Abstract
Here we demonstrate a new paradigm in redox signaling, whereby oxidants resulting from metabolic stress directly alter protein palmitoylation by oxidizing reactive cysteine thiolates. In mice fed a high-fat, high-sucrose diet and in cultured endothelial cells (ECs) treated with high palmitate and high glucose (HPHG), there was decreased HRas palmitoylation on Cys181/184 (61±24% decrease for cardiac tissue and 38±7.0% in ECs). This was due to oxidation of Cys181/184, detected using matrix-assisted laser desorption/ionization time of flight (MALDI TOF)-TOF. Decrease in HRas palmitoylation affected its compartmentalization and Ras binding domain binding activity, with a shift from plasma membrane tethering to Golgi localization. Loss of plasma membrane-bound HRas decreased growth factor-stimulated ERK phosphorylation (84±8.6% decrease) and increased apoptotic signaling (24±6.5-fold increase) after HPHG treatment that was prevented by overexpressing wild-type but not C181/184S HRas. The essential role of HRas in metabolic stress was made evident by the similar effects of expressing an inactive dominant negative N17-HRas or a MEK inhibitor. Furthermore, the relevance of thiol oxidation was demonstrated by overexpressing manganese superoxide dismutase, which improved HRas palmitoylation and ERK phosphorylation, while lessening apoptosis in HPHG treated ECs.—Burgoyne, J. R., Haeussler, D. J., Kumar, V., Ji, Y., Pimental, D. R., Zee, R. S., Costello, C. E., Lin, C., McComb, M. E., Cohen, R. A., Bachschmid, M. M. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis.
Keywords: growth factor signaling, high-fat high-glucose diet, oxidative post-translational modifications, reactive oxygen species, reactive nitrogen species
The stable tethering of HRas and NRas to the plasma membrane required for growth factor signaling is regulated by C-terminal palmitoylation. Several stages of post-translational processing are required for exocytic transport of Ras from the Golgi to the plasma membrane. The C-terminal CAAX motif that is conserved in all forms of Ras is sequentially farnesylated and proteolysed, and then undergoes carboxyl methylation (1). The addition of a farnesyl lipid moiety to the C-terminal cysteine is insufficient in providing a stable interaction of HRas or NRas with membranes (2). In addition, Ras must undergo acylation in order for it to be incorporated into lipid bilayers and transported by the Golgi secretory pathway to become stably anchored to the plasma membrane (3). The addition of a palmitoyl moiety to Ras in the form of palmitoyl-CoA is catalyzed by the recently identified palmitoyl acyltransferase DHHC9/GCP16 (4). This modification is reversible, as the thio-ester bond can be hydrolyzed by acyl-protein thioesterases located at the plasma membrane (5). The half-life for HRas acylation has been determined as 2.4 h (6), although a recent publication highlights that this process may actually be much more rapid (7). The acylation cycle allows dynamic regulation of Ras localization and function with its dysregulation following mutation of palmitoylated cysteines leading to aberrant cell signaling (8–10).
HRas plays a prominent role in regulating the growth, differentiation, and death of endothelial cells through its ability to potentiate growth factor signaling. In the vasculature, the binding of vascular endothelial growth factor (VEGF) to its corresponding receptor on endothelial cells initiates a downstream signaling cascade where HRas is an early mediator. When activated by GTP, HRas binds to the Ras binding domain (RBD) and stimulates the activity of c-Raf, which phosphorylates MEK1 and MEK2, which in turn phosphorylate ERK1 and ERK2 (11, 12). Activated ERK stimulates cell survival and proliferation by enhancing DNA transcription (13).
The palmitoylation of HRas occurs at 2 vicinal cysteines, designated Cys181 and Cys184. A prerequisite for cysteines that undergo palmitoylation is that they are in the reactive deprotonated thiolate form (14). In accordance with these cysteines being reactive, it has been previously reported that Cys181 and Cys184 in HRas can be oxidatively modified in vitro by oxidized glutathione or S-nitrosoglutathione and that Cys184 can be modified by 15-deoxy-Δ-12,14-prostaglandin J2, leading to increased HRas activity (15, 16). In addition, treatment of 3T3 cells with the transnitrosylating agent S-nitrosocysteine decreases the palmitoylation of HRas (17). Furthermore, oxidation of the vicinal cysteines by phenylarsine oxide prevents HRas plasma membrane localization (18), but whether this occurs as a result of physiological or pathological levels of oxidants is not yet known.
This study was undertaken to determine whether the oxidant increase caused by metabolic stress could alter the palmitoylation status of HRas. It was found that palmitoylation of HRas was decreased in cardiac tissue from mice fed a high-fat, high-sucrose (HFHS) diet or cultured endothelial cells treated to mimic metabolic stress. In accordance with these findings, there was a loss in HRas-dependent signaling and plasma membrane localization. Furthermore, the perturbation of the HRas acylation cycle appears to contribute to an observed increase in endothelial cell apoptosis mediated by metabolic stress and could be abrogated by manganese superoxide dismutase (MnSOD).
MATERIALS AND METHODS
Affinity capture of palmitoylated proteins
Bovine aortic endothelial cells (BAECs) were lysed into 100 mM Tris (pH 7.4) buffer containing 1% SDS and 100 mM maleimide, or hearts were homogenized to a 10% (w/v) homogenate in 100 mM Tris (pH 7.4) buffer containing 100 mM maleimide and then supplemented with 1% SDS. Samples were incubated at room temperature overnight to block free thiols. The maleimide was then removed from the soluble fraction after centrifugation using Zeba desalting spin columns (Pierce, Rockford, IL, USA). These samples were then supplemented with 400 mM hydroxylamine and 0.25 mM biotin-HPDP (Pierce) in the presence of 0.5% SDS and incubated for 1 h at room temperature. Reactions were terminated by a second desalting step using Zeba spin-desalting columns. Biotinylated proteins were purified by incubating samples for 3 h with streptavidin-agarose in the presence of 1% Triton-X100. The agarose beads were then rinsed 5 times with buffer (100 mM Tris, pH 7.4, and 1% Triton-X100) after being placed into empty spin columns. Proteins modified by biotin-HPDP were eluted from the streptavidin-agarose beads by the addition of 100 mM 2-mecaptoethanol.
