Abstract
Pulmonary fibrosis, characterized by excess deposition of extracellular matrix by myofibroblasts, is a serious component of chronic lung diseases. Cadherin-11 (CDH11) is increased in wound healing and fibrotic skin. We hypothesized that CDH11 is increased in pulmonary fibrosis and contributes its development. CDH11 expression was assessed in lung tissue from idiopathic pulmonary fibrosis patients. The role of CDH11 in lung fibrosis was determined using the bleomycin model of pulmonary fibrosis, and in vitro analyses were performed on A549 cells during the process of epithelial to mesenchymal transition (EMT). Immunohistochemical studies demonstrated CDH11 expression on fibroblasts, epithelial cells, and alveolar macrophages of patients with pulmonary fibrosis and mice given bleomycin. Interestingly, CDH11-deficient mice had decreased fibrotic endpoints in the bleomycin model of pulmonary fibrosis compared to wild-type mice. Furthermore, anti-CDH11-neutralizing monoclonal antibodies successfully treated established pulmonary fibrosis induced by bleomycin. TGF-β levels were reduced in bronchoalveolar lavage (BAL) fluid, BAL cells, and primary alveolar macrophages from CDH11-deficient mice. Mechanistic studies demonstrated that TGF-β up-regulated CDH11 expression on A549 cells, and inhibition of CDH11 expression using siRNA reduced TGF-β-induced EMT. Together, these results identify CDH11 as a novel therapeutic target for pulmonary fibrosis. Schneider, D. J., Wu, M., Le, T. T., Cho, S.-H., Brenner, M. B., Blackburn, M. R., Agarwal, S. K. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGFβ production and epithelial to mesenchymal transition.
Keywords: macrophage, extracellular matrix, lung, alveolar, fibroblast
Fibrosis is the principal feature of a group of chronic lung diseases termed interstitial lung diseases (ILDs). Idiopathic pulmonary fibrosis (IPF) is a particularly deadly form of ILD, which carries a mean survival time of 2 to 3 yr from the time of diagnosis (1). This condition affects 200,000 Americans (2), and its incidence is projected to increase(3, 4). In addition, more prevalent chronic lung conditions, such as asthma and chronic obstructive pulmonary disease, also exhibit features of fibrosis and share common pathways leading to its development (5–7).
It is postulated that mechanisms of fibrosis resemble processes activated during wound healing(8, 9). These include inflammation; protease activation; expression of profibrotic mediators, such as transforming growth factor (TGF)-β; the appearance of myofibroblasts; and deposition of extracellular matrix proteins, such as collagen. In the setting of fibrosis, these processes are amplified, resulting in enhanced formation and survival of myofibroblasts, the principle source of extracellular matrix proteins. Suggested sources of myofibroblasts include differentiation of resident fibroblasts (10), bone marrow-derived precursors (11), and epithelial to mesenchymal transition (EMT) (12, 13).
EMT occurs in physiological scenarios, such as development and wound healing, but also has demonstrated pathogenic roles, such as malignant transformation and, in the setting of organ fibrosis, as a source of myofibroblasts(12, 13). Cadherins are thought to play a role in EMT, as cells classically display loss of epithelial characteristics and markers, such as epithelial (E) cadherin (14, 15), and the gain of a mesenchymal phenotype and markers, such as neural (N) cadherin (16, 17).
Cadherins are a group of transmembrane glycoproteins that mediate calcium-dependent homophilic cell-to-cell adhesion at adherens junctions (18). During embryogenesis, cadherins dictate patterns of cell differentiation, morphogenesis, migration, and invasion, in part, through the regulation of EMT. Emerging studies suggest a role for cadherin-11 (CDH11) in the process of wound healing, corroborated by detecting increased CDH11 levels in subcutaneous and lung fibroblasts stimulated to differentiate into myofibroblasts (19). Consistent with the hypothesis that wound healing and fibrosis share common pathways, two independent microarray studies demonstrated increased CDH11 in biopsies taken from patients with scleroderma vs. normal skin (20, 21). These findings suggest that CDH11 may promote the process of fibrosis by facilitating the differentiation of resident tissue fibroblasts into myofibroblasts.
CDH11 is also associated with a mesenchymal and invasive phenotype. Namely, CDH11 expression correlates with developmental cell migration (22) and promotes invasive behavior of synovial fibroblasts (23). Further, epithelial breast cancer cells often up-regulate CDH11, which correlates with a highly invasive behavior (24). If extrapolated to lung epithelial cells, these findings may be consistent with the process of EMT. However, the role of CDH11 in the process of EMT and pulmonary fibrosis has not been investigated.
These findings led to the hypothesis that CDH11 is a mediator in EMT and the development of pulmonary fibrosis. To address this hypothesis, utilizing both genetic and pharmacologic approaches, we examined the contribution of CDH11 to bleomycin-induced pulmonary fibrosis. Further, we examined the expression of CDH11 in samples obtained from patients with ILD. CDH11 expression was demonstrated in two cell types in lungs of the bleomycin model and patients with ILD: alveolar macrophages and hyperplastic alveolar epithelial cells (AECs). The results of this study suggest that both of these cell types contribute to CDH11-dependent pulmonary fibrosis through the regulation of TGF-β production and EMT in AECs.
MATERIALS AND METHODS
Human lung samples
Deidentified human lung tissue was obtained from the Lung Tissue Research Consortium. Patients were classified as mild and severe IPF according to spirometry, pathological specimen, and high resolution CT scan, as described previously (25).
