Abstract
New strategies for expression, purification, functional characterization, and structural determination of membrane-spanning G-protein-coupled receptors (GPCRs) are constantly being developed because of their importance to human health. Here, we report a Caenorhabditis elegans heterologous expression system able to produce milligram amounts of functional native and engineered GPCRs. Both bovine opsin [(b)opsin] and human adenosine A2A subtype receptor [(h)A2AR] expressed in neurons or muscles of C. elegans were localized to cell membranes. Worms expressing these GPCRs manifested changes in motor behavior in response to light and ligands, respectively. With a newly devised protocol, 0.6–1 mg of purified homogenous 9-cis-retinal-bound bovine isorhodopsin [(b)isoRho] and ligand-bound (h)A2AR were obtained from C. elegans from one 10-L fermentation at low cost. Purified recombinant (b)isoRho exhibited its signature absorbance spectrum and activated its cognate G-protein transducin in vitro at a rate similar to native rhodopsin (Rho) obtained from bovine retina. Generally high expression levels of 11 native and mutant GPCRs demonstrated the potential of this C. elegans system to produce milligram quantities of high-quality GPCRs and possibly other membrane proteins suitable for detailed characterization.—Salom, D., Cao, P., Sun, W., Kramp, K., Jastrzebska, B., Jin, H., Feng, Z., Palczewski, K. Heterologous expression of functional G-protein-coupled receptors in Caenorhabditis elegans.
Keywords: native GPCR, purification, membrane proteins
Modern pharmaceutical research relies increasingly on structural information about protein targets. Both lead compound generation and optimization are greatly facilitated if a 3-dimensional structure of the biological target is known. Although membrane proteins (MPs) comprise the majority of druggable targets (1), they account for <1% of structures in the Protein Data Base (PDB). Human G-protein-coupled receptors (GPCRs) in particular are targets for about half of therapeutic drugs currently sold or in development (1–5). Despite the biomedical significance of GPCRs, bovine rhodopsin [(b)Rho] is the only vertebrate GPCR with an unaltered native sequence whose structure has been solved to atomic resolution (6–8). Rhodopsin can be obtained in reasonable amounts from its natural source, whereas other vertebrate GPCRs are expressed at low levels in their native tissues.
A number of expression systems have been developed that yield GPCRs in quantities sufficient for crystallization. Cell-free (9), cell-based, and animal-based (10) systems each have several advantages and disadvantages (4, 5, 11), but, despite the number of expression systems currently available, no native human GPCR has yet been crystallized. Common problems with eukaryotic expression systems are their low yield, high cost, and either lack or heterogeneity of post-translational modifications.
Decades of research on GPCRs with diffusible ligands are starting to yield important structural information (12). Advances range from improvements in GPCR expression and purification, stabilizing mutations, and identification of new ligands and detergents, to new methods for GPCR crystallization and X-ray diffraction collection (13–15). However, to date these improvements have been made at the expense of important changes in the target protein. Such changes include chemical modifications, deglycosylation, multiple point mutations, long deletions, insertions of entire proteins, etc., producing GPCRs with altered pharmacological and functional properties compared to those of their natural human counterparts. High-resolution structures of native proteins remain the most reliable templates for precise structure-based drug design. Therefore, much effort is still needed to develop new strategies for the expression and purification of milligram amounts of native GPCRs in a homogeneous, functional form.
Here we report a new expression system for heterologous human GPCRs in Caenorhabditis elegans that is potentially applicable to other MPs. This nematode expresses ∼1100 GPCRs (5% of its genome; ref. 16) in neurons to detect bacteria and environmental compounds. Heterologous expression of human GPCRs in C. elegans has certain advantages over other in vivo and in vitro expression systems because of this animal's relatively facile genetic manipulation, short life cycle, scalability, phenotypic diversity, and potential tissue-specific MP expression. Thus, C. elegans combines the advantages of in vivo animal expression and conventional single-cell expression systems. The demonstrated expression of several native and engineered GPCRs in C. elegans and simple purification of (b)isoRho and (h)A2AR illustrate the potential of this organism to become a major expression system for functional and structural studies of eukaryotic MPs in general and human GPCRs in particular.
MATERIALS AND METHODS
GPCR gene constructs
GPCR expression constructs were generated as follows: promoters myo-3 (17) or H20 (18) were inserted into a pBluscript KS(+) vector at HindIII/Xbal or Pstl, respectively. Synthesized cDNAs (Genescript, Piscataway, NJ, USA), either codon optimized (19) or unoptimized, encoding a GPCR followed by a tobacco etch virus (TEV) protease site, T7 and Rho9 tags (20), and the polyadenine [poly(A)] tail of unc-54 (21), were engineered between Notl and Xhol. Several constructs with modifications were exceptions, based on the following reasons: the TEV cleavage site and T7 and Rho9 tags were not introduced into the (b)opsin construct because it contained a Rho9 tag at the end of its C terminus; previously crystallized constructs of mutated (m) GPCRs contained no TEV site, T7 nor Rho9, because they already had His tags; and the codon-unoptimized (h)A2AR construct contained just the Rho9 tag (see Table 1). The entire GPCR fusion protein coding region for each construct was sequenced to confirm the absence of random mutations.
Table 1.
Heterologous expression of vertebrate GPCRs in C. elegans
| Abbreviation | GPCR molecular identity | Expression level (μg/ml wet worms) |
|
|---|---|---|---|
| Pmyo-3 driven | PH20 driven | ||
| (b)opsin | Bovine rod cell opsin | 0.5–1a | 0.5–1a |
| (h)A2ARb | Human adenosine receptor type A2A | 0.5–1a | N.A. |
| (h)A2AR | Human adenosine receptor type A2A | 0.1–0.5 | 0.1–0.5 |
| (h)A2ARm-T4L | Mutant human adenosine receptor type A2A, T4 lysozyme chimeric protein | 0.5–1 | 0.1–0.5 |
| (h)5-HT4Rnb | Human 5-HT receptor type 4, isoform n | 0.1–0.5 | N.A. |
| (h)5-HT4Rb-DsRedb | Human 5-HT receptor type 4, isoform b, fused with DsRed | 0.5–1 | 0.1–0.5 |
| (h)5-HT2AR | Human 5-HT receptor type 2A | <0.1 | N.A. |
| (h)β2AR | Human β2-adrenergic receptor | 0.1–0.5 | <0.1 |
| (h)β2ARm -T4L | Mutant β2-human adrenergic receptor, T4 lysozyme chimeric protein | 0.1–0.5 | 0.1–0.5 |
| (t)β1AR | Turkey β1-adrenergic receptor | <0.1 | – |
| (t)β1ARm | Mutant turkey β1-adrenergic receptor | 0.1–0.5 | 0.1–0.5 |
Expression levels were quantified by comparing immunoblots of each heterologous GPCR with the band produced by 5 ng of control protein loaded on the same gel directly or indirectly (mutated GPCRs or GPCR-T4Ls) as described in Materials and Methods. N.A., integrated lines are not available; −, no significant immunoblot signal was detected. Immunoblotting analyses were done with 1D4 mAb (native GPCRs) or monoclonal antibodies against the His tag (mutant or chimeric GPCRs).
Expression levels of (b)opsin and (h)A2AR were estimated to be ∼1.5 μg/ml wet pellet from analyzing silver-stained SDS-PAGE gels of purified protein.
Codon unoptimized.
Sequence references for GPCRs were as follows: (b)opsin, NP_001014890; (h)A2AR, NP_000666; (h)5-HT4Rb, NP_000861; (h)5-HT4Rn, ref. 22; (h)5-HT2AR, NP_000612; (h)β2AR, NP_000015; (t)β1AR, P07700; (h)A2ARm-T4L, PDB 3eml; (h)β2ARm-T4L, PDB 3d4s; and (t)β1ARm, PDB 2vt4.