Immunoblotting
The input before streptavidin-agarose capture and the proteins purified following the hydroxylamine-dependent biotin switch assay were resolved on SDS-PAGE gels, Western blotted, and then immunostained with either a Ras (05-516; Millipore, Bedford, MA, USA) or endothelial nitric oxide synthase (eNOS) antibody (610296; BD Transduction Laboratories, Franklin Lakes, NJ, USA). This allowed the amount of protein eluted to be normalized against the input to account for any variation in protein concentration that may be generated by the many desalting steps required for this protocol. Unprocessed cell lysates and heart homogenates were also immunostained with antibodies to phosphorylated (9101; Cell Signaling, Danvers, MA, USA) or total ERK (9102; Cell Signaling) and antibodies to GAPDH (2118; Cell Signaling), cleaved caspase-3 (9664; Cell Signaling), and MnSOD (SOD-111; Stressgen, San Diego, CA, USA).
Animal model
C57BL6 mice (0000664; Jackson Laboratories, Bar Harbor, ME, USA), obtained at 7 wk of age, were acclimatized for 1 wk on chow diet, and at 8 wk of age were fed either chow diet containing 6% fat (2018; Harlan Teklad, Madison, WI, USA) or HFHS diet containing 35.5% fat (58% of calories, primarily as lard) and 36.6% carbohydrate (primarily sucrose) without added cholesterol for 4 mo (F1850; Bioserve, Frenchtown, NJ, USA). This diet, the composition of which reflects a typical Western diet, induces insulin resistance and accelerated atherosclerosis (19). The investigation conforms to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 1996) and was approved by the Institutional Animal Care and Use Committee of Boston University Medical Center.
Cell treatments
Adenoviral vectors were generated using previously published protocols (20, 21). BAECs were infected with adenoviruses for 48 h before further treatment. Infected or noninfected cells were treated for 16 h with 5 mM glucose and 0.67% BSA (control), 25 mM glucose, and 0.67% BSA (high glucose), 25 mM glucose, 0.4 mM oleic acid, and 0.67% BSA (high oleate and glucose) or 25 mM glucose, 0.4 mM palmitic acid, and 0.67% BSA [high palmitate and high glucose (HPHG)]. This gives a molar ratio of 4 to 1 between the fatty acid and BSA. After treatment, cells were rinsed with PBS before being lysed into either Laemmli buffer or buffer for purification of palmitoylated proteins or fixed in paraformaldehyde for imaging. To attenuate MEK activity, the inhibitor U0126 was added at a concentration of 10μM to BAECs in serum-free medium.
Immunohistochemistry
BAECs for immunohistochemistry were grown to 80% confluence on 8-well chamber slides (Nunc, Rochester, NY, USA) before being infected with either Xpress-tagged wild-type HRas, Xpress-tagged C181/184S HRas, RBD-RFP [Addgene, Cambridge, MA, USA; plasmid 18664: mRFP-RBD(R59A)-mRFP] or MnSOD adenovirus. At 48 h after infection, cells were rinsed 3 times with PBS and then fixed with 4% fresh paraformaldehyde for 15 min. After incubation, cells were washed 3 times and then permeabilized in 2 mg/ml saponin for 10 min before being blocked in 5% BSA for 1 h. After another 3 washes, fixed cells were incubated overnight at 4°C with a combination of antibodies to either Xpress tag (R91025; Invitrogen, Carlsbad, CA, USA), RFP, or the Golgi protein syntaxin-6 (2869; Cell Signaling). The next day, cells were rinsed 3 times in PBS, then incubated with the secondary antibodies anti-mouse Alexa Fluor 568 and anti-rabbit Alexa Fluor 488 for 1 h at room temperature before being washed a further 3 times. Mounting medium containing DAPI stain was placed onto the surface of each slide, followed by a coverslip that was then sealed using nail polish. Slides were viewed using a Perkin Elmer spinning-disk confocal microscope (Perkin Elmer, Wellesley, MA, USA) to generate colocalization images for overexpressed Xpress-tagged HRas and syntaxin-6. A fluorescence microscope (Nikon Eclipse 80i; Nikon, Tokyo, Japan) was also used to image overexpressed HRas localization and DAPI nuclear staining in infected BAECs. Colocalization between expressed HRas and the Golgi marker syntaxin-6 was determined using the ImageJ plugin Intensity Correlation Analysis downloaded from the Wright Cell Imaging Facility (Toronto Western Research Institute, Toronto, ON, Canada; http://www.uhnresearch.ca/facilities/wcif/download.php).
Mass spectrometry (MS) analysis
Confluent BAECs infected for 48 h with a low titer of wild-type His-tagged HRas adenovirus were treated either with control cell culture medium or medium containing HPHG for 16 h. Cells were then lysed, and palmitoylated proteins were labeled using the hydroxylamine-dependent biotin-switch assay. Overexpressed HRas was purified from each sample using Ni-charged magnetic agarose beads (Qiagen, Valenica, CA, USA). Purified HRas was resolved by nonreducing SDS-PAGE and visualized using colloidal Coomassie stain. Bands corresponding to HRas were excised, diced into small pieces, and then subjected to trypsin (Promega, Madison, WI, USA) in-gel digestion using the ProteaseMAX (Promega)-based method for enhanced extraction of peptides. Extracted peptides were then desalted using C18 zip-tips (Millipore), and a small fraction was spotted along with α-cyano-4-hydroxycinnamic acid or 2,5-dihydroxybenzoic acid matrix onto a matrix-assisted laser desorption/ionization (MALDI) target plate. An ABI 4800 (Applied Biosystems, Foster, City, CA, USA) or Buker Daltonics (Billerica, MA, USA) MALDI time-of-flight (TOF)/TOF instrument was used to acquire MS or MS/MS data from the samples. Peptide mass fingerprinting, MS/MS search, and de novo sequencing were performed using mMass (Martin Strohalm, Prague, Czech Republic; http://www.mmass.org), flexAnalysis (Bruker Daltonics), Mascot (Matrix Science, Boston, MA, USA), and Data Explorer (Applied Biosystems). Mascot searches were performed with a mass error of 50 ppm against the SwissProt database version 57.15, (ftp://ftp.expasy.org/databases/uniprot/knowledgebase/uniprot_sprot.fasta.gz) including a maximum of 3 missed cleavages and as variable modifications methionine oxidation, biotin-HPDP, maleimide, glutathione, and 4-hydroxynonenal (HNE).