Mice
These studies were reviewed and approved by the University of Texas Health Science Center Animal Welfare Committee. Cadherin 11-null (cadherin-11−/−) mice on the C129 background have been backcrossed onto the C57/B6 background (26, 27), and wild-type (WT) littermates were used as controls in this study. Mice received 50 μl intratracheal (i.t.) sterile saline or bleomycin (Blenoxane; Teva Pharmaceuticals, Petach Tikva, Israel) at 3.5 U/kg diluted in sterile saline. Cadherin-11-blocking antibody hybridoma clones 13C2 and 23C6 were cultured and isolated in LPS-free conditions, as described previously (27). Delivery schedule for systemic cadherin-11-neutralizing antibodies was based on this same previous report (27). For our purposes, 8- to 12-wk-old WT C57BL/6J female mice (Harlan, Indianapolis, IN, USA) were given i.t. bleomycin, as above, and treated with an intraperitoneal (i.p.) loading dose of 500 μg 23C6, 13C2, or isotype control antibody (mouse monoclonal, MOPC 31C clone; Sigma, St. Louis, MO, USA) in 100 μl PBS at 10 d after bleomycin exposure. Mice were subsequently administered 100-μg antibody in 100 μl PBS every other day until d 20. All endpoints were collected on d 21.
Bronchoalveolar lavage (BAL) inflammatory cell counts and histology
These endpoints were collected and processed as described previously (28). BAL cell pellets and supernatants were portioned into aliquots and saved for protein quantification (Sircol collagen assay, Biocolor Assays, Carrickfergus, UK; Mouse TGF-β Quantikine ELISA, R&D Systems, Minneapolis, MN, USA). Fibrosis was quantified using the Ashcroft scoring method (29) on 20 images per hematoxylin-and-eosin (H&E)-stained mouse lung. Analyses were performed in a blind fashion with regard to animal genotype and/or treatment. For immunostaining, slides were incubated with primary antibodies for αSMA (1:500 dilution, 4°C overnight; mouse monoclonal antibody; Sigma-Aldrich, St. Louis, MO, USA), cadherin-11 (10 μg/ml, 4°C overnight; clone 5B2H5, Invitrogen, Carlsbad, CA, USA).
BAL cytospin immunofluorescence (IF)
BAL cell pellets were cytospun onto microscope slides, allowed to air dry, fixed in 2% paraformaldehyde, and permeabilized with 0.2% saponin in PBS. Slides were incubated with antibodies for 1 h at room temperature. Antibodies used included isotype control antibody (MOPC 31C; Sigma-Aldrich), anti-mouse cadherin-11 (clone 23C6 or 13C2; ref. 27), anti-human/mouse cadherin-11 (clone 5B2H5; Invitrogen), anti-human/mouse N-cadherin (clone 3B9; Invitrogen), or anti-human E-cadherin (clone HECD-1; Invitrogen), anti-mouse E-cadherin (clone 4A2C7; Invitrogen). Primary antibodies were followed by secondary antibody (1:1000, 1 h at room temperature, donkey anti-mouse IgG Cy3; Jackson Immunoresearch Laboratories, West Grove, PA, USA) and covered with Prolong Gold antifade medium with DAPI (Invitrogen). Confocal images were obtained using a TCS SP5 confocal laser microscope (Leica, Wetzlar, Germany).
Western blot detection of cadherin-11
Protein lysates were prepared from homogenization of frozen tissue in standard lysis buffer containing protease inhibitor (Roche, Kaiseraugst, Switzerland). Equal protein quantities were separated by electrophoresis on 10% Tris-HCl SDS Ready-Gels (Bio-Rad, Hercules, CA, USA) and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% dehydrated milk in TBS/T, incubated with primary antibody (cadherin-11, 1:500, mouse monoclonal antibody, Invitrogen; α-tubulin, 1:5000, mouse monoclonal antibody, Sigma-Aldrich), incubated with secondary antibodies conjugated to HRP (1:5000; eBiosciences, San Diego, CA, USA), and detected with ECL reagents (Thermo Fisher Scientific, Waltham, MA, USA).
Analysis of whole-lung mRNA
RNA was obtained from lung tissue as described previously (28). Transcripts for mouse and human cadherin-11 were obtained with corresponding TaqMan probes (Applied Biosystems, Foster City, CA, USA) and measured in parallel with peptidylprolyl isomerase A (PPIA) using quantitative real time PCR. Values are presented as mean normalized transcript levels using the comparative Ct method (2ΔΔCt) (30).
Analysis of A549 cells
A549 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in 96-well plates and grown overnight in DMEM containing 10% FBS and 1% antibiotics. For siRNA knockdown of cadherin-11, cells were washed with antibiotic-free medium and subsequently incubated with Optimem (Invitrogen) with Lipofectamine RNAiMax (Invitrogen) and either nontargeting siRNA or human cadherin-11 siRNA (antisense sequence: 5′-UUUGAAUGGAGUCAUAAGGUU; Dharmacon RNAi Technologies; Thermo Fisher Scientific, Lafayette, CO, USA) for 48 h. Cells were then trypsinized and cultured in antibiotic-free medium. After 6 h, cells were serum starved overnight in DMEM containing 0.1% BSA. Cells were then stimulated with or without TGF-β at 10 ng/ml in DMEM plus 0.1% BSA for 24 h. RNA was isolated using cell to Ct reagents (Applied Biosystems), according to the manufacturer's instructions. Transcripts for cadherin-11, Col1a1, N-cadherin, and E-cadherin were obtained with corresponding Taqman probes (Applied Biosystems) and are presented as mean fold change vs. control. Imaging of A549 cells was performed with a BX60 inverted-phase contrast microscope (Olympus, Center Valley, PA, USA).
Analysis of alveolar macrophages in vitro
MHS alveolar macrophages (American Type Culture Collection) were cultured overnight in 96-well plates containing RPMI plus 10% FBS and 1% antibiotics. Cells were stained as above for BAL cytospins. Cells were also stimulated with 10 μg/ml LPS for 24 h and RNA was analyzed as above for A549 cells.
Primary alveolar macrophages were obtained from BAL fluid of WT and cadherin-11−/−, as described previously (25). Briefly, BAL from 6 mice were pooled for each experiment, washed, and cultured in RPMI 1640 with 10% FBS for 4 h to allow adherence. Nonadeherent cells were removed, and adherent cells were cultured for 18 h at 37°C/5% CO2. Supernatants were assessed for TGF-β levels by ELISA.
