collected to assess neutrophil differentiation and numbers by direct visualization of stained cells or via flow cytometry, as described below. Cell viability was assessed by vital dye (trypan blue) exclusion. Stimulated neutrophils from P. aeruginosa-infected lung were purified from BALF by centrifugation in Percoll, as described above for purifying naïve neutrophils from BM.
Measurement of HDC mRNA
Total RNA from PAO1-stimulated neutrophils purified from naïve BM was prepared using Qiagen RNeasy kits and incubated with DNase (Promega) to remove residual genomic DNA. Expression of mRNA was quantified by real-time RT-PCR and analyzed by a Ct method. Primer-probe sets were purchased from MWG Biotech (High Point, NC, USA) with sequences as follows: HDC (primers 5′-TGAGGAAGACAAGCAACAGG-3′ and 5′-GCCTGTCAAATGCACAGACT-3′; probe 5′-CGTTGCACAGACAAACACAGGCA-3′ with 5′ carboxyfluorescein and 3′ black-hole quencher-1; amplimer=83 bp) and HGPRT (primers 5′-AGGTTGCAAGCTTGCTGGT-3′ and 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′; probe 5′-TGTTGGATACAGGCCAGACTTTGTTGGAT-3′ with 5′ 6-carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein; amplimer =123 bp). Amplifications were carried out simultaneously for 40 cycles of 95°C for 15 s and 60°C for 15 s on a Chromo 4 PTC-200 thermal cycler (MJ Research, Canada) using SuperScript III Platinum RT-PCR kits (Invitrogen). Ct values of samples of interest were compared with those of a reference sample of naïve neutrophils, which was also used to control for interassay (plate-to-plate) variation. Relative differences between a given experimental sample (of naïve or Pseudomonas-exposed neutrophils) and the reference sample were calculated using the equation 2–ΔΔCt, where ΔCt = Ct HDC – Ct HGPRT, and ΔΔCt = ΔCt Sample – ΔCt Reference.
Measurement of histamine, LTB4, LTC4, and PGD2
Histamine concentrations in lung homogenates, BALF, mast cells, and neutrophils were determined by Immunotech ELISA (Beckman Coulter, Fullerton, CA, USA). In mast cell preparations, LTB4, LTC4, and PGD2 were detected by ELISA (Cayman Chemical, Ann Arbor, MI, USA).
Flow cytometry
Cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA). Antibodies were purchased from BD PharMingen (San Diego, CA, USA), unless indicated otherwise. In studies of cells obtained by BAL, neutrophils were detected, based on double-positive expression of Mac-1 and Gr-1, using PE-conjugated anti-Mac-1 and FITC-conjugated anti-Gr-1. Rat antimouse monoclonal Ig CD16/32 was used to block nonspecific binding.
Statistical analysis
Results are expressed as the mean ± se. Data were normally distributed as assessed by Shapiro-Wilk tests implemented in SPSS (version 16) and were compared by two-tailed Student′s t tests, and P < 0.05 is considered significant.
RESULTS
P. aeruginosa strain PAO1 provokes neutrophil histamine production, whereas strain PA103 kills neutrophils and depresses histamine production
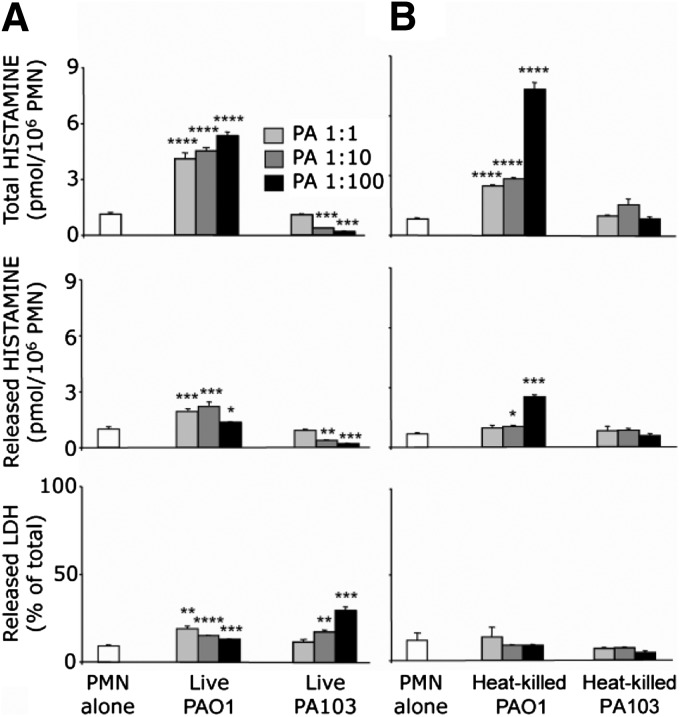
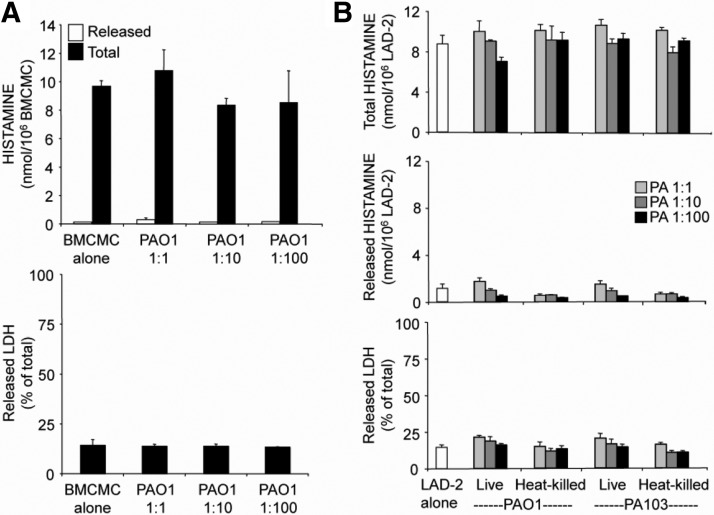
To test the hypothesis that Pseudomonas provokes neutrophils to produce histamine, naïve mouse neutrophils (purity at least 97%) were incubated with two laboratory strains of P. aeruginosa originating from human clinical isolates and representing the two broad categories of pathogenic P. aeruginosa: invasive (strain PAO1) and cytotoxic (strain PA103). The PAO1 strain is epithelial cell-invasive and features type III secretion system phenotype ExoS+/ExoT+/ExoU–/ExoY+ [34]. On the other hand, PA103 is cytotoxic to epithelial cells with distinct type III secretion system phenotype ExoS–/ExoT+/ExoU+/ExoY+ [34]. PAO1, which in addition to the other known differences with PA103, exhibits an enhanced ability to form biofilms [35], greatly increased neutrophil histamine content and secretion (Fig. 1A), independently of cytotoxicity, but PA103 had no effect or decreased histamine content, depending on its concentration. Indeed, PA103 killed neutrophils at a stoichiometry of 10 bacteria/neutrophil or greater, as reflected by extracellular release of LDH. At a ratio of 1:100, PA103 was significantly more cytotoxic than PAO1 (P<0.01). Neither PAO1 nor PA103 alone produced detectable histamine in the titers used in these assays (data not shown). To test whether viable organisms are required for stimulation of histamine production or cytotoxicity, heat-killed PAO1 and PA103 were coincubated with neutrophils, revealing that cytotoxicity, but not stimulation of histamine production, is heat-sensitive and may be a property solely of living cells (Fig. 1).