Generation and maintenance of transgenic (TG) worm lines
Standard protocols were used to generate TG worm lines. In brief, TG worm lines transiently expressing GPCRs were generated by injecting GPCR constructs (10 ng/μl) and DNA encoding the coral-derived red fluorescent protein (DsRed; 3 ng/μl) under the control of the same promoter (either Pmyo-3 or PH20) and selected by DsRed visualization. Identified stable worm lines were then exposed to 350 × 100 μJ/cm2 ultraviolet light (Spectrolinker XL-1500; Spectronics Corp., Westbury, NY, USA), to break up the chromosomal DNA and facilitate heterologous DNA to stably integrate into future progeny. F3 progeny of integrated TG lines were then screened and backcrossed to wild-type (WT) worms 3 times.
Bristol N2 strain worms used for this study were maintained by standard methods that included culture on nematode growth medium (NGM; 0.25% peptone, 51 mM NaCl, 25 mM K3PO4, 5 μg/ml cholesterol, 1 mM CaCl2, and 1 mM MgCl2) plates with OP50 bacteria, cryostorage, and recovery from stocks. Compositions of media and solutions, as well as detailed protocols for their use, were previously described (23).
Immunohistochemistry (IHC)
In vivo IHC was performed according to Gottschalk and Schafer (24). In brief, young adult (d 1) animals from transient or integrated TG worm lines were scored and mounted with halocarbon oil on 2% agarose pads. Alexa-488 (Molecular Probes, Eugene, OR, USA)-conjugated 1D4 antibody (purified from hybridoma supernatant by DEAE anion exchange chromatography; ref. 25) in 0.4–0.6% Triton X-100-containing injection buffer (20 mM K3PO4, 3 mM potassium citrate, and 2% PEG 6000, pH 7.5) was injected into the pseudocoelom. Animals were next transferred from agarose pads with M9 buffer onto NGM plates. At 6 h after recovery, live worms were examined for 1D4 antibody immunoreactivity under a confocal microscope. Under these conditions, 1D4 antibody entered cells and bound to target membrane proteins, whereas cytosolic DsRed could not be visualized due to cell membrane permeability.
For staining fixed worms, age-synchronized d 1 or larval stage 4 (L4) animals from integrated TG worm lines were sandwiched between 2 cover glasses, buried in dry ice for 30 min, and then fixed with 100% methanol (10 min), followed by 100% acetone (10 min). Then, worms were washed with PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4·2H2O, and 1.76 mM KH2PO4, pH 7.4) for 0.5 h and incubated with PBS containing Alexa-488-conjugated 1D4 antibody and 0.1% Triton X-100 overnight at 4°C. Stained worms were subsequently washed 3 times with PBS and examined by confocal microscopy. All experiments were done with a Leica TCS SP2 confocal microscope (Leica Microsystems, Bannockburn, IL, USA). Either live worms immobilized with 10 mM NaN3 on 2% agarose pads or methanol/acetone-fixed worms were used. Stains employed were DsRed (λex=543 nm; λem=580–630 nm) and Alexa-488 (λex=488 nm; λem=510–530 nm).
Large-scale worm cultures
Worm fermentation was carried out according to a previously published protocol (26), with slight modifications to accommodate our specific fermentation system (BioFlo/CelliGen 115; New Brunswick Scientific, Edison, NY, USA). Worms were first cultured on twenty-five 150-mm high-growth-medium (HGM) plates seeded with HB101 bacteria for 2 to 3 generations (1–2 wk) and then transferred into a fermentor in a final volume of 10 L S medium (23). Next, worms were cultured for 2 generations (∼1 wk) in the fermentor (pH 7.0, 20°C, 50% dissolved oxygen, 300 rpm agitation with a low-shear pitched blade impeller) until most reached the young adult stage. For worm harvesting, a liquid culture was first centrifuged at 6000 g (JA-10; Beckman, Brea, CA, USA) for 15 min, and the pellet, resuspended in a minimum volume of S medium, was vigorously shaken. The resulting worm suspension (3 ml aliquots) was carefully overlaid onto 30 ml of ice-cold 35% sucrose-containing M9 buffer (23) in a 50-ml Falcon tube, followed by a 10-min, 1000-g centrifugation (Allegra 6KR; Beckman) at 4°C. The live worm-containing top layer and interface were carefully collected and then diluted with an equal volume of ice-cold M9 buffer, followed by a 2500-g centrifugation (Allegra 6KR) to remove the sucrose. This M9 wash was repeated once, and the clean worm pellet was resuspended in an equal volume of buffer containing 20 mM HEPES (pH 7.4), 2 mM EDTA, and protease inhibitor cocktail (cOmplete Mini, EDTA-free; Roche, Branchburg, IN, USA). The preparation, defined as “wet worms,” was frozen at −80°C until further use.
GPCR purification
Wet worms from the −80°C stock were thawed and suspended in 1 vol of 50 mM bis-tris-propane (BTP) buffer (pH 7.0) supplemented with protease inhibitor cocktail, followed by homogenization (120 psi, 4 cycles) with a microfluidizer (M-110Y Microfluidizer Processor; Microfluidics, Newton, MA, USA). The homogenate was centrifuged (1 h, 100,000 g), and the membrane pellet was resuspended in 50 mM BTP (pH 7.0) and 250 mM NaCl plus protein inhibitor cocktail to achieve the same final volume as the homogenate. For (b)opsin, the preparation at this point was incubated in the dark with an excess of 9-cis-retinal (Toronto Research Chemicals, Toronto, ON, Canada) to obtain ground-state (b)isoRho. For (h)A2AR, the preparation was incubated with an excess of the (h)A2AR antagonist ZM241385 (Tocris Bioscience, Ellisville, MO, USA). Next, the suspension was incubated with porcine pancreas phospholipase PLA2 (50 U/ml wet worms; Sigma-Aldrich, St. Louis, MO, USA) and 1 mM CaCl2 (30 min, 4°C) and then solubilized with 20 mM n-dodecylmaltoside (DDM) on a rotator for 1 h at 4°C. The resulting detergent extract was clarified by centrifugation (30 min, 48,400 g, 4°C), passed through a 0.8-μm filter, and then incubated with agarose-immobilized anti-rhodopsin 1D4 antibody (ref. 25; 5–10 μl of settled gel per milliliter of starting wet worms) on a rotator for 1 h, 4°C. The resulting gel was loaded onto a column, washed with ≥20 column volumes of washing buffer (1 mM DDM in 50 mM BTP, pH 7.0, containing 250 mM NaCl). Bound GPCR then was eluted with 5 column volumes of 1 mg/ml of competing peptide in the washing buffer.
Purified recombinant (b)isoRho in a quartz cuvette was scanned with a Varian Cary 50 Bio UV-Vis spectrophotometer (Varian, Santa Clara, CA, USA). For spectra, an aliquot of recombinant (b)isoRho was diluted in hydroxylamine containing washing buffer.
