Abstract
Activation of G-protein-coupled receptors (GPCRs) initiates signal transduction cascades that affect many physiological responses. The worm Caenorhabditis elegans expresses >1000 of these receptors along with their cognate heterotrimeric G proteins. Here, we report properties of 9-cis-retinal regenerated bovine opsin [(b)isoRho] and human melanopsin [(h)Mo], two light-activated, heterologously expressed GPCRs in the nervous system of C. elegans with various genetically engineered alterations. Profound transient photoactivation of Gi/o signaling by (b)isoRho led to a sudden and transient loss of worm motility dependent on cyclic adenosine monophosphate, whereas transient photoactivation of Gq signaling by (h)Mo enhanced worm locomotion dependent on phospholipase Cβ. These transgenic C. elegans models provide a unique way to study the consequences of Gi/o and Gq signaling in vivo with temporal and spatial precision and, by analogy, their relationship to human neuromotor function.—Cao, P., Sun, W., Kramp, K., Zheng, M., Salom, D., Jastrzebska, B., Jin, H., Palczewski, K., Feng, Z. Light-sensitive coupling of rhodopsin and melanopsin to Gi/o and Gq signal transduction in Caenorhabditis elegans.
Keywords: optogenetics, G-protein signaling
Membrane-bound G-protein-coupled receptors (GPCRs) transduce extracellular signals into intracellular physiological responses via their functional heterotrimeric G-protein partners. In the nervous system, these G-protein signals mediate neurotransmission that affects subsequent animal behaviors. However, the underlying molecular and cellular mechanisms of G-protein signaling in vivo are still poorly understood.
The soil-dwelling nematode Caenorhabditis elegans uses ∼1000 GPCRs (5% of its genome; ref. 1) expressed in its neurons to respond to environmental chemical, mechanical, and thermal stimuli, mediate synaptic function, reshape neural circuits, and modulate muscle activity, all of which affect its motor behavior. Components of the anciently evolved heterotrimeric G-protein-signaling pathways are highly conserved in C. elegans with respect to their protein sequences, functions, and signaling mechanisms. For example, there are 21 Gα, 2 Gβ, and 2 Gγ subunits in G proteins of C. elegans. Among the 21 α subunits, GSA-1, GOA-1, EGL-30, and GPA-12 are orthologs of the corresponding mammalian Gα families Gs, Gi/o, Gq and G12, respectively (2, 3). Together with its fully described shape, position, and connectivity of >300 neurons, C. elegans provides a unique model to study the cellular and molecular mechanisms of G-protein signaling (4, 5).
Because of its relatively simple genetics, C. elegans has been used to develop several models that allowed us to identify, quantify, and analyze many signaling components that regulate neuromuscular behaviors (6–8). In recent years, bacterial photoactivated channels (channel rhodopsin or ChR2; refs. 9–12), ion pumps (halorhodopsin or Halo/NpHR; refs. 13–16), and enzymes (photoactivated adenylyl cyclase or PAC; refs. 17, 18) were introduced into C. elegans to exert spatiotemporal control over excitation and inhibition of neurons or the onset of intra- and intercellular processes affecting specific behaviors. Approaches that introduced engineered protein chimeras of mammalian Rho and GPCRs (optoXRs; ref. 19), vertebrate rhodopsin (vRh; ref. 20), and a synthetic optogenetic transcription device (21) into mice were also used to control GPCR-mediated physiological processes. These optogenetic tools provide additional convenient experimental means and unparalleled opportunities to dissect cellular and molecular mechanisms regulating such behaviors (for review, see ref. 22). However, some intrinsic properties of native or engineered photoreceptive proteins can also limit their applications. For example, ChR2 and NpHR directly depolarize or hyperpolarize host neurons rather than indirectly affecting neuronal activity through other cellular processes. Whether optogenetic studies of PAC-induced increase in cytosolic concentrations of cyclic adenosine monophosphate (cAMP)- and OptoXR-stimulated Gs or Gq signaling apply to endogenous signaling pathways in live host organisms has yet to be shown. Therefore, establishing optogenetic approaches that can directly monitor heterogeneous GPCRs that functionally couple to specific C. elegans G proteins to affect downstream motor behavior would be highly desirable.
The mammalian opsin family members rhodopsin (Rho) and melanopsin (Mo) are photoreceptive GPCRs found in specialized rod cells or photoreceptive ganglion cells (ipGCs), respectively (23). On photoactivation, Rho couples to transducin (Gt) for visual signal transduction in vivo (24) and also to Gi/o in vitro (25), both of which belong to the Gi/o subfamily. In contrast, Mo is believed to couple to Gq for signaling that regulates circadian rhythms (23). Although C. elegans avoids lethal exposure to short-wavelength light (26, 27), this soil-inhabiting nematode does not possess vision. There are no known orthologs of Rho, Mo, or Gαt in the genome of C. elegans. Because Gi/o and Gq are conserved in the transparent body of C. elegans, it is possible to heterologously express photoreceptors and directly activate endogenous Gi/o and Gq pathways in this live organism, thus identifying G protein signaling pathway components with high spatial and temporal precision.
Here, we expressed bovine (b)opsin and human (h)Mo in the nervous system of C. elegans and used optogenetic tools to directly monitor (b)Rho and (h)Mo coupling to Gi/o and Gq signaling in vivo. We found that exposure to a pulse of low-dose visible light sufficed to trigger a sudden and transient loss of motility in (b)opsin-expressing animals, or initiate increased locomotion of (h)Mo-expressing worms. Both light-mediated motor behaviors depended on added 9-cis-retinal, an active chromophore of Rho, and required the presence of endogenous worm Gi/o and Gq-signaling components.
MATERIALS AND METHODS
C. elegans strains and maintenance
Bristol N2 strain C. elegans worms were used for this study. The loss-of-function mutants goa-1(sa734), egl-8(md1971), egl-30(md186), gsa-1(pk75), gpa-12(pk322), gpa-3(pk35), pde-6(ok3410), tax-2(ok3403), tax-4(p678), cng-1(jh111), and cng-3(jh113), the triple mutant cng-1(jh111);cng-3(jh113);tax-4(p678), and the quadruple mutant pde-1(nj57);pde-2(tm3098);pde-3(nj59);pde-5(nj49) were obtained from the Caenorhabditis Genetics Center (CGC; University of Minnesota, Minneapolis, MN, USA). Pde-4(nj60) was generated by crossing wild-type (WT) worms with the double-mutant pde-4(nj60);pde-6(ok3410) (provided by Dr. X. Z. Xu, University of Michigan, Ann Arbor, MI, USA). The quadruple mutant cng-1(jh111);cng-3(jh113);tax-2(ok3403);tax-4(p678) was generated by crossing the tax-2(ok3403) mutant with the triple mutant cng-1(jh111);cng-3(jh113);tax-4(p678). Each mutant worm line was crossed 3 times with WT if it had not been reported to be outcrossed extensively by the CGC.
Primers used in mutation screens and the mutated segments were as follows: goa-1(sa734): GCTGCACCACATACAGTGAGTGA (forward), ACGAAATATTCGGACGTTCTATGG (reverse), with an early stop mutation in aa52; egl-30(md186): CTGGCGGTGCCAGACTATCT (forward), TCGCTTAAAGCCACTATCTCCCT (reverse), with a D to E mutation in aa201; gpa-12(pk322): TCTGGAACGACGCTGCGA (forward), GTCTGTCTTCGAGAATCACCTGA (reverse), with a deletion spanning exon 2–8; gpa-3(pk35): CGGATTTACTGCAGAAGAGGC (forward), CATTCCATAATGCTTGAATGGC (reverse), with a deletion spanning exon 2–6 and a transposon insertion, Tc1; pde-4(nj60): GGGATATCACGTGGCTTTGGAG (forward), CCTTGACGCTAACACCGAACAC (reverse), with a deletion of exon 4 (isoform a) causing a frameshift; pde-6(ok3410): CAACTTAAAGATCTCGGCCACC (forward), GCTGACACAATCCCCACTCTC (reverse), with a deletion in exon 8 encoding most of the catalytic domain; tax-2(ok3403): GCAAATGCTTCAAAAGAGCC (forward), GAGTCCGAGCAATTCTGAAAA (reverse), with a deletion of ∼400 bp; tax-4(p678): TGCATACGACTACGGCTCAG (forward), GTAAAACTCACCACACACGTCC (reverse), with an amber stop mutation in aa82; cng-1(jh111): TCGGCTTGAGCACTGGATAT (forward), GAGCTCATAACAGAGGGACATACA (reverse), with a deletion spanning exon 8–13; cng-3(jh113): CAGTGACACTTCGTCGTGGGA (forward), TTTACGGGAATGAGATCGTGAG (reverse), with a deletion spanning exon 4–8; gsa-1(pk75): TTCTCACTTGCTGGAAAGACAA (forward), GGAGGAATAGGAGTGCTGTGTT (reverse), with a transposon-induced deletion of exon 7 causing a frameshift; The egl-8(md1971) mutant expressing (h)Mo in neurons was obtained by screening for the specific egl-8 phenotype.