Figure 1. Strain-selective stimulation of mouse PMN histamine production and cytotoxicity by live and heat-killed P. aeruginosa.
(A and B) Effects of living and heat-killed P. aeruginosa strains PAO1 and PA103 on PMN total production of histamine (extracted from cells plus secreted into medium) and on release of histamine as well as LDH (as a measure of cytotoxicity), respectively. PMNs were incubated with bacteria at ratios of PMN:P. aeruginosa (PA), ranging from 1:1 to 1:100. PMNs incubated without bacteria served as controls. Values represent mean ± se; n = 3 versus corresponding PMN-alone group: *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.00001.
Pseudomonas-specific toxins ExoS and pyocyanin do not account for the histamine-stimulating effect of PAO1 on neutrophils
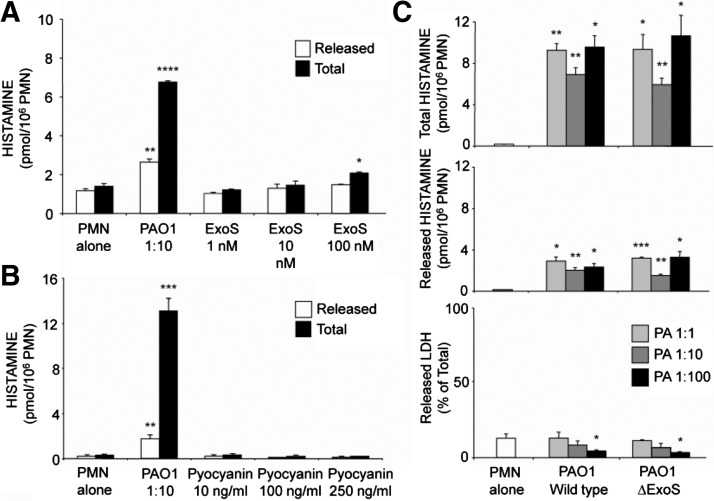
To identify specific Pseudomonas products responsible for the observed stimulation of histamine production by neutrophils, purified ExoS (a major type III secretion system exotoxin expressed by PAO1 but not by PA103) and pyocyanin were tested in the mouse neutrophil histamine production and release assay. Results, shown in Fig. 2A, reveal that ExoS, which is an enzymatic toxin which ADP ribosylates a variety of proteins in target cells [36], was a modest, concentration-dependent inducer of neutrophil histamine production when incubated in recombinant form with neutrophils. Experiments shown in Fig. 2B further reveal that the nonenzymatic pigment/toxin pyocyanin, in concentrations up to 0.25 μg/ml, had no effect on histamine production or release. Separate experiments, shown in Fig. 2C, found no difference in histamine-stimulating capacity between WT PAO1 and an isogenic strain lacking the ExoS gene, suggesting that ExoS does not play a significant role in the observed histamine-stimulating effect of intact organisms.
Figure 2. Effects of Pseudomonas ExoS and pyocyanin on neutrophil histamine production and release.
(A) Effects of a range of concentrations of recombinant ExoS in comparison with effects of incubating naïve mouse PMNs alone or with live PAO1 strain of P. aeruginosa at a 1:10 ratio of PMN:bacteria. (B) Effects of incubating purified pyocyanin in experiments of similar design. (A and B) Black and white bars, amounts of total and extracellularly released histamine, respectively. (C) Results of incubating neutrophils with a range of ratios of WT PAO1 or isogenic mutant PAO1 bacteria containing genetic deletion of the ExoS gene (ΔExoS). For data in all panels, values represent mean ± se; n = 3 versus corresponding PMN-alone group: *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
LPS endotoxin weakly induces histamine production in neutrophils
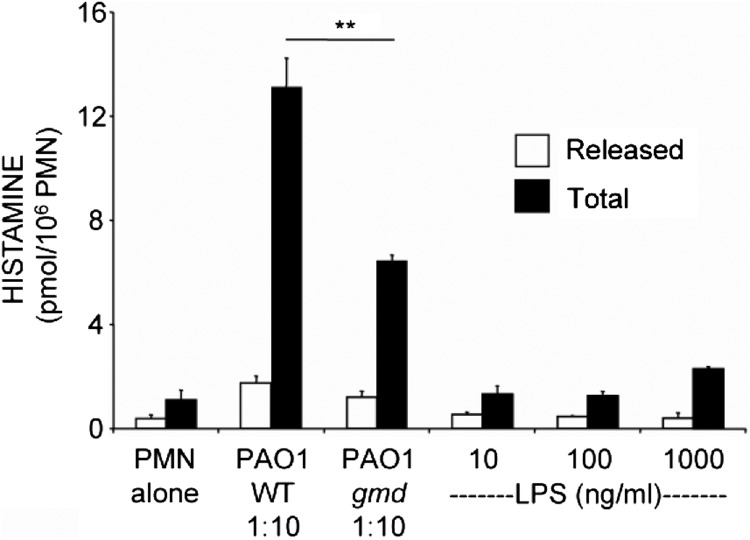
To test the possibility of whether LPS (which influences behavior of neutrophils and of several other inflammatory cells) is a source of histamine-stimulating activity in PAO1 bacteria, we incubated naïve mouse neutrophils with purified LPS and with a LPS-defective strain of PAO1. Results shown in Fig. 3 reveal that although there was a concentration-dependent trend for an increase in neutrophil histamine production provoked by LPS alone, these changes did not reach statistical significance. Furthermore, as shown in Fig. 3, LPS-defective PAO1 [30] retains capacity to stimulate neutrophil production and release of histamine, albeit with reduced potency compared with WT LPS-positive PAO1. These findings suggest that LPS is not the principal inducer of histamine production but may play a secondary role.