RESULTS
HFHS diet in mice and HPHG in BAECs decreases Ras and eNOS palmitoylation and attenuates growth factor signaling
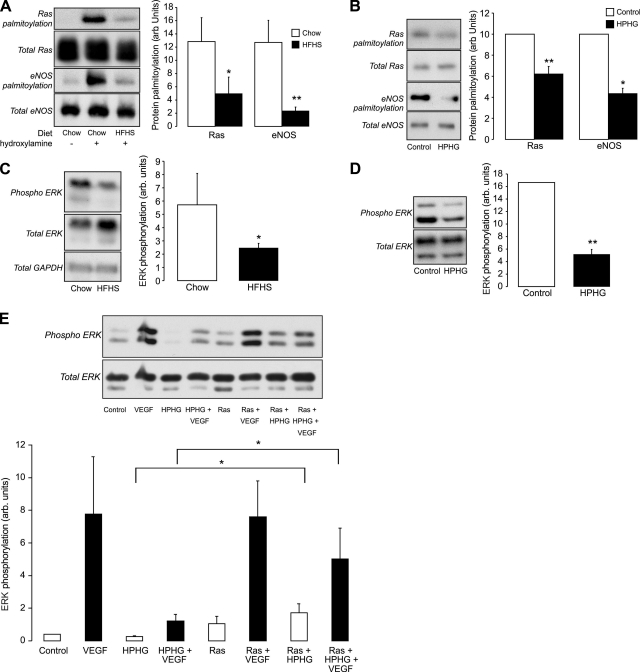
The hydroxylamine-dependent biotin-switch assay is specific for protein palmitoylation, as demonstrated by the fact that the Ras cysteines reduced by hydroxylamine were nearly eliminated in the Ras C181/184S mutant (Supplemental Fig. S1A, B). With the use of this assay, a loss in Ras and eNOS palmitoylation was detected in cardiac tissue from mice fed an HFHS diet for 4 mo compared with mice fed standard chow (Fig. 1A). The effect of diet in vivo was mimicked in BAECs treated with culture medium containing HPHG for 16 h (Fig. 1B). In cardiac tissue from HFHS-fed mice and in BAECs treated with HPHG, there was a loss in basal ERK phosphorylation (Fig. 1C, D). Furthermore, the ability of VEGF to increase ERK phosphorylation in BAECs was attenuated by treatment with HPHG (Fig. 1E). This effect was partially reversed by the overexpression of wild-type HRas, which restored basal and improved VEGF-induced ERK phosphorylation.
Figure 1.
Effect on protein palmitoylation and growth factor signaling in BAECs treated with HPHG and hearts of mice fed HFHS diet. A) Ras (total Ras antibody) and eNOS palmitoylation is lower in cardiac tissue from mice fed a HFHS diet for 4 mo compared with a chow diet, as detected using the hydroxylamine dependent biotin-switch assay (n=4). B) Ras (total Ras antibody) and eNOS palmitoylation is also decreased in BAECs treated with medium containing HPHG for 16 h compared with control medium (n=6). C) In animals fed an HFHS diet, there is a substantial loss in basal ERK phosphorylation compared with mice fed chow (n=4). D) In BAECs treated with HPHG, there is also decreased basal ERK phosphorylation compared with control medium-treated cells (n=6). E) Basal and VEGF-induced ERK phosphorylation that was attenuated by preincubation of BAECs with HPHG was prevented in cells that overexpressed wild-type HRas (n=6). All graphs have been normalized for protein loading. Error bars = se. *P < 0.05, **P < 0.01; Student's t test.
Treatment of BAECs with HPHG alters HRas localization, which is restored by overexpression of MnSOD
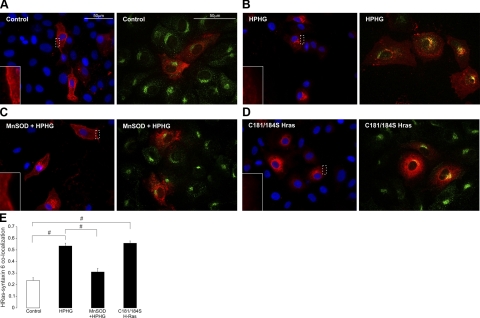
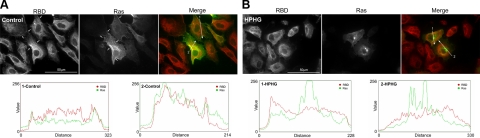
The detection of a low titer of overexpressed HRas in BAECs displayed homogenous cytosolic and plasma membrane staining with very little Golgi localization in control medium-treated cells (Fig. 2A). In contrast, cells that had been treated for 16 h with HPHG had a dramatic change in HRas localization, with loss in plasma membrane staining and intense colocalization with the Golgi marker syntaxin-6 (Fig. 2B). In BAECs in which MnSOD was overexpressed and treated with HPHG, HRas compartmentalization had improved plasma membrane staining and less localization at the Golgi similar to control (Fig. 2C). The palmitoyl cysteine mutant of Ras (C181/184S) displayed no plasma membrane localization and instead localized around the outside of the nucleus and to a large extent at the Golgi (Fig. 2D). In BAECs supplemented with control medium and treated for 5 min with VEGF, HRas activity was detected at the plasma membrane, indicated by colocalization of this GTPase with RBD (Fig. 3A). In contrast, in BAECs treated with HPHG and stimulated with VEGF, there was less HRas activity at the membrane compared with controls, and accumulated HRas at the Golgi failed to interact with RBD (Fig. 3B).
Figure 2.