Worms were maintained by standard methods that included culture on nematode growth medium (NGM; 0.25% peptone, 51 mM NaCl, 25 mM K3PO4, 5 μg/ml cholesterol, 1 mM CaCl2, and 1 mM MgCl2) plates with OP50 bacteria, cryostorage, and recovery from stocks. Compositions of media and solutions, as well as detailed protocols for their use, were previously described (28).
GPCR gene constructs and generation of transgenic (TG) worm lines
For GPCR expression constructs, the promoter of myo-3 (29) or H20 (30) was inserted into a pBluscript KS(+) vector at HindIII/Xbal or Pstl, respectively. Synthesized (Genescript USA, Piscataway, NJ, USA) codon-optimized (31) cDNAs encoding (b)opsin and (h)Mo or unoptimized cDNA encoding (h)5HT4Rb were engineered between Notl and Xhol. A Rho9 tag (32) was linked to the C-terminal of (h)Mo and (h)5HT4Rb. The ptx gene (provided by Dr. X. Z. Xu, University of Michigan; ref. 33) was subcloned into a PH20 construct with BamHI/NotI. The entire GPCR fusion protein coding region of each construct was sequenced to confirm the presence of the GPCR and the absence of random mutations. Sequence references for GPCRs are as follows: (b)opsin, NP_001014890; (h)Mo, NP_150598.1; and 5HT4Rb, NP_000861.
TG worm lines transiently expressing GPCRs and selected by DsRed visualization were generated by injecting GPCR constructs (10 ng/μl) and DNA encoding coral-derived red fluorescent protein DsRed (3 ng/μl) under control of the same promoter (either Pmyo-3 or PH20). The ptx gene was coinjected with green fluorescent protein driven by the same promoter (PH20). To integrate GPCR cDNA into the worm genome, identified TG worm lines were exposed to 350 × 100 μJ/cm2 ultraviolet light (Spectrolinker XL-1500; Spectronics Corp., Westbury, NY, USA) and F3 progeny of integrated TG lines were then screened and backcrossed to WT worms 3 times.
In vivo light-response assays
One day before these experiments, larval stage 4 (L4) animals raised at 20°C were transferred onto NGM plates seeded with 100 μl OP50 bacteria culture containing either DMSO vehicle control (no retinal), 10 μM 9-cis-retinal, or 10 μM all-trans-retinal (Toronto Research Chemicals, Toronto, ON, Canada). The resulting plates were wrapped with aluminum foil and stored in a cardboard box overnight at 20°C. Light-response experiments were carried out at 22°C in a dark room by using a Zeiss Stemi SV11-Apo microscope (Carl Zeiss, Oberkochen, Germany) mounted with a Kramer Universal Stereo Fluorescence Attachment and Cubes (USFAC) unit (Kramer Scientific, Amesbury, MA, USA), an Andor iXon DV897 electron-multiplying charge-coupled device (EMCCD) camera (Andor, South Windsor, CT, USA), and a ProScan II H117 motorized stage (Prior Scientific, Rockland, MA, USA). A ×1.6 objective lens in combination with a ×2.5 magnifying lens and 7 lux of transmitted white light was used for (b)opsin-expressing animals, or 5 lux of such light for (h)Mo-expressing animals, at the above hardware settings to visualize and track worms during experiments. For each light-response assay, a d 1 worm with an embedded platinum wire (an L4 worm raised overnight, becomes a young adult) was transferred onto an unseeded NGM plate (tracking plate); worms crawled vigorously under these conditions (34).
To measure the motor response to light of worms expressing (b)opsin in neurons, 1000 lux blue light (488±20 nm) was delivered after ∼5 s of control imaging to animals from a metal halide short arc bulb housed in an EXFO X-Cite 120PC-Q unit (Lumen Dynamics, Mississauga, ON, Canada) through a Kramer USFAC for 1 s, and animals were continuously imaged for another 6 min. To measure the light response of thrashing worms, 2 μl ddH2O was used to immerse animals, a procedure that initiated thrashing within 1 s (35). To measure the light response during reversals of direction, an anterior nose touch with a platinum wire was applied to trigger this response (36), if a spontaneous reversal did not occur. Before measuring the light response of worms expressing (h)Mo, animals on tracking plates (seeded with OP50 bacteria with or without 9-cis-retinal) were kept in the dark for 2 h. Worms then were imaged for 1 min, exposed to blue light (1000 lux, 488±20 nm) for 15 s, and then tracked for another 5 min. To measure the light response of egl-30 mutant worms expressing control, (b)opsin, or (h)Mo, such worms were pretreated with 2.5 μM PMA (LC Laboratories, Woburn, MA, USA) for 2 h before tracking. Worm locomotion before and after illumination was recorded in AVI movies at 30 Hz with a self-developed software package to capture images, control the onset and duration of illumination, and integrate this information. Light intensity output of the EXFO unit was calibrated to reach a targeted intensity (±5%) at the microscopic field, measured with a Macam L203 Photometer (MacamPhotometrics, Livingston, UK). Worm locomotion velocities were computed by a previously published algorithm (37). To score the degree of motor activity loss, images were analyzed frame by frame. A light-response index was defined and used as follows: 5 = complete lack of motion >10 s; 4 = complete lack of motion >10 s except for head shaking; 3 = lack of motion 2–10 s; 2 = lack of motion ≤2 s; 1= changed locomotion speed or direction; and 0 = no change noted in motor activity.
Spectrum of isoRho and Gt coupling assays
Reconstitution and purification of isoRho are described in the accompanying article (38). Purified recombinant (b)isoRho was scanned in a quartz cuvette with a Varian Cary 50 Bio UV-vis spectrophotometer (Varian, Santa Clara, CA, USA). The function of (b)isoRho purified from bovine retinas or worms was evaluated by a Gt activation fluorescence assay. At respective concentrations of 250 and 25 nM, the molar ratio of Gt to Rho was 10:1. Protein samples were diluted in 20 mM bis-tris-propane (BTP) buffer (pH 7.0), 120 mM NaCl, 2 mM MgCl2 and 1 mM n-dodecyl-β-d-maltoside (DDM), and then exposed to light for 15 s with a fiber light covered with a bandpass wavelength filter (480–520 nm). Reactions were carried out at 20°C in a continuously stirred cuvette. After 300 s of incubation, 5 μM GTPγS was added. Pseudo-first order kinetic rates (k) were derived from the function A(t) = Amax(1 − exp−kt), where Amax is the maximal Gαt fluorescence change, and A(t) is the relative fluorescence change at time t. The intrinsic fluorescence increase emanating from Gαt was measured with a Perkin Elmer L55 luminescence spectrophotometer (Perkin Elmer, Wellesley, MA, USA), by using excitation and emission wavelengths of 300 and 345 nm, respectively (39–41). No changes in tryptophan fluorescence were detected in control experiments without GTPγS.