Figure 3. Limited effect of LPS on neutrophil histamine production and release.
Live WT P. aeruginosa strain PAO1 bacteria or live LPS-defective mutant strain PAO1-gmd bacteria were incubated with naïve PMNs at 1:10 ratios of PMN:bacteria. Alternatively, PMNs were incubated with medium alone or with purified LPS at concentrations ranging from 10 to 1000 ng/mL, as shown. Values represent mean ± se; n = 3; **P < 0.01; values obtained by incubating with purified LPS were not significantly different from values obtained with PMN alone (P>0.05).
TLRs do not play major roles in Pseudomonas-induced production of histamine by neutrophils
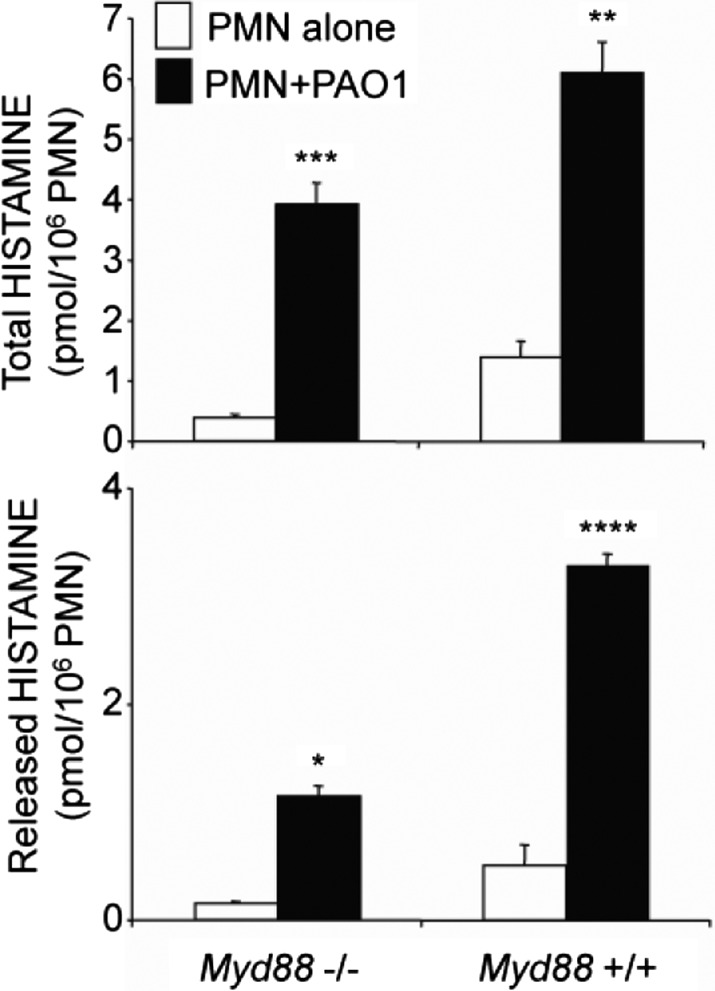
Innate immune cells, such as neutrophils, recognize P. aeruginosa and other microbes via PRRs [37], especially TLRs. To determine whether TLRs play important roles in P. aeruginosa-stimulated production of neutrophil histamine, purified neutrophils from Myd88−/− mice lacking most TLR signaling were cocultured with PAO1 for 3 h. Additionally, WT mouse neutrophils were precultured with anti-TLR4 for 30 min and then cocultured for 3 h with PAO1. As revealed by the data in Fig. 4, PAO1 induced production of histamine by Myd88−/− neutrophils. Although histamine content was lower in Myd88−/− neutrophils than in WT cells at baseline and after incubation with PAO1, the induction of histamine was similar in Myd88−/− and WT neutrophils compared with their control groups, respectively. Furthermore, anti-TLR4 failed to suppress Pseudomonas-induced production of histamine by WT neutrophils (data not shown). These findings suggest that classical, Myd88-dependent TLRs—including TLR4—do not make major contributions to stimulation of histamine production.
Figure 4. P. aeruginosa stimulates Myd88-null neutrophils to produce and release histamine.
The upper panel shows total histamine (released plus extracted) produced by TLR signaling-defective Myd88−/− or by WT (+/+) PMNs incubated with medium alone or with P. aeruginosa strain PAO1 at a ratio of PMN:bacteria of 1:10. The lower panel shows amounts of histamine released extracellularly by PMNs incubated with medium alone or PAO1 strain bacteria. Values represent mean ± se; n = 3 versus corresponding PMN-alone control groups: *P < 0.05; **P < 0.01; ***P < 0.0001; and ****P < 0.00001.
Mast cells do not respond to PAO1 by producing or releasing histamine, LTB4/C4, or PGD2
Mast cells are the usual major source of tissue histamine. Our prior studies showed that >99% of histamine in normal, uninflamed mouse airway is from mast cells [10]. To determine whether P. aeruginosa stimulates mast cells to produce and release histamine, mouse C57BL/6 BMCMC (Fig. 5A) and human mast cell line LAD-2 (Fig. 5B) were cocultured with PAO1 for 1 h, revealing no stimulation of histamine production or release. Additional studies showed that live and heat-killed PAO1 and PA103 also failed to stimulate de novo production by LAD-2 cells of LTB4, LTC4, and PGD2 (data not shown).
Figure 5. P. aeruginosa does not stimulate histamine production or release by mast cells.
(A) The graphs show results of incubating mouse BMCMCs with live PAO1 strain bacteria in ratios of BMCMC:bacteria, ranging from 1:1 to 1:100. PAO1 was not cytotoxic, as reflected by the lack of a change in LDH release from mast cells over the interval of observation. (B) The graphs show results of similar experiments with cells of the human mast cell line LAD-2, which was incubated separately with live and heat-killed Pseudomonas strains PAO1 and PA103, also without significant cytotoxicity or effects on histamine at ratios of mast cells:bacteria, shown in Fig. 1, to stimulate histamine production and release and/or cytotoxicity in neutrophils. Values represent mean ± se; n = 3.
Mast cells resist cytotoxic effects of PA103
As shown in Fig. 5B, LAD-2 mast cells reveal no toxic responses when incubated with PA103 strain P. aeruginosa, using ratios of cells:bacteria, which degrade plasma membrane integrity in neutrophils, as assessed by extracellular release of LDH.
