Abstract
Neural agrin plays a pleiotropic role in skeletal muscle innervation and maturation, but its specific effects on the contractile function of aneural engineered muscle remain unknown. In this study, neonatal rat skeletal myoblasts cultured within 3-dimensional engineered muscle tissue constructs were treated with 10 nM soluble recombinant miniagrin and assessed using histological, biochemical, and functional assays. Depending on the treatment duration and onset time relative to the stage of myogenic differentiation, miniagrin was found to induce up to 1.7-fold increase in twitch and tetanus force amplitude. This effect was associated with the 2.3-fold up-regulation of dystrophin gene expression at 6 d after agrin removal and enhanced ACh receptor (AChR) cluster formation, but no change in cell number, expression of muscle myosin, or important aspects of intracellular Ca2+ handling. In muscle constructs with endogenous ACh levels suppressed by the application of α-NETA, miniagrin increased AChR clustering and twitch force amplitude but failed to improve intracellular Ca2+ handling and increase tetanus-to-twitch ratio. Overall, our studies suggest that besides its synaptogenic function that could promote integration of engineered muscle constructs in vivo, neural agrin can directly promote the contractile function of aneural engineered muscle via mechanisms distinct from those involving endogenous ACh.—Bian, W., Bursac, N. Soluble miniagrin enhances contractile function of engineered skeletal muscle.
Keywords: synaptogenesis, myogenesis, twitch force, acetylcholine receptors
The transplantation of tissue-engineered muscle substitutes is a promising therapeutic option for the repair of irreversible skeletal muscle loss due to various muscular diseases, traumatic injuries, and congenital defects (1). However, successful in vitro engineering of highly functional 3-dimensional (3D) muscle tissues starting from dissociated cells (e.g., myoblasts or satellite cells) requires a complex set of biochemical and biophysical stimuli to recapitulate myogenic processes during development or regeneration. Specifically, nerve-derived signals are known to play important roles during skeletal muscle development, including enhancing and sustaining muscle growth, promoting muscle differentiation (2), and facilitating the formation of functional neuromuscular junctions (NMJs; ref. 3). Previously, coculturing primary nerve explants with engineered muscle tissues has been shown to yield a 2- and 1.7-fold increase in muscle twitch and tetanus force, respectively, as well as the formation of functional NMJs (4, 5). However, potential skeletal muscle therapies using this approach would be limited by difficulties in obtaining and culturing autologous nerve explants and integrating obtained cocultured tissues into the host neuromuscular system. In contrast, the use of single or a combination of trophic factors secreted by motor neurons at NMJs could provide apparent advantages for the engineering of aneural functional muscle (e.g., long-term culture and differentiation without loss of viability); however, the potential benefit of this approach remains largely unexplored. Therefore, in this report, we set to study the effects of agrin, a nerve-secreted synaptogenic factor, on contractile function of engineered skeletal muscle.
Agrin, a 220-kDa heparin sulfate proteoglycan (6), contains multiple domains that interact with different downstream targets in skeletal muscle and elicit pleiotropic effects on the muscle innervation and maturation. Specifically, the C terminus of agrin has been shown to induce the aggregation and stabilization of acetylcholine receptors (AChRs) in muscle sarcolemma (7–9) by activating the muscle-specific receptor tyrosine kinase (MuSK) and binding to α-dystroglycan (10), an extracellular component of the large membrane-bound dystrophin-associated protein complex involved in lateral force transmission between the extracellular matrix (ECM) and the cytoskeleton (11). The N terminus of agrin, on the other hand, binds extracellularly to several isoforms of laminin (laminin-1, laminin-2, and laminin-4; ref. 12) and can indirectly engage integrins to affect both the stabilization of AChR clusters and the lateral force transmission through the cell-ECM linkage (11). In addition, agrin has recently been shown to promote the maturation of the excitation-contraction (E-C) coupling apparatus (13) and to alter Na+/K+-ATPase and ion channel expression (14) in developing human myotubes in vitro via yet unexplained mechanisms. Intensive research in the past decade has established the canonical role of neural agrin in NMJ development via its influence on the aggregation of AChRs (15–18), but it remains unknown whether agrin could directly augment the muscle force production via noncanonical mechanisms involving the effects on dystrophin-associated protein complex and/or E-C coupling. Of special interest for muscle tissue engineering is the question of whether this noncanonical function of agrin could mimic the increase of contractile force generation observed in the cocultures of engineered muscle and primary nerve explants (4, 5).
Previous studies in transgenic mice lacking the ACh-synthesizing enzyme, choline acetyltransferase (ChAT; refs. 15, 19), have suggested that ACh can counter the synaptogenic effects of agrin. Specifically, AChRs were found to cluster more extensively on the muscle sarcolemma in mice with reduced ACh synthesis than the wild-type controls. However, in this specific mouse model, it was impossible to separate the individual effects of the nerve-secreted and muscle endogenous ACh. Thus, it is still unclear whether a change in only the muscle endogenous ACh can modulate the canonic effects of agrin on AChR clustering, a question of importance for the optimal design of engineered muscle tissues that would efficiently integrate with the host neuromuscular system. In addition, aneural myotubes in 2-dimensional (2D) in vitro cultures have been shown to self-synthesize and secrete ACh or ACh-like compound (ACh-lc; ref. 20), which promoted the myotube survival and Ca2+-mediated spontaneous activity (21, 22). Whether this autocrine ACh stimulation, or lack of it, could influence contractile force generation in aneural myotubes and modulate potential noncanonical effects of agrin on contractile force generation remains unknown, in part, because it is difficult to measure generated contractile forces in 2D cultures attached to a rigid substrate.
Therefore, in the current study, we utilized a 3D engineered aneural muscle tissue system (23, 24) that allows measurements of contractile force to investigate the potential noncanonical effects of agrin on contractile force generation, the role of endogenous ACh in this noncanonical function of agrin, and the effect of endogenous ACh on the canonical effect of agrin on AChR clustering. Miniagrin (90 kDa), a recombinant C-terminal fragment of agrin, was utilized in the experiments due to its small size, which could facilitate diffusion through 3D engineered tissue, solubility in culture medium, and the ability to reproduce major functions of the full-length agrin. Contractile forces were first assessed in agrin-treated muscle constructs relative to those of nontreated controls. Cell numbers, muscle myosin expression level, and mRNA and protein expression levels of dystrophin, as well as amplitude and kinetics of intracellular Ca2+ transient, were examined to determine the main noncanonical mechanisms underlying the agrin-induced change in contractile force. Muscle endogenous ACh levels were suppressed by α-NETA (a specific ChAT inhibitor; ref. 25) in control and agrin-treated constructs, and contractile force generation, intracellular Ca2+ transients, and AChR clustering were assessed to elucidate the modulatory effects of reduced endogenous ACh levels on both the noncanonical (contractile force) and canonical (postsynaptic differentiation) roles of agrin in the aneural engineered muscle tissue.
MATERIALS AND METHODS
Isolation of neonatal rat skeletal myoblasts (NRSKMs)
NRSKMs were isolated as described previously (23). Briefly, muscle tissue from the lower hindlimbs of 2- to 3-d-old Sprague-Dawley rats was dissected, minced, and digested with 1 mg/ml collagenase (Worthington, Lakewood, NJ, USA) in Wyles solution (137 mM NaCl, 5 mM KCl, 21 mM HEPES, 0.7 mM Na2HPO4, 100 mM glucose, and 0.1 mg/ml BSA) for 2 h at 37°C. The isolated cells were resuspended in growth medium (DMEM, 10% v/v FBS, 50 U/ml penicillin G, 50 μg/ml streptomycin, and 5 μg/ml gentamicin), preplated for 2 h at 37°C to enrich the myoblast population, and subsequently mixed with hydrogel solution to form engineered muscle tissues. All experiments involving animals conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 1996) and an animal protocol approved by the Duke University Animal Care and Use Committee.
