Abstract
To explore the structural mechanisms underlying the assembly and activation of family A GPCR dimers, we used the rat M3 muscarinic acetylcholine receptor (M3R) as a model system. Studies with Cys-substituted mutant M3Rs expressed in COS-7 cells led to the identification of several mutant M3Rs that exclusively existed as cross-linked dimers under oxidizing conditions. The cross-linked residues were located at the bottom of transmembrane domain 5 (TM5) and within the N-terminal portion of the third intracellular loop (i3 loop). Studies with urea-stripped membranes demonstrated that M3R disulfide cross-linking did not require the presence of heterotrimeric G proteins. Molecular modeling studies indicated that the cross-linking data were in excellent agreement with the existence of a low-energy M3R dimer characterized by a TM5-TM5 interface. [35S]GTPγS binding/Gαq/11 immunoprecipitation assays revealed that an M3R dimer that was cross-linked within the N-terminal portion of the i3 loop (264C) was functionally severely impaired (∼50% reduction in receptor-G-protein coupling, as compared to control M3R). These data support the novel concept that agonist-induced activation of M3R dimers requires a conformational change of the N-terminal segment of the i3 loop. Given the high degree of structural homology among family A GPCRs, these findings should be of broad significance.—Hu, J., Thor, D., Zhou, Y., Liu, T., Wang, Y., McMillin, S. M., Mistry, R., Challiss, R. A. J., Costanzi, S., Wess, J. Structural aspects of M3 muscarinic acetylcholine receptor dimer formation and activation.
Keywords: G-protein-coupled receptor, GPCR, disulfide-scanning mutagenesis
G-protein-coupled receptors (GPCRs) regulate the activity of virtually all physiological functions and serve as excellent targets for drug therapy (1). Class A GPCRs represent by far the largest subfamily of GPCRs, consisting of ∼670 members in humans (2). Various experimental approaches have demonstrated that class A GPCRs are able to form dimers and/or higher-order oligomeric complexes (3–5). Most notably, a recent