Statistics
Values are expressed as means ± se. As appropriate, groups were compared by ANOVA, and follow-up comparisons between groups were conducted using 2-tailed Student's t test. Values of P ≤ 0.05 were considered to be significant.
RESULTS
Expression and localization of CDH11 in patients with ILD
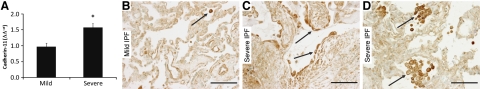
To determine whether CDH11 expression is increased in pulmonary fibrosis, transcripts were examined in lung tissue from IPF patients with severe airway restriction vs. lung tissue in regions with relatively normal appearance on high-resolution, lung-computed tomography scans and histology from IPF patients with mild restriction in lung function (25). Results demonstrate increased CDH11 mRNA expression in patients with severe IPF (Fig. 1A). Immunohistochemistry was performed to identify cell types expressing CDH11 in lungs of patients with IPF. Expression of CDH11 was below the limits of detection of immunohistochemistry in lung parenchyma of a normal human lung and in histologically normal regions of lung from patients with IPF (Fig. 1B and Supplemental Fig. S1A, B). In patients with severe IPF, CDH11 immunoreactivity was noted on fibroblast-like cells in fibrotic foci (Fig. 1C and Supplemental Fig. S1C). Interestingly, strong immunoreactivity also was noted in hyperplastic AECs adjacent to fibrotic foci in the severe IPF group (Fig. 1C). In addition, CDH11 expression was localized to alveolar macrophages in normal human lung (Supplemental Fig. S1A, B), mild IPF lung (Fig. 1B), and severe IPF lung (Fig. 1D). These results indicate that CDH11 expression is increased in severe IPF.
Figure 1.
CDH11 expression and localization in human IPF lungs. A) Quantitative RT-PCR measuring CDH11 transcripts present in RNA isolates from biopsies taken from patients with IPF showing mild and severe airway restriction. Transcripts for CDH11 were measured in parallel with PPIA, and values are presented as mean ± se normalized transcript levels (Δ−Ct). *P < 0.05 vs. mild IPF. B–D) Immunolocalization for CDH11 in representative lung sections of patients with mild IPF (B) and severe IPF (C, D). Arrows denote staining present in alveolar macrophages (B, D) and hyperplastic AECs (C). Scale bars = 100 μm. Transcripts and displayed sections are representative of n = 10 (mild IPF; severe IPF).
Contribution of CDH11 to features of pulmonary fibrosis
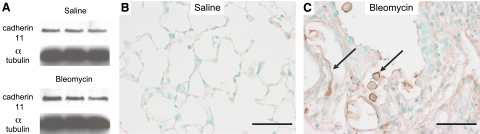
The i.t. bleomycin model is the most commonly utilized animal model to study mechanisms of pulmonary fibrosis (31). Similar to findings in patients with IPF, lungs from mice given bleomycin display increased CDH11 expression (Fig. 2A) and CDH11 immunoreactivity on fibroblasts, hyperplastic AECs, and alveolar macrophages (Fig. 2C). To examine the contribution of CDH11 to pulmonary fibrosis, mice lacking CDH11 (Cdh11−/−) and WT mice were compared in the i.t. bleomycin model. Results of H&E staining display no detectable difference in pulmonary histology between WT and Cdh11−/− mice given saline (Fig. 3A). Examination of lung sections from WT mice given bleomycin displays standard histopathologic features consistent with pulmonary fibrosis, including inflammation, disruption of normal alveolar architecture (Fig. 3A), increased collagen (Fig. 3B), and myofibroblasts (Fig. 3C) in the alveolar air spaces. These histological endpoints are substantially reduced in Cdh11−/− mice (Fig. 3A–C). Quantifiable endpoints consistent with pulmonary fibrosis, such as BAL-soluble collagen (25, 28, 32, 33) (Fig. 3D) and Ashcroft scoring (29) (Fig. 3E) display typical increases in WT mice given bleomycin vs. saline, and significant reductions in these endpoints in Cdh11−/− mice given bleomycin. Collectively, these results demonstrate that CDH11 is a critical mediator in development of pulmonary fibrosis.
Figure 2.
CDH11 expression and localization in mouse lungs with bleomcyin-induced fibrosis. A) Western blot for CDH11 from lung homogenates isolated from mice given bleomycin and saline. B, C) Immunolocalization for CDH11 in representative lung sections of mice treated with saline (B) and bleomycin (C). Arrows denote staining present in alveolar macrophages and hyperplastic AECs. Scale bars = 50 μm. Displayed sections are representative of n = 12 (saline) and n = 20 (bleomycin).
Figure 3.
Histopathological changes in bleomcyin-induced pulmonary fibrosis associated with the genetic removal of CDH11. A) Lungs were taken from mice 21 d after 3.5-U bleomycin installation and processed for sectioning and H&E staining (A). B, C) Collagen deposition (B) and myofibroblasts (C) in distal air-spaces were assessed with Masson's trichrome and α SMA immunohistochemistry. D, E) Fibrosis was quantified by determination of collagen present in BAL fluid (D) and Ashcroft scores (E) on H&E-stained lung sections. Scores were determined on 20 images/mouse lung. n = 6 (WT saline; Cdh11 −/− saline), n = 13 (WT bleomycin), and n = 11 (Cdh11 −/− bleomycin). Scale bars = 500 μm (A); 100 μm (B, C).*P < 0.05 vs. WT saline; #P < 0.05 vs. WT bleomycin.