Immunohistochemistry (IHC)
Age-synchronized d 1 or L4 animals from TG worm lines were sandwiched between two cover glasses, buried in dry ice for 30 min, and then fixed with 100% methanol (10 min), followed by 100% acetone (10 min). Worms then were washed with PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4·2H2O, and 1.76 mM KH2PO4, pH 7.4) for 0.5 h and incubated overnight at 4°C with PBS containing Alexa-488-conjugated anti-Rho 1D4 antibody and 0.1% Triton X-100. Stained worms were subsequently washed 3 times with PBS before examination by confocal microscopy. All experiments were done with a Leica TCS SP2 confocal microscope (Leica Microsystems, Bannockburn, IL, USA). Either live worms immobilized with 10 mM NaN3 on 2% agarose pads or methanol/acetone-fixed worms were used. Stains employed were DsRed (λex=543 nm; λem=580–630 nm) and Alexa-488 (λex=488 nm; λem=510–530 nm).
Statistical analyses
Statistical significance was analyzed with Statistica software (StatSoft, Tulsa, OK, USA), using t tests and ANOVA with Bonferroni corrections or Dunnet's post hoc analyses, as indicated in the figure legends.
RESULTS
Light induces a 9-cis-retinal-dependent sudden and transient loss of motility in C. elegans expressing (b)opsin in neurons
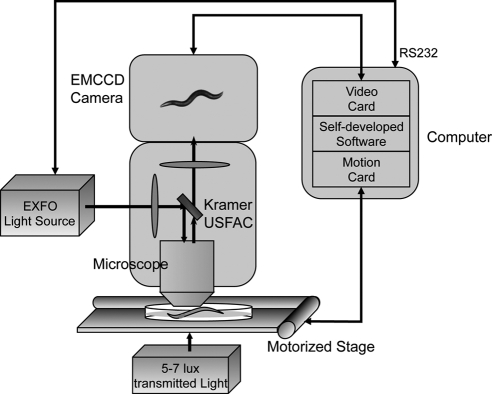
Because Rho and Mo are known to activate Gi/o and Gq in vitro, we introduced these photoreceptors into the nervous system of C. elegans, to determine whether worm Gi/o and Gq can couple to these ectopically expressed GPCRs in vivo. To detect directly any light-induced behavioral response of these GPCR TG animals, we modified the automated quantitative analysis of behavior of nematode (AQUABN) system, which simultaneously quantifies many aspects of worm locomotion (6–8), to facilitate delivery of light at a specific wavelength, intensity, and duration, and record worm motor activity under extremely dim illumination (Fig. 1).
Figure 1.
Diagram of the modified AQUABN system used to quantify the motor behavior of heterologous (b)Rho- and (h)Mo-expressing C. elegans. Light-response experiments were done with a Zeiss Stemi SV11-Apo fluorescent dissecting microscope mounted with a Kramer USFAC, an Andor iXon DV897 EMCCD camera, and a ProScan II H117 motorized stage. A ×1.6 objective lens combined with ×2.5 magnification lens, and 5 or 7 lux of transmitted white light was used with the listed hardware to visualize and track worms for motor functional analyses. Illumination of selected intensity and wavelength was delivered from a metal halide short-arc bulb housed in an EXFO X-Cite 120PC-Q unit through the Kramer USFAC. Worm movements before and after illumination were recorded as AVI movies at 30 Hz with a self-developed software package used to capture images, control the onset and duration of illumination, and integrate this information. The camera and stage were connected with peripheral component interconnect (PCI) cards located in the computer, and the light source was connected via a recommended standard (RS) 232 port.
TG worms expressing (b)opsin in neurons ([N] (b)opsin) were used initially to examine worm locomotion behavior. As described in the accompanying article (38), expression of (b)opsin was confirmed by IHC and immunoblots of worm cell lysates. Similar to WT animals, when these worms were touched with a platinum wire applied to their anterior or posterior, they responded with a vigorous reversal of direction (anterior) or increased forward locomotion (posterior), respectively (36). We then carried out a behavioral analysis to determine whether these TG worms exhibit any in vivo behavioral phenotype in response to light. Blue light (488±20 nm) was chosen as the stimulus because (b)Rho (11-cis-retinal-bound opsin) and (b)isoRho (9-cis-retinal-bound opsin) have a maximum absorbance at ∼500 nm and 485 nm, respectively (42). Moreover, it had been reported that exposure to low-energy 1000-lux blue light (∼1.5×10−3 mW/mm2) per se did not modify normal worm behavior (26, 27). Therefore, any behavioral change of TG worms in response to this stimulus should result from their expression of (b)opsin.
Before reaching the young adult d 1 stage, TG worms expressing (b)opsin in neurons were preincubated overnight in the dark with either 10 μM 9-cis-retinal (an active chromophore for opsin), 10 μM all-trans-retinal (the photoactivated isomerized product) or vehicle control (no retinal). Worms then were transferred to new NGM tracking plates for examination. Similar to WT animals that exhibit an initial locomotion acclimation period after changing conditions (7), these worms moved rapidly on transfer to the tracking plates and then slowed down. Strikingly, immediately after exposure to 1000 lux blue light for 1 s, fast-moving worms preincubated with 9-cis-retinal instantly were immobilized at their last body position on the plates and remained stationary for 20 min (Supplemental Movie S1A). This light-induced complete lack of motor activity and slow recovery was observed even when worms were either moving rapidly after stimulation by harsh touch before light exposure, crawling forward or backward, or changing direction.
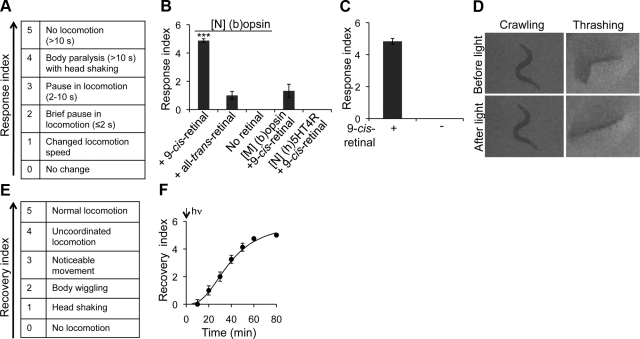
To quantify this behavioral phenotype, we used our response index to quantify the degree of lost motor function (Fig. 2A). If an animal completely ceased moving and held its posture for >10 s after light exposure, it was scored as a 5. If it stopped moving for >10 s except for wiggling its head, it was ranked as a 4. An animal that did not move for >2 but ≤10 s was given a 3, and a brief pause ≤2 s was scored as a 2. An animal that exhibited a change in motor behavior (speed or direction) but did not cease moving within 2 s after light exposure was rated as 1. Animals with no detectable motility change within 2 s were scored as 0. Compared to those with 9-cis-retinal pretreatment, TG worms expressing (b)opsin in neurons preincubated with control vehicle (no retinal) did not respond to blue light exposure (Fig. 2B and Supplemental Movie S1B). Moreover, worms from the same line preincubated with all-trans-retinal or those expressing (b)opsin in muscles preincubated with 9-cis-retinal failed to cease locomotion after light exposure (Fig. 2B). These results indicate that the sudden loss of motility in worms expressing (b)opsin was dependent on preincubation with 9-cis-retinal and specific to neurons. Moreover, worms expressing human serotonin receptor subtype 4 in neurons ([N] (h)5HT4R) preincubated with 9-cis-retinal did not display any obvious motor response under the same experimental conditions (Fig. 2B and Supplemental Movie S1C). Expression of (h)5HT4R in neurons was confirmed by immunoblotting of worm cell lysates (see accompanying article; ref. 38). This result further indicates that the sudden loss of worm motility was specifically due to the expression of (b)opsin rather than other GPCRs in neurons.
Figure 2.