Fabrication of tissue-engineered muscle constructs
Porous engineered muscle tissue constructs consisting of 3D aligned cross-striated myotubes were fabricated using the mesoscopic hydrogel-molding approach, as described previously (23, 24). Briefly, a silicon template containing an array of staggered hexagonal posts was first created using a high-aspect-ratio soft lithography technique. Then, polydimethylsiloxane (PDMS) was double-casted off the silicon template to yield a tissue mold with the template geometry. The freshly isolated NRSKMs (107/ml) were mixed with bovine fibrinogen (4 mg/ml; Sigma, St. Louis, MO, USA), Matrigel (10% v/v; BD Biosciences, Franklin Lakes, NJ, USA), bovine thrombin (0.2 U/mg fibrinogen; Sigma) and 2× growth medium, injected in the tissue mold, and incubated at 37°C for 45–60 min to allow gel polymerization. The constructs were cultured for 4 d in high-serum-containing growth medium and subsequently for 10 d in low-serum-containing differentiation medium (DMEM, 3% v/v horse serum, 50 U/ml penicillin G, 50 μg/ml streptomycin, 5 μg/ml gentamicin) to facilitate myoblast fusion and the maturation of multinucleated myotubes. The resulting elliptical pores in the engineered tissue served to enhance oxygen and nutrient transport to muscle cells and promote uniform cell alignment throughout the entire tissue volume (Supplemental Fig. S1).
Treatment of muscle constructs with rat miniagrin and α-NETA
Recombinant rat agrin was purchased from R&D systems (Minneapolis, MN, USA). This ∼90-kDa protein contains the C-terminal part of rat agrin (∼220 kDa), including 4 EGF-like repeats and 3 laminin globular G domains. It is the neural isoform of agrin identified by the 9-aa insert at the Z site, which is capable of inducing clustering of AChRs in differentiating murine myotubes (12, 26). This recombinant agrin, termed miniagrin, was applied at a concentration of 10 nM to muscle constructs over varying time periods, ranging from 2 to 8 d, and different onset times, at d 0 or 4 of differentiation. The ChAT inhibitor, α-NETA (Sigma) was applied at a concentration of 50 μM between d 0 and 10 of differentiation to decrease the level of endogenous ACh in engineered muscle. Both miniagrin and α-NETA were applied fresh every other day when changing culture medium.
Quantification of AChR clustering
Engineered muscle tissues were fixed in 4% formaldehyde for 2 h at 4°C and incubated with 1 μg/ml Alexa Fluor 488-conjugated α-bungarotoxin (Invitrogen, Carlsbad, CA, USA) to label the membrane-bound AChRs and DAPI to simultaneously stain nuclei. Stained tissue constructs were imaged at ×20 view with a Zeiss confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany). A total of 9 images in 3 tissue samples from 3 independent isolations were manually analyzed for each experimental group, as follows. Background fluorescence was increased to a minimum level that allowed the visualization of myotube boundaries. The percentage of myotubes containing AChR clusters was measured by dividing the number of positively stained myotubes by the total number of myotubes. The number of AChR clusters per positively stained myotube was also quantified. In addition, the cluster length (longest diameter in the cluster) was measured for all AChR clusters and classified into one of the 3 ranges: 0–20, 20–40, and 40–60 μm. The percentage of AChR clusters in each range and the average cluster length were also quantified for each experimental group.
DNA content quantification
Engineered muscle constructs were rapidly frozen in liquid nitrogen. The total DNA in each tissue sample was isolated using a DNeasy blood and tissue kit (Qiagen, Valencia, CA, USA) and eluted in 200 μl AE buffer (10 mM Tris-HCl and 0.5 mM EDTA, pH 9.0). DNA concentration was then measured using a spectrometer (Nanodrop 1000, NanoDrop Products, Wilmington, DE, USA) to obtain the total DNA content of a tissue construct.
Quantitative RT-PCR
The total RNA from frozen muscle constructs was isolated using an RNeasy mini kit (Qiagen) per the manufacturer's instructions. The quality of isolated RNA was checked using a Nanodrop 1000 spectrophotometer, and only RNA with A260/A280 > 1.8 was used in the 1-step quantitative RT-PCR (qRT-PCR) analysis. To prepare each 30-μl qRT-PCR reaction, 50 ng total RNA (5 ng/μl in 10 μl) was mixed with 15 μl 2× 1-step qRT-PCR master mix (ABgene, Epsom, UK) and 5 μl aqueous solution containing 5 U reverse transcriptase (SuperScript II; Invitrogen), primers (0.05 μg for genes of medium abundance, e.g., GAPDH; 0.1 μg for genes of low abundance, e.g., Chrne and Dmd) and 20 pm Taqman probes (primers and Taqman probes shown in Supplemental Table S1 were kindly provided by Dr. Hyung-Suk Kim, University of North Carolina, Chapel Hill, NC, USA). The efficiency of primer/probe sets for the target genes was comparable to that for the reference gene, and all primer/probe sets had >90% efficiency. Reactions were then loaded in a 96-well MicroAmp plate (Applied Biosystems, Carlsbad, CA, USA) and run on an ABI7300 real-time PCR system (Applied Biosystems) with a 30-min RT cycle at 48°C, followed by a 10-min initial PCR at 95°C and 50 repeated 2-step PCR cycles (15 s at 95°C, then 1 min at 60°C). The fold changes of target genes relative to the reference gene (GAPDH) were calculated using the 2−ΔΔCt method (27).
Western blot analysis
Engineered muscle constructs were digested with 20 μg/ml bovine plasmin (Innovative Research, Novi, MA, USA) until fully dissolved. Dissociated cells were collected by centrifugation and lysed in RIPA buffer (Sigma). Total protein was collected, and its concentration was measured using a BCA kit (Pierce, Rockford, IL, USA). The isolated protein was then mixed with Laemmli buffer (Bio-Rad, Hercules, CA, USA; ref. 28), boiled, and loaded on Tris-HCl gels (Bio-Rad; 7.5% for total myosin and β-tubulin; 5% for total myosin and fast myosin). After electrophoresis and transfer, membrane was blocked, incubated with primary antibodies [mouse anti-total myosin, MF 20, Developmental Studies Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA, USA; mouse anti-fast myosin, F59, DSHB; rabbit anti-β-tubulin, Abcam, Cambridge, MA, USA] overnight at 4°C, followed by HRP-conjugated secondary antibodies (Pierce) for 1 h at room temperature, and probed with chemiluminescence. The band intensity was measured using ImageQuant TL software (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Isometric contractile force measurements
The use of hydrogel-based muscle tissue constructs allowed us to perform standard physiological tests of contractile force generation, otherwise difficult to accomplish in the traditional 2D muscle cultures on rigid substrates. On d 14 of culture, engineered tissue constructs were removed from PDMS molds, transferred into a temperature-controlled chamber, and immersed in culture medium at 36 ± 1°C. The constructs were separated from the surrounding nylon frame on opposite sides and cut to allow uniaxial tissue stretch (Supplemental Fig. S2A). One of the two uncut sides was then attached to a fixed tissue holder in the chamber, and the other was attached to a floating PDMS holder, which was connected to a sensitive force transducer (provided by Dr. Robert Dennis, University of North Carolina, Chapel Hill, NC, USA). A motorized linear actuator (Thorlabs, Newton, NJ, USA) controlled the position of the force transducer and the length of tissue construct, which was independently recorded using a complementary metal oxide semiconductor camera (The Imagesource, Charlotte, NC, USA) mounted on the top of the chamber. Force signals were amplified with a DAM50 differential amplifier (World Precision Instruments, Sarasota, FL, USA) and recorded using a custom-designed program written in LabView (National Instruments, Austin, TX, USA). A pair of parallel platinum electrodes was used to apply electrical stimuli and elicit isometric muscle contraction.