Systemic delivery of CDH11-blocking antibody improves established pulmonary fibrosis
Our results show that Cdh11 −/− mice have reduced fibrosis in response to bleomycin challenge. Well-documented studies indicate that pulmonary fibrosis in the bleomycin model is present at least 7 d after bleomycin installation (31). Therefore, to determine whether established fibrosis can be successfully treated by targeting CDH11, WT mice were administered CDH11-blocking antibodies (clone 23C6 or 13C2) via the i.p. route beginning 10 d after i.t. bleomycin installation. Lung sections stained with H&E (Fig. 4A), Masson's trichrome (Fig. 4B), and α smooth muscle actin (SMA) immunohistochemistry (Fig. 4C) all demonstrate markedly reduced histopathological features of pulmonary fibrosis in mice treated with 23C6 or 13C2 compared to mice receiving isotype control antibody. Similarly, soluble collagen levels in mice treated with CDH11 antibodies were significantly reduced vs. mice treated with isotype control (Fig. 4D). These results confirm findings in Cdh11 −/− mice that CDH11 contributes to pulmonary fibrosis and demonstrate that systemic delivery of CDH11-blocking antibody successfully treats established fibrosis.
Figure 4.
CDH11-blocking antibody improves established pulmonary fibrosis. A–C) Mice received antibody every other day beginning 10 d after bleomycin exposure. Lungs were then taken at d 21 and processed for sectioning and H&E staining (A), collagen deposition (B), and myofibroblasts in distal air spaces (C). D) Fibrosis was quantified by determination of collagen present in BAL fluid. n = 6 (saline; bleomycin+23C6), n = 7 (bleomycin+isotype), n = 8 (bleomycin+13C2). Scale bars = 500 μm (A); 100 μm (B, C). *P < 0.05 vs. saline; #P < 0.05 vs. bleomycin + isotype.
CDH11 contributes to EMT in AECs
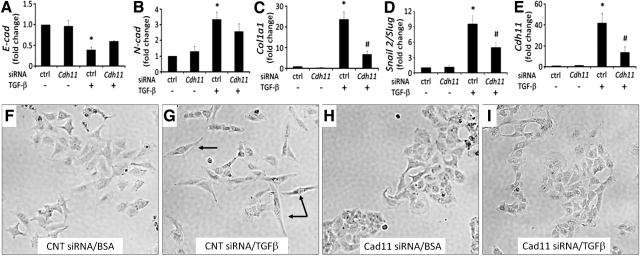
Hyperplasia is a characteristic of AECs undergoing the profibrotic process of EMT in vivo (34–36). Interestingly, the above results demonstrating CDH11 expression on hyperplastic AECs in patients with IPF (Fig. 1C) and mice given bleomycin (Fig. 2C) suggest that CDH11 may regulate EMT. Alveolar carcinoma A549 cells stimulated with TGF-β were used to investigate the role of CDH11 in EMT as one potential mechanism for the reduction in pulmonary fibrosis in the absence of CDH11. Consistent with the process of EMT, TGF-β decreased E-cadherin transcript levels and increased N-cadherin, Col1a1, and Snail2/Slug levels in A549 cells (Fig. 5A–D). In parallel with these changes consistent with EMT, Cdh11 expression was also increased (Fig. 5E). To determine whether CDH11 is a regulator of TGF-β-induced EMT, A549 cells were transfected with siRNA specific for Cdh11 followed by TGF-β stimulation. The expression of Cdh11 was decreased with ∼75% efficiency (Fig. 5E). Cdh11 knockdown showed a slight reversal of TGF-β-induced decreased E-cadherin and increased N-cadherin expression, although not statistically significant (Fig. 5A, B). Interestingly, Cdh11 knockdown substantially reduced TGF-β-induced Col1a1 and Snail2/Slug expression (Fig. 5C, D). Furthermore, Cdh11 knockdown prevented the development of mesenchymal morphology in TGF-β-stimulated A549 cells (Fig. 5F). These results demonstrate that CDH11 is a key regulator of TGF-β-induced EMT in A549 cells and provide a potential mechanism of reduced pulmonary fibrosis observed with CDH11 inhibition.
Figure 5.
CDH11 up-regulation promotes TGF-β-induced EMT in lung epithelial cells. A–E) A549 lung epithelial cells were transfected with Cdh11 or control (ctrl) siRNA and subsequently stimulated with TGF-β (10 ng/ml); 24 h later, RNA was isolated and transcripts were determined for E-cadherin (A), N-cadherin (B), Col1a1 (C), Snail2/Slug (D), and Cdh11 (E). Data are mean ± se fold change transcripts vs. BSA plus ctrl siRNA. *P < 0.05 vs. BSA + Cdh11 siRNA; #P < 0.05 vs. TGF-β + ctrl siRNA. F–I) Cell morphology was also assessed using phase contrast microscopy. Data are representative of 3 separate experiments.
Detection of CDH11 on alveolar macrophages
Findings in lung sections of patients with IPF and mice given bleomycin indicate immunolocalization of CDH11 to alveolar macrophages (Figs. 1B, D and 2C). This is the first report of CDH11 expression on a macrophage population. Therefore, to carefully examine CDH11 expression on alveolar macrophages, IF studies were performed (Supplemental Figs. S2 and S3). In addition, confocal IF microscopy of BAL alveolar macrophages from patients with ILD (Fig. 6A), patients with IPF (Fig. 6B), and mice given bleomycin (Fig. 6C) demonstrates specific CDH11 expression predominantly localized to the cell membrane. Additional confirmation of CDH11 expression on macrophages was demonstrated in the mouse MHS alveolar macrophage cell line by IF, which demonstrated immunolocalization for CDH11 (Fig. 6D), and by Western blot, which demonstrated a band of ∼116 kDa that corresponds to CDH11, as seen in WT dermal fibroblasts, a cellular population known to express CDH11 (Fig. 6F). Similar to what has been observed in IF studies, the expression of CDH11 on macrophages is lower than CDH11 expression on fibroblasts. Finally, MHS cells display a relative increase in Cdh11 transcripts when activated with LPS in vitro (Fig. 6E). LPS-induced activation of MHS cells was verified by a parallel fold increase in IL-6 transcripts (5139±938). Collectively, these results confirm the presence of CDH11 on alveolar macrophages.