PAO1 causes airway and lung parenchymal neutrophilia and increases lung and infected lung neutrophil histamine levels in mice
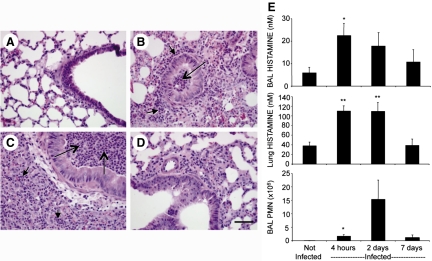
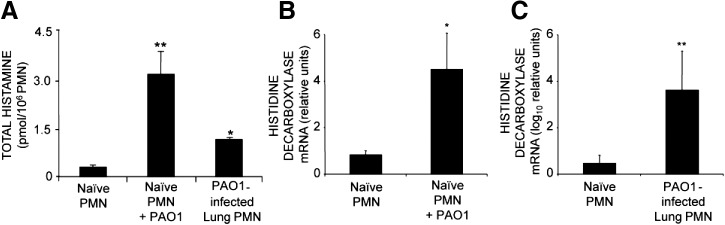
As noted, the in vitro studies with isolated mouse neutrophils suggested that strain PAO1 enhanced neutrophil production of histamine. To assess whether this occurs in vivo, we infected mouse airways with PAO1. Tracheal inoculation with bacteria, as shown in Fig. 6A–D, caused neutrophilic bronchitis and pneumonia. Neutrophils were prominent in airway and alveolar lumen and interstitium within 4 h and remained prominent 2 days after inoculation. Cell viability, assessed by vital dye exclusion in neutrophils, collected by BAL, was 93% at 4 h and 32% at 2 days. By 7 days, bronchitis and pneumonia persisted but were less intense and widespread than at 2 days. As shown in Fig. 6E, histamine levels rose up to 3.7-fold in BALF and 2.9-fold in whole lung by 4 h after infection when lung and airway were heavily burdened with neutrophils and remained elevated 2 days after infection but were subsiding by 7 days. Furthermore, as revealed in Fig. 7A, histamine production by neutrophils (purity of 98–99%) from PAO1-infected lung is elevated in comparison with production by naïve neutrophils purified from BM. These findings suggest that stimulation of neutrophil histamine production by P. aeruginosa occurs in vivo as well as in vitro.
Figure 6. Correlations between neutrophilia and histamine concentration in PAO1 P. aeruginosa-infected lungs.
(A–D) Representative lung and airway histology in H&E-stained sections of uninfected (PBS-exposed) mice and mice 4 h, 2 days, and 7 days after infection with 5 × 106 live PAO1 strain bacteria. All micrographs are at the same magnification. (D) Original scale bar is 50 μm. Examples of collections of neutrophils in airways and in alveoli or interstitium are identified by long and short arrows, respectively. (E) Graphs compare histamine and PMN concentrations at the same intervals after infection. Values represent mean ± se; n = 4–6 animals/group; *P < 0.05; **P < 0.01 compared with values observed in uninfected mice.
Figure 7. P. aeruginosa strain PAO1 stimulates neutrophil production of histamine and of transcripts encoding histamine-synthesizing histidine decarboxylase in vitro and in vivo.
(A) Comparison of total histamine content in naïve PMNs purified from BM of uninfected mice, naïve PMNs incubated with P. aeruginosa strain PAO1 in vitro at a 1:10 ratio of PMN:bacteria, and PMNs purified from PAO1-infected lungs 4 h after infection (n=3 animals; *P<0.05 and **P<0.01 compared with naïve PMNs). (B) Effects on HDC transcript levels (relative to HGPRT transcript levels) of incubating PMNs purified from mouse BM with P. aeruginosa strain PAO1 in vitro at a 1:10 ratio of PMN:bacteria. (C) Measurements of HDC transcript levels in PMN obtained in vivo from PAO1-infected mouse lungs. Values were log10 transformed to facilitate graphical comparison of HDC transcript expression in naïve and in vivo-stimulated PMNs. Values represent mean ± se ΔΔCt values, determined by real-time RT-PCR (n=3; *P<0.05 and **P<0.01 compared with relative HDC transcript levels in PAO1-naïve control PMNs).
PAO1 provokes neutrophils to produce histamine by up-regulating expression of HDC mRNA
To establish whether Pseudomonas-stimulated histamine production by neutrophils is a result of increased expression of mRNA encoding the rate-limiting enzyme in histamine synthesis, expression of mRNA-encoding HDC was examined in C57BL/6 mouse BM-derived neutrophils (incubated for 200 min with or without P. aeruginosa PAO1) or with neutrophils purified from lungs of PAO1-infected mice. Results establish that PAO1 strongly up-regulates neutrophil HDC mRNA expression in vitro (Fig. 7B) and in vivo (Fig. 7C), and the increase in HDC expression is greater in vivo (1400-fold) than in vitro (5.4-fold).