After 10 min of equilibration, the length of the tissue construct was set to the cultivation length (L0=6 mm). The construct was then stimulated by a single electrical pulse (amplitude: 3.6 V/cm; duration: 5 ms) to elicit isometric muscle contraction (twitch). Parameter of a single twitch, including amplitude (At), the time to peak twitch (TPT, from the onset of electrical stimulus to the time of peak twitch) and half-relaxation time (RT1/2, from the time of peak twitch to 50% recovery) were derived from the recorded force traces, as described previously (29). The construct was then stimulated by a 1-s-long pulse train with frequency that was increased every 5 min (i.e., to 5, 10, 20, 40, and 60 Hz) until tetanus was reached. Parameters of the tetanus, including peak amplitude (AT) and the tetanus-to-twitch ratio (TtR=AT/At), were calculated from the recorded traces.
Sensitivity of contractile force amplitude to extracellular Ca2+ concentration
Engineered muscle constructs were first incubated at resting length in Ca2+-free, 1 mM EDTA containing Tyrode's solution (in mM: 135 NaCl, 5.4 KCl, 1 MgCl2, 0.33 NaH2PO4, 5 HEPES, 5 glucose, and 1 EDTA) to prevent active force generation. Subsequently, the extracellular Ca2+ concentration ([Ca2+]) was elevated every 20 min to a higher value (in mM: 0.1, 0.2, 0.4, 0.8, 1.2, 1.6, 2.4, 3.2, and 4.8), and a 5-ms-long, 3.6-V/cm electrical pulse was applied to elicit isometric contraction (twitch). The twitch amplitude At was expressed as a function of pCa = −log10([Ca2+]), and At(pCa) curves were compared among different groups after normalization with the respective At0 at the highest [Ca2+] of 4.8 mM. The At(pCa) curves were then fitted using the Hill equation to obtain the Hill coefficient (h) and pCa50, the pCa value when At = ½ At0 (30):
Statistical analysis was applied to the pCa50 values and the log form of Hill coefficient, log10(h), both of which were previously shown to exhibit a normal distribution in physiological data (30).
Nonratiometric measurements of intracellular Ca2+ transients
Engineered muscle constructs were incubated in serum-free medium containing 5 μM calcium-sensitive dye Rhod-2 (Invitrogen) for 30 min at 37°C, followed by 20-min incubation in dye-free medium. The stained constructs were then transferred into a custom chamber mounted on a Nikon TE2000-inverted fluorescence microscope (Nikon,Tokyo, Japan) and perfused with warm (36±1°C) Tyrode's solution (in mM: 135 NaCl, 5.4 KCl, 1.8 CaCl, 1 MgCl, 0.33 NaHPO, 5 HEPES, and 5 glucose). Blebbistatin (5 μM; Sigma) was added to inhibit contractions and eliminate motion artifacts during recording (31). The stained constructs were electrically stimulated (5-ms, 3.6-V/mm pulses) with a pair of parallel platinum electrodes, illuminated by green light (520±25 nm), and emitted fluorescence signals (605±30 nm) were simultaneously recorded from 504 sites within a circular field of view (diameter: 5 mm) with 1.2-kHz sampling rate and 187-μm resolution using a photodiode array (RedShirt Imaging, Decatur, GA, USA; refs. 29, 32). Signals in the pore regions of the tissue construct were identified by thresholding (<20% of maximum signal) and discarded from further analysis.
To quantify the kinetics of Ca2+ transients, the mean Ca2+ transient trace was first obtained by normalizing Ca2+ transient trace in each tissue site with its respective maximum amplitude and then averaging the normalized Ca2+ transient traces over all tissue sites. The upstroke time (tup) was measured as the interval from the onset to the peak of the mean Ca2+ transient. The decay times (τ) were measured at different percentages of the Ca2+ transient recovery (i.e., between the time of peak and the time of particular recovery level of the mean transient). The average amplitude of Ca2+ transient (ACaT) in tissue constructs was estimated by averaging fractional fluorescence changes at all tissue sites (Supplemental Fig. S3). The measured ACaT values in treated groups were normalized for each cell isolation to the corresponding ACaT value measured in the untreated group. Because of the nonratiometric nature of the measurements, the underlying assumption for these comparisons was that the average basal intracellular Ca2+ levels were similar among different groups.
Statistical analysis
Data are expressed as means ± sd. Statistical significance was determined by Student's t test for 2 groups and by 1-way ANOVA followed by Tukey post hoc test for ≥3 groups. Differences were considered significant at values of P < 0.05.
RESULTS
Miniagrin augments contractile force generation of engineered muscle tissue
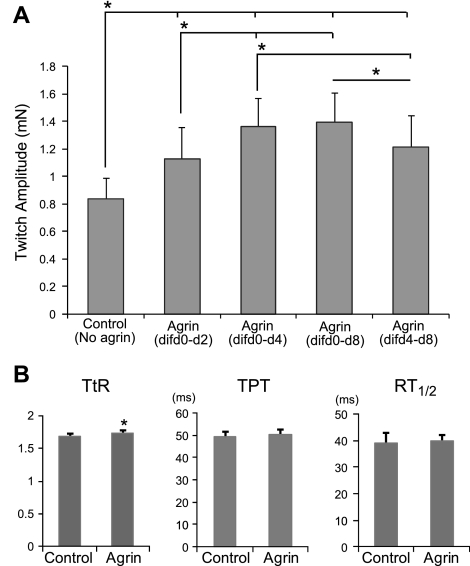
Engineered muscle constructs were exposed to 10 nM soluble miniagrin for 2, 4, or 8 d starting on d 0 of differentiation (d 4 of culture). A lower concentration of miniagrin (1 nM) was also tested, but no effect on the contractile force generation was observed. The average amplitude of twitch force generated by agrin-treated constructs was compared to the average among constructs with no agrin treatment (controls) for each cell isolation to obtain the agrin-induced fold changes. At d 10 of differentiation, the measured twitch force amplitudes At for 2-, 4-, and 8-d agrin exposure (1.13±0.23, 1.36±0.20, and 1.39±0.21 mN; 9 constructs from 3 cell isolations/group) were increased relative to controls (0.83±0.15 mN) by 1.4 ± 0.1-, 1.7 ± 0.2-, and 1.7 ± 0.2-fold, respectively (Fig. 1A; example force traces in Supplemental Fig. S2B). When the 4-d agrin exposure was started on d 4 of differentiation, instead of d 0, the resulting increase in force amplitude was reduced to 1.5 ± 0.2-fold (At=1.21±0.22 mN) relative to controls. These results showed that both the duration of exposure to miniagrin and its onset relative to the stage of myogenic differentiation affected the level of increase in contractile force amplitude of engineered muscle. On the basis of these results, all subsequent experiments were performed using 4-d agrin exposure starting on d 0 of differentiation.
Figure 1.