Worms expressing (b)opsin in neurons evidence a sudden transient loss of motility upon light exposure. A) Light-response index employed. B) L4 TG animals expressing (b)opsin in neurons ([N] (b)opsin) or muscles ([M] (b)opsin), and TG animals expressing (h)5HT4R in neurons ([N] (h)5HT4R) were preincubated with either 10 μM 9-cis-retinal ([N] (b)opsin, [M] (b)opsin, and [N] (h)5HT4R), all-trans-retinal ([N] (b)opsin), or no retinal ([N] (b)opsin) overnight and then transferred onto unseeded NGM plates (tracking plates). Vigorously crawling TG animals were then exposed to blue light (1000 lux, 488±20 nm) for 1 s. Light-responsive motor behaviors of these animals were recorded and scored according to the response index. Data were derived from 3 independent experiments with 5–10 animals each. C) Vigorously thrashing TG animals expressing (b)opsin in neurons pretreated with or without 10 μM 9-cis-retinal were exposed to 1 s of blue light (1000 lux, 488±20 nm). Light-response behaviors were recorded and scored. Data were derived from 3 independent experiments with 4–10 animals each. D) Vigorously crawling or thrashing TG animals expressing (b)opsin in neurons pretreated with 10 μM 9-cis-retinal were exposed to blue light (1000 lux, 488±20 nm) for 1 s. Images were obtained just before light exposure (top panels) and immediately (0.5 s) after light exposure (bottom panels). E) Recovery index employed. F) Vigorously crawling TG animals expressing (b)opsin in neurons and preincubated with 10 μM 9-cis-retinal were exposed to blue light (1000 lux, 488±20 nm) for 1 s. Locomotion recovery of initially motionless animals was then scored at times indicated. Data were derived from 3 independent experiments with 3–8 animals each. Error bars indicate means ± se. ***P < 0.01; 1-way ANOVA with Bonferroni correction.
To determine the relationship between the degree of light exposure and the loss of mobility, TG worms expressing (b)opsin in neurons preincubated with 9-cis-retinal were exposed to blue light with illuminance ranging from 10 to 1000 lux for 1 s, and their motor responses were scored. As shown in Supplemental Fig. S1A, the loss of motility of these worms positively correlated with increasing blue light intensity, such that intensities approaching 1000 lux caused complete cessation of movement. In addition, preincubation with 10 μM 9-cis-retinal for a minimum of 40–80 min was sufficient to produce this maximal response to 1000 lux blue light (Supplemental Fig. S1B, C). These studies further support the relationship between the light-induced behavior of TG worms and the photoactivation of (b)isoRho, thereby establishing an experimental paradigm for subsequent mechanistic studies.
C. elegans exhibits two fundamentally different types of motor activity: crawling on a solid surface with an S-shaped movement pattern (slow muscle activity) and thrashing (swimming) in liquid medium with a C-shaped movement (fast muscle activity) (35). The underlying mechanisms controlling these two types of muscle activity are unknown. We found that blue light (1000 lux, 488±20 nm, 1 s) also effectively triggered an instant cessation of thrashing in TG worms expressing (b)opsin in neurons after their preincubation with 9-cis-retinal, but not with the vehicle control (no retinal; Fig. 2C and Supplemental Movie S1D, E). These data indicate that the sudden loss of motility exhibited by these TG worms was independent of their initial type of motor activity.
Both contracted or relaxed muscles can lead to apparent loss of muscle activity but by distinctly different underlying physiological and molecular mechanisms. Crawling worms expressing (b)opsin in neurons and preincubated with 9-cis-retinal promptly ceased motor activity upon light exposure and maintained their S-shaped body posture on the solid agar support (Fig. 2D, left panel). Whether this behavior resulted from muscle rigidity or relaxation was unclear. However, worms in liquid medium that promptly lost their thrashing motor behavior on light exposure gradually straightened and lengthened (Fig. 2D, right panel, and Supplemental Movie S1D). The latter behavior indicates that light-induced loss of motility of these TG worms in liquid medium resulted from muscle relaxation.
To learn whether these light-exposed inactive TG worms were seriously injured or even dead, we continuously observed completely inactive TG worms after 1 s blue light exposure every 10 min for total of 80 min under 7 lux transmitted white light. These animals started to shake their heads (scored as 1 by the recovery index) within 20 min, then wiggled their bodies (scored as 2), evidenced noticeable movement (scored as 3), exhibited uncoordinated locomotion (scored as 4), and finally, displayed normal locomotion (scored as 5) by 80 min (Fig. 2E, F, and Supplemental Movie S1A). Fully recovered worms also exhibited a touch response similar to WT animals, and no obvious behavioral, developmental, or reproductive defect was noted for the rest of their lives. Therefore, the light-induced sudden loss of motility in these TG worms was transient.
Taken together, worms expressing (b)opsin in neurons, when preincubated with 9-cis-retinal, exhibited a striking cessation of motility when exposed to 1000 lux blue light. This sudden loss of motility was transient and independent of the initial type of motor activity.
Characterization of (b)isoRho in vivo and in vitro
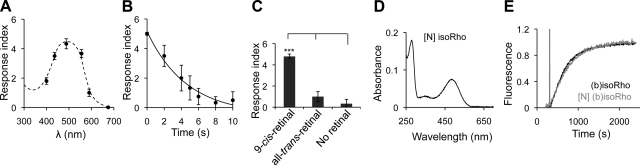
The above rapid motor response of 9-cis-retinal-pretreated TG worms expressing (b)opsin in neurons to blue light suggested that functional (b)isoRho was present in worm neurons. This encouraged us to characterize [N] (b)isoRho further by comparing its biochemical properties with those of (b)isoRho from its native source. To determine the wavelength that triggered the maximum light response, TG worms expressing (b)opsin in neurons and preincubated with 9-cis-retinal were exposed to 250 lux light at wavelengths ranging from 300–700 nm for 1 s, and their neuromotor responses were scored. The light-induced motility defect displayed a bell-shaped curve with a maximum at a wavelength of ∼490 nm, matching the absorbance spectrum of (b)isoRho purified from bovine retina (Fig. 3A).
Figure 3.
In vivo and in vitro functional analysis of recombinant (b)isoRho. A) Vigorously crawling TG animals expressing (b)opsin in neurons and preincubated with 10 μM 9-cis-retinal were exposed to light (250 lux) at indicated wavelengths, and their motor responses were recorded and scored (y axis). Data were derived from 3 independent experiments with 5–10 animals each. B) TG animals expressing (b)opsin in neurons and preincubated with 10 μM 9-cis-retinal were exposed to 1000 lux blue light for 0, 2, 4, 5, 6, 8, and 10 s. Control (0 s) and motionless animals then were transferred to regular NGM plates seeded with OP50 bacteria to recover for 2 h. Recovered animals were exposed to blue light again (1000 lux, 488±20 nm) for 1 s, and their light responses were recorded as shown. Data were derived from 3 independent experiments with 3–8 animals each. C) TG animals expressing (b)opsin in neurons and preincubated with 10 μM 9-cis-retinal were exposed to blue light (1000 lux, 488±20 nm) for 10 s. Motionless animals then were transferred to a OP50 bacteria seeded NGM plate containing 10 μM 9-cis-retinal, 10 μM all-trans-retinal, or no retinal to recover for 2 h. Recovered animals were tested for their responses to blue light (1000 lux, 488±20 nm, 1 s). Data were derived from 3 independent experiments with 5–10 animals each. D) Absorbance spectrum of recombinant (b)isoRho purified from TG worms expressing (b)opsin in neurons ([N] (b)isoRho). E) Fluorescence assay of Gαt activation by (b)isoRho and [N] (b)isoRho purified from bovine retina or from TG worms expressing (b)opsin in neurons, respectively. Fluorescence change was normalized for both traces. Error bars indicate means ± se. ***P < 0.001; 1-way ANOVA with Bonferroni correction.
In rod cells, 11-cis-retinal binds to opsin to form ground-state Rho in the dark (24). Absorption of a photon of light by Rho causes photoisomerization of 11-cis-retinal to all-trans-retinal, resulting in a major conformational change of the photopigment that leads to activation of Gt within seconds and release of all-trans-retinal in minutes (43, 44). Within hours, the resulting apo-(b)opsin binds 11-cis-retinal produced by the retinoid cycle, regenerating Rho (24). To further test whether worm [N] (b)isoRho can be regenerated after light exposure, TG worms expressing (b)opsin in neurons and preincubated with 9-cis-retinal were exposed to blue light (1000 lux, 488±20 nm) for up to 10 s and then transferred to freshly seeded NGM plates without a retinal supplement for 2 h, to allow complete recovery of locomotor function. These recovered TG worms were next exposed to blue light again for 1 s, and their motor responses were scored. As shown in Fig. 3B, this light-response index displayed an inverse relationship to their period of previous exposure to blue light. That is, a 10-s previous exposure to light completely abolished a locomotion response to the second brief light stimulus. This finding indicates that once a TG worm takes up 9-cis-retinal and converts it to all-trans-retinal in response to light, it is unable to convert this retinoid back into the photosensitive cis form under these experimental conditions. Consistent with this observation, if worms recovered from the first light exposure in plates that contained 9-cis-retinal, but not all-trans-retinal, they did respond to the second light stimulus (Fig. 3C). These findings further confirm that (b)opsin expressed in worms is fully functional in vivo.