Figure 6.
CDH11 is present on alveolar macrophages. A–C) Representative images of confocal microscopy on BAL cells stained with CDH11 (23C6) antibody isolated from patients with ILD (A), patients with IPF (B), and mice given bleomycin (C). Images representative of n = 2 (ILD; IPF). D) Cultured MHS alveolar macrophages stained with CDH11 (23C6) antibody. E) Cdh11 transcripts measured in MHS alveolar macrophages were stimulated with 10 μg/ml LPS. Data are representative of 5 separate experiments and are presented as mean ± se fold change vs. medium alone. F) Protein lysates from MHS cells and WT dermal fibroblasts demonstrate CDH11 expression by Western blot using anti-CDH11 monoclonal antibody (clone 5B2H5). Scale bars = 50 μm (A–C); 20 μm (D).
CDH11-dependent regulation of TGF-β
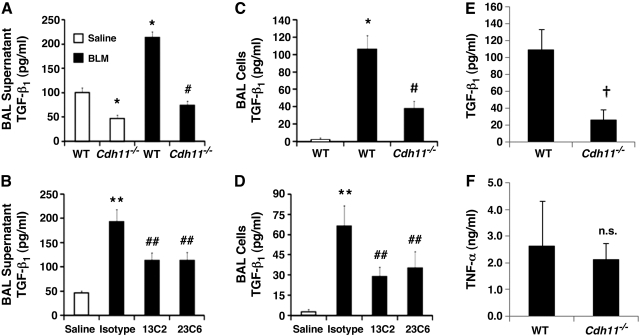
TGF-β is a well-recognized profibrotic mediator, in part due to its ability to induce EMT(12, 37) and the differentiation of myofibroblasts (38), resulting in excess collagen production (39). Also, TGF-β is elevated in BAL fluid from mice given bleomycin (40) and patients with pulmonary fibrosis (41). To determine CDH11-dependent effects on TGF-β, BAL fluid levels of soluble TGF-β were determined in Cdh11 −/− and CDH11 antibody-treated mice given bleomycin. Results demonstrate typical increases in BAL TGF-β from WT mice given bleomycin (Fig. 7A, B). In contrast, Cdh11−/− mice and CDH11 antibody-treated mice given bleomycin displayed markedly reduced levels of BAL TGF-β. Further, Cdh11−/− mice demonstrated significantly lower levels of TGF-β at baseline compared to WT mice given saline (Fig. 7A). Localization studies in pulmonary fibrosis demonstrate that the majority of TGF-β is produced in the alveolar macrophage(40, 42). Our results indicate that alveolar macrophages are ∼85% of the inflammatory cell population isolated from BAL fluid of mice given bleomycin (Supplemental Fig S4). Therefore, to isolate and quantify TGF-β at its source in the model, total protein was isolated from inflammatory cells isolated from BAL fluid. Findings display expected increases in cell pellet TGF-β isolated from mice given bleomycin, but significant decreases in Cdh11−/− and CDH11 antibody-treated mice given bleomycin (Fig. 7C, D). Although alveolar macrophage numbers are substantially increased with bleomycin administration, macrophage numbers are unaffected by CDH11 (Supplemental Fig S4B, E).
Figure 7.
CDH11 promotes TGF-β expression from alveolar macrophages. A–D) TGF-β levels were quantified in BAL supernatants (A, B) and protein lysates (C, D) from BAL cell pellets utilizing a standard TGF-β ELISA. Data are expressed as means ± se. Number of mice in each group is as reported in Figs. 2 and 3. E, F) In vitro TGF-β (E) and TNF-α (F) production from primary alveolar macrophages cultured from WT and Cdh11−/− mice. Results are means ± se from 3 independent experiments. n.s. = not statistically significant vs. WT. *P < 0.05, **P < 0.05 vs. WT or WT saline; #P < 0.05, ##P < 0.05 vs. WT bleomycin or bleomycin + isotype; †P < 0.05 vs. WT.
To further demonstrate that CDH11 plays a role in modulating TGF-β production by alveolar macrophages, primary alveolar macrophages were isolated and cultured from BAL of WT and Cdh11−/− mice. Supernatants of WT mice have significantly greater amounts of TGF-β relative to Cdh11−/− mice (Fig. 7E), but no significant difference was observed with TNF-α production (Fig. 7F). Together with the results from BAL fluid and cell pellets, these results suggest that CDH11 present on alveolar macrophages regulates the production of TGF-β by alveolar macrophages.
DISCUSSION
In the current report, we identified CDH11 as a mediator of EMT and pulmonary fibrosis. We demonstrate that CDH11 expression is increased in human IPF samples and localizes to AECs and alveolar macrophages. Notably, Cdh11−/− and WT mice treated with CDH11-blocking antibody had decreased pulmonary fibrosis endpoints in the i.t. bleomcyin model. Mechanistically, CDH11 regulated the process of EMT in a lung epithelial cell line, and its expression on alveolar macrophages was associated with production of TGF-β. These data support a pivotal role of CDH11 in pulmonary fibrosis and implicate CDH11 as a critical mediator of TGF-β production and EMT, two processes that are well recognized in the generation of myofibroblasts and pulmonary fibrosis.
One aim of this study was to investigate the feasibility of specifically treating established pulmonary fibrosis by targeting CDH11. Mice given bleomycin and subsequently treated 10 d later with CDH11-blocking antibody displayed substantial reductions in typical pulmonary fibrotic endpoints. Mechanistically, how the anti-cadherin-11 antibodies function at the molecular level is not known. At the cellular levels, reductions in lymphocytes were observed; this treatment effect was otherwise independent of any changes in inflammatory cell counts. While this may be attributable to the strategic delivery of CDH11-targeted therapy after the inflammatory phase, Cdh11 −/− mice receiving bleomycin displayed virtually identical inflammatory cell counts and differentials. A review by Moeller et al. (31) specifically emphasizes the lack of studies in the bleomycin model that aim to treat existing pulmonary fibrosis. Therefore, our findings point to the promise and novelty of CDH11 as a therapeutic target for established pulmonary fibrosis.