Effect of miniagrin on contractile force generation in engineered muscle constructs. A) Effect of miniagrin exposure duration and onset time on twitch force amplitude At in engineered muscle constructs. dif d0-d2, d 0–2 of differentiation. B) Effect of miniagrin on tetanus-to-twitch ratio TtR and twitch kinetics (TPT and RT1/2) in engineered muscle networks. Agrin (10 nM) was applied between differentiation d 0 and 4 of differentiation. n = 9 constructs/group, from 3 independent cell isolations. *P < 0.05.
In addition to the increase in twitch amplitude, the 4-d exposure of muscle networks to agrin starting on d 0 of differentiation yielded a slight, but statistically significant, increase in twitch-to-tetanus ratio, TtR, (1.74±0.04 vs. 1.68±0.05 in controls, n=9), but no significant change in either the TPT (50.4±2.3 vs. 49.3±2.4 ms in controls, n=9) or the RT1/2 (39.0±3.8 vs. 39.9±2.3 ms in controls, n=9) (Fig. 1B).
Miniagrin does not affect muscle myosin expression or total cell number
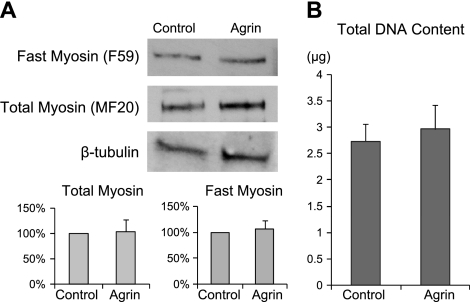
Previous studies using coculture of engineered muscle and primary nerve explants have shown a positive trend but no significant increase in the expression of adult myosin isoforms (4). Similarly, the application of recombinant agrin did not affect the expression level of muscle myosin in cultured mouse C2 myotubes (33). Consistent with these results, the application of miniagrin to engineered muscle construct in this study caused no detectable change in the expression level of either total sarcomeric myosin or the fast myosin isoform (n=9 from 3 cell isolations; Fig. 2A). Furthermore, the total DNA content in the agrin-treated and control constructs were comparable (n=9 from 3 isolations; Fig. 2B), suggesting that the exposure of engineered muscle networks to miniagrin had negligible effect on the cell number. Taken together, these results suggest that neither cellular up-regulation of major contractile proteins nor cellular hyperplasia was a likely contributor to the agrin-induced increase in contractile force.
Figure 2.
Effect of miniagrin on the expression of muscle myosin and total DNA content in engineered muscle constructs. A) Protein levels of fast myosin and total myosin were normalized in agrin-treated cells relative to control constructs in each cell isolation. β-Tubulin served as the loading control; n = 6 constructs/group, from 3 independent cell isolations. B) Total DNA content in agrin-treated and control constructs; n = 9 constructs/group, from 3 independent cell isolations. No significant difference was found between the groups.
Miniagrin does not affect sensitivity of contractile force to extracellular Ca2+ concentration or intracellular Ca2+ transient amplitude and shape in engineered muscle
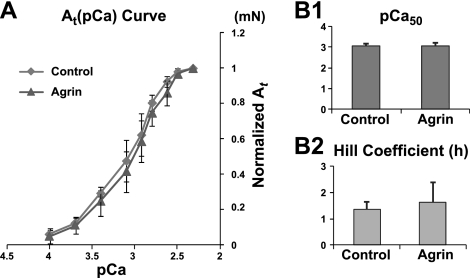
Recombinant neural agrin has been shown to promote the maturation of the E-C coupling apparatus in cultured human (but not mouse) myotubes by up-regulating functional ryanodine receptors and L-type Ca2+ channels (13). In this study with neonatal rat engineered muscle, treatment with miniagrin did not alter the sensitivity of generated contractile force to extracellular Ca2+ concentration (i.e., the shape of the twitch force amplitude-pCa curve, n=6; Fig. 3), suggesting that the observed increase in contractile force by agrin was not a result of altered E-C coupling or Ca2+-dependent regulation of acto-myosin interaction in myofilaments (34, 35). In addition, optical mapping of intracellular Ca2+ transients revealed no significant difference in the transient amplitude, upstroke time tup and decay times τ between the agrin-treated and control constructs (n=9; Supplemental Fig. S4), further indicating that the potential alterations in important aspects of intracellular Ca2+ handling did not significantly contribute to the agrin-induced increase in muscle force production.
Figure 3.
Effect of miniagrin on sensitivity of twitch force amplitude At to extracellular Ca2+ concentration ([Ca2+]) in muscle constructs. A) Average At (pCa) curves in untreated (control) and agrin-treated constructs. Forces at each [Ca2+] are normalized relative to the force at the highest tested [Ca2+]. B1, 2) pCa50 value (at normalized At=0.5; B1) and Hill coefficient h (B2) obtained from Hill equation (30) fits of normalized At (pCa) curves; n = 6 constructs/group, from 3 independent cell isolations.
Miniagrin up-regulates dystrophin gene expression in engineered muscle tissue
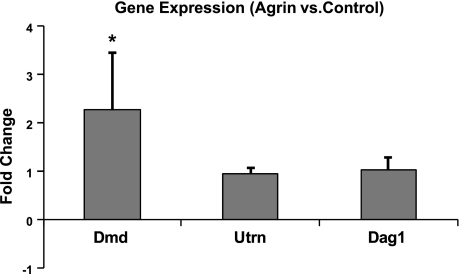
The miniagrin contains a binding domain for α-dystroglycan, one of the molecules of dystrophin-associated protein complex. By binding to α-dystroglycan, miniagrin may strengthen the linkage between the ECM and the myotube cytoskeleton, potentially enhancing the lateral force transmission and total force generation in engineered muscle constructs (11, 36). Therefore, the gene expression levels of dystrophin, utrophin (a structural homologue of dystrophin primarily located at NMJs), and α-/β-dystroglycan in agrin-treated and control muscle constructs were assessed on d 10 of differentiation (when force tests were also performed) using 1-step qRT-PCR. As shown in Fig. 4, agrin treatment resulted in 2.3 ± 1.2-fold increase in dystrophin gene expression and no significant changes in the expression of utrophin or α-/β-dystroglycan (n=10). Interestingly, the dystrophin expression was up-regulated, although miniagrin had been already removed from the culture medium for 6 d (after d 4 of differentiation).
Figure 4.
Effect of miniagrin on gene expression levels of dystrophin (Dmd), utrophin (Utrn), and dystroglycan (Dag1) in engineered muscle constructs. Gene expression levels are shown relative to the untreated controls; n = 10 constructs/group, from 5 independent cell isolations. *P < 0.05 vs. control.