Localization of HRas (Xpress-tag antibody, shown in red) in control medium or HPHG-treated BAECs. Left panels: fluorescence microscopy images of overexpressed HRas localization with DAPI nuclear staining in BAECs, including a magnified region of HRas plasma membrane staining. Right panels: confocal images showing colocalization of overexpressed HRas (Xpress-tag antibody, red) and the Golgi marker syntaxin-6 (green) in BAECs. A) In control medium, endothelial cell HRas is homogenously distributed throughout much of the cell and is also found at the plasma membrane. B) In BAECs treated with HPHG, HRas displays very little localization at the plasma membrane and instead is mostly found at the Golgi, which is evident from a high level of colocalization with syntaxin-6. C) Overexpression of MnSOD in BAECs before HPHG treatment prevents, to some extent, the redistribution of HRas by improving plasma membrane and cytosolic localization while decreasing that which is located at the Golgi. D) Overexpressed C181/184S HRas displays very little plasma membrane staining and instead colocalizes to a high extent with syntaxin-6 and also localizes around the outside of the nucleus. E) Quantification of the colocalization of HRas with the Golgi marker syntaxin-6 shows increased colocalization in BAECs treated with HPHG that is reversed by overexpression of MnSOD. Colocalization ranges between −1 for perfect exclusion and 1 for maximum correlation (n=20–25). Error bars = se. #P < 0.005; Student's t test.
Figure 3.
Intracellular activity of HRas in treated BAECs. This was determined by analyzing the colocalization of RBD with HRas overexpressed (Xpress-tag antibody) in BAECs that had been stimulated for 5 min with VEGF before being fixed. A) In control medium-treated cells, there is some colocalization of HRas with RBD at the plasma membrane (indicated by the white arrows), which is also apparent in the fluorescence intensity scans generated along the white lines indicated for each analyzed cell. B) In HPHG-treated BAECs, there is less membrane colocalization and also no increase in RBD staining with the accumulated HRas at the Golgi as indicated in the intensity scan for each analyzed cell.
MS analysis of HRas shows increased S-glutathiolation of terminal cysteines following treatment of BAECs with HPHG
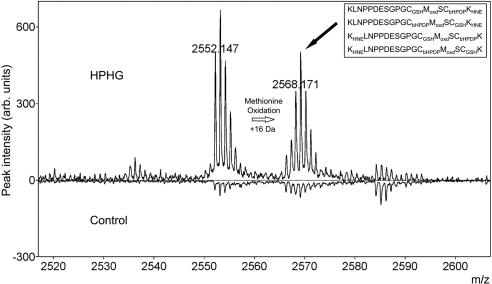
Overexpressed His-tagged HRas purified from BAECs treated with control or HPHG medium was processed using the hydroxylamine-dependent biotin-switch assay to label palmitoylated cysteine residues with biotin-HPDP. After the protein was trypsinized, fragments were analyzed by MALDI MS to look for differences in peak intensities between the two experimental groups. Two peaks of interest were identified in HRas purified from BAECs treated with HPHG (Fig. 4 and Supplemental Fig. S2). These peaks were then further analyzed using MALDI TOF-TOF for analysis of sequence and post-translational modifications (Supplemental Fig. S3A, B). This led to the discovery that HRas from HPHG-treated BAECs was S-glutathiolated at either Cys181 or Cys184 with the other cysteine labeled with biotin-HPDP as evidence that it had been palmitoylated. In addition, Lys168 or 185 was modified by HNE, and in some of the HRas there was oxidation of Met182, which supports the presence of an oxidizing environment in BAECs treated with HPHG.
Figure 4.
MS analysis of HRas from BAECs treated with control or HPHG medium. Comparison between the MALDI TOF spectra for overexpressed HRas purified from the cell lysate of BAECs treated with either control or HPHG medium after having been processed by the hydroxylamine dependent biotin-switch assay. In the spectrum for HRas from HPHG-treated cells, there were 2 peaks of interest that were more abundant than in the control group that had masses which equated to HNE, methionine oxidized and S-glutathiolated C-terminal fragments of HRas. MALDI TOF/TOF spectra of these peaks (Supplemental Fig. S2) revealed a complex mixture of oxidized HRas where either Cys181 or 184 was S-glutathiolated, with the other cysteine being palmitoylated (biotin-HPDP). In addition, Lys168 or 185 was modified by HNE, and in the right hand peaks, Met182 was also oxidized. Inset: various modifications of the C-terminal peptide identified using MALDI TOF/TOF in the highlighted peak (solid black arrow) of HRas from BAECs treated with HPHG.
Treatment of BAECs with HPHG induces apoptotic signaling that is modulated by HRas
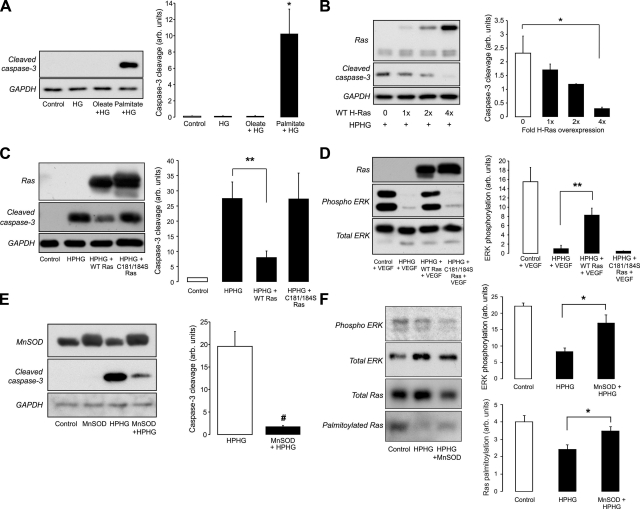
Treatment of BAECs with high glucose alone or high glucose with the unsaturated fatty acid oleate did not induce caspase-3 cleavage (Fig. 5A). However, treatment for the same period of time with high glucose and the saturated fatty acid palmitate did significantly enhance caspase-3 cleavage. The increase in this marker of apoptotic signaling could be attenuated in a titer-dependent manner by adenoviral overexpression of wild-type HRas in BAECs before treatment with HPHG (Fig. 5B). However, it is likely that to induce protection from HPHG, the overexpressed HRas needs to be palmitoylated, because overexpression of the C181/184S form of HRas failed to prevent caspase-3 cleavage (Fig. 5C). This supposition is supported by the fact that overexpression of C181/184S, unlike the wild-type form, did not restore VEGF-dependent ERK phosphorylation (Fig. 5D). The role for oxidants in mediating the apoptotic signaling induced by HPHG was demonstrated by the protective effect of the overexpression of MnSOD (Fig. 5E). In addition, overexpression of MnSOD improved HRas palmitoylation and basal ERK phosphorylation in BAECs treated with HPHG (Fig. 5F). The formation of oxidants by the mitochondria was detected in BAECs using MitoTracker Red CM-H2XRos, which had increased fluorescence in cells treated with HPHG (Supplemental Fig. S4).