SDS-PAGE and immunoblotting
To confirm GPCR expression in worm TG lines, either worm membranes or intact-worm pellets were vortexed in electrophoresis loading buffer and centrifuged briefly, and samples were analyzed by immunoblotting after SDS-PAGE on 4–12% Bis-Tris polyacrylamide gels (Invitrogen, Carlsbad, CA, USA). Worm crude membranes or samples from each GPCR purification step were examined by SDS-PAGE to assess the degree of protein purification. In some experiments, detergent-solubilized receptors were deglycosylated with peptide:N-glycosidase F (PNGase F; New England Biolabs, Ipswich, MA, USA). For silver staining, SDS-PAGE gels were washed with ddH2O (twice, 5 min each) and fixed with 30% ethanol and 10% acetic acid (twice, 15 min each). Fixed gels were washed with 10% ethanol (twice, 5 min each) and then with ddH2O (twice, 5 min each), followed by soaking in 2 mM sodium thiosulfate (1 min). Gels were washed again with ddH2O (twice, 1 min each) and then stained with 50 ml of 12 mM silver nitrate containing 4 μl of 37% formaldehyde (5 min). Stained gels were washed with ddH2O (twice, 30 s each) and then developed with 0.4 M sodium carbonate, 80 μM sodium thiosulfate, and 0.02% formaldehyde. Gel development was terminated with a 5% acetic acid soak (5 min) followed by 5 ddH2O washes. Quantification of signals for both immunoblots and silver-stained gels was done with ImageJ software (27), used to analyze digital pictures of gels or membranes. Area values of bands were measured and compared with the area of purified (b)Rho loaded on the same gel. SimplyBlue SafeStain (Invitrogen) was used for Coomassie staining of gels.
Radioligand binding assays
Day 1 worms were collected from eight 100-mm NGM plates with an M9 wash followed by a 5-min 200-g centrifugation (Allegra 6KR; Beckman). The worm pellet (0.5 ml) was resuspended in 20 ml buffer A (25 mM HEPES, pH 7.4; 1 mM EDTA; and 2 mM MgCl2) and sonicated on ice (70% amplitude, 30 s, 6 times) with a 150T Ultrasonic Dismembrator (Fisher Scientific, Pittsburgh, PA, USA), followed by a brief centrifugation (5 min at 500 g) to remove worm debris. The supernatant was centrifuged for 20 min at 48,000 g to pellet the membranes, which then were resuspended in 500 μl buffer A to yield worm crude membranes. For binding saturation studies, a volume of 20 μl (h)A2AR crude membranes was added to buffer A containing 0.5% BSA and various concentrations of isotopic [3H]-CGS21680 (Perkin Elmer, Waltham, MA, USA) with or without competing ligand to reach a total volume of 50 μl, and then the mixture was incubated at room temperature for 1 h (20). For nonspecific binding, 10 mM caffeine (Sigma-Aldrich) was included in the reaction mixtures. Reaction mixtures were filtered through GF/B glass microfiber filters (Whatman, Piscataway, NJ, USA) in a vacuum manifold, and filters were washed with 15 ml cold buffer A multiple times. Washed filters were next soaked in 5 ml of scintillation cocktail (Perkin-Elmer), and radioactivity was measured in a LS-6500 Beckman scintillation counter. A 1-site-saturation curve was fitted to each data set by using SigmaPlot 11 (SyStat Software, Inc., San Jose, CA, USA) to determine kd values.
In vivo light-response assays
One day before these experiments, L4 animals raised at 20°C were transferred to NGM plates. Plates then were seeded with 100 μl OP50 bacterial culture containing 10 μM 9-cis-retinal or all-trans-retinal diluted from 10 mM stocks in DMSO. The resulting plates were wrapped with aluminum foil and stored overnight at 20°C. Light-response experiments were carried out at 22°C in a dark room by using a Zeiss Stemi SV11-Apo microscope mounted with a Kramer Universal Stereo Fluorescence Attachment and Cubes unit (USFAC; Kramer Scientific, Amesbury, MA, USA), and an Andor iXon DV897 electron-multiplying charge-coupled device (EMCCD) camera (Andor, South Windsor, CT, USA). A ×1.6 objective lens combined with a ×2.5 magnification lens and 7 lux transmitted white light was used at the above hardware settings to visualize and manually track worms during the analysis. For each light-response assay, a d 1 worm with an embedded platinum wire was transferred onto a NGM plate freshly seeded with OP50 (tracking plate). Under these conditions, worms crawled vigorously (28). After 2 min of imaging, 1500 lux of blue light (488±20 nm) was delivered to the animals from a metal halide short arc bulb housed in an EXFO X-Cite 120PC-Q unit (Lumen Dynamics, Mississauga, ON, Canada) through a Kramer USFAC, and the animals were continuously imaged for another 4 min. Worm locomotion before and after illumination was recorded in AVI movies at 30 Hz with a self-developed software package to capture images and control the onset and duration of illumination and integrate this information. Light output of the EXFO unit was calibrated to reach a targeted intensity (±5%) at the microscopic field, as measured with a Macam L203 Photometer (MacamPhotometrics, Livingston, UK). Worm locomotion velocities were computed by a previously published algorithm (29).
Analyses of worm locomotion in response to ligands
Locomotion experiments were done with the automated and quantitative analysis of behavior of nematode (AQUABN) worm behavioral quantification system, according to a protocol described previously (30). Day 1 adult (h)A2AR worms were randomly transferred from stock plates to tracking plates and tracked for 10 min. Under these conditions, animals initially moved vigorously and then entered a steady locomotion state after several minutes (30). Therefore, we computed the average speed from 7–10 min as the control locomotion velocity to eliminate the early acclimation phase. In these experiments, concentrated ligands (CGS21680 or adenosine; Acros Organics, Morris Plains, NJ, USA) were added to liquid NGM, to reach targeted concentrations, and the resulting NGM-containing ligand medium was used to produce ligand-containing plates.
Gt coupling assays
The functionality of (b)Rho or (b)isoRho purified from bovine retinas or worms was evaluated by a transducin (Gt) activation fluorescence assay. The molar ratio of Gt to Rho was 10:1 at concentrations of 250 and 25 nM, respectively. Protein samples were diluted in 20 mM BTP buffer (pH 7.0) containing 120 mM NaCl, 2 mM MgCl2, and 1 mM DDM, and then exposed to light for 15 s with a fiber light covered with a bandpass wavelength filter (480–520 nm). The reaction was carried out at 20°C in a continuously stirred cuvette. After 300 s of incubation, 5 μM GTPγS was added. Pseudo-first-order kinetic rates (k) were derived from the function A(t) = Amax(1 − exp−kt), where Amax is the maximal Gt fluorescence change, and A(t) is the relative fluorescence change at time t. The intrinsic fluorescence increase emanating from Gt was measured with a Perkin Elmer L55 luminescence spectrophotometer, employing excitation and emission wavelengths of 300 and 345 nm, respectively (31–33). No changes in intrinsic tryptophan fluorescence were detected in control experiments without GTPγS.
Statistical analysis
Statistical significance was analyzed with Statistica software (StatSoft, Tulsa, OK, USA), using t tests and ANOVA, with Bonferroni corrections or Dunnet's post hoc analyses, as indicated in the figure legends.
RESULTS
Heterologous expression of (b)opsin in C. elegans
We reasoned that heterologous GPCRs expressed in certain worm tissues rather than the whole body could more likely be purified to near homogeneity, a prerequisite for in vitro structural studies. Therefore, the promoters myo-3 (Pmyo-3; ref. 17) and H20 (PH20; ref. 18), which drive strong gene expression in muscles and the nervous system, respectively, were chosen to control heterologous expression of vertebrate GPCRs in these 2 worm tissues. Muscles comprise the greatest portion of the worm body mass, and the nervous system has the largest numbers of a specific cell type; e.g., 302 of the 959 total somatic cells in an adult are neurons. Notably, endogenous worm GPCR biosynthetic machinery in these tissues could promote the expression of vertebrate GPCRs and assist with their folding, post-translational modification, and stability, to allow a high production of functional GPCRs.