As described in the accompanying article (38), [N] (b)isoRho can be purified from TG worms. To examine whether [N] (b)isoRho is functional in vitro, the absorption spectrum of purified worm recombinant [N] (b)isoRho was determined and found to display its expected ∼485-nm maximum absorbance (Fig. 3D). Because purified (b)isoRho from bovine retina can activate Gt (45), a fluorescence-based assay was used to monitor guanyl-nucleotide exchange in the α subunit of Gt that occurs on its activation. As shown in Fig. 3E, recombinant [N] (b)isoRho purified from worms did activate Gt. Moreover, the rates of Gt activation promoted by reconstituted/purified (b)isoRho from bovine retina and recombinant [N] (b)isoRho from worms were similar, k = 2.64 ± 0.08 × 10−3 · s−1 and 2.07 ± 0.06 × 10−3 · s−1, respectively. These observations strongly indicate that the (b)isoRho expressed in worm neurons was functional in vivo and in vitro.
Photoactivated (b)isoRho activates Gi/o in vivo
C. elegans contains only one known ortholog of the mammalian Gi/o α subunit, GOA-1(NP_492108), which shares an 83% identical amino acid sequence with both the a and b isoforms of human Go subunit α (GNAO1, NP_066268.1, and NP_620073.2, respectively). Thus, we hypothesized that expressed (b)isoRho would activate endogenous GOA-1, leading to a sudden and transient loss of worm motility.
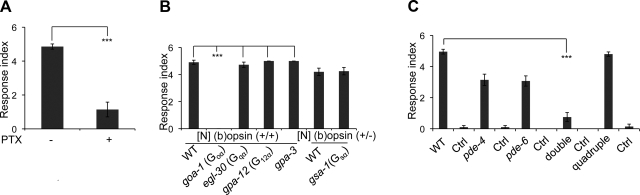
To test this idea, we first determined whether blocking GOA-1 function would inhibit the light-induced motility response. cDNA encoding pertussis toxin (PTX), a peptide that selectively inhibits Gi/o function (33, 46), was introduced into the nervous system of TG worms expressing (b)opsin. PTX incorporation then was found to abolish the light response of these worms preincubated with 9-cis-retinal (Fig. 4A), indicating that photoactivation of (b)isoRho in worm neurons had indeed stimulated worm Gi/o signaling. To further examine whether this signaling occurred specifically through Gi/o, the [N] (b)opsin TG worm line was crossed with loss-of-function mutants of goa-1, egl-30 (47, 48), gpa-12 (49, 50), and gsa-1 (51), with gene products that encode the worm α subunits of Go, Gq, G12, and Gs, respectively. Because the homozygous gsa-1 loss-of-function mutant is a larval lethal mutant, a heterozygous (gsa-1+/−) worm was produced instead. The resulting [N] (b)opsin-expressing-heterozygous (gsa-1) and homozygous (goa-1, egl-30, and gpa-12) worms were then tested for their responses to light. Because worms bearing the loss-of-function egl-30 mutation were lethargic, they were supplemented with phorbol 12-myristate 13-acetate (PMA), an activator of Gq downstream protein kinase C (PKC), 2 h before tracking (52) to promote WT- or near WT-locomotive behavior. As expected, [N] (b)opsin-expressing-goa-1 mutant worms did not respond to the light stimulus, indicating that disruption of Gi/o function inhibited (b)isoRho signaling (Fig. 4B and Supplemental Movie S1F). In contrast, light induced a marked and robust loss of motility in [N] (b)opsin-expressing egl-30, gsa-1, and gpa-12 mutant animals, comparable to that seen in (b)opsin-expressing WT worms (Fig. 4B). This observation supports the idea that Gi/o signaling was specifically activated. Additional experiments revealed that worms expressing (b)opsin in loss-of-function mutant gpa-3, whose gene product encodes a weak mammalian Gi/o α subunit homologue that mediates the response to lethal doses of short-wavelength light (33), responded to light similarly to WT animals (Fig. 4B). The above pharmacological and genetic evidence indicates that photoactivation of (b)isoRho in neurons specifically activates worm Gi/o, and not other G proteins.
Figure 4.
Light induces activation of Go/i and cAMP-specific PDEs in TG worms expressing (b)opsin in neurons. A) TG animals ([N] (b)opsin) with transient pan-neuronal expression of PTX (+) or control vector (−) were pretreated with 10 μM 9-cis-retinal and tested for their motor response to blue light (1 s, 1000 lux, 488±20 nm). Data were derived from 3 independent experiments with 10 animals each. ***P < 0.001; t test. B) TG animals ([N] (b)opsin) were crossed with loss-of-function mutants goa-1 (Gαo), egl-30 (Gαq), gpa-12 (Gα12), and gsa-1 (Gαs) worms and gpa-3 worms, which share less homology with mammalian Gi/o. The resulting homozygous (+/+) or heterozygous (+/−) progeny were tested for their response to blue light (1 s, 1000 lux, 488±20 nm). Data were derived from 3 independent experiments with 5–10 animals each. ***P < 0.001; 1-way ANOVA with Dunnett's correction. C) TG animals with transient pan-neuronal expression of (b)opsin in WT, pde-4 loss-of-function mutant, pde-6 loss-of-function mutant, pde-4;pde-6 loss-of-function mutant, and pde-1;pde-2;pde-3;pde-5 loss-of-function mutant worms were pretreated with 10 μM 9-cis-retinal and tested for their responses to blue light (1 s, 1000 lux, 488±20 nm). Controls for each sample were worms cultured in the same plate that did not express the coinjected marker, DsRed. Data were derived from 3 independent experiments with 10–15 animals each. ***P < 0.001; t test. Error bars indicate means ± se.
cAMP-specific PDEs are required for Gi/o-mediated signaling
cAMP and cyclic guanosine monophosphate (cGMP) are important second messengers that enable downstream G-protein signaling. Levels of cAMP and cGMP are regulated by phosphodiesterases (PDEs) with activities that are, in turn, regulated by G proteins. In photoreceptor cells, Rho activates Gt, which then stimulates PDEs to cleave cGMP, thereby causing decreased levels of cGMP that then lead to closure of cyclic nucleotide-gated (CNG) channels (53).
The pathway Gi/o employed for downstream signaling in [N] (b)opsin expressing TG worms had not been identified. In C. elegans, there are 4 cGMP-specific PDEs (PDE-1, PDE-2, PDE-3, and PDE-5) and 2 cAMP-specific PDEs (PDE-4 and PDE-6), all of which share high identity with human PDEs (33, 54). To examine which pathway is responsible for Gi/o-mediated signaling in these TG worms, a quadruple(pde-1;pde-2;pde-3;pde-5) loss-of-function mutant, a double(pde-4;pde-6) loss-of-function mutant, a pde-4 loss-of-function mutant, and a pde-6 loss-of-function mutant worm line, each expressing (b)opsin in its nervous system, were generated. This was accomplished by injecting the (b)opsin DNA construct into double and quadruple mutants, or by genetic crossing the single mutants with the neuronal (b)opsin expressing TG worm line (see Materials and Methods). As shown in Fig. 4C, [N] (b)opsin expressing-pde-4;pde-6 double mutant worms exhibited a significantly reduced light-stimulated response, indicating that inhibition of cAMP disrupted Gi/o-mediated signaling. IHC staining confirmed that (b)opsin was expressed in mutant pde-4;pde-6 worm neurons (data not shown). Consistently, [N] (b)opsin expressing-pde-4 or pde-6 single mutants also displayed reduced light responses, but to a lesser extent (Fig. 4C). In contrast, the [N] (b)opsin-expressing pde-1;pde-2;pde-3;pde-5 quadruple mutant line displayed a light-triggered response similar to that of [N] (b)opsin-expressing WT worms (Fig. 4C), demonstrating that cGMP does not play a role in Gi/o-mediated signaling. Therefore, cAMP-specific PDEs are involved in (b)isoRho coupled Gi/o signaling in [N] (b)opsin-expressing worms.