Figure 5.
Effect of HPHG on caspase-3 cleavage in treated BAECs. A) Treatment of BAECs with HG or HG with 0.4 mM of the unsaturated fatty acid oleate did not induce caspase-3 cleavage. However, treatment of BAECs with HF and 0.4 mM palmitate increased accumulation of this apoptotic signaling marker (n=3). B) Wild-type HRas overexpression (shown using total Ras antibody) prevented caspase-3 cleavage in BAECS treated with HPHG in a titer-dependent manner (n=3). C) Although the wild-type HRas prevented caspase-3 cleavage, the C181/184S was unable to protect cells from the apoptotic effect of HPHG (n=6). D) In BAECs, VEGF-dependent ERK phosphorylation was inhibited by HPHG, an effect that was prevented by overexpressing wild-type HRas but not the C181/184S mutant (n=3). E) Overexpression of the mitochondrial form of SOD, MnSOD, prevented caspase-3 cleavage in BAECs treated with HPHG (n=3). F) In BAECs overexpressing MnSOD and treated with HPHG, there is increased basal ERK phosphorylation and HRas palmitoylation compared with HPHG treatment alone (variations in amount of input protein is due to protein loss during multiple sample desalting steps during the biotin switch assay, n=3–4). All graphs have been normalized for protein loading. Error bars = se. *P < 0.05, **P < 0.01, #P < 0.005; Student's t test.
Effects of HPHG are mimicked by inhibiting MEK or expressing an inactive dominant negative HRas (N17HRas) mutant
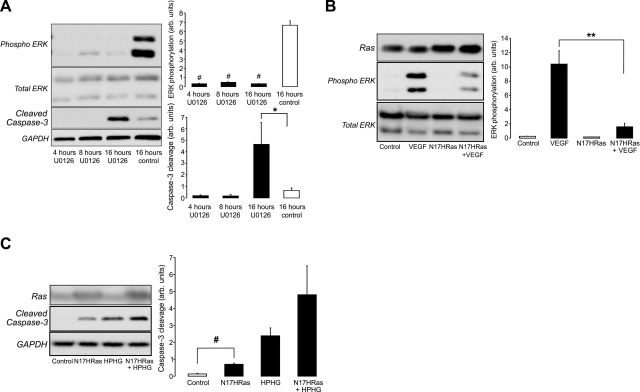
The inhibition of MEK by U0126 rapidly attenuated basal ERK phosphorylation and increased caspase-3 cleavage after 16 h (Fig. 6A). The effect of HPHG on HRas function also was mimicked by expressing an inactive dominant negative form of HRas (N17HRas), which is known to inhibit endogenous GTPase activity at the plasma membrane. The overexpression of N17HRas attenuated VEGF-dependent phosphorylation of ERK (Fig. 6B). In addition, BAECs expressing N17HRas had increased caspase-3 cleavage and were not protected from the apoptotic effects of HPHG (Fig. 6C). This further supports the hypothesis that HRas normally prevents apoptotic signaling induced by HPHG by Ras GTPase activity and plasma membrane signaling, which is disrupted by the relocalization of HRas to the Golgi by oxidation and prevention of palmitoylation of cysteines 181/184.
Figure 6.
Effect of ERK inhibition and blocking HRas plasma membrane signaling in BAECs on growth factor and apoptotic signaling. A) Inhibition of MEK by U0126 attenuated basal ERK phosphorylation and increased caspase-3 cleavage 16 h after treatment. B) Overexpression of inactive dominant negative N17-HRas (total Ras antibody) in BAECs attenuated VEGF-dependent ERK phosphorylation (n=3). C) Overexpression of N17-HRas also increased cell apoptotic signaling in control medium-treated BAECs and did not protect cells from the apoptotic effect of HPHG (n=3). All graphs have been normalized for protein loading. Error bars = se. *P < 0.05, **P < 0.01, #P < 0.005; Student's t test.
DISCUSSION
Oxidation of reactive cysteine thiolates has the propensity to alter protein function by adding a distinct shape and charge characteristic (22). However, whether such modifications can affect protein palmitoylation is unclear, especially in vivo. Because we found such profound suppression of growth factor-mediated ERK signaling, our aim here was to establish whether the oxidizing environment generated by metabolic stress might alter HRas palmitoylation and therefore HRas-mediated plasma membrane signaling. The two C-terminal palmitoylation sites are cycled physiologically, allowing HRas to transit from the Golgi to the plasma membrane (2). We first noted suppression of HRas palmitoylation and ERK signaling in an in vivo model of metabolic disease and accelerated vascular disease (19). To mirror the in vivo metabolic effects in these animals, we used a cell culture system with BAECs that were treated with HPHG. Our findings are likely to be broadly applicable because HPHG treatment of cultured cells has frequently been used to simulate metabolic stress in vascular endothelial and smooth muscle cells (23), cardiac myocytes (24), pancreatic β cells (25, 26), and retinal pericytes (27) and is also known to induce oxidant formation and apoptosis in some.