Suppression of endogenous ACh level decreases force production in agrin-treated and untreated engineered muscle
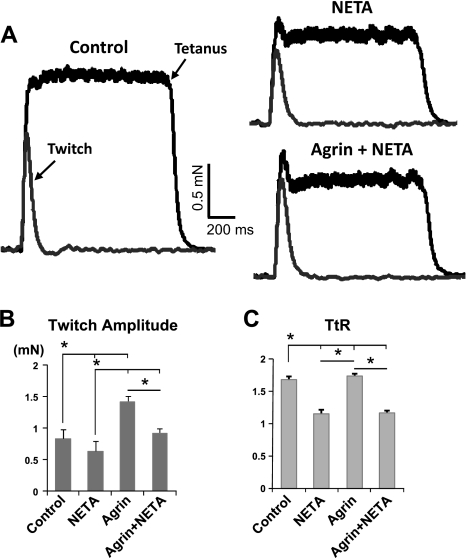
The autocrine stimulation of AChRs by endogenously produced ACh or ACh-lc has been previously suggested to underlie spontaneous Ca2+ spikes and twitching activity in cultured aneural myotubes and by sustaining muscle activity, possibly promoting myotube survival (21, 22). In this study, we further assessed whether altered levels of endogenous ACh can also affect force generation capacity of aneural engineered muscle and/or modulate the force-enhancing effects of agrin observed in this study. A specific ChAT inhibitor, α-NETA (20, 25, 37) was applied at a concentration of 50 μM during d 0–10 of differentiation to pharmacologically reduce levels of muscle endogenous ACh. We found that α-NETA completely abolished the spontaneous twitching (n=9; Supplemental Fig. S5) and significantly reduced twitch force amplitude in both control and agrin-treated muscle constructs (0.64±0.10 mN with α-NETA vs. 0.83±0.15 mN in controls, and 0.92± 0.08 mN with α-NETA and miniagrin vs. 1.41±0.16 mN with miniagrin, n=9/group; Fig. 5A, B). This finding revealed the importance of sustaining spontaneous twitching by autocrine ACh stimulation to contractile function of aneural muscle constructs. Furthermore, the α-NETA treatment did not alter the total DNA content of muscle constructs (n=9/group, Supplemental Fig. S6A), resulting in the preserved trends in the normalized force per microgram DNA (i.e., generated force per cell, n=9/group; Supplemental Fig. S6B). Moreover, α-NETA disproportionately reduced tetanus amplitude in both control and agrin-treated constructs (introducing an initial dip in the tetanus trace; Fig. 5A), and consequently, significantly decreased the tetanus-to-twitch ratio TtR (n=9/group; Fig. 5C).
Figure 5.
Effect of α-NETA on twitch and tetanus force in engineered muscle constructs without and with miniagrin treatment. NETA denotes α-NETA treatment, applied between d 0 and 10 of differentiation. A) Representative force traces of a single twitch and tetanus from the same isolation in control, α-NETA-treated, and α-NETA-treated constructs exposed to miniagrin. B, C) Twitch force amplitude At (B) and tetanus-to-twitch ratio TtR (C) are decreased by α-NETA in both control and agrin-treated constructs; n = 9 constructs/group, from 3 independent cell isolations. *P < 0.05.
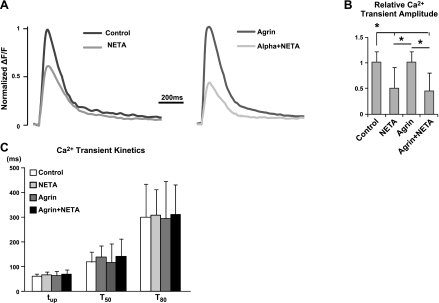
The facts that α-NETA significantly altered Ca2+-dependent parameters of muscle constructs (e.g., spontaneous twitching rate, TtR) while agrin had much less significant effect on these parameters or on Ca2+ handling, prompted us to record Ca2+ transients in α-NETA-treated constructs to determine whether the impaired contractile function by α-NETA was a result of altered Ca2+ signaling. We found that in both agrin-treated and control constructs, suppression of endogenous ACh levels with α-NETA reduced the amplitude of Ca2+ transients by ∼50%, without altering the transient kinetics (i.e., upstroke time tup and decay times at different recovery levels, τ50 and τ80; Fig. 6). The fact that miniagrin treatment did not exert any positive effect on the reduced amplitude of Ca2+ transient by α-NETA suggested independent mechanisms of action by agrin (dystrophin up-regulation) and endogenous ACh levels (E-C coupling) on the generation of contractile force in aneural engineered muscle tissue.
Figure 6.
Effect of α-NETA on intracellular Ca2+ transients in engineered muscle constructs without and with miniagrin treatment. A) Representative traces of mean Ca2+ transients from the same isolation showing relative effect of α-NETA treatment in control and agrin-exposed constructs. B) Relative changes of Ca2+ transient amplitude induced by α-NETA in control and agrin-treated constructs. C) Application of α-NETA caused no significant change of Ca2+ transient kinetics, including the upstroke time tup and decay time τ at 50 and 80% recovery. n = 12 constructs/group, from 6 independent cell isolations. *P < 0.05.
Suppression of endogenous ACh level enhances agrin-induced AChR clustering in engineered muscle
ACh has been shown to oppose agrin-induced AChR clustering in developing skeletal muscle (15). Specifically, while agrin-knockout mice exhibited aberrant NMJ development, NMJs differentiated extensively in mice with suppressed ACh synthesis due to ChAT deficiency. Moreover, ChAT deficiency partially rescued the abnormal NMJ formation in agrin-knockout mice. In addition to their noncanonic roles in force generation, understanding the interdependent effects of muscle endogenous ACh and agrin on AChR clustering is of particular interest to promote postsynaptic differentiation and integration of aneural engineered muscle constructs, which may improve their survival and contractile function under host neural control. Therefore, we immunostained with α-bungarotoxin (Fig. 7A) engineered muscle constructs (at d 10 of differentiation) to quantify AChR clustering in the aneural myotubes with and without miniagrin treatment during normal and α-NETA suppressed levels of endogenous ACh.
Figure 7.
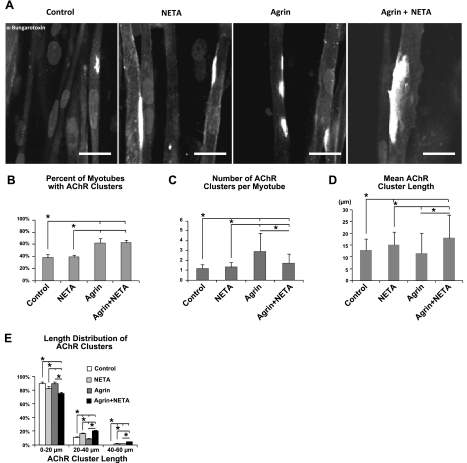
Effect of miniagrin and α-NETA on AChR clustering in engineered muscle constructs. A) Representative confocal images of α-bungarotoxin-labeled AChR clusters in tissue constructs under different treatments. Scale bars = 20 μm. B–E) Effect of treatments on the percentage of myotubes containing AChR clusters (B), the average number of AChR clusters per myotube (C), distribution of AChR cluster lengths (D), and mean length of AChR cluster (E). Note that the number of >40-μm clusters in control group is 0. n = 9 images/group, from 3 constructs/group (each from an independent cell isolation). *P < 0.05.
As shown in Fig. 7B, miniagrin treatment increased the percentage of myotubes with AChR clusters regardless of the endogenous ACh levels in the muscle constructs (62.2±4.5% in agrin-treated vs. 38.1±3.6% in control constructs, 62.8±3.6% in agrin/α-NETA-treated vs. 39.6±2.3% in α -NETA-treated constructs; a total of 48 myotubes in 3 different constructs analyzed per treatment group). Suppressing endogenous ACh levels by α-NETA decreased the number of AChR clusters per myotube (1.7±1.0 in agrin/α-NETA-treated vs. 2.9±1.8 in agrin-treated; Fig. 7C) but induced a significant increase in the average length of AChR clusters in agrin-treated constructs (17.9±9.8 μm in agrin/α-NETA-treated vs. 11.5±8.5 μm in agrin-treated constructs, Fig. 7D), suggesting the enhancing effect of α-NETA on agrin-induced AChR clustering activity. In contrast, α-NETA caused no change in the cluster number per myotube (1.3±0.5 in α-NETA-treated vs. 1.2±0.4 in controls; Fig. 7C) and slightly increased cluster length (15.1±5.5 μm in α-NETA-treated vs. 12.8±4.9 μm in controls; Fig. 7D) in untreated controls. The agrin/α-NETA-treated constructs had the highest fraction of medium and large AChR clusters (>40 μm; Fig. 7E). These results collectively show that miniagrin promoted AChR clustering activity in aneural myotubes and that suppressing muscle endogenous ACh facilitated the stabilization of larger AChR clusters, especially those induced by miniagrin.