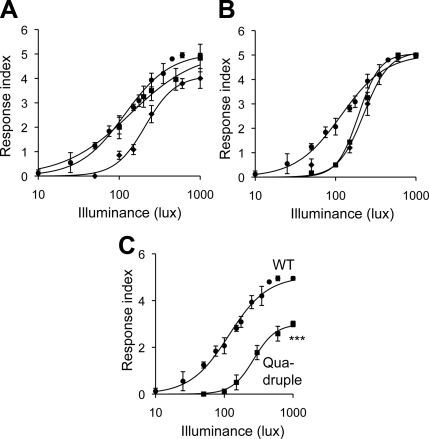
But which CNG is specifically regulated by cAMP? To test the possible role of CNG ion channels in Gi/o-mediated signaling, we introduced (b)opsin DNA into cng-1, cng-3, tax-2, or tax-4 loss-of-function mutant, and quadruple(cng-1;cng-3;tax-2;tax-4) loss-of-function mutant worms. All four genes are currently known to encode CNGs that are homologues of their mammalian counterparts (55). Functional absence of either cng-1, cng-3, or tax-2 partially blocked the light-induced loss of motility (Fig. 5A, B); moreover, light-induced loss of motility was markedly reduced in the [N] (b)opsin-expressing quadruple mutant (cng-1;cng;3;tax2;tax4) worm (Fig. 5C). These data indicate that CNG channels play a role in (b)isoRho coupled Gi/o signaling.
Figure 5.
Light responses of loss-of-function tax-2, tax-4, cng-1, and cng-3 mutant worms expressing (b)opsin in neurons. A, B) WT worms with neuronal transgenic expression of (b)opsin (circles; A, B) or worms with loss-of-function mutants tax-2 (diamonds; A), tax-4 (squares; A), cng-1 (diamonds; B) or cng-3 (squares; B) expressed in neurons were preincubated with 9-cis-retinal and tested for their responses to blue light (1 s, 1000 lux, 488±20 nm). Data were derived from 3 independent experiments with 5–11 animals each. C) WT worms and quadruple loss-of-function mutant of cng-1;cng-3;tax-2;tax-4 worms, both with pan-neuronal expression of (b)opsin, were tested for their response to blue light (1 s, 1000 lux, 488±20 nm). Data represent 3 independent experiments with 3–10 animals each. Error bars indicate means ± se. ***P < 0.001; 2-way ANOVA.
Enhanced light-induced locomotion is mediated by Gq in worms expressing (h)Mo in neurons
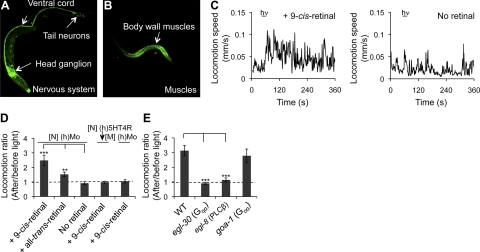
By simply monitoring this on and off phenotypic motility switch, we were able to identify heterotrimeric Gi/o proteins in live worms that directly coupled to (b)isoRho activated by light. In addition, this protocol allowed us to dissect the involved Gi/o downstream signaling pathways in vivo. To examine whether the same optogenetic tool was sufficiently sensitive to carry out functional studies of other heterologously expressed GPCRs, we then tested the light-induced responses of TG worms expressing (h)Mo, a GPCR in the opsin family believed to couple to Gq. Similar to (b)opsin, (h)Mo was expressed in the C. elegans nervous system under control of PH20 and in muscles under control of Pmyo-3 (Fig. 6A, B). TG worms expressing (h)Mo in neurons were slightly hyperactive in locomotion, whereas TG worms expressing (h)Mo in muscles did not display a phenotype obviously different from WT controls. To determine the light-induced response, L4 TG larvae expressing (h)Mo in neurons were preincubated with either 10 μM 9-cis-retinal, 10 μM all-trans-retinal, or control vehicle (no retinal) overnight in the dark and then transferred to a new NGM tracking plate. Unlike worms expressing (b)opsin in neurons that ceased motor activity in response to blue light, TG worms expressing (h)Mo in neurons and preincubated with 9-cis-retinal failed to show any significant change in their rapid locomotion on light exposure. However, if these TG worms were incubated on the tracking plates seeded with OP50 bacteria for 2 h in dark before light stimulation, most worms slowed their motor activity during this period. On stimulation by blue light (1000 lux, 488±20 nm, 15 s), locomotion of worms preincubated with 10 μM 9-cis-retinal but not control vehicle (no retinal), was enhanced (Fig. 6C and Supplemental Movie S2A, B). Compared to [N] (h)Mo worms, [M] (h)Mo and [N] (h)5HT4R worms preincubated with 9-cis-retinal did not respond to the light stimulus under these experimental conditions (Fig. 6D and Supplemental Movie S2C), indicating that this response was specific to (h)Mo in neurons. Interestingly, [N] (h)Mo worms partially responded to preincubation with all-trans-retinal (Fig. 6D), possibly because Mo either has intrinsic isomerase activity and/or can react with both cis- and trans-retinoids (56).
Figure 6.
Worms expressing (h)Mo in neurons display enhanced locomotion via Gq signaling. A, B) Day 1 or L4 TG animals were fixed with methanol and acetone, permeabilized with TritonX-100, stained with Alexa-488-conjugated 1D4 mAb, and subjected to confocal microscopy. A) Representative Alexa-488-conjugated 1D4 mAb fluorescent image resulting from IHC of an L4 TG animal expressing (h)Mo in neurons. 1D4 mAb stained the ventral cord, as well as many neurons in the head ganglion, tail ganglion, and middle sections. 1D4 mAb also highlighted the nose tip. B) Representative Alexa-488-conjugated 1D4 mAb fluorescent image resulting from IHC analysis of a L4 TG animal expressing (h)Mo in muscles. 1D4 mAb-stained body wall muscles. C–E) Day 1 TG animals expressing human Mo in neurons ([N] (h)Mo) or muscles ([M] (h)Mo) with or without 9-cis-retinal pretreatment were transferred onto OP50-containing NGM plates seeded or not seeded with 9-cis-retinal and kept in dark for 2 h. Animals then were imaged for 1 min, exposed to blue light (1000 lux, 488 ± 20 nm) for 15 s, and tracked for another 5 min. C) Left panel: locomotion speed trace of a TG animal ([N] (h)Mo) with 9-cis-retinal treatment. Right panel: locomotion speed trace of a TG animal ([N] (h)Mo) without retinal treatment. D) Locomotion ratios (average locomotion speed of an animal at 1 min after vs. 1 min before light exposure) of TG animals expressing either Mo in neurons ([N] (h)Mo) or muscles ([M] (h)Mo), or (h)5HT4R in neurons ([N] (h)5HT4R) and preincubated with 10 μM 9-cis-retinal, 10 μM all-trans-retinal, or no retinal. Data were derived from 3 independent experiments with 4–6 animals each. E) Locomotion ratios of WT and loss-of-function egl-30, egl-8, and goa-1 mutant animals expressing Mo in neurons and preincubated with 10 μM 9-cis-retinal. Data were derived from 3 independent experiments with 4–6 animals each. Error bars indicate means ± se. **P < 0.01, ***P < 0.001; t test.