As shown in Fig. 1A, cDNAs encoding WT vertebrate GPCRs were linked to Pmyo-3 or PH20, followed by a DNA sequence encoding a TEV protease cleavage site, a sequence encoding the initial 11 aa of the leader sequence of T7 bacteriophage gene10 [an epitope for a T7 monoclonal antibody (mAb)], a sequence encoding the C-terminal 9 aa of (b)Rho (an epitope for 1D4 mAb; ref. 34), and the unc-54 polyadenylation signal (21). Both T7 and Rho9 tags are used to detect and purify GPCRs, and both can be subsequently removed from the GPCRs by TEV protease treatment. Because the C termini of mammalian GPCRs are unstructured or deleted in all but one of the GPCR crystal structures available to date (35), these introduced tags are unlikely to affect the folding of heterologously expressed GPCRs. Unlike WT constructs, engineered GPCR constructs contained a His tag instead, based on information about previously crystallized constructs of mutated (m) GPCRs. (b)Opsin did not have any extra tag because it already contained the Rho9 tag (end of its C terminus), and the codon-unoptimized A2AR construct only contained a Rho9 tag.
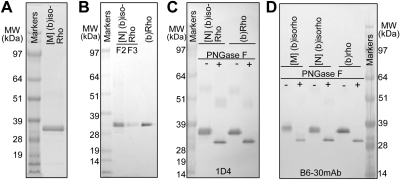
Figure 1.
Heterologous expression of GPCRs in C. elegans. A) Diagram of a generic vertebrate GPCR-expressing construct. In this construct, Pmyo-3 or PH20 is followed by a multiple cloning sequence (MCS), GPCR cDNA (GPCR), a TEV protease cleavage site (TEV), and T7 and Rho9 tags. A stop codon (stop) was engineered between these tags and the unc-54 polyadenylation signal [poly(A)]. B, C) L4 or d 1 TG animals were fixed with methanol and acetone, permeabilized with Triton X-100, stained with Alexa-488-conjugated 1D4 mAb, and subjected to confocal microscopy. B) Representative Alexa-488-coinjugated 1D4 mAb fluorescent image of an L4 TG animal expressing (b)opsin in neurons. 1D4 mAb stained ventral and dorsal cord, motor neurons (arrowheads), and many neurons in the head ganglion, tail ganglion, and middle sections. 1D4 mAb also clustered in the nose tip. C) representative Alexa-488-conjugated 1D4 mAb fluorescent image resulted from IHC analysis of a d 1 TG animal expressing (b)opsin in muscles. 1D4 mAb was visualized in body wall muscles, as well as muscles in the neck and head. D) 1D4-mAb immunoprecipitated lysates from 4 TG worm lines, including those with muscular expression of (b)opsin ([M] (b)opsin), muscular expression of DsRed only ([M] control), pan-neuronal expression of (b)opsin ([N] (b)opsin), pan-neuronal expression of DsRed only ([N] control), and 2 ng (b)Rho were subjected to immunoblotting against alkaline phosphatase-conjugated 1D4 mAb. Molecular weight (MW) markers are at left.
We first used (b)opsin to develop a heterologous C. elegans GPCR expression system because of our experience in purifying and characterizing native (b)Rho (6). cDNA constructs encoding (b)opsin, either under Pmyo-3 or PH20, were injected into the gonads of d 1 WT worms along with a different DNA encoding the coral-derived red fluorescent protein DsRed as a selective marker driven by the same promoter. DsRed fluorescent signals then were visualized under a microscope, and several (∼4) TG worm lines that manifested strong DsRed expression in target tissues were selected. This selection was based on the assumption that expression levels of heterologous (b)opsin positively correlated with those of independently expressed DsRed.
In vivo IHC analyses (24) were also performed to confirm that (b)opsin was expressed in the selected TG worm lines. Alexa-488-conjugated 1D4 mAb together with an optimal concentration of Triton X-100 was injected into the worm pseudocoelom. Triton X-100 permeabilizes worm cells, thereby allowing antibodies to enter cells and bind to the C terminus of (b)opsin. As predicted, cytosolic DsRed signals in neuronal cells or muscles were no longer detected after this injection (Supplemental Fig. S1C, G, J), indicating that these cells had indeed been permeabilized. Independently, the green fluorescence of Alexa-488-conjugated 1D4 mAb was visualized in neurons located in the head ganglion, motor neurons, and ventral cord of TG animals expressing (b)opsin under the control of PH20 (Supplemental Fig. S1B, F), or head muscles of TG animals expressing (b)opsin under the control of Pmyo-3 (Supplemental Fig. S1I). These results demonstrate that (b)opsin was expressed in these targeted tissues. Furthermore, the 1D4 mAb stain revealed hollow shapes in head neurons and motor neurons (Supplemental Fig. S1D, F) and stripped shapes in muscles (Supplemental Fig. S1I), indicating that the recombinant (b)opsin associated with plasma membranes in these worm cells. Thus, it is highly likely that the recombinant (b)opsin was correctly folded and inserted into the lipid membrane.
These in vivo IHC analyses were employed not only to confirm the presence of (b)opsin in targeted worm tissues but also to compare transient expression levels of (b)opsin between TG worm lines. Worms displaying the strongest IHC signals were then selected, and the extrachomosomal GPCR DNAs were integrated into their genomes. Each integration experiment generated multiple (3–4) integrated TG worm lines, all of which superficially resembled WT animals with respect to their behavior and brood size (number of progeny per hermaphrodite). TG worm lines expressing (b)opsin in muscles exhibited a WT growth rate, whereas those expressing (b)opsin in neurons grew slower than WT worms, leading to an ∼50% longer reproductive life cycle (eggs to egg-producing adult hermaphrodites). These observations suggest that the integrated TG lines obtained were suitable for large-scale liquid culture.
To examine further the localization of (b)opsin, IHC analysis with Alexa-488-conjugated 1D4 mAb was done with L4 or young adult d 1 fixed animals from selected integrated TG worm lines expressing (b)opsin. As expected, (b)opsin was found exclusively in the entire worm nervous system (driven by PH20) or muscles (driven by Pmyo-3) (Fig. 1B, C). Finally, to determine the relative expression levels of (b)opsin in these TG worm lines, immunoprecipitated worm lysates along with purified (b)Rho from bovine retina were loaded onto SDS-PAGE gels and subsequently blotted with 1D4 mAb (Fig. 1D). TG worm lines exhibiting the highest levels of (b)opsin then were selected as candidates for further analysis and large-scale worm culture.
Purification of (b)isoRho
TG worm lines with the highest (b)opsin expression in muscles or neurons were initially cultured on twenty-five 150-mm HGM plates for 2 generations and then in a 10-L fermentor for 2 additional generations. Worms were harvested when most were young adults and had the highest levels of opsin expression, as assessed by immunoblotting with 1D4 mAb against lysates sampled at different culture stages. After collection and separation from bacteria, these worms were defined as wet worms. About 400 ml of wet TG worms expressing a mammalian GPCR in either neurons or muscles was consistently produced from each fermentation.
For purification of (b)isoRho, wet (b)opsin TG worms were homogenized in a microfluidizer. Worm crude membranes were collected by centrifugation, and (b)isoRho was generated by incubation with excess 9-cis-retinal chromophore. (9-Cis-retinal was used to recombine with opsin instead of the native 11-cis-isomer because it is commercially available and more photostable than 11-cis-retinal, and both Rho and isoRho isomerize to all-trans-retinal.) The resulting (b)isoRho then was isolated by solubilization of this membrane preparation in DDM, followed by binding to agarose-immobilized 1D4 mAb and elution with a competing peptide. (b)IsoRho purified from the selected worm lines expressing (b)opsin occasionally displayed a double band (Fig. 2). Treatment of this purified (b)isoRho with PNGase F led to a narrow protein band, demonstrated by immunoblotting with either 1D4 mAb (Fig. 2C) or B6–30 mAb that specifically recognizes the N terminus of opsin (ref. 36; Fig. 2D). These data suggest that differing N-glycosylation of (b)opsin observed in worm neurons and muscles occurs in the living animal. Nevertheless, purification to homogeneity of recombinant (b)isoRho expressed in both worm neurons and muscles is achievable in a single chromatographic step. Based on silver-stained SDS-PAGE gels and spectroscopic measurements, the yield of pilot purifications for purified recombinant (b)isoRho from either neurons or muscles was ∼1.5 μg/ml of wet worms. Thus, at least 0.6 mg of total (b)isoRho can be obtained from only one (10 L) worm fermentation. Further optimization of methods presented here will likely allow a milligram scale production per 10-L fermentation.