Together with the substantial decrease in HRas palmitoylation observed in our in vivo animal and cellular model of metabolic stress, we observed a concomitant decrease in palmitoylation of eNOS, suggesting that both in vivo and in vitro there is a widespread and important decrease in this protein modification. It has been previously reported that palmitoylation of eNOS is required for optimal stimulated release of nitric oxide (28), which may have an important effect on cardiac function in HFHS-fed mice. This loss in eNOS palmitoylation would certainly warrant an independent study to investigate the pathophysiological effects that this may have. However, our focus was to ascertain whether loss in protein palmitoylation during metabolic stress was due to oxidant modifications of the reactive thiols that normally are palmitoylated on HRas. This was demonstrated with MALDI-TOF-TOF MS on His-tagged HRas purified from infected BAECs treated with or without HPHG. These results highlighted that treatment of BAECs with HPHG increased the S-glutathiolation of HRas at either Cys181 or Cys184, with the other site being palmitoylated. These findings highlight that HRas must be processed before it gets oxidized, as one cysteine is palmitoylated, with the other being oxidized. S-glutathiolation is a reversible thiol modification that is relatively stable but provides for the possibility that oxidation of Ras, and therefore Ras localization could be additionally regulated. Studies using individual cysteine mutants have demonstrated that loss in palmitoylation at either site causes dysfunctional HRas localization (9, 10). Generally, mutation at either site leads to HRas being predominantly located at the Golgi, with no plasma membrane staining for the C181S form and only a small amount of plasma membrane staining for C184S HRas, which is enhanced when protein synthesis is inhibited by cycloheximide (9, 10). In cells treated with HPHG, there is a mixed population of monopalmitoylated HRas, with it being palmitoylated at either Cys181 or Cys184, with both forms likely being unable to signal via Raf at the Golgi, as there was no colocalization with RBD shown by confocal imaging. This is supported by a previous report showing that mutation at either palmitoylation site leads to a loss in HRas activity at the Golgi (10). However, it has also been reported that there is still some activity of monopalmitylated C181 at the plasma membrane (9, 10). Therefore, it may be predominantly the loss in palmitoylation at C181 due to thiol oxidation that is responsible for the attenuation in HRas signaling seen in our studies of HPHG treated BAECs.
Our findings were further substantiated by showing, with confocal and fluorescence imaging, that treatment with HPHG caused marked relocalization of HRas, which was no longer found at the plasma membrane but instead in the Golgi. This pattern of localization was similar to that of the C181/184S mutant of HRas, demonstrating the magnitude of the terminal cysteine oxidation induced by metabolic stress. The fact that the C181/184S mutant HRas was more diffuse than the HRas in BAECs treated with HPHG might be explained by the single-site palmitoylation of the protein being capable of anchoring the protein to the Golgi membrane but being insufficient for exocytic transport to the plasma membrane.
Likely due to its relocalization away from the plasma membrane, the loss in HRas palmitoylation and displacement from the plasma membrane had a confounding effect on growth factor signaling. The basal phosphorylation of the HRas downstream target ERK was substantially lower in hearts of mice with metabolic stress, and basal and VEGF-induced phosphorylation of ERK was abrogated in endothelial cells treated with HPHG. The overexpression of wild-type HRas restored basal and VEGF signaling by likely augmenting the total amount of HRas that remained at the plasma membrane despite HPHG treatment. The fact that C181/184S HRas was unable to restore impaired ERK phosphorylation in metabolically stressed cells further substantiates the importance of altered HRas palmitoylation and plasma membrane localization and signaling.
In BAECs treated with HPHG, there was increased cell apoptosis that was not a detergent-like effect or caused by micelle formation, as the fatty acid oleate did not induce cell death (27, 29). Here we attribute this increase in apoptotic cell signaling to the loss in HRas plasma membrane localization due to prevention of normal protein palmitoylation by cysteine oxidation. This was demonstrated by the overexpression of wild-type HRas, which, presumably by providing additional HRas that was less oxidized, restored growth factor signaling and prevented the increase in the accumulation of the apoptotic cell marker caspase-3 cleavage in BAECs treated with HPHG. The C181/184S mutant was unable to protect cells from the apoptotic effects of HPHG, demonstrating the significance of HRas palmitoylation and plasma membrane anchoring in maintaining cell viability. Assessment for the role of basal ERK phosphorylation in maintaining endothelial cell viability using a MEK inhibitor demonstrated that MEK/ERK activity under basal conditions is required to prevent cell apoptosis. In addition to ERK, other targets of HRas may also play a role in maintaining cell viability and may include the PI3K and AKT. Further evidence for the importance of HRas signaling at the plasma membrane in preventing programmed cell death was demonstrated by overexpressing a dominant negative form of HRas (N17-HRas), which blocked VEGF-induced ERK phosphorylation and also increased caspase-3 cleavage. This makes it likely that the loss in HRas-mediated growth factor signaling at the plasma membrane following HPHG treatment of endothelial cells explains the switch from a survival to a proapoptotic phenotype. This is supported by several studies (30–32) that show that ERK activation is antiapoptotic and that its specific inhibition can increase programmed cell death. The treatment of BAECs with HPHG for 16 h to induce a loss in HRas palmitoylation and apoptotic signaling likely reflects the time required to accumulate oxidized monolipidated HRas at the Golgi, thus depleting the dipalmitoylated HRas from the plasma membrane. This likely defines a temporal relationship controlled by the extent and period of oxidant formation coupled with the rate of depalmitoylation and cycling of HRas between the plasma membrane and the Golgi.
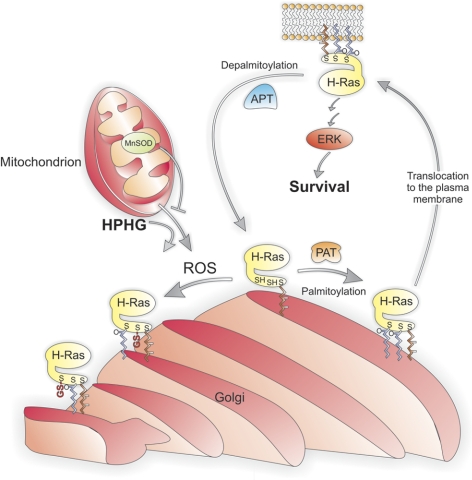
The significance of oxidant formation in the cellular dysfunction generated by HPHG was evident in cells overexpressing MnSOD, which had less caspase-3 cleavage. This demonstrates not only that oxidants generated in this system are detrimental but, because MnSOD is located in the mitochondria, that these organelles are the likely source of oxidants. Furthermore, MnSOD overexpression restored, to some extent, the localization of HRas in HPHG-treated BAECs by improving plasma membrane localization and decreasing the accumulation at the Golgi. In addition, the overexpression of MnSOD improved HRas palmitoylation and basal ERK phosphorylation in BAECs treated with HPHG, providing an explanation for its ability to prevent apoptosis. Therefore, in this model of metabolic stress, enhanced formation of superoxide by the mitochondria is responsible for HRas oxidation. However, due to the relative stability and rate of chemical reactivity, it is highly unlikely that this oxidant directly modifies the cysteines on HRas. Instead, superoxide likely acts as an intermediate by being converted to peroxynitrite or hydrogen peroxide after diffusing out of the mitochondria. These oxidants have the propensity to modify reactive thiols, such as Cys181 or Cys184, on HRas. The findings of this study are summarized in Fig. 7.