Mammalian Mo activates Gq, which, in turn, activates PLCβ (57, 58). C. elegans Gqα EGL-30 (NP_001021574.1) shares 82% identical amino acid sequence with human GNAQ (NP_002063.2). To determine whether activated (h)Mo specifically couples to Gq and subsequently activates PLCβ, (h)Mo was expressed in neurons of egl-30 (Gqα), egl-8 (PLCβ), and goa-1(Goα) loss-of-function mutant worms, and the ensuing light-response assays were analyzed. As with the protocol described above, [N] (h)Mo-expressing egl-30 mutant worms were supplemented with PMA for 2 h before tracking to achieve a locomotor behavior similar to WT. As seen in Fig. 6E, light exposure enhanced the locomotion of both (h)Mo-expressing WT and goa-1 mutant worms, but not (h)Mo-expressing egl-30 or egl-8 mutant worms (Fig. 6E and Supplemental Movie S2D, E), thereby demonstrating that (h)Mo expressed in C. elegans neurons directly activated Gq and subsequently PLCβ in response to light.
DISCUSSION
In this study, (b)opsin and (h)Mo were expressed in the nervous system of C. elegans, and activation of these photoreceptors led to their coupling to Gi/o and Gq signaling pathways, respectively, with temporal precision. Activation of Gi/o signaling in worm neurons resulted in a sudden but transient loss of motility of rapidly moving TG animals, whereas activation of Gq signaling in neurons enhanced locomotion of acclimated slow-moving TG animals. Notably, our optogenetic tools combined with behavioral, genetic, and biophysical evidence demonstrated conservation of photochemical and signaling mechanisms in C. elegans. For example, the so-called Rho cycle of visual transduction could be detected in [N] opsin-expressing worms by analysis of their motor function in vivo and by the absorbance spectrum and Gt coupling experiments with recombinant (b)isoRho purified from these worms in vitro. In [N] (h)Mo-expressing TG worms, 9-cis-retinal was also observed to be required for robust Mo-mediated initiation of locomotion. This finding is consistent with a recent report that Mo obtained from murine ipGCs in the dark state bound to 11-cis-retinal, which was converted into all-trans-retinal after light exposure (56). The functional output induced by endogenous Gi/o or Gq signaling in host animals was tightly associated with precise coupling of the G protein with the ectopically expressed photoreceptor. Thus, we conclude that photoreceptive Rho and Mo can be used to elicit specific Gi/o or Gq signaling pathways in C. elegans.
More than 800 human GPCRs are involved in many key biological processes. However, humans only have 16 distinct Gα genes encoding 23 different isoforms that belong to 4 major classes of heterotrimeric G proteins, Gi/o, Gs, G12, and Gq. A common GPCR-activated mechanism is shared by all heterotrimeric G proteins, although each Gα protein engages distinct downstream effectors. Recent studies indicate that the exact downstream effectors and the physiological outputs of the same G-protein signaling pathway can vary, depending on the physiological properties of the cells where G-protein signals are elicited, as well as the cellular/signaling connectivity of these cells (59). Identification of downstream effectors and the consequent physiological output of a specific Gα signal in a given context is critical for understanding how a variety of GPCRs regulate distinct biological processes through a limited number of heterotrimeric G proteins. C. elegans uses >1000 GPCRs to sense a variety of environmental stimuli, mediate synaptic function, reshape neural circuits and modulate muscle activity (1). Similar to mammals, worms have only 21 Gα proteins that presumably couple to all these GPCRs (3). Although worms with loss-of-function or gain-of-function mutations in genes encoding components of Gi/o and Gq pathways provide useful models to study signaling by these highly conserved G proteins, transient and specific activation of their pathways with spatiotemporal precision provides an unparalleled approach to dissect the mechanisms of GPCR and G protein signaling at both cellular and molecular levels. Our optogenetic tools present just such an opportunity. In the future, we will express mammalian opsins in specific C. elegans cells to manipulate neuromuscular activity. Combined with classical genetics and electrophysiological methods (60), this approach will greatly facilitate understanding of G-protein signaling mechanisms.
Here we found that exogenous 9-cis-retinal, but not all-trans-retinal, is required for a full light-mediated neuromuscular response in both [N] (b)opsin- and [N] (h)Mo-expressing TG worms. Therefore, it appears unlikely that worms contain visual cycle components that convert all-trans-retinal to a retinal with a cis double bond. But unlike Rho, the photochemistry of Mo, an important retinylidene GPCR that regulates circadian rhythms, the pupillary light reflex and other nonvisual responses to light, is poorly understood. Our [N] Mo-expressing TG worms and neuromotor quantification paradigm provide an in vivo experimental means to investigate certain photochemical aspects of [N] Mo signaling. For example, this model could be used to test the hypothesis that Mo is a bistable photopigment with intrinsic photoisomerase activity that uses light to convert trans-cis and cis-trans isomers of the chromophore. Partial activity of [N] (h)Mo-expressing TG animals treated with all-trans-retinal could indicate that (h)Mo is a bistable photopigment. Recombinant (h)Mo expressed in C. elegans could be purified to test this possibility as well as to pursue other biophysical studies (see accompanying article; ref. 38).
Notably, the onset of Gi/o and downstream cAMP signaling in worm neurons resulted in a dramatic, sudden, and transient loss of motility. This on-and-off behavior was independent of the previous type of motor activity. More studies are needed to characterize further the molecular and cellular mechanisms underlying this intriguing phenotype. Genetic and pharmacological screens will also be performed to identify downstream Gi/o signaling components that regulate this motor activity along with small molecules that affect it.
Supplementary Material
Acknowledgments
The authors thank L. T. Webster for critical comments on the manuscript.
This research was supported in part by grants GM083241 (to Z.F.), EY008061, EY009339 and P30 EY11373 (to K.P., co-principal investigator) from the U.S. National Institutes of Health, and Mt. Sinai Health Care Foundation Scholars Program in the Basic Science (to Z. F.). Research was also supported by a U54 award to the New York SGX Research Center for Structural Genomics (NYSGXRC) from the National Institute of General Medical Sciences (GM074945; principal investigator: Stephen K. Burley) under a contract to Polgenix, Inc. D.S. and W.S. are employees of Polgenix, Inc. K.P. is CSO at Polgenix, Inc. P.C., K.K., B.J., H.J., and Z.F. report no conflicts of interest. The Z.F. laboratory received support from Polgenix, Inc.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Bargmann C. I. (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282, 2028–2033 [DOI] [PubMed] [Google Scholar]
- 2. Lochrie M. A., Mendel J. E., Sternberg P. W., Simon M. I. (1991) Homologous and unique G protein alpha subunits in the nematode Caenorhabditis elegans. Cell Regul. 2, 135–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bastiani C., Mendel J. (2006) Heterotrimeric G proteins in C. elegans. WormBook doi: 10.1895/wormbook.1.75.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mendel J. E., Korswagen H. C., Liu K. S., Hajdu-Cronin Y. M., Simon M. I., Plasterk R. H., Sternberg P. W. (1995) Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 267, 1652–1655 [DOI] [PubMed] [Google Scholar]
- 5. Segalat L., Elkes D. A., Kaplan J. M. (1995) Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science 267, 1648–1651 [DOI] [PubMed] [Google Scholar]
- 6. Li W., Feng Z., Sternberg P. W., Xu X. Z. (2006) A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng Z., Li W., Ward A., Piggott B. J., Larkspur E. R., Sternberg P. W., Xu X. Z. (2006) A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 127, 621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu A. L., Feng Z., Hsieh M. Y., Xu X. Z. (2009) Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol. Aging 30, 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E. (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U. S. A. 100, 13940–13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyden E. S., Zhang F., Bamberg E., Nagel G., Deisseroth K. (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 [DOI] [PubMed] [Google Scholar]
- 11. Zhang F., Wang L. P., Boyden E. S., Deisseroth K. (2006) Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3, 785–792 [DOI] [PubMed] [Google Scholar]