The expression of CDH11 on several cellular populations in the fibrotic lung suggests that CDH11 regulates lung fibrosis through multiple mechanisms. Given the expression of CDH11 on the fibroblasts, it is likely that CDH11 regulates the behavior of the lung fibroblast. Indeed, previous studies have demonstrated that CDH11 is a critical regulator of fibroblast invasion (27, 43). However, in the current report, CDH11 expression in the lung also was observed on AECs and alveolar macrophages, two cells that potentially contribute to the development of pulmonary fibrosis. The data in this report do not address the question of whether the AEC, the alveolar macrophage, or both are the critical step regulated by CDH11 during the development of fibrosis. These studies are currently under way.
CDH11 is associated with a mesenchymal phenotype in cells during development (22) and in various forms of pathology, including cancer (24) and rheumatoid arthritis (27). Though referred to as a marker of EMT (44–47), the role of CDH11 in promoting this process has not been investigated. In the context of pulmonary fibrosis, hyperplastic AECs are found in fibrotic regions in patients with IPF (48, 49), commonly express epithelial and mesenchymal markers, and thus, are thought to represent a transitional epithelial cell undergoing EMT. Though its significance to human pulmonary fibrosis is still under investigation, the contribution of epithelial cells to fibroblast and myofibroblast populations in the lung is well documented (12, 13, 37, 50). We report CDH11 expression on AECs in the bleomycin model of pulmonary fibrosis and patients with IPF. Furthermore, in vitro studies of epithelial cells stimulated with TGF-β exhibit substantial up-regulations in CDH11. In addition, knockdown of CDH11 significantly reduces A549 cell EMT characterized by reduced TGF-β-dependent mesenchymal morphogenesis and expression of Col1a1 and Snail2/Slug. While, most studies have focused on E-cadherin-dependent inhibition of EMT (14–17) in pathological scenarios, our novel findings demonstrate that CDH11 is promoting features of EMT.
Patients with IPF and mice given bleomycin demonstrate CDH11 on alveolar macrophages, a major source of TGF-β in clinical and experimental pulmonary fibrosis (40, 42). TGF-β induces differentiation of myofibroblasts (38) and stimulates epithelial cells to undergo EMT(12, 37). The heralded critical regulatory step of (patho)biological TGF-β activity is the activation of TGF-β protein through cleavage of the LAP protein fragment by various proteases (51–53). However, we demonstrate for the first time that CDH11 dramatically influences TGF-β production from alveolar macrophages in bleomycin-induced fibrosis. In the context of TGF-β activity in pathological scenarios, regulation of substrate availability upstream of protein cleavage would influence its overall activity, as evidenced by our study demonstrating paralleled reductions in fibrotic endpoints in vivo. These endpoints include known TGF-β-mediated fibroproliferative processes, such as myofibroblast production and EMT. As knowledge of TGF-β-mediated pathology continues to expand, the results of this study are of considerable value given the dramatic regulation of TGF-β by CDH11.
There is extensive potential for the influence of cadherins on profibrotic intracellular signaling pathways. It is well recognized that cadherin-dependent formation of adherens junctions regulates the actin cytoskeleton through Rho GTPases (54). Of note, activation of Rho GTPases is increased in fibroblasts of patients with IPF and promotes their migratory phenotype (55). Furthermore, increased activation of Rho GTPase was detected during TGF-β-dependent myofibroblast differentiation in vitro (56). These studies support the in vivo evidence of CDH11-dependent formation of myofibroblasts and suggest direct influence of CDH11 on fibroblast migration and myofibroblast differentiation through the action of Rho GTPases. While myofibroblast formation in our study may be the result of CDH11-directed up-regulation in TGF-β, other investigations indicate that this may also be mediated by Rho activity. Interestingly, one study found that Rho GTPase activation in macrophages positively regulates TGF-β translation (57). In addition, inhibition of signaling downstream of Rho activity reduced experimental kidney fibrosis in association with reduced expression of TGF-β (58). We speculate these studies provide insight into potential mechanisms of CDH11-dependent regulation of TGF-β expression and further suggest details of CDH11-mediated fibrosis.
Alternatively, previous studies indicate the role of the Wnt pathway in promoting endpoints consistent with pulmonary fibrosis such as EMT (35), lung fibroblast proliferation, and extracellular matrix production (59). Other reports demonstrate increased nuclear localization of β-catenin in type II AECs and fibroblasts of patients with IPF indicative of increased Wnt signaling (35, 60, 61). Classically, β-catenin binds to the cytoplasmic tail of cadherins at the catenin binding site (18). Interestingly, a recent study demonstrates that cadherin-bound β-catenin is released on adherens junction dissociation and becomes part of the intracellular pool available to signal within the canonical Wnt/β-catenin pathway (62). Epithelial cadherins, especially E-cadherin, are critical in maintaining epithelial cell-to-cell junctions through adherens junctions. Loss of these adherens junctions, mainly through down-regulation of E-cadherin, is a fundamental and critical step in the process of EMT (63). As demonstrated previously, CDH11 promotes EMT in lung epithelial cells, including the expression of Snail2/Slug, which is a known repressor of E-cadherin expression (61). Therefore, it is possible that CDH11-dependent transition to mesenchymal morphology may mobilize intracellular β-catenin pools increasing substrate for the profibrotic Wnt intracellular signaling pathway. Thus, we speculate the Rho and Wnt intracellular signaling pathways warrant further investigation to uncover detailed mechanisms of CDH11-dependent pathology.