We also examined the expression of specific AChR subunits since previous in vivo studies (38, 39) have suggested possible roles of agrin in postsynaptic differentiation through the up-regulation of AChR ε-subunit, which during the maturation of NMJs replaces the fetal γ-subunit. However, we found no significant change in the expression level of AChR α-, γ-, or ε-subunit genes in agrin-treated vs. control muscle constructs using quantitative RT-PCR analysis (AChR α-subunit: P=0.29; AChR γ-subunit: P=0.26; AChR ε-subunit: P=0.40, n=10; Supplemental Fig. S7).
DISCUSSION
One of the main challenges in engineering highly functional skeletal muscle is the lack of knowledge of what biochemical and biophysical factors are required to recapitulate normal muscle development in vitro. In this study, we hypothesized that neural agrin, one of the well-known synaptogenic factors involved in the early assembly of functional neuromuscular junctions, can directly affect the contractile function of aneural developing myotubes. On the basis of this hypothesis, we systematically investigated the potential of soluble recombinant miniagrin to improve the contractile function of tissue-engineered skeletal muscle in vitro. The main findings were that miniagrin treatment induced up to 1.7-fold increase in twitch and tetanus force amplitudes of engineered muscle constructs, similar to the force increase previously reported in engineered muscle constructs cocultured with primary nerve explants (4); the agrin-induced effect was dependent on both the exposure duration and onset time relative to the stage of myogenic differentiation; miniagrin treatment did not significantly change the total DNA content, the protein expression levels of muscle myosins, sensitivity of twitch force amplitude to extracellular Ca2+ concentration, Ca2+ transient amplitude and kinetics, or dystroglycan and utrophin gene expression, but induced a 2.3 ± 1.2-fold increase in dystrophin gene expression; and miniagrin also promoted the occurrence of the AChR clusters without significantly increasing their average size. In addition, in light of the suggested role of endogenous ACh or ACh-lc in sustaining muscle activity and promoting survival of aneural myotubes in previous studies (21, 22), we examined the effect of suppressed endogenous ACh synthesis on contractile force generation and postsynaptic differentiation in aneural muscle constructs with and without agrin treatment. Our results suggest that suppressed levels of endogenous ACh by α-NETA significantly reduced the twitch and even more so the tetanus force amplitude in both control and agrin-treated constructs; this effect was, at least in part, caused by the impaired Ca2+ handling, including the significantly reduced Ca2+ transient amplitude; and suppressed endogenous ACh levels facilitated the enhanced AChR clustering on muscle sarcolemma induced by agrin. These results warrant future studies on optimizing the effects of agrin and endogenous ACh on muscle contractile function and synaptogenesis to promote in vitro conditioning of aneural engineered muscle and facilitate its function and neural integration in vivo.
While neural agrin has been known to play critical roles in the formation and maturation of postsynaptic structures by triggering and stabilizing the aggregation of AChRs at the muscle sarcolemma (16, 26, 40), its specific effects on the muscle force generation have been difficult to examine in vivo (independent from other secreted factors) or in vitro using conventional 2D cultures grown on rigid substrate. We, therefore utilized in vitro engineered muscle tissues, as a well-controlled and more natural 3D cell culture environment to show for the first time that soluble recombinant miniagrin can directly augment contractile force of developing aneural rat primary myotubes. This effect was most pronounced when miniagrin was applied at initial stages of differentiation (d 0–4) and was at least partially attributable to measured dystrophin up-regulation on d 10 of differentiation, i.e., 6 d after removal of miniagrin from the culture medium (Fig. 4). This important finding suggests that a brief exposure to miniagrin during early myogenesis of aneural muscle cells may trigger downstream signaling events to up-regulate dystrophin expression in more mature myotubes, which, in turn, would enhance muscle force production by strengthening bonds between cells and extracellular matrix (11, 36) (without altering Ca2+-related mechanisms of force generation, Fig. 3 and Supplemental Fig. S4, or expression of muscle contractile proteins, Fig. 2). Interestingly, although Gramolini et al. (33) have previously shown that 1 nM agrin treatment of murine C2 myotubes cultured on 2D substrates starting on d 3 and 4 of differentiation up-regulated utrophin expression 2 d after the treatment, we found no change in utrophin gene expression at 6 d after applying 10 nM agrin on d 0–4 of differentiation. We also found no effect of 1 nM agrin on force production, likely because higher agrin doses were needed to overcome diffusion barriers within 3D muscle constructs. In addition to obvious differences in cell species, timing of agrin treatment, dose and type of recombinant agrin, and differences in 2D vs. 3D culture setting between the two studies, one potential explanation for this apparent discrepancy is that utrophin may be the developmental precursor of dystrophin (41, 42); therefore, its potential early up-regulation by agrin could directly or indirectly lead to the later up-regulation of dystrophin. Furthermore, the agrin-induced up-regulation of dystrophin in our muscle constructs did not significantly affect contraction kinetics, including TPT and RT1/2, but the previous findings in mdx mice showed that the loss of dystrophin caused slowing of contraction kinetics (43). This apparent discrepancy could result from differences in dystrophin expression in vitro and in vivo, different developmental stages of studied muscle fibers, the presence of innervation in vivo, and the potential differences in other factors influencing contraction kinetics (e.g., calcium homeostasis, ref. 44; ECM composition, etc.). For example, dystrophin gene expression in our study was up-regulated 2.3-fold, and although we have not assessed the corresponding change in the protein expression, it may be possible that altered twitch kinetics in wild-type vs. mdx mice requires significantly higher changes in dystrophin expression (45, 46).
On the other hand, our findings that agrin treatment did not alter force sensitivity to extracellular Ca2+ concentration, or Ca2+ transient amplitude and kinetics (Fig. 3 and Supplemental Fig. S4) in engineered muscle are in a good agreement with previous reports, showing no effect of agrin on maturation of E-C coupling and Ca2+ handling (e.g., up-regulation of functional ryanodine receptors and L-type Ca2+ channels) in murine myotubes (13). Notably, in contrast to results in rodent cells, neural agrin has been shown to affect maturation of the E-C coupling in human myotubes (13), which requires caution when extrapolating our findings on the functional effect of agrin on engineered neonatal rat muscle to human settings. Systematic comparison between engineered tissues made of rodent and human muscle cells will be needed in the future to clarify the species-related differences. To this end, an important aspect of our future work will be optimization of methods to engineer functional skeletal muscle tissues from human primary or pluripotent stem cell-derived myoblasts (47, 48).