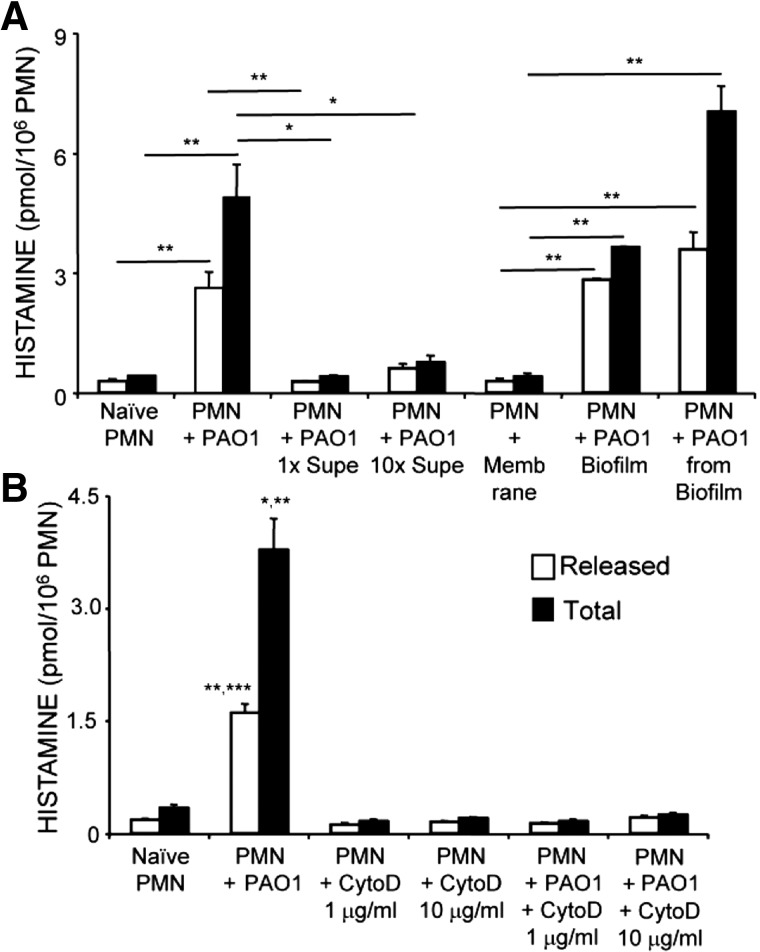
Neutrophils increase histamine production in response to PAO1 P. aeruginosa cultured in planktonic or biofilm conditions
As shown by the data in Fig. 8A, the stimulatory effect of PAO1 on naïve neutrophils is seen with bacteria grown in liquid culture or on membranes as a biofilm. The stimulatory effect of PAO1 cultured as a biofilm is present whether the bacteria remain on the membrane or are removed and solubilized in medium. However, medium conditioned by planktonic PAO1 has little or no ability to stimulate neutrophil histamine production, suggesting that the stimulant is not a soluble-secreted factor and that stimulation may require direct contact between bacteria and neutrophils.
Figure 8. Stimulation of PMN histamine production by PAO1 P. aeruginosa in biofilms and prevention of activation by inhibition of phagocytosis.
(A) Comparison of released and total histamine in PMNs purified from uninfected mouse BM (“Naïve PMN”) with histamine produced by naïve PMNs incubated separately with each of the following: live PAO1 strain P. aeruginosa (1:10 ratio; “PMN+PAO1”), PAO1 culture supernatants (“1× Supe” contains a dilution of supernatant equivalent to the amount conditioned by the number of bacteria used in the “PMN+PAO1” condition; “10× Supe” is a tenfold-higher concentration of supernatant), uninfected biofilm membrane (“PMN+Membrane”), infected biofilm membrane (“PMN+PAO1 Biofilm”), and PAO1 removed from biofilm and suspended in liquid (“PMN+PAO1 from Biofilm”). Values represent mean ± se; n = 3/experimental condition; *P < 0.05 and **P < 0.01. (B) Comparison of histamine in naïve PMN with histamine in naïve PMN incubated with PAO1 or with cytochalasin D (CytoD; 1 or 10 μg/ml), with or without PAO1. Values represent mean ± se; n = 3/experimental condition; *P < 0.05 (total histamine PMN+PAO1 vs. Naïve PMN or PMN+PAO1+CytoD 1 μg/ml); **P < 0.01 (total histamine PMN+PAO1 vs. PMN+PAO1+CytoD 10 μg/ml, released histamine PMN+PAO1 vs. naïve PMN or PMN+PAO1+CytoD 1 μg/ml); ***P < 0.001 (released histamine PMN+PAO1 vs. PMN+PAO1+CytoD 10 μg/ml).
Inhibition of phagocytosis prevents stimulation of neutrophil histamine production by PAO1 P. aeruginosa
As shown in Fig. 8B, stimulation of released and total histamine by naïve neutrophils upon exposure to PAO1 bacteria was entirely prevented by incubation of neutrophils with cytochalasin D, a cell-permeable inhibitor of intracellular polymerization of actin. This finding suggests that a neutrophil behavior (such as phagocytosis), which depends on actin polymerization, is required for PAO1-mediated stimulation of histamine production.
DISCUSSION
The findings reported here provide the first evidence that a strain of P. aeruginosa stimulates HDC and histamine expression by neutrophils in cultured cells and in the respiratory tract of infected mice, suggesting that bacterial products can provoke neutrophils to become sources of histamine in Pseudomonas-infected airways. Although mast cells are major sources of tissue histamine, they were not stimulated to produce or release histamine when incubated with the same strain of Pseudomonas that evoked this behavior in neutrophils. Intriguingly, the effect on neutrophils was strain-specific. It may be significant that the P. aeruginosa strain PAO1 that enhanced total neutrophil histamine content, as well as its release into medium, is also the strain that forms biofilms, which are linked to persistent infections, and thus, possibly to chronic stimulation of neutrophil histamine production. In contrast, the PA103 strain, which is more cytotoxic than PAO1 at higher ratios of bacteria:neutrophils, does not cause histamine release. PAO1 and PA103 are laboratory strains derived from human clinical isolates. The data in this work indicate that PA103 bacteria killed neutrophils without stimulating them. The capacity to inflict damage severe enough to induce leak of cytosolic LDH likely explains the observed suppression of histamine production and secretion. This cytotoxic potential was selective for neutrophils in comparison with mast cells, suggesting the possibility that neutrophils, among leukocytes, are especially vulnerable to cytotoxic strains of P. aeruginosa. There is of course the potential that the cytotoxicity of PA103 masks an intrinsic, histamine-stimulating activity. However, if present, this activity is much weaker than that of PAO1. Overall, these results suggest that neutrophils are differentially affected by bacterial virulence factors and that discriminating among P. aeruginosa strains on this basis could be useful for making therapeutic choices and establishing prognosis.
Although PA103 bacteria were much more cytotoxic than PAO1 bacteria, both strains possessed cytotoxic potential, which may require bacteria to be alive, as heat-killed preparations failed to provoke LDH release. Alternatively, one or more heat-labile toxins were inactivated by the killing procedure. Regardless, the observed lack of histamine-stimulating activity in heat-killed PA103 bacteria further rules against the possibility that effects of putative, histamine-stimulating products (such as those in heat-killed preparations of PAO1) are masked by intrinsic cytotoxicity. It is more likely the case for PAO1 that its cytotoxicity curbs histamine-stimulating activity, which could explain why histamine-stimulating activity of heat-killed PAO1 is almost twice that of live bacteria relative to histamine production and release from unstimulated neutrophils, as shown in Fig. 1.