Figure 7.
Proposed model for the regulation of HRas palmitoylation under physiological conditions and in the presence of HPHG. HRas that has been processed by being sequentially farnesylated, proteolysed, and carboxyl methylated is palmitoylated at Cys181 and Cys184 at the Golgi membrane by the palmitoyl acyltransferase (PAT) under physiological conditions. Palmitoylation of HRas at both sites induces exocytic translocation of this GTPase from the Golgi to the plasma membrane, where it mediates growth factor signaling, including regulating the ERK pathway that is involved in endothelial cell survival. At the plasma membrane, HRas is depalmitoylated by the acyl protein thioesterase (APT), which causes the GTPase to return to the Golgi, where it can then be repalmitoylated and begin the transport cycle again. This cycle is disrupted by reactive oxygen species (ROS) generated by mitochondria, which are elevated by HPHG and can be attenuated by increased MnSOD activity. The increased ROS from the mitochondria in endothelial cells treated with HPHG oxidize either Cys181 or Cys184 on HRas, leading to S-glutathiolation on either cysteine, preventing palmitoylation at these sites. This results in the formation of monopalmitoylated HRas, which becomes trapped at the Golgi, as single-site palmitoylation is insufficient to mediate efficient exocytic transport to the plasma membrane.
We have previously reported that the activity of HRas can be modulated through oxidation of C118, with S-glutathiolation leading to increased catalytic activity (33, 34). In this study, we found no evidence of C118 oxidation of HRas when analyzing MS data, although, because of technical shortcomings of MS related to the small tryptic peptide involved, we cannot exclude it entirely. In any case, we found that accumulated HRas at the Golgi was inactive; therefore, based on this finding, the oxidation of C118 is unlikely to play a role in mediating endothelial cell death in this model of metabolic stress. Therefore, while C118 plays an important role in acute physiological redox signaling at the plasma membrane, under pathological conditions in the presence of high levels of oxidants, the oxidation of Cys181 or Cys184 renders HRas inactive by trapping it at the Golgi, preventing physiological signaling through Raf.
In summary, a novel paradigm in redox signaling is revealed here, whereby oxidants generated in vascular endothelial cells treated with HPHG prevent HRas palmitoylation by enhancing oxidative S-glutathione adduct formation on C-terminal cysteines 181 and 184. This loss in HRas acylation prevents its transport to the plasma membrane, thus decreasing growth factor signaling that is required for maintaining cell homeostasis. This highlights a pathophysiological mechanism of cellular dysfunction caused by metabolic stress, whereby oxidants disrupt normal HRas palmitoylation and plasma membrane signaling. This underscores the clinical relevance of our study by providing a potential molecular mechanism to explain impaired angiogenesis and growth factor resistance in patients with vascular disease associated with metabolic syndrome, as observed in the VIVA, FIRST, and AGENT clinical trials (35–37). In addition, these findings draw attention to the potential widespread regulation of protein palmitoylation by metabolic disease via increased protein thiol oxidation. We demonstrate here a pathophysiological mechanism involving protein palmitoylation that can be regulated by cellular antioxidant systems that include MnSOD, providing a molecular mechanism for therapeutic intervention.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grants PO1-HL-068758 and R37-HL-04017 and NIH predoctoral training grant HL-007969-06A1, as well as by the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services, under contract HHSN268201000031C. This work was also supported by a Sir Henry Wellcome postdoctoral fellowship from The Wellcome Trust (sponsor reference 085483/Z/08/Z; to J.R.B.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the awarding offices.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Wright L. P., Philips M. R. (2006) Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 47, 883–891 [DOI] [PubMed] [Google Scholar]
- 2. Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P. I. (2005) An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307, 1746–1752 [DOI] [PubMed] [Google Scholar]
- 3. Hancock J. F., Paterson H., Marshall C. J. (1990) A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63, 133–139 [DOI] [PubMed] [Google Scholar]
- 4. Swarthout J. T., Lobo S., Farh L., Croke M. R., Greentree W. K., Deschenes R. J., Linder M. E. (2005) DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280, 31141–31148 [DOI] [PubMed] [Google Scholar]
- 5. Duncan J. A., Gilman A. G. (1998) A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J. Biol. Chem. 273, 15830–15837 [DOI] [PubMed] [Google Scholar]
- 6. Baker T. L., Zheng H., Walker J., Coloff J. L., Buss J. E. (2003) Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic H-ras. J. Biol. Chem. 278, 19292–19300 [DOI] [PubMed] [Google Scholar]
- 7. Ahearn I. M., Tsai F. D., Court H., Zhou M., Jennings B. C., Ahmed M., Fehrenbacher N., Linder M. E., Philips M. R. (2011) FKBP12 binds to acylated H-ras and promotes depalmitoylation. Mol. Cell 41, 173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cadwallader K. A., Paterson H., Macdonald S. G., Hancock J. F. (1994) N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol. Cell. Biol. 14, 4722–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roy S., Plowman S., Rotblat B., Prior I. A., Muncke C., Grainger S., Parton R. G., Henis Y. I., Kloog Y., Hancock J. F. (2005) Individual palmitoyl residues serve distinct roles in H-ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25, 6722–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Misaki R., Morimatsu M., Uemura T., Waguri S., Miyoshi E., Taniguchi N., Matsuda M., Taguchi T. (2010) Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis. J. Cell Biol. 191, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wellbrock C., Karasarides M., Marais R. (2004) The RAF proteins take centre stage. Nat. Rev. Mol. Cell. Biol. 5, 875–885 [DOI] [PubMed] [Google Scholar]