Figure 2.
Purification of recombinant (b)isoRho from C. elegans. A) Representative Coomassie-stained SDS-PAGE gel of (b)isoRho purified from TG worms expressing (b)opsin in muscles ([M] (b)isoRho). B) Representative silver-stained SDS-PAGE gel of (b)isoRho purified from TG worms expressing (b)opsin in neurons ([N] (b)isoRho) compared with 500 ng of Rho purified from bovine retina [(b)Rho]. F2 and F3 indicate fractions of recombinant (b)isoRho eluted from the 1D4 mAb immunoaffinity column. C) Representative immunoblot of purified [N] (b)isoRho and (b)Rho, with or without PNGase F treatment, recognized by alkaline phosphatase-conjugated 1D4 mAb. D) Representative immunoblot of purified (b)isoRho from TG worm muscles ([M] isoRho) or neurons ([N] isoRho) and (b)Rho, either treated or not treated with PNGase F and detected with alkaline phosphatase-conjugated B6–30 mAb that recognizes the N terminus of opsin.
Characterization of (b)isoRho in vivo and in vitro
Considering that (b)isoRho and (b)Rho obtained from bovine retina have a maximum absorbance at ∼485 and 500 nm, respectively (37), we first determined whether worms expressing (b)opsin in muscles respond to blue light (488±20 nm). It had been reported that exposure to low-energy blue light (∼2.3×10−3 mW/mm2) per se does not modify worm behavior (38). Therefore, any behavioral change of TG worms in response to this stimulus should result from the presence of (b)isoRho.
Before reaching the young adult d 1 stage, TG worms were preincubated overnight in the dark with either 9-cis-retinal, a light-sensitive, active chromophore for opsin, or all-trans-retinal, the light-insensitive isomerized photoproduct. Worms then were transferred to a new NGM plate for behavioral examination. Similar to WT animals that exhibit an initial locomotion acclimation period after changing conditions (30), these animals initially moved rapidly and then slowed. However, after exposure to 1500 lux blue light, TG animals preincubated with 9-cis-retinal increased their locomotion speed, whereas those preincubated with all-trans-retinal were apparently unaffected (Fig. 3A).
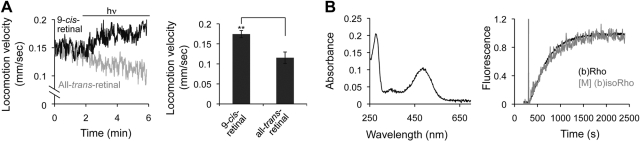
Figure 3.
In vivo and in vitro functional analysis of recombinant (b)isoRho. A) Day 1 TG animals expressing (b)opsin in muscles and preincubated with 10 μM 9-cis-retinal or all-trans-retinal were transferred and tracked on OP50-seeded plates under 7 lux transmitted white light for 2 min. Blue light (1500 lux; 488±20 nm; hν) was then applied, and animals were continuously tracked for another 4 min. Left panel: sample traces of locomotion speed as a function of time for TG animals expressing (b)opsin in muscles and preincubated with either 9-cis-retinal or all-trans-retinal. Right panel: bar graph of averaged locomotion speed from 4 to 6 min (defined as locomotion velocity). Error bars indicate means ± se; n = 10. **P < 0.01; t test. B) Left panel: absorbance spectrum of recombinant (b)isoRho purified from TG worms expressing (b)opsin in muscles. Right panel: fluorescence assay of Gt activation by (b)Rho and (b)isoRho purified from bovine retina or from TG worms expressing (b)opsin in muscles, respectively. Fluorescence change was normalized for both traces.
The above observation suggested that heterologous (b)opsin expressed in worm muscles was functional, thus encouraging us to characterize its biophysical and signaling properties. First, the absorption spectrum of purified recombinant (b)isoRho was determined and found to display the signature ground-state ∼485-nm maximum absorbance (Fig. 3B, left panel).
Because purified (b)Rho from bovine retina can activate Gt (39), a fluorescence-based approach was used to monitor guanyl-nucleotide exchange in the α subunit of Gt that occurs upon its activation. As seen in Fig. 3B (right panel), recombinant (b)isoRho purified from worm muscles did activate Gt. Notably, rates of Gt activation promoted by purified (b)Rho from bovine retina and recombinant (b)isoRho from worms were similar, k = 2.64 ± 0.04 × 10−3 s−1 for (b)Rho and 2.35 ± 0.09 × 10−3 s−1 for (b)isoRho from worms. (b)IsoRho purified from worm neurons displayed a yield, absorption spectrum, and Gt activation properties similar to (b)isoRho purified from worm muscles (see accompanying article; ref. 40).
The above data indicate that (b)isoRho purified from worms possesses properties similar to those of (b)Rho purified from its native source. Thus, a heterologous C. elegans expression system that can produce a highly purified native form of (b)opsin in milligram quantities was established.
Expression and purification of (h)A2AR
The next challenge was to determine whether the C. elegans heterologous expression system could be adapted to other native mammalian GPCRs. (h)A2AR was chosen for this effort because of its biomedical importance and the available functional and structural data. Using the same methodology described for (b)opsin, we obtained several integrated TG worm lines for each type of (h)A2AR expressed, either from codon-optimized or unoptimized cDNA (Table 1). Among these, one TG line expressing codon-optimized (h)A2AR in neurons and another codon unoptimized for (h)A2AR expression in muscles were selected for further study, based on their relatively high expression levels (Table 1).
Alexa-488-conjugated 1D4 mAb injected into the pseudocoelom of d 1 animals with (h)A2AR expressed in neurons (Fig. 4A) or muscles (Fig. 4B) specifically stained either neurons located in the head ganglion or muscles, indicating the presence of heterologous (h)A2AR in these host tissues. Expression of (h)A2AR in worm neurons, but not muscles, delayed the worm growth rate by ∼40% and significantly suppressed locomotion of host animals (Supplemental Fig. S2).
Figure 4.
Expression, functional analysis, and purification of heterologous (h)A2AR. A, B) Representative IHC images of live d 1 TG animals with neuronal (A) or muscular (B) expression of (h)A2AR. Alexa-488-conjugated1D4 mAb was injected into the pseudocoelom of the worm head (A) or middle body section (B) together with TritonX-100, as described in Materials and Methods. Live worms were imaged by confocal microscopy. A) In a TG animal expressing (h)A2AR in neurons, 1D4 mAb was observed solely in the nervous system, including the ventral cord and many neurons in the head ganglion. B) In a TG worm expressing (h)A2AR in muscles, 1D4 mAb was observed in a body wall muscle (stripped rhomboid) and 2 vulva muscles (dumbbell shapes). C) Day 1 TG animals with muscular expression of DsRed only (control) or (h)A2AR ([M] (h)A2AR) were transferred to OP50-seeded NGM plates containing 10 mM adenosine. Locomotion speed of these animals was quantified for 10 min at 30 Hz. The first 6 min of locomotion data were discarded to eliminate the acclimation phase, and the averaged speed from 7 to 10 min was calculated as the animal's locomotion velocity. Error bars indicate means ± se. *P < 0.05; t test; n = 12. D) Radioligand-binding assays indicate that membranes obtained from TG animals expressing (h)A2AR in muscles bind CGS21680, an (h)A2AR specific ligand. Circles, total bound CGS21680; squares, nonspecifically bound CGS21680; triangles, CGS21680 specifically bound to (h)A2AR. One-site-saturation curves were fitted to the data to determine Kd (Kd=70.1±5.8 nM). E) Left lane: silver-stained gel of (h)A2AR purified with immobilized 1D4 mAb. Middle lane: detergent-solubilized membranes were treated with PNGase-F before purification.