In summary, the findings of this study reveal that CDH11 is a critical regulator of pulmonary fibrosis. The data suggest that CDH11 may regulate TGF-β production by alveolar macrophages and EMT in AECs. As continuing investigation supports the role of EMT in the development of organ fibrosis, this research indicates that CDH11 is a central participant in these conditions, which include but are not limited to idiopathic pulmonary fibrosis, autoimmune diseases, such as scleroderma, hepatic fibrosis, and renal fibrosis. Our studies also reveal CDH11 as a newly identified candidate therapeutic target for fibrosis.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grants HL095401 and HL70952 (M.R.B), NIH training awards TL1RR024147 (D.J.S.) and 5T32AR052283-03 (M.W.), and NIH grant K08AR054404 and a Scleroderma Foundation New Investigator Award (S.K.A.). The authors declare that M.B.B. owns equity options and has received consulting fees from Synovex Corporation.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. American Thoracic Society (2000) Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am. J. Respir. Crit. Care Med. 161, 646–664 [DOI] [PubMed] [Google Scholar]
- 2. Coultas D. B., Zumwalt R. E., Black W. C., Sobonya R. E. (1994) The epidemiology of interstitial lung diseases. Am. J. Respir. Crit. Care Med. 150, 967–972 [DOI] [PubMed] [Google Scholar]
- 3. Raghu G., Weycker D., Edelsberg J., Bradford W. Z., Oster G. (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 174, 810–816 [DOI] [PubMed] [Google Scholar]
- 4. Olson A. L., Swigris J. J., Lezotte D. C., Norris J. M., Wilson C. G., Brown K. K. (2007) Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 176, 277–284 [DOI] [PubMed] [Google Scholar]
- 5. Elias J. A., Lee C. G., Zheng T., Ma B., Homer R. J., Zhu Z. (2003) New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jeffery P. K. (1999) Differences and similarities between chronic obstructive pulmonary disease and asthma. Clin. Exp. Allergy. 29(Suppl. 2), 14–26 [PubMed] [Google Scholar]
- 7. Ward P. A., Hunninghake G. W. (1998) Lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 157, S123–S129 [DOI] [PubMed] [Google Scholar]
- 8. Wynn T. A. (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phan S. H. (2002) The myofibroblast in pulmonary fibrosis. Chest 122, 286S–289S [DOI] [PubMed] [Google Scholar]
- 11. Phillips R. J., Burdick M. D., Hong K., Lutz M. A., Murray L. A., Xue Y. Y., Belperio J. A., Keane M. P., Strieter R. M. (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 114, 438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim K. K., Kugler M. C., Wolters P. J., Robillard L., Galvez M. G., Brumwell A. N., Sheppard D., Chapman H. A. (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 103, 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willis B. C., duBois R. M., Borok Z. (2006) Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 3, 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frixen U. H., Behrens J., Sachs M., Eberle G., Voss B., Warda A., Lochner D., Birchmeier W. (1991) E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113, 173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takao S., Che X., Fukudome T., Natsugoe S., Ozawa M., Aikou T. (2000) Down-regulation of E-cadherin by antisense oligonucleotide enhances basement membrane invasion of pancreatic carcinoma cells. Hum. Cell 13, 15–21 [PubMed] [Google Scholar]
- 16. Islam S., Carey T. E., Wolf G. T., Wheelock M. J., Johnson K. R. (1996) Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J. Cell Biol. 135, 1643–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieman M. T., Prudoff R. S., Johnson K. R., Wheelock M. J. (1999) N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 147, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wheelock M. J., Johnson K. R. (2003) Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19, 207–235 [DOI] [PubMed] [Google Scholar]
- 19. Hinz B., Pittet P., Smith-Clerc J., Chaponnier C., Meister J. J. (2004) Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol. Biol. Cell 15, 4310–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gardner H., Shearstone J. R., Bandaru R., Crowell T., Lynes M., Trojanowska M., Pannu J., Smith E., Jablonska S., Blaszczyk M., Tan F. K., Mayes M. D. (2006) Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 54, 1961–1973 [DOI] [PubMed] [Google Scholar]
- 21. Whitfield M. L., Finlay D. R., Murray J. I., Troyanskaya O. G., Chi J. T., Pergamenschikov A., McCalmont T. H., Brown P. O., Botstein D., Connolly M. K. (2003) Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl. Acad. Sci. U. S. A. 100, 12319–12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borchers A., David R., Wedlich D. (2001) Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128, 3049–3060 [DOI] [PubMed] [Google Scholar]
- 23. Valencia X., Higgins J. M., Kiener H. P., Lee D. M., Podrebarac T. A., Dascher C. C., Watts G. F., Mizoguchi E., Simmons B., Patel D. D., Bhan A. K., Brenner M. B. (2004) Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J. Exp. Med. 200, 1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pishvaian M. J., Feltes C. M., Thompson P., Bussemakers M. J., Schalken J. A., Byers S. W. (1999) Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 59, 947–952 [PubMed] [Google Scholar]
- 25. Zhou Y., Murthy J. N., Zeng D., Belardinelli L., Blackburn M. R. (2010) Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One 5, e9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horikawa K., Radice G., Takeichi M., Chisaka O. (1999) Adhesive subdivisions intrinsic to the epithelial somites. Dev. Biol. 215, 182–189 [DOI] [PubMed] [Google Scholar]
- 27. Lee D. M., Kiener H. P., Agarwal S. K., Noss E. H., Watts G. F., Chisaka O., Takeichi M., Brenner M. B. (2007) Cadherin-11 in synovial lining formation and pathology in arthritis. Science 315, 1006–1010 [DOI] [PubMed] [Google Scholar]
- 28. Schneider D. J., Lindsay J. C., Zhou Y., Molina J. G., Blackburn M. R. (2010) Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB J. 24, 70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashcroft T., Simpson J. M., Timbrell V. (1988) Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 41, 467–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 31. Moeller A., Ask K., Warburton D., Gauldie J., Kolb M. (2008) The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 40, 362–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chunn J. L., Molina J. G., Mi T., Xia Y., Kellems R. E., Blackburn M. R. (2005) Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J. Immunol. 175, 1937–1946 [DOI] [PubMed] [Google Scholar]