- 12. Chong H., Vikis H. G., Guan K. L. (2003) Mechanisms of regulating the Raf kinase family. Cell. Signal. 15, 463–469 [DOI] [PubMed] [Google Scholar]
- 13. Ballif B. A., Blenis J. (2001) Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 12, 397–408 [PubMed] [Google Scholar]
- 14. Dietrich L. E., Ungermann C. (2004) On the mechanism of protein palmitoylation. EMBO Rep. 5, 1053–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mallis R. J., Buss J. E., Thomas J. A. (2001) Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem. J. 355, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oliva J. L., Perez-Sala D., Castrillo A., Martinez N., Canada F. J., Bosca L., Rojas J. M. (2003) The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J2 binds to and activates H-Ras. Proc. Natl. Acad. Sci. U. S. A. 100, 4772–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker T. L., Booden M. A., Buss J. E. (2000) S-Nitrosocysteine increases palmitate turnover on Ha-Ras in NIH 3T3 cells. J. Biol. Chem. 275, 22037–22047 [DOI] [PubMed] [Google Scholar]
- 18. Oeste C. L., Diez-Dacal B., Bray F., Garcia de Lacoba M., de la Torre B. G., Andreu D., Ruiz-Sanchez A. J., Perez-Inestrosa E., Garcia-Dominguez C. A., Rojas J. M., Perez-Sala D. (2011) The C-terminus of H-Ras as a target for the covalent binding of reactive compounds modulating Ras-dependent pathways. PLoS One 6, e15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schreyer S. A., Lystig T. C., Vick C. M., LeBoeuf R. C. (2003) Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis 171, 49–55 [DOI] [PubMed] [Google Scholar]
- 20. Zee R. S., Yoo C. B., Pimentel D. R., Perlman D. H., Burgoyne J. R., Hou X., McComb M. E., Costello C. E., Cohen R. A., Bachschmid M. M. (2010) Redox regulation of sirtuin-1 by S-glutathiolation. Antioxid. Redox Signal. 13, 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haeussler D. J., Evangelista A. M., Burgoyne J. R., Cohen R. A., Bachschmid M. M., Pimental D. R. (2011) Checkpoints in adenoviral production: cross-contamination and E1A. PLoS One 6, e23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charles R. L., Eaton P. (2008) Redox signalling in cardiovascular disease. Proteomics Clin. Appl. 2, 823–836 [DOI] [PubMed] [Google Scholar]
- 23. Inoguchi T., Li P., Umeda F., Yu H. Y., Kakimoto M., Imamura M., Aoki T., Etoh T., Hashimoto T., Naruse M., Sano H., Utsumi H., Nawata H. (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49, 1939–1945 [DOI] [PubMed] [Google Scholar]
- 24. Miller T. A., LeBrasseur N. K., Cote G. M., Trucillo M. P., Pimentel D. R., Ido Y., Ruderman N. B., Sawyer D. B. (2005) Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem. Biophys. Res. Commun. 336, 309–315 [DOI] [PubMed] [Google Scholar]
- 25. Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. (1998) Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. U. S. A. 95, 2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briaud I., Harmon J. S., Kelpe C. L., Segu V. B., Poitout V. (2001) Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 50, 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cacicedo J. M., Benjachareowong S., Chou E., Ruderman N. B., Ido Y. (2005) Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes 54, 1838–1845 [DOI] [PubMed] [Google Scholar]
- 28. Liu J., Garcia-Cardena G., Sessa W. C. (1996) Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry 35, 13277–13281 [DOI] [PubMed] [Google Scholar]
- 29. Wu Y., Song P., Xu J., Zhang M., Zou M.-H. (2007) Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J. Biol. Chem. 282, 9777–9788 [DOI] [PubMed] [Google Scholar]
- 30. Gupta K., Kshirsagar S., Li W., Gui L., Ramakrishnan S., Gupta P., Law P. Y., Hebbel R. P. (1999) VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp. Cell Res. 247, 495–504 [DOI] [PubMed] [Google Scholar]
- 31. Xia Z., Dickens M., Raingeaud J., Davis R. J., Greenberg M. E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331 [DOI] [PubMed] [Google Scholar]
- 32. Kumar P., Coltas I. K., Kumar B., Chepeha D. B., Bradford C. R., Polverini P. J. (2007) Bcl-2 protects endothelial cells against gamma-radiation via a Raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res. 67, 1193–1202 [DOI] [PubMed] [Google Scholar]
- 33. Adachi T., Pimentel D. R., Heibeck T., Hou X., Lee Y. J., Jiang B., Ido Y., Cohen R. A. (2004) S-Glutathiolation of ras mediates redox-sensitive signaling by angiotensin ii in vascular smooth muscle cells. J. Biol. Chem. 279, 29857–29862 [DOI] [PubMed] [Google Scholar]
- 34. Clavreul N., Adachi T., Pimental D. R., Ido Y., Schöneich C., Cohen R. A. (2006) S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 20, 518–520 [DOI] [PubMed] [Google Scholar]
- 35. Henry T. D., Annex B. H., McKendall G. R., Azrin M. A., Lopez J. J., Giordano F. J., Shah P. K., Willerson J. T., Benza R. L., Berman D. S., Gibson C. M., Bajamonde A., Rundle A. C., Fine J., McCluskey E. R. (2003) The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107, 1359–1365 [DOI] [PubMed] [Google Scholar]
- 36. Grines C. L., Watkins M. W., Helmer G., Penny W., Brinker J., Marmur J. D., West A., Rade J. J., Marrott P., Hammond H. K., Engler R. L. (2002) Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation 105, 1291–1297 [DOI] [PubMed] [Google Scholar]
- 37. Simons M., Annex B. H., Laham R. J., Kleiman N., Henry T., Dauerman H., Udelson J. E., Gervino E. V., Pike M., Whitehouse M. J., Moon T., Chronos N. A. (2002) Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.