In vivo ligand response assays were performed to determine whether (h)A2ARs expressed in worms were functional. As shown in Fig. 4C, d 1 animals from the TG worm line expressing (h)A2AR in muscles responded to exogenous adenosine with enhanced locomotion, whereas the same pharmacological treatment suppressed locomotion of control animals that expressed only DsRed. Similarly, locomotion of d 1 animals from the TG worm line expressing (h)A2AR in neurons was stimulated by the exogenous A2AR agonist, CGS21680 (27 μM; Supplemental Fig. S2A), and the increase in locomotion velocity was dose dependent (Supplemental Fig. S2B). Interestingly, worms expressing A2AR in muscles did not respond to CGS21680 at dosages used to treat worms expressing A2AR in neurons (Supplemental Fig. S2A). The reasons for these different responses to CGS21680 remain to be clarified. Nevertheless, the above in vivo data suggest that heterologous (h)A2AR expressed either in worm muscles or neurons is functional.
To evaluate further the properties of (h)A2AR, radioligand binding assays with crude membranes prepared from the TG worm line expressing (h)A2AR in muscles were performed. Saturation binding with increasing concentrations of [3H]-CGS21680 incubated with membranes prepared from TG worms is shown in Fig. 4D. Incubation of the radioligand together with nonradioactive caffeine demonstrates that the ligand specifically bound to the recombinant (h)A2AR with an affinity constant of 70.1 ± 5.8 nM, consistent with previously reported values for other expression systems and human platelet membrane preparations (41–43).
Taken together, these results indicate that heterologous (h)A2AR expressed in worms was functional, as documented by both changes in worm locomotion in response to specific ligands and proper ligand binding activity. Because of success in purifying (b)isoRho, a similar protocol was used to purify codon-unoptimized (h)A2AR from worms expressing this receptor in muscles (Table 1). Under optimal fermentation conditions (Supplemental Fig. S3), we produced and purified to apparent homogeneity heterologous (h)A2AR expressed in worm muscles in 1 single chromatographic step with agarose-immobilized 1D4 mAb (Fig. 4E). The potent (h)A2AR antagonist ZM241385 was used at all times during the purification. About 20% of the receptor was N-glycosylated (Fig. 4E, left lane), but treatment of the DDM-solubilized membranes with PNGase F before purification was sufficient to obtain a single deglycosylated band (Fig. 4E, middle lane). The identity of the receptor was confirmed by immunoblotting with 1D4 mAb (Supplemental Fig. S4). Based on this and other immunoblotting results, the yield of (h)A2AR was similar to (b)isoRho purified from worm muscles. Therefore, C. elegans can be used to produce milligram quantities of high-quality heterologous functional (b)isoRho and (h)A2AR.
Expression of other vertebrate GPCRs
To test the versatility of this C. elegans heterologous expression system further, we generated integrated TG lines expressing native forms of several vertebrate GPCRs with biomedical importance. These included human serotonin receptor type 4 isoforms n and b [(h)5-HT4Rn and (h)5-HT4Rb], human serotonin receptor type 2A [(h)5-HT2AR], human β2-adrenergic receptor [(h)β2AR], and turkey β1-adrenergic receptor [(t)β1AR; Table 1]. Integrated TG lines expressing (h)5-HT4R-DsRed fusion protein were produced to visualize heterologous GPCR expression directly. Integrated TG lines expressing previously crystallized constructs, namely mutated (t)β1AR [(t)β1ARm], mutated (h)A2AR and (h)β2AR with T4 lysozyme chimeras [(h) A2ARm-T4L and (h)β2ARm-T4L] were also made to compare the C. elegans heterologous expression system with existing insect cell expression systems (Table 1). Constructs and TG worm lines expressing these GPCRs were generated by methods previously described, except that the T7 and Rho9 tags were replaced with a C-terminal His tag for previously crystallized constructs to allow comparisons with previous studies. In addition, the T4L chimeras had an N-terminal Flag tag.
The presence and cellular localization of these heterologous GPCRs were then confirmed either by IHC analyses recognizing Alexa-488-conjugated 1D4 mAb, by monoclonal antibody against the His tag (His-tag mAb), or by direct microscopic visualization of (h)5HT4R-DsRed (Table 1). For estimation of heterologous protein expression levels, HGM plate-cultured d 1 animals from the above TG worm lines along with known amounts of purified control proteins were subjected to immunoblotting against 1D4 mAb, His-tag mAb, or DsRed mAb. Protein levels for each GPCR-expressing TG line were estimated directly or indirectly by comparing immunoblots of each heterologous GPCR with bands produced by 5 ng of purified control proteins (Table 1).
In summary, recombinant (b)isoRho and (h)A2AR were successfully purified using C. elegans as a heterologous expression system. Most important, the recombinant (b)isoRho and (h)A2AR retained the expected properties of native receptors, including (b)isoRho's reaction to light and (h)A2AR's ligand binding. The yield of highly purified (b)isoRho and (h)A2AR was ∼0.6 mg/10 L of worm culture. This system also was used successfully to express several other avian and mammalian GPCRs in C. elegans (summarized in Table 1).
DISCUSSION
GPCRs are involved in many key biological processes and participate both directly and indirectly in the pathology of numerous diseases. These membrane-embedded receptors are especially susceptible to pharmacological ligands, making them the target of half of these therapeutic drugs either on the market or in current development (4, 44). Thus purified native and pathogenic mutant functional GPCRs are highly prized by biomedical investigators and the pharmaceutical industry for pharmacological and biochemical studies. Many mammalian GPCRs have been successfully purified, and >30 crystal structures of mutated GPCRs or GPCR-T4L chimeras have been determined, including (t)β1AR, (h)D3 dopamine receptor, (h)β2-AR, (h)A2AR, (h)H1 histamine receptor, and (h)CXCR4 chemokine receptor. However, all these mutant GPCRs/GPCR-T4Ls exhibit pharmacologic properties distinct from their WT counterparts, stemming from structural artifacts caused by their sequence modifications (45–48). The multicellular organism C. elegans expresses >1000 endogenous GPCRs. Considering the foregoing information, we tested whether this endogenous worm GPCR biosynthesis machinery could facilitate the correct post-translational modification, folding, membrane insertion, and stabilization of TG vertebrate GPCRs and allow large-scale purification of high-quality native or mutant vertebrate GPCRs.
Heterologous expression systems are needed for GPCR purification because nearly all GPCRs are expressed in small amounts in their native tissues. Thus, a strategy to express these mammalian GPCRs in worm muscles or neurons was developed. Specifically, this approach combined a set of in vivo IHC, behavioral, and biochemical techniques to create a multistep procedure to select several integrated TG worm lines with increased expression of recombinant GPCRs that also possessed in vivo function (Fig. 5). Furthermore, in vitro functional analyses, such as absorbance and/or G-protein coupling, were performed to determine whether the purified GPCRs were properly folded and functional. Through this approach, TG worm lines expressing (b)opsin in neurons or muscles or (h)A2AR in muscles enabled the purification of more than half a milligram of these GPCRs from just one 10-L worm fermentation. Notably, the resulting purified receptors were shown to be N-glycosylated, a post-translational modification often critical for correct protein folding and/or membrane transport and insertion (49, 50). These data strongly support our hypothesis that C. elegans contains conserved mechanisms that facilitate proper folding of heterologously expressed mammalian GPCRs. Moreover, heterologous bovine isoRho purified from C. elegans evidenced both in vivo and in vitro function and displayed the same Gt coupling kinetics as (b)Rho obtained from its native source.