The data in Fig. 2 suggest that heat-stable, secreted products of P. aeruginosa PAO1 may account for the observed stimulation of histamine production and release by live and heat-killed preparations of bacteria. As PAO1 and PA103 have opposing effects on histamine production and release by neutrophils, it is likely that stimulatory effects of PAO1 are a result of one or more factors not produced by PA103, which lacks the capacity to stimulate histamine, even when rendered nontoxic by exposure to heat, which does not reduce the histamine-stimulating potential of PAO1 bacteria. As PAO1 and PA103 differ in type III secretion phenotype, with PAO1 secreting ExoS and PA103 secreting ExoU and not ExoS [34], we explored effects of recombinant ExoS on neutrophils and compared histamine-stimulating activities of an isogenic strain of PAO1, with and without genetic deletion of ExoS. Although high concentrations of recombinant ExoS stimulated histamine production (Fig. 2), this effect was small, as might be expected of a toxin that is thought to require direct injection into target cells via the type III secretion apparatus. Moreover, the toxic effects of ExoS and other type III exotoxins are mediated by enzymatic (e.g., ADP-ribosylating) activity that is heat-labile. Failure to detect a major difference between WT and ExoS null strains of PAO1 in histamine-stimulating activity is further evidence that ExoS is not a major stimulating factor of the PAO1 strain. By similar reasoning, none of the other three known type III exotoxins (ExoT, ExoU, and ExoY) contributes to the effect, given that PAO1 lacks ExoU and that the stimulatory effect in heat-killed preparations of bacteria is observed only in PAO1 (and not in PA103, despite elimination of toxicity), although both strains express ExoT and ExoY. Potentially, type III exotoxins synergize with yet-to-be-identified factors to stimulate histamine production in neutrophils.
Chemically and biologically diverse stimuli cause neutrophils to increase histamine production. For example, neutrophils increase histamine production in response to nonbacterial inflammation [12] and to an extracellular bacterium (M. pulmonis) without a type III secretion system or ExoS-related exotoxins [10]. It may be important that Mycoplasma and Pseudomonas species, although different in obvious ways, share a capacity for eluding or subverting host immune responses. It is tempting to speculate that sustained airway survival of these bacteria benefits from enhanced production of histamine, which (by promoting vascular leakage of Igs and other immune proteins) helps them to out-compete microbes with lesser ability to evade immune responses. In any case, as neutrophil histamine-producing machinery responds to diverse stimuli, we explored other P. aeruginosa products known to affect neutrophils.
Pyocyanin is a redox-active, pigmented phenazine proposed to play a pathogenic role in airway disease in cystic fibrosis [38], to favor persistence of bacterial infection by accelerating neutrophil apoptosis [39], and to contribute to pathogenesis of P. aeruginosa lung infection in mice [40]. However, as shown in Fig. 2B, no effects of pyocyanin on histamine production or secretion were found at concentrations up to 250 ng/mL, suggesting that pyocyanin's effects, if present, are not potent. However, it remains possible that pyocyanin augments the effects of other factors in combination or that pyocyanin released by phagocytosed bacteria has a greater effect than pyocyanin exposed to the neutrophil surface.
LPS, which is well known as an endotoxin and as an abundant nonprotein structural component of cell walls, is another immunostimulatory factor produced by gram-negative bacteria, including P. aeruginosa. Like ExoS and pyocyanin, LPS is absent in wall-less bacteria, such as Mollicutes (e.g., Mycoplasma species). LPS interacts with TLR4 to activate signaling pathways leading to changes in immune cells, including neutrophils. We tested whether LPS affected the PAO1 effects on neutrophils using several nonredundant approaches: measuring histamine production and release by neutrophils incubated directly with LPS, comparing neutrophil responses to WT PAO1 bacteria with responses to LPS O-saccharide-deficient PAO1-gmd bacteria, and comparing responses of WT and Myd88−/− neutrophils lacking TLR signaling machinery. We also explored effects of decreasing neutrophil responsiveness to LPS by incubation with anti-TLR4. The data in Fig. 3 reveal a statistically insignificant trend toward an increase in total histamine in neutrophils incubated with escalating concentrations of LPS and a significant but partial decrease in histamine-stimulating potency of LPS-deficient PAO1-gmd bacteria in comparison with WT bacteria. On the other hand, as shown by Fig. 4, Myd88−/− neutrophils respond robustly to PAO1, although they lack a major component of TLR4 signaling machinery mediating responses to LPS. Although TLR4 responses can be mediated by MyD88-independent as well as MyD88-dependent pathways, the failure of anti-TLR4 (which blocks LPS effects proximal to the generation of intracellular signals) to reduce histamine responses further supports the conclusions drawn from responses of Myd88−/− neutrophils. These data—although not ruling out a contribution by LPS to stimulation of histamine production by neutrophils—suggest that LPS is neither the sole nor the major stimulatory factor.
P. aeruginosa is widely distributed in nature and is an opportunistic pathogen causing acute as well as chronic infections. Neutrophils are typically the most abundant immune cell in bacterially infected airways. Thus, it may be significant that a strain encoding the invasive type III secretion (ExoS+/ExoU–) phenotype—which predominates in isolates linked to clinical airway disease in humans [41]—stimulates histamine production. PAO1 and PA103 themselves are not significant sources of histamine in these experiments (data not shown). Mast cells generally are considered to be the major source of histamine in a noninflamed airway, where they act as sentinels and form a first line of defense against invading microorganisms. To test whether P. aeruginosa induces mast cells to release stores of histamine and provoke de novo synthesis of lipid mediators, we exposed human and mouse mast cells to P. aeruginosa in vitro. The results show that the bacteria are not toxic to mast cells and do not stimulate histamine production or release, nor do they enhance production of cysteinyl LTs, LTB4/C4, and the principal mast cell PG, PGD2. Therefore, stimulation by P. aeruginosa of histamine production and release in immune cells are cell-specific—and do not extend to all cells with the capability of making histamine. Whether P. aeruginosa strains have the capacity to stimulate histamine in other immune cells, like macrophages, remains to be determined. It is clear from the lung tissue sections in Fig. 6, however, that neutrophil numbers far exceed those of macrophages in clinically significant P. aeruginosa infections of lung and airway and that neutrophils are positioned to make much larger aggregate contributions to local levels of histamine.