- 12. Stirman J. N., Crane M. M., Husson S. J., Wabnig S., Schultheis C., Gottschalk A., Lu H. (2011) Real-time multimodal optical control of neurons and muscles in freely behaving Caenorhabditis elegans. Nat. Methods 8, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han X., Boyden E. S. (2007) Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One 2, e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang F., Wang L. P., Brauner M., Liewald J. F., Kay K., Watzke N., Wood P. G., Bamberg E., Nagel G., Gottschalk A., Deisseroth K. (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 [DOI] [PubMed] [Google Scholar]
- 15. Chow B. Y., Han X., Dobry A. S., Qian X., Chuong A. S., Li M., Henninger M. A., Belfort G. M., Lin Y., Monahan P. E., Boyden E. S. (2010) High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leifer A. M., Fang-Yen C., Gershow M., Alkema M. J., Samuel A. D. (2011) Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat. Methods 8, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weissenberger S., Schultheis C., Liewald J. F., Erbguth K., Nagel G., Gottschalk A. (2011) PACalpha- an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J. Neurochem. 116, 616–625 [DOI] [PubMed] [Google Scholar]
- 18. Ryu M. H., Moskvin O. V., Siltberg-Liberles J., Gomelsky M. (2010) Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J. Biol. Chem. 285, 41501–41508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Airan R. D., Thompson K. R., Fenno L. E., Bernstein H., Deisseroth K. (2009) Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 [DOI] [PubMed] [Google Scholar]
- 20. Gutierrez D. V., Mark M. D., Masseck O., Maejima T., Kuckelsberg D., Hyde R. A., Krause M., Kruse W., Herlitze S. (2011) Optogenetic control of motor coordination by Gi/o protein-coupled vertebrate rhodopsin in cerebellar Purkinje cells. J. Biol. Chem. 286, 25848–25858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye H., Daoud-El Baba M., Peng R. W., Fussenegger M. (2011) A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332, 1565–1568 [DOI] [PubMed] [Google Scholar]
- 22. Deisseroth K. (2011) Optogenetics. Nat. Methods 8, 26–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terakita A. (2005) The opsins. Genome Biol. 6, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palczewski K. (2006) G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stryer L., Bourne H. R. (1986) G proteins: a family of signal transducers. Annu. Rev. Cell Biol. 2, 391–419 [DOI] [PubMed] [Google Scholar]
- 26. Ward A., Liu J., Feng Z., Xu X. Z. (2008) Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 11, 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edwards S. L., Charlie N. K., Milfort M. C., Brown B. S., Gravlin C. N., Knecht J. E., Miller K. G. (2008) A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6, e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stiernagle T. (2006) Maintenance of C. elegans. WormBook doi: 10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A. (1993) Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135, 385–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yabe T., Suzuki N., Furukawa T., Ishihara T., Katsura I. (2005) Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development 132, 3197–3207 [DOI] [PubMed] [Google Scholar]
- 31. Stenico M., Lloyd A. T., Sharp P. M. (1994) Codon usage in Caenorhabditis elegans: delineation of translational selection and mutational biases. Nucleic Acids Res. 22, 2437–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salom D., Wu N., Sun W., Dong Z., Palczewski K., Jordan S., Salon J. A. (2008) Heterologous expression and purification of the serotonin type 4 receptor from transgenic mouse retina. Biochemistry 47, 13296–13307 [DOI] [PubMed] [Google Scholar]
- 33. Liu J., Ward A., Gao J., Dong Y., Nishio N., Inada H., Kang L., Yu Y., Ma D., Xu T., Mori I., Xie Z., Xu X. Z. (2010) C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat. Neurosci. 13, 715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sawin E. R., Ranganathan R., Horvitz H. R. (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 [DOI] [PubMed] [Google Scholar]
- 35. Pierce-Shimomura J. T., Chen B. L., Mun J. J., Ho R., Sarkis R., McIntire S. L. (2008) Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc. Natl. Acad. Sci. U. S. A. 105, 20982–20987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Way J. C., Chalfie M. (1989) The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 3, 1823–1833 [DOI] [PubMed] [Google Scholar]
- 37. Zhang S., Jin W., Huang Y., Yang J., Feng Z. (2011) Profiling a Caenorhabditis elegans behavioral parametric dataset with a supervised K-means clustering algorithm identifies genetic networks regulating locomotion. J. Neurosci. Methods 197, 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salom D., Cao P., Sun W., Kramp K., Jastrzebska B., Jin H., Feng Z., Palczewski K. (2011) Heterologous expression of functional G-protein-coupled receptors in Caenorhabditis elegans. [E-pub ahead of print] FASEB J. doi: 10.1096/fj.11–197780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fahmy K., Sakmar T. P. (1993) Regulation of the rhodopsin-transducin interaction by a highly conserved carboxylic acid group. Biochemistry 32, 7229–7236 [DOI] [PubMed] [Google Scholar]
- 40. Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 41. Heck M., Hofmann K. P. (2001) Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J. Biol. Chem. 276, 10000–10009 [DOI] [PubMed] [Google Scholar]
- 42. Spalink J. D., Reynolds A. H., Rentzepis P. M., Sperling W., Applebury M. L. (1983) Bathorhodopsin intermediates from 11-cis-rhodopsin and 9-cis-rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 80, 1887–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kiser P. D., Golczak M., Maeda A., Palczewski K. (2011) Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim. Biophys. Acta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jastrzebska B., Fotiadis D., Jang G. F., Stenkamp R. E., Engel A., Palczewski K. (2006) Functional and structural characterization of rhodopsin oligomers. J. Biol. Chem. 281, 11917–11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Darby C., Falkow S. (2001) Mimicry of a G protein mutation by pertussis toxin expression in transgenic Caenorhabditis elegans. Infect. Immun. 69, 6271–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brundage L., Avery L., Katz A., Kim U. J., Mendel J. E., Sternberg P. W., Simon M. I. (1996) Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron 16, 999–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hajdu-Cronin Y. M., Chen W. J., Patikoglou G., Koelle M. R., Sternberg P. W. (1999) Antagonism between G (o) alpha and G (q) alpha in Caenorhabditis elegans: the RGS protein EAT-16 is necessary for G (o) alpha signaling and regulates G (q) alpha activity. Genes Dev. 13, 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yau D. M., Yokoyama N., Goshima Y., Siddiqui Z. K., Siddiqui S. S., Kozasa T. (2003) Identification and molecular characterization of the G alpha12-Rho guanine nucleotide exchange factor pathway in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 100, 14748–14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Linden A. M., Moorman C., Cuppen E., Korswagen H. C., Plasterk R. H. (2003) Hyperactivation of the G12-mediated signaling pathway in Caenorhabditis elegans induces a developmental growth arrest via protein kinase C. Curr. Biol. 13, 516–521 [DOI] [PubMed] [Google Scholar]
- 51. Korswagen H. C., Park J. H., Ohshima Y., Plasterk R. H. (1997) An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev. 11, 1493–1503 [DOI] [PubMed] [Google Scholar]
- 52. Lackner M. R., Nurrish S. J., Kaplan J. M. (1999) Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24, 335–346 [DOI] [PubMed] [Google Scholar]
- 53. Polans A., Baehr W., Palczewski K. (1996) Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends Neurosci. 19, 547–554 [DOI] [PubMed] [Google Scholar]
- 54. Charlie N. K., Thomure A. M., Schade M. A., Miller K. G. (2006) The Dunce cAMP phosphodiesterase PDE-4 negatively regulates G alpha (s)-dependent and G alpha (s)-independent cAMP pools in the Caenorhabditis elegans synaptic signaling network. Genetics 173, 111–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cho S. W., Cho J. H., Song H. O., Park C. S. (2005) Identification and characterization of a putative cyclic nucleotide-gated channel, CNG-1, in C. elegans. Mol. Cells 19, 149–154 [PubMed] [Google Scholar]
- 56. Walker M. T., Brown R. L., Cronin T. W., Robinson P. R. (2008) Photochemistry of retinal chromophore in mouse melanopsin. Proc. Natl. Acad. Sci. U. S. A. 105, 8861–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Panda S., Nayak S. K., Campo B., Walker J. R., Hogenesch J. B., Jegla T. (2005) Illumination of the melanopsin signaling pathway. Science 307, 600–604 [DOI] [PubMed] [Google Scholar]
- 58. Qiu X., Kumbalasiri T., Carlson S. M., Wong K. Y., Krishna V., Provencio I., Berson D. M. (2005) Induction of photosensitivity by heterologous expression of melanopsin. Nature 433, 745–749 [DOI] [PubMed] [Google Scholar]
- 59. Tesmer J. J. (2010) The quest to understand heterotrimeric G protein signaling. Nat. Struct. Mol. Biol. 17, 650–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richmond J. E., Jorgensen E. M. (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 2, 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.