In this study, we also found that the suppression of autocrine AChR stimulation by α-NETA efficiently abolished spontaneous twitching activity (Supplemental Fig. S5), a result that can be likely attributed to drug-induced change in membrane depolarization and generation of spontaneous Ca2+ spikes (21, 22). The α-NETA treatment also had detrimental effect on the contractile force generation in both control and agrin-treated engineered muscle constructs (Fig. 5), which was associated with the significant reduction of Ca2+ transient amplitude (Fig. 6). Our results thus suggest an important direct role of autocrine AChR stimulation in the maturation of muscle E-C machinery in developing aneural myotubes. Interestingly, for both normal and reduced levels of autocrine AChR stimulation, application of miniagrin significantly increased twitch (Fig. 5B) and tetanus amplitudes but had no effect on tetanus-to-twitch ratio (Fig. 5C). This was consistent with our finding that the increase in force generation by agrin was a result of up-regulated dystrophin expression (Fig. 4), with no induced alterations in intracellular Ca2+ handling (Figs. 3 and 6 and Supplemental Fig. S4). Taken together, agrin and endogenous ACh appear to exert their noncanonical influence on the contractile function of engineered rodent muscle constructs via distinct mechanisms involving dystrophin expression and intracellular Ca2+ handling, respectively.
The potential therapeutic effect of engineered muscle constructs will depend not only on their state of functional differentiation but also on their ability to successfully integrate into the host neuromuscular system. While the canonical actions of agrin and endogenous ACh levels on postsynaptic differentiation of aneural engineered muscle do not directly determine the muscle force output due to electrical stimulation, they are likely to provide trophic support for long-term spontaneous activity and differentiation of aneural engineered muscle tissue in vitro and promote the integration and contractile function of implanted muscle constructs in vivo. Specifically, our results showed that miniagrin treatment enhanced the formation of the AChR clusters in engineered muscle constructs, consistent with the previous findings in transgenic mice and cultured muscle cells (15, 49), while the decrease of endogenous ACh levels further augmented this effect (Fig. 7). Unlike the expected effect on the AChR clustering, soluble miniagrin in this study did not alter the expression of individual AChR subunits, in contrast to previous studies that showed agrin-induced switch of AChR γ-subunit to ε-subunit in denervated rat soleus muscle (38) and the up-regulation of AChR ε-subunit in engineered muscle constructs cocultured with nerve explants (5). This result may be due to the fact that soluble miniagrin lacks laminin-binding N-terminal fragment, which has been previously suggested as necessary for the induction of AChR ε-subunit expression (50). Incorporating full-length recombinant agrin into the hydrogel matrix may be a viable strategy to further promote the maturation of postsynaptic structures in myotube membrane and to potentially facilitate the innervation of tissue-engineered muscle after implantation.
In summary, through the use of 3D tissue culture and a combination of histological, biochemical, and functional assays, for the first time, we demonstrated that the transient application of soluble recombinant miniagrin can directly augment the contractile force generation in aneural engineered muscle tissues. This effect may be primarily or partially attributed to the up-regulation of dystrophin expression that likely enhanced lateral force transmission of muscle cells by strengthening their binding to extracellular matrix. Furthermore, we showed that reducing endogenous ACh levels can significantly decrease contractile force generation in aneural engineered muscle by impairing their intracellular Ca2+ handling, a mechanism apparently different from that of the agrin action. On the other hand, miniagrin and decreased endogenous ACh levels were found to synergize with respect to their stimulatory effect on AChR clustering in myotubes. Overall, our results suggest that further functionalization of recombinant miniagrin and/or use of a cocktail of synaptogenic and neurotrophic factors may serve to sustain or promote muscle viability and function in the absence of nerve-derived stimulation. This and similar strategies may be used in the future to augment function and integration of engineered muscle tissue grafts and promote contractile function of diseased or injured muscle.
Supplementary Material
Acknowledgments
The authors thank Dr. Robert Dennis (University of North Carolina, Chapel Hill, NC, USA) for providing the force transducer and Dr. Hyung-Suk Kim (University of North Carolina, Chapel Hill) for designing qRT-PCR gene expression assays. The authors also acknowledge invaluable technical assistance from Mark Juhas, Dr. Wayne Pfeiler, and Dr. Dennis Himmel.
This work was supported by Lew's fellowship from the Center for Biomolecular and Tissue Engineering at Duke University to W.B. and U.S. National Institutes of Health grant AR055226 from the National Institute of Arthritis and Musculoskeletal and Skin Disease to N.B.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. DiEdwardo C. A., Petrosko P., Acarturk T. O., DiMilla P. A., LaFramboise W. A., Johnson P. C. (1999) Muscle tissue engineering. Clin. Plast. Surg. 26, 647–656, ix–x [PubMed] [Google Scholar]
- 2. Midrio M. (2006) The denervated muscle: facts and hypotheses. A historical review. Eur. J. Appl. Physiol. 98, 1–21 [DOI] [PubMed] [Google Scholar]
- 3. Sanes J. R., Lichtman J. W. (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 [DOI] [PubMed] [Google Scholar]
- 4. Larkin L. M., Van der Meulen J. H., Dennis R. G., Kennedy J. B. (2006) Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev. Biol. Anim. 42, 75–82 [DOI] [PubMed] [Google Scholar]
- 5. Bach A. D., Beier J. P., Stark G. B. (2003) Expression of Trisk 51, agrin and nicotinic-acetycholine receptor epsilon-subunit during muscle development in a novel three-dimensional muscle-neuronal co-culture system. Cell Tissue Res. 314, 263–274 [DOI] [PubMed] [Google Scholar]
- 6. Nitkin R. M., Smith M. A., Magill C., Fallon J. R., Yao Y. M., Wallace B. G., McMahan U. J. (1987) Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol. 105, 2471–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glass D. J., Bowen D. C., Stitt T. N., Radziejewski C., Bruno J., Ryan T. E., Gies D. R., Shah S., Mattsson K., Burden S. J., DiStefano P. S., Valenzuela D. M., DeChiara T. M., Yancopoulos G. D. (1996) Agrin acts via a MuSK receptor complex. Cell 85, 513–523 [DOI] [PubMed] [Google Scholar]
- 8. Herbst R., Avetisova E., Burden S. J. (2002) Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development 129, 5449–5460 [DOI] [PubMed] [Google Scholar]
- 9. Jacobson C., Cote P. D., Rossi S. G., Rotundo R. L., Carbonetto S. (2001) The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J. Cell Biol. 152, 435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopf C., Hoch W. (1996) Agrin binding to alpha-dystroglycan. Domains of agrin necessary to induce acetylcholine receptor clustering are overlapping but not identical to the alpha-dystroglycan-binding region. J. Biol. Chem. 271, 5231–5236 [DOI] [PubMed] [Google Scholar]
- 11. Berthier C., Blaineau S. (1997) Supramolecular organization of the subsarcolemmal cytoskeleton of adult skeletal muscle fibers. A review. Biol. Cell 89, 413–434 [DOI] [PubMed] [Google Scholar]
- 12. Denzer A. J., Brandenberger R., Gesemann M., Chiquet M., Ruegg M. A. (1997) Agrin binds to the nerve-muscle basal lamina via laminin. J. Cell Biol. 137, 671–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bandi E., Jevsek M., Mars T., Jurdana M., Formaggio E., Sciancalepore M., Fumagalli G., Grubic Z., Ruzzier F., Lorenzon P. (2008) Neural agrin controls maturation of the excitation-contraction coupling mechanism in human myotubes developing in vitro. Am. J. Physiol. Cell Physiol. 294, C66–C73 [DOI] [PubMed] [Google Scholar]