In obstructive airway diseases, neutrophils may be more injurious than protective and eventuate in irreversible pathological changes. A paradox in lungs of individuals with cystic fibrosis is that bacterial infections often persist, despite an abundance of neutrophils, which fail to clear or eliminate some bacteria, especially P. aeruginosa, associated with long-term colonization [42] and is a pathogen in other causes of bronchiectasis and chronic obstructive bronchitis [18, 43], with or without acute exacerbation [44, 45]. The present work raises the possibility that histamine originating from Pseudomonas-stimulated neutrophils contributes to bronchoconstriction, airflow obstruction, airway edema, and persistence of inflammation. The potential for substantial elevations of airway and lung tissue histamine to occur during Pseudomonas infection is supported by the data in Figs. 6 and 7. Baseline levels of BALF and lung histamine in uninfected mice are largely from mast cells, as established by studies in mast cell-deficient mice [10]. Lung histamine may be secreted at a basal rate from mast cells in the lung or delivered to lung via the bloodstream from extrapulmonary sites. In pneumonia, however, local production of histamine by neutrophils can be an important source of increased histamine [10]. The neutrophil contribution can be significant, although on a per-cell basis, neutrophils store far less histamine than mast cells, as is evident from the present data comparing histamine content of BMCMC and LAD-2 mast cells in Fig. 5 with neutrophil content in Figs. 1–4. The data in the present work suggest that despite storing much less histamine than mast cells, Pseudomonas-stimulated neutrophils substantially boost lung and airway concentrations of histamine, as neutrophils far outnumber mast cells in infected lungs and as Pseudomonas-stimulated neutrophils tend to release rather than store histamine. Mast cell granules store large amounts of cationic histamine by co-packaging with high-density polyanions such as heparin. Neutrophil granules may lack this capability. Figure 6 reveals that augmentation of lung histamine content occurs within 4 h of infection and has faded by 7 days when infection and inflammation have largely cleared. This course is consistent with observed, strong stimulation of histamine production in naïve BM-derived neutrophils exposed to PAO1 for fewer than 4 h (i.e., 200 min for the data in Fig. 1). Finally, studies depicted in Fig. 7 suggest that the mechanism of increase in histamine production by neutrophils in vitro and in Pseudomonas-infected lungs, involves induction of transcription of the gene encoding the rate-limiting enzyme in endogenous synthesis, HDC. Further studies are needed to establish the intramolecular sequence of events by which Pseudomonas-derived stimulants activate HDC transcription.
In summary, these studies reveal strain-dependent stimulation or suppression of neutrophil histamine production by isolates of the Gram-negative bacterial respiratory pathogen, P. aeruginosa. Stimulation by PAO1 strain bacteria in vitro and in vivo is a result of genetic up-regulation of mRNA encoding the rate-limiting enzyme in histamine production by heat-stable factors, including but not limited to LPS. Stimulation appears to require direct contact between bacteria and neutrophils, is caused by bacteria cultured in planktonic or biofilm modes of growth, and may require phagocytosis of bacteria by stimulated neutrophils. On the other hand, PA103 strain bacteria kill neutrophils without stimulating histamine production. These effects of P. aeruginosa do not extend to mast cells, which are the major sources of histamine in uninflamed airway and resist histamine stimulation by PAO1 and cell killing by PA103. Further studies will be required to determine the extent to which these findings apply to human neutrophils and to humans with P. aeruginosa infections of lung and airways.
ACKNOWLEDGMENTS
This work was supported by grant PO1 HL024136 from NIH, by the Diamond Family Foundation, and by Cystic Fibrosis Research. The authors thank Baidong Hou for assistance in obtaining Myd88–/– mice and Drs. Weidong Kong and Ramya Srinivasan for assistance in growing mutant strains of PAO1 and in culturing PAO1 as biofilms.
Footnotes
- BALF
- BAL fluid
- BMCMC
- bone marrow-derived cultured mast cell
- Ct
- cycle threshold
- ExoA/S/T/U/Y
- exotoxin A/S/T/U/Y
- HDC
- histidine carboxylase
- HGPRT
- hypoxanthine guanine phosphoribosyl transferase
- LT
- leukotriene
- Mac-1
- macrophage antigen 1
AUTHORSHIP
X.X. conceived of, designed, and performed experiments, analyzed data, and wrote the manuscript. H.Z., Y.S., and S.V.L. performed experiments, provided technical support, and contributed reagents and materials. C.A.L. provided laboratory support, contributed reagents and materials, and edited the manuscript. J.P.W-K. contributed reagents and materials and edited the manuscript. G.H.C. conceived of, designed, supervised experiments, analyzed data, and wrote the manuscript.
REFERENCES
- 1. Pappas G., Saplaoura K., Falagas M. E. (2009) Current treatment of pseudomonal infections in the elderly. Drugs Aging 26, 363–379 [DOI] [PubMed] [Google Scholar]
- 2. Costerton J. W., Stewart P. S., Greenberg E. P. (1999) Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322 [DOI] [PubMed] [Google Scholar]
- 3. Singh P. K., Schaefer A. L., Parsek M. R., Moninger T. O., Welsh M. J., Greenberg E. P. (2000) Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407, 762–764 [DOI] [PubMed] [Google Scholar]
- 4. Worlitzsch D., Tarran R., Ulrich M., Schwab U., Cekici A., Meyer K. C., Birrer P., Bellon G., Berger J., Weiss T., Botzenhart K., Yankaskas J. R., Randell S., Boucher R. C., Döring G. (2002) Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109, 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kang P. J., Hauser A. R., Apodaca G., Fleiszig S. M., Wiener-Kronish J., Mostov K., Engel J. N. (1997) Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 24, 1249–1262 [DOI] [PubMed] [Google Scholar]
- 6. Yahr T. L., Goranson J., Frank D. W. (1996) Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22, 991–1003 [DOI] [PubMed] [Google Scholar]
- 7. Finck-Barbançon V., Goranson J., Zhu L., Sawa T., Wiener-Kronish J. P., Fleiszig S. M., Wu C., Mende-Mueller L., Frank D. W. (1997) ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25, 547–557 [DOI] [PubMed] [Google Scholar]