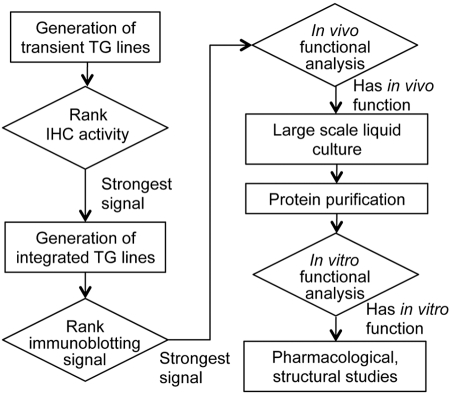
Figure 5.
Flow chart for expression and purification of mammalian GPCRs in C. elegans. The biology of C. elegans allowed expression and isolation of functional GPCRs in milligram amounts. After transient TG worm lines were obtained, IHC assays were performed to select worm lines exhibiting the strongest immunoreactivity against 1D4 mAb for each GPCR expressed in either neurons or muscles. Selected transient TG worm lines were next integrated for GPCR expression, resulting in multiple integrated TG lines from each transient TG line. These integrated TG worm lines were subjected to immunoblotting against 1D4 mAb to select those with the highest levels of GPCR expression. Chosen TG worm lines then were assessed for heterologous GPCR-regulated motor behaviors, indicating that these GPCRs were functional. Selected functional TG worm lines were next used for large-scale liquid culture and consequent GPCR purification. Finally, functional assays were performed to confirm that the purified receptors were still properly folded after purification.
A number of diffusible ligand-activated native GPCRs, mutated GPCRs, and mutated GPCR-T4L chimeras were also expressed in worms. Protein expression levels for most of these GPCRs were slightly lower than (b)opsin or (h)A2AR. In general, muscles exhibited higher heterologous protein expression than neurons. Whether codon optimization helped to increase heterologous GPCR expression was inconclusive, as judged by comparing unoptimized and codon-optimized (h)A2AR expression in worm muscles (Table 1). Higher expression of codon-unoptimized (h)A2AR was unexpected and could simply be an artifact of TG line generation and selection.
Levels of heterologous GPCR expression estimated from worm homogenates by immunoblotting were consistently lower than protein levels estimated for purified (h)A2AR and (b)opsin expressed in either neurons or muscles. One possible reason for this underestimation is that lipids and worm debris present in membrane preparations negatively affected the quality of the receptors' electrophoretic migration. Nonetheless, several forms of avian and mammalian GPCRs were successfully expressed in worms, although the expression of (h)5-HT2AR and (t)β1AR was noticeably lower than other tested GPCRs. Given the low cost of large-scale worm cultures and the rapid growth rate of C. elegans, obtaining TG animals that produce sufficient quantities of (h)5-HT2AR and (t)β1AR for biophysical and pharmacological studies still appears feasible.
Interestingly, mutated GPCRs and mutated GPCR chimeras with T4L did not consistently exhibit higher expression levels than native GPCRs. For example, native forms of (h)A2AR and (h)5HT4R were among those GPCRs with high levels of expression. This observation suggests that G-protein signaling machinery in worm host cells is more effective in enhancing heterologous WT GPCR production than mutant or chimeric GPCRs with structural modifications introduced for stabilization and crystallization.
In summary, C. elegans can serve as an excellent host for heterologous GPCR expression. It takes little time at relatively low cost to generate TG worm lines, maintain these lines, and culture TG worms in large quantities in suspension, because these hermaphrodites have a short reproductive life cycle (∼3.5 d at 20°C), relatively simple genetics, and ability to survive cryostorage. Thus, it required less than a month starting from plate-culturing TG worms to collecting wet worms from a fermentor, with reagent costs < $1000. The period needed to generate TG worm lines expressing mammalian GPCRs varied, depending on the specific GPCR. Typically, it took ∼2 mo to obtain an integrated TG worm line expressing functional GPCRs. In the future, this C. elegans-based expression system may be expanded to express pathogenic GPCRs, channels, transporters, and receptors other than GPCRs, for which heterologous expression is problematic in existing single-cell based systems (51).
Supplementary Material
Acknowledgments
The authors thank L.T. Webster, Jr., and members of the K.P. laboratory for critical comments on the manuscript. The authors thank Dr. I. Katsura (Graduate University for Advanced Studies, Mishima, Japan) for PH20 DNA and M. H. Zheng for technical assistance.
This research was supported in part by grants EY-008061, EY-009339, and P30 EY-11373 (to K.P.) from the U.S. National Institutes of Health, and Mt. Sinai Health Care Foundation Scholars Program in the Basic Science (to Z.F.). Research was also supported by a U54 award to the New York SGX Research Center for Structural Genomics (NYSGXRC) from the National Institute of General Medical Sciences (GM074945; principal investigator: Stephen K. Burley) under a contract to Polgenix, Inc. D.S. and W.S. are employees of Polgenix, Inc. K.P. is CSO at Polgenix Inc. P.C., K.K., B.J., H.J., and Z.F. report no conflicts of interest. The Z.F. laboratory received support from Polgenix, Inc.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Overington J. P., Al-Lazikani B., Hopkins A. L. (2006) How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 [DOI] [PubMed] [Google Scholar]
- 2. Parrill A. L. (2008) Crystal structures of a second G protein-coupled receptor: triumphs and implications. Chem. Med. Chem. 3, 1021–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Milligan G., Canals M., Pediani J. D., Ellis J., Lopez-Gimenez J. F. (2006) The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found. Symp. Proc. 2, 145–161 [DOI] [PubMed] [Google Scholar]
- 4. Lundstrom K., Wagner R., Reinhart C., Desmyter A., Cherouati N., Magnin T., Zeder-Lutz G., Courtot M., Prual C., Andre N., Hassaine G., Michel H., Cambillau C., Pattus F. (2006) Structural genomics on membrane proteins: comparison of more than 100 GPCRs in 3 expression systems. J. Struct. Funct. Genomics 7, 77–91 [DOI] [PubMed] [Google Scholar]
- 5. Lundstrom K. (2005) Structural genomics of GPCRs. Trends Biotechnol. 23, 103–108 [DOI] [PubMed] [Google Scholar]
- 6. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 7. Li J., Edwards P. C., Burghammer M., Villa C., Schertler G. F. (2004) Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 343, 1409–1438 [DOI] [PubMed] [Google Scholar]
- 8. Okada T., Sugihara M., Bondar A. N., Elstner M., Entel P., Buss V. (2004) The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J. Mol. Biol. 342, 571–583 [DOI] [PubMed] [Google Scholar]
- 9. Klammt C., Schwarz D., Dotsch V., Bernhard F. (2007) Cell-free production of integral membrane proteins on a preparative scale. Methods Mol. Biol. 375, 57–78 [DOI] [PubMed] [Google Scholar]
- 10. Panneels V., Kock I., Krijnse-Locker J., Rezgaoui M., Sinning I. (2011) Drosophila photoreceptor cells exploited for the production of eukaryotic membrane proteins: receptors, transporters and channels. PLoS One 6, e18478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lundstrom K. (2007) Structural genomics and drug discovery. J. Cell. Mol. Med. 11, 224–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sprang S. R. (2011) Cell signalling: Binding the receptor at both ends. Nature 469, 172–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker M. (2010) Making membrane proteins for structures: a trillion tiny tweaks. Nat. Methods 7, 429–434 [DOI] [PubMed] [Google Scholar]
- 14. Baker M. (2010) Structural biology: The gatekeepers revealed. Nature 465, 823–826 [DOI] [PubMed] [Google Scholar]