- 14. Jurdana M., Fumagalli G., Grubic Z., Lorenzon P., Mars T., Sciancalepore M. (2009) Neural agrin changes the electrical properties of developing human skeletal muscle cells. Cell. Mol. Neurobiol. 29, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Misgeld T., Kummer T. T., Lichtman J. W., Sanes J. R. (2005) Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. U. S. A. 102, 11088–11093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bezakova G., Helm J. P., Francolini M., Lomo T. (2001) Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo. J. Cell Biol. 153, 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin S., Maj M., Bezakova G., Magyar J. P., Brenner H. R., Ruegg M. A. (2008) Muscle-wide secretion of a miniaturized form of neural agrin rescues focal neuromuscular innervation in agrin mutant mice. Proc. Natl. Acad. Sci. U. S. A. 105, 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campanelli J. T., Hoch W., Rupp F., Kreiner T., Scheller R. H. (1991) Agrin mediates cell contact-induced acetylcholine receptor clustering. Cell 67, 909–916 [DOI] [PubMed] [Google Scholar]
- 19. Brandon E. P., Lin W., D'Amour K. A., Pizzo D. P., Dominguez B., Sugiura Y., Thode S., Ko C. P., Thal L. J., Gage F. H., Lee K. F. (2003) Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J. Neurosci. 23, 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamann M., Chamoin M. C., Portalier P., Bernheim L., Baroffio A., Widmer H., Bader C. R., Ternaux J. P. (1995) Synthesis and release of an acetylcholine-like compound by human myoblasts and myotubes. J. Physiol. 489, 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bandi E., Bernareggi A., Grandolfo M., Mozzetta C., Augusti-Tocco G., Ruzzier F., Lorenzon P. (2005) Autocrine activation of nicotinic acetylcholine receptors contributes to Ca2+ spikes in mouse myotubes during myogenesis. J. Physiol. 568, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cognard C., Constantin B., Rivet-Bastide M., Imbert N., Besse C., Raymond G. (1993) Appearance and evolution of calcium currents and contraction during the early post-fusional stages of rat skeletal muscle cells developing in primary culture. Development 117, 1153–1161 [DOI] [PubMed] [Google Scholar]
- 23. Bian W., Bursac N. (2009) Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials 30, 1401–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bian W., Liau B., Badie N., Bursac N. (2009) Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat. Protoc. 4, 1522–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sastry B. V., Jaiswal N., Owens L. K., Janson V. E., Moore R. D. (1988) 2-(alpha-Naphthoyl)ethyltrimethylammonium iodide and its beta-isomer: new selective, stable and fluorescent inhibitors of choline acetyltransferase. J. Pharmacol. Exp. Ther. 245, 72–80 [PubMed] [Google Scholar]
- 26. McMahan U. J. (1990) The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 55, 407–418 [DOI] [PubMed] [Google Scholar]
- 27. Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 28. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 29. Hinds S., Bian W., Dennis R. G., Bursac N. (2011) The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32, 3575–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker J. S., Li X., Buttrick P. M. (2010) Analysing force-pCa curves. J. Muscle Res. Cell Motil. 31, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fedorov V. V., Lozinsky I. T., Sosunov E. A., Anyukhovsky E. P., Rosen M. R., Balke C. W., Efimov I. R. (2007) Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4, 619–626 [DOI] [PubMed] [Google Scholar]
- 32. Badie N., Bursac N. (2009) Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys. J. 96, 3873–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gramolini A. O., Burton E. A., Tinsley J. M., Ferns M. J., Cartaud A., Cartaud J., Davies K. E., Lunde J. A., Jasmin B. J. (1998) Muscle and neural isoforms of agrin increase utrophin expression in cultured myotubes via a transcriptional regulatory mechanism. J. Biol. Chem. 273, 736–743 [DOI] [PubMed] [Google Scholar]
- 34. Brandt P. W., Cox R. N., Kawai M. (1980) Can the binding of Ca2+ to two regulatory sites on troponin C determine the steep pCa/tension relationship of skeletal muscle? Proc. Natl. Acad. Sci. U. S. A. 77, 4717–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacIntosh B. R. (2003) Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol. Sci. 18, 222–225 [DOI] [PubMed] [Google Scholar]
- 36. Bloch R. J., Gonzalez-Serratos H. (2003) Lateral force transmission across costameres in skeletal muscle. Exerc. Sport Sci. Rev. 31, 73–78 [DOI] [PubMed] [Google Scholar]
- 37. Entwistle A., Zalin R. J., Warner A. E., Bevan S. (1988) A role for acetylcholine receptors in the fusion of chick myoblasts. J. Cell Biol. 106, 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rimer M., Mathiesen I., Lomo T., McMahan U. J. (1997) gamma-AChR/epsilon-AChR switch at agrin-induced postsynaptic-like apparatus in skeletal muscle. Mol. Cell. Neurosci. 9, 254–263 [DOI] [PubMed] [Google Scholar]
- 39. Schaeffer L., de Kerchove d'Exaerde A., Changeux J. P. (2001) Targeting transcription to the neuromuscular synapse. Neuron 31, 15–22 [DOI] [PubMed] [Google Scholar]
- 40. Gautam M., Noakes P. G., Moscoso L., Rupp F., Scheller R. H., Merlie J. P., Sanes J. R. (1996) Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535 [DOI] [PubMed] [Google Scholar]
- 41. Lin S., Burgunder J. M. (2000) Utrophin may be a precursor of dystrophin during skeletal muscle development. Brain Res. Dev. Brain Res. 119, 289–295 [DOI] [PubMed] [Google Scholar]
- 42. Radojevic V., Lin S., Burgunder J. M. (2000) Differential expression of dystrophin, utrophin, and dystrophin-associated proteins in human muscle culture. Cell Tissue Res. 300, 447–457 [DOI] [PubMed] [Google Scholar]
- 43. Deconinck N., Rafael J. A., Beckers-Bleukx G., Kahn D., Deconinck A. E., Davies K. E., Gillis J. M. (1998) Consequences of the combined deficiency in dystrophin and utrophin on the mechanical properties and myosin composition of some limb and respiratory muscles of the mouse. Neuromuscul. Disord. 8, 362–370 [DOI] [PubMed] [Google Scholar]
- 44. Mallouk N., Jacquemond V., Allard B. (2000) Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc. Natl. Acad. Sci. U. S. A. 97, 4950–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DelloRusso C., Scott J. M., Hartigan-O'Connor D., Salvatori G., Barjot C., Robinson A. S., Crawford R. W., Brooks S. V., Chamberlain J. S. (2002) Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc. Natl. Acad. Sci. U. S. A. 99, 12979–12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharp P. S., Bye-a-Jee H., Wells D. J. (2011) Physiological characterization of muscle strength with variable levels of dystrophin restoration in mdx mice following local antisense therapy. Mol. Ther. 19, 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barberi T., Bradbury M., Dincer Z., Panagiotakos G., Socci N. D., Studer L. (2007) Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med. 13, 642–648 [DOI] [PubMed] [Google Scholar]
- 48. Stavropoulos M. E., Mengarelli I., Barberi T. (2009) Differentiation of multipotent mesenchymal precursors and skeletal myoblasts from human embryonic stem cells. Curr. Protoc. Stem Cell Biol. Chap. 1, Unit 1F 8 [DOI] [PubMed] [Google Scholar]
- 49. Tourovskaia A., Li N., Folch A. (2008) Localized acetylcholine receptor clustering dynamics in response to microfluidic focal stimulation with agrin. Biophys. J. 95, 3009–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones G., Herczeg A., Ruegg M. A., Lichtsteiner M., Kroger S., Brenner H. R. (1996) Substrate-bound agrin induces expression of acetylcholine receptor epsilon-subunit gene in cultured mammalian muscle cells. Proc. Natl. Acad. Sci. U. S. A. 93, 5985–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.