- 8. Fleiszig S. M., Wiener-Kronish J. P., Miyazaki H., Vallas V., Mostov K. E., Kanada D., Sawa T., Yen T. S., Frank D. W. (1997) Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yahr T. L., Vallis A. J., Hancock M. K., Barbieri J. T., Frank D. W. (1998) ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95, 13899–13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X., Zhang D., Zhang H., Wolters P. J., Killeen N. P., Sullivan B. M., Locksley R. M., Lowell C. A., Caughey G. H. (2006) Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J. Exp. Med. 203, 2907–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiraishi M., Hirasawa N., Oikawa S., Kobayashi Y., Ohuchi K. (2000) Analysis of histamine-producing cells at the late phase of allergic inflammation in rats. Immunology 99, 600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka S., Deai K., Konomi A., Takahashi K., Yamane H., Sugimoto Y., Ichikawa A. (2004) Expression of L-histidine decarboxylase in granules of elicited mouse polymorphonuclear leukocytes. Eur. J. Immunol. 34, 1472–1482 [DOI] [PubMed] [Google Scholar]
- 13. Barnes P. J. (2000) Chronic obstructive pulmonary disease. N. Engl. J. Med. 343, 269–280 [DOI] [PubMed] [Google Scholar]
- 14. Sethi S., Murphy T. F. (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 359, 2355–2365 [DOI] [PubMed] [Google Scholar]
- 15. Simpson J. L., Phipps S., Gibson P. G. (2009) Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol. Ther. 124, 86–95 [DOI] [PubMed] [Google Scholar]
- 16. Stănescu D., Sanna A., Veriter C., Kostianev S., Calcagni P. G., Fabbri L. M., Maestrelli P. (1996) Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 51, 267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis S. A., Pavord I. D., Stringer J. R., Knox A. J., Weiss S. T., Britton J. R. (2001) The relation between peripheral blood leukocyte counts and respiratory symptoms, atopy, lung function, and airway responsiveness in adults. Chest 119, 105–114 [DOI] [PubMed] [Google Scholar]
- 18. Wilson R., Dowling R. B. (1998) Lung infections. 3. Pseudomonas aeruginosa and other related species. Thorax 53, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White M. V., Slater J. E., Kaliner M. A. (1987) Histamine and asthma. Am. Rev. Respir. Dis. 135, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 20. Goldie R. G., Pedersen K. E. (1995) Mechanisms of increased airway microvascular permeability: role in airway inflammation and obstruction. Clin. Exp. Pharmacol. Physiol. 22, 387–396 [DOI] [PubMed] [Google Scholar]
- 21. Braude S., Royston D., Coe C., Barnes P. J. (1984) Histamine increases lung permeability by an H2-receptor mechanism. Lancet 2, 372–374 [DOI] [PubMed] [Google Scholar]
- 22. Chan T. B., Eiser N., Shelton D., Rees P. J. (1987) Histamine receptors and pulmonary epithelial permeability. Br. J. Dis. Chest 81, 260–267 [DOI] [PubMed] [Google Scholar]
- 23. Morgan D. J. R., Moodley I., Davies R. J. (1983) Sputum histamine in acute asthma, chronic bronchitis and pneumonia. Thorax 38, 712 [Google Scholar]
- 24. Bryant D. H., Pui A. (1982) Histamine content of sputum from patients with asthma and chronic bronchitis. Clin. Allergy 12, 19–27 [DOI] [PubMed] [Google Scholar]
- 25. Sheinman B. D., Devalia J. L., Davies R. J., Crook S. J., Tabaqchali S. (1986) Synthesis of histamine by Haemophilus influenzae. Br. Med. J. (Clin. Res. Ed.) 292, 857–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolters P. J., Mallen-St Clair J., Lewis C. C., Villalta S. A., Baluk P., Erle D. J., Caughey G. H. (2005) Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin. Exp. Allergy 35, 82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu X., Zhang D., Lyubynska N., Wolters P. J., Killeen N. P., Baluk P., McDonald D. M., Hawgood S., Caughey G. H. (2006) Mast cells protect mice from mycoplasma pneumonia. Am. J. Respir. Crit. Care Med. 173, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pereira S., Lowell C. (2003) The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J. Immunol. 171, 1319–1327 [DOI] [PubMed] [Google Scholar]
- 29. Cisz M., Lee P. C., Rietsch A. (2008) ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J. Bacteriol. 190, 2726–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Augustin D. K., Song Y., Baek M. S., Sawa Y., Singh G., Taylor B., Rubio-Mills A., Flanagan J. L., Wiener-Kronish J. P., Lynch S. V. (2007) Presence or absence of lipopolysaccharide O antigens affects type III secretion by Pseudomonas aeruginosa. J. Bacteriol. 189, 2203–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kulich S. M., Frank D. W., Barbieri J. T. (1995) Expression of recombinant exoenzyme S of Pseudomonas aeruginosa. Infect. Immun. 63, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Essar D. W., Eberly L., Hadero A., Crawford I. P. (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172, 884–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirshenbaum A. S., Akin C., Wu Y., Rottem M., Goff J. P., Beaven M. A., Rao V. K., Metcalfe D. D. (2003) Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk. Res. 27, 677–682 [DOI] [PubMed] [Google Scholar]
- 34. Neely A. N., Holder I. A., Wiener-Kronish J. P., Sawa T. (2005) Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 31, 153–158 [DOI] [PubMed] [Google Scholar]
- 35. Kirov S. M., Webb J. S., O'May C. Y., Reid D. W., Woo J. K., Rice S. A., Kjelleberg S. (2007) Biofilm differentiation and dispersal in mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 153, 3264–3274 [DOI] [PubMed] [Google Scholar]
- 36. Engel J., Balachandran P. (2009) Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 12, 61–66 [DOI] [PubMed] [Google Scholar]
- 37. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 38. Caldwell C. C., Chen Y., Goetzmann H. S., Hao Y., Borchers M. T., Hassett D. J., Young L. R., Mavrodi D., Thomashow L., Lau G. W. (2009) Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am. J. Pathol. 175, 2473–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prince L. R., Bianchi S. M., Vaughan K. M., Bewley M. A., Marriott H. M., Walmsley S. R., Taylor G. W., Buttle D. J., Sabroe I., Dockrell D. H., Whyte M. K. (2008) Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J. Immunol. 180, 3502–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lau G. W., Ran H., Kong F., Hassett D. J., Mavrodi D. (2004) Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 72, 4275–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feltman H., Schulert G., Khan S., Jain M., Peterson L., Hauser A. R. (2001) Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147, 2659–2669 [DOI] [PubMed] [Google Scholar]
- 42. Gómez M. I., Prince A. (2007) Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr. Opin. Pharmacol. 7, 244–251 [DOI] [PubMed] [Google Scholar]
- 43. Rivera M., Nicotra M. B. (1982) Pseudomonas aeruginosa mucoid strain. Its significance in adult chest diseases. Am. Rev. Respir. Dis. 126, 833–836 [DOI] [PubMed] [Google Scholar]
- 44. Sethi S. (2000) Infectious etiology of acute exacerbations of chronic bronchitis. Chest 117, 380S–385S [DOI] [PubMed] [Google Scholar]
- 45. Murphy T. F. (2009) Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 15, 138–142 [DOI] [PubMed] [Google Scholar]