- 33. Sun C. X., Zhong H., Mohsenin A., Morschl E., Chunn J. L., Molina J. G., Belardinelli L., Zeng D., Blackburn M. R. (2006) Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 116, 2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Homer R. J., Zheng T., Chupp G., He S., Zhu Z., Chen Q., Ma B., Hite R. D., Gobran L. I., Rooney S. A., Elias J. A. (2002) Pulmonary type II cell hypertrophy and pulmonary lipoproteinosis are features of chronic IL-13 exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L52–L59 [DOI] [PubMed] [Google Scholar]
- 35. Konigshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O. V., Jahn A., Rose F., Fink L., Seeger W., Schaefer L., Gunther A., Eickelberg O. (2009) WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 119, 772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pozharskaya V., Torres-Gonzalez E., Rojas M., Gal A., Amin M., Dollard S., Roman J., Stecenko A. A., Mora A. L. (2009) Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One 4, e7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Willis B. C., Borok Z. (2007) TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L525–L534 [DOI] [PubMed] [Google Scholar]
- 38. Roy S. G., Nozaki Y., Phan S. H. (2001) Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int. J. Biochem. Cell Biol. 33, 723–734 [DOI] [PubMed] [Google Scholar]
- 39. Cutroneo K. R. (2007) TGF-beta-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen. 15(Suppl. 1), S54–S60 [DOI] [PubMed] [Google Scholar]
- 40. Phan S. H., Kunkel S. L. (1992) Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp. Lung Res. 18, 29–43 [DOI] [PubMed] [Google Scholar]
- 41. Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. (1991) Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 5, 155–162 [DOI] [PubMed] [Google Scholar]
- 42. Khalil N., Bereznay O., Sporn M., Greenberg A. H. (1989) Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J. Exp. Med. 170, 727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kiener H. P., Niederreiter B., Lee D. M., Jimenez-Boj E., Smolen J. S., Brenner M. B. (2009) Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 60, 1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forino M., Torregrossa R., Ceol M., Murer L., Della V. M., Del P. D., D'Angelo A., Anglani F. (2006) TGFbeta1 induces epithelial-mesenchymal transition, but not myofibroblast transdifferentiation of human kidney tubular epithelial cells in primary culture. Int. J. Exp. Pathol. 87, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reneker L. W., Bloch A., Xie L., Overbeek P. A., Ash J. D. (2010) Induction of corneal myofibroblasts by lens-derived transforming growth factor beta1 (TGFbeta1): a transgenic mouse model. Brain Res. Bull. 81, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sarrio D., Rodriguez-Pinilla S. M., Hardisson D., Cano A., Moreno-Bueno G., Palacios J. (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 68, 989–997 [DOI] [PubMed] [Google Scholar]
- 47. Vered M., Dayan D., Yahalom R., Dobriyan A., Barshack I., Bello I. O., Kantola S., Salo T. (2010) Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int. J. Cancer 127, 1356–1362 [DOI] [PubMed] [Google Scholar]
- 48. Kasper M., Haroske G. (1996) Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol. Histopathol. 11, 463–483 [PubMed] [Google Scholar]
- 49. Katzenstein A. L., Zisman D. A., Litzky L. A., Nguyen B. T., Kotloff R. M. (2002) Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am. J. Surg. Pathol. 26, 1567–1577 [DOI] [PubMed] [Google Scholar]
- 50. Willis B. C., Liebler J. M., Luby-Phelps K., Nicholson A. G., Crandall E. D., du Bois R. M., Borok Z. (2005) Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 166, 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crawford S. E., Stellmach V., Murphy-Ullrich J. E., Ribeiro S. M., Lawler J., Hynes R. O., Boivin G. P., Bouck N. (1998) Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93, 1159–1170 [DOI] [PubMed] [Google Scholar]
- 52. Munger J. S., Huang X., Kawakatsu H., Griffiths M. J., Dalton S. L., Wu J., Pittet J. F., Kaminski N., Garat C., Matthay M. A., Rifkin D. B., Sheppard D. (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 [DOI] [PubMed] [Google Scholar]
- 53. Yu Q., Stamenkovic I. (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176 [PMC free article] [PubMed] [Google Scholar]
- 54. Noren N. K., Niessen C. M., Gumbiner B. M., Burridge K. (2001) Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305–33308 [DOI] [PubMed] [Google Scholar]
- 55. Cai G. Q., Zheng A., Tang Q., White E. S., Chou C. F., Gladson C. L., Olman M. A., Ding Q. (2010) Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp. Cell Res. 316, 1600–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kono Y., Nishiuma T., Nishimura Y., Kotani Y., Okada T., Nakamura S., Yokoyama M. (2007) Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am. J. Respir. Cell Mol. Biol. 37, 395–404 [DOI] [PubMed] [Google Scholar]
- 57. Xiao Y. Q., Freire-de-Lima C. G., Schiemann W. P., Bratton D. L., Vandivier R. W., Henson P. M. (2008) Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J. Immunol. 181, 3575–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagatoya K., Moriyama T., Kawada N., Takeji M., Oseto S., Murozono T., Ando A., Imai E., Hori M. (2002) Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 61, 1684–1695 [DOI] [PubMed] [Google Scholar]
- 59. Salazar K. D., Lankford S. M., Brody A. R. (2009) Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1002–L1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chilosi M., Poletti V., Zamo A., Lestani M., Montagna L., Piccoli P., Pedron S., Bertaso M., Scarpa A., Murer B., Cancellieri A., Maestro R., Semenzato G., Doglioni C. (2003) Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 162, 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Konigshoff M., Balsara N., Pfaff E. M., Kramer M., Chrobak I., Seeger W., Eickelberg O. (2008) Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One 3, e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kam Y., Quaranta V. (2009) Cadherin-bound beta-catenin feeds into the Wnt pathway upon adherens junctions dissociation: evidence for an intersection between beta-catenin pools. PLoS One 4, e4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bolos V., Peinado H., Perez-Moreno M. A., Fraga M. F., Esteller M., Cano A. (2003) The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116, 499–511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.