- 15. Topiol S., Sabio M. (2009) X-ray structure breakthroughs in the GPCR transmembrane region. Biochem. Pharmacol. 78, 11–20 [DOI] [PubMed] [Google Scholar]
- 16. Bargmann C. I. (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282, 2028–2033 [DOI] [PubMed] [Google Scholar]
- 17. Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A. (1993) Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135, 385–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yabe T., Suzuki N., Furukawa T., Ishihara T., Katsura I. (2005) Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development 132, 3197–3207 [DOI] [PubMed] [Google Scholar]
- 19. Stenico M., Lloyd A. T., Sharp P. M. (1994) Codon usage in Caenorhabditis elegans: delineation of translational selection and mutational biases. Nucleic Acids Res. 22, 2437–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salom D., Wu N., Sun W., Dong Z., Palczewski K., Jordan S., Salon J. A. (2008) Heterologous expression and purification of the serotonin type 4 receptor from transgenic mouse retina. Biochemistry 47, 13296–13307 [DOI] [PubMed] [Google Scholar]
- 21. Fire A., Waterston R. H. (1989) Proper expression of myosin genes in transgenic nematodes. EMBO J. 8, 3419–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vilaro M. T., Domenech T., Palacios J. M., Mengod G. (2002) Cloning and characterization of a novel human 5-HT4 receptor variant that lacks the alternatively spliced carboxy terminal exon. RT-PCR distribution in human brain and periphery of multiple 5-HT4 receptor variants. Neuropharmacology 42, 60–73 [DOI] [PubMed] [Google Scholar]
- 23. Stiernagle T. (2006) Maintenance of C. elegans. WormBook, doi: 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gottschalk A., Schafer W. R. (2006) Visualization of integral and peripheral cell surface proteins in live Caenorhabditis elegans. J. Neurosci. Methods 154, 68–79 [DOI] [PubMed] [Google Scholar]
- 25. Salom D., Li N., Zhu L., Palczewski K. (2005) Purification of the G protein-coupled receptor rhodopsin for structural studies. In G Protein-Coupled Receptor–Protein Interactions (George S. R., O'Dowd B. F., eds) pp. 1–17, John Wiley & Sons, New York [Google Scholar]
- 26. Fabian T. J., Johnson T. E. (1994) Production of age-synchronous mass cultures of Caenorhabditis elegans. J. Gerontol. 49, B145–156 [DOI] [PubMed] [Google Scholar]
- 27. Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 28. Sawin E. R., Ranganathan R., Horvitz H. R. (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 [DOI] [PubMed] [Google Scholar]
- 29. Zhang S., Jin W., Huang Y., Su W., Yang J., Feng Z. (2011) Profiling a Caenorhabditis elegans behavioral parametric dataset with a supervised K-means clustering algorithm identifies genetic networks regulating locomotion. J. Neurosci. Meth. 197, 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng Z., Li W., Ward A., Piggott B. J., Larkspur E. R., Sternberg P. W., Xu X. Z. (2006) A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 127, 621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fahmy K., Sakmar T. P. (1993) Regulation of the rhodopsin-transducin interaction by a highly conserved carboxylic acid group. Biochemistry 32, 7229–7236 [DOI] [PubMed] [Google Scholar]
- 32. Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 33. Heck M., Hofmann K. P. (2001) Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J. Biol. Chem. 276, 10000–10009 [DOI] [PubMed] [Google Scholar]
- 34. MacKenzie D., Arendt A., Hargrave P., McDowell J. H., Molday R. S. (1984) Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry 23, 6544–6549 [DOI] [PubMed] [Google Scholar]
- 35. Mustafi D., Palczewski K. (2009) Topology of class A G protein-coupled receptors: insights gained from crystal structures of rhodopsins, adrenergic and adenosine receptors. Mol. Pharmacol. 75, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adamus G., Zam Z. S., Arendt A., Palczewski K., McDowell J. H., Hargrave P. A. (1991) Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 31, 17–31 [DOI] [PubMed] [Google Scholar]
- 37. Spalink J. D., Reynolds A. H., Rentzepis P. M., Sperling W., Applebury M. L. (1983) Bathorhodopsin intermediates from 11-cis-rhodopsin and 9-cis-rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 80, 1887–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ward A., Liu J., Feng Z., Xu X. Z. (2008) Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 11, 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jastrzebska B., Fotiadis D., Jang G. F., Stenkamp R. E., Engel A., Palczewski K. (2006) Functional and structural characterization of rhodopsin oligomers. J. Biol. Chem. 281, 11917–11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cao P., Sun W., Kramp K., Zheng M., Salom D., Jastrzebska B., Jin H., Palczewski K., Feng Z. (2011) Light-sensitive coupling of rhodopsin and melanopsin to Gi/o and Gq signal transduction in Caenorhabditis elegans. [E-pub ahead of print] FASEB J. doi: 10.1096/fj.11-197798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robeva A. S., Woodard R., Luthin D. R., Taylor H. E., Linden J. (1996) Double tagging recombinant A1- and A2A-adenosine receptors with hexahistidine and the FLAG epitope. Development of an efficient generic protein purification procedure. Biochem. Pharmacol. 51, 545–555 [DOI] [PubMed] [Google Scholar]
- 42. O'Malley M. A., Lazarova T., Britton Z. T., Robinson A. S. (2007) High-level expression in Saccharomyces cerevisiae enables isolation and spectroscopic characterization of functional human adenosine A2a receptor. J. Struct. Biol. 159, 166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varani K., Gessi S., Dalpiaz A., Borea P. A. (1996) Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. Br. J. Pharmacol. 117, 1693–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lundstrom K. (2006) Latest development in drug discovery on G protein-coupled receptors. Curr. Protein Pept. Sci. 7, 465–470 [DOI] [PubMed] [Google Scholar]
- 45. Dror R. O., Arlow D. H., Borhani D. W., Jensen M. O., Piana S., Shaw D. E. (2009) Identification of two distinct inactive conformations of the beta2-adrenergic receptor reconciles structural and biochemical observations. Proc. Natl. Acad. Sci. U. S. A. 106, 4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lodowski D. T., Angel T. E., Palczewski K. (2009) Comparative analysis of GPCR crystal structures. Photochem. Photobiol. 85, 425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanni S., Neri M., Tavernelli I., Rothlisberger U. (2009) Observation of “ionic lock” formation in molecular dynamics simulations of wild-type beta 1 and beta 2 adrenergic receptors. Biochemistry 48, 4789–4797 [DOI] [PubMed] [Google Scholar]
- 48. Provasi D., Filizola M. (2010) Putative active states of a prototypic g-protein-coupled receptor from biased molecular dynamics. Biophys. J. 98, 2347–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Markkanen P. M., Petaja-Repo U. E. (2008) N-glycan-mediated quality control in the endoplasmic reticulum is required for the expression of correctly folded delta-opioid receptors at the cell surface. J. Biol. Chem. 283, 29086–29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaushal S., Ridge K. D., Khorana H. G. (1994) Structure and function in rhodopsin: the role of asparagine-linked glycosylation. Proc. Natl. Acad. Sci. U. S. A. 91, 4024–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee J. K., Stroud R. M. (2010) Unlocking the eukaryotic membrane protein structural proteome. Curr. Opin. Struct. Biol. 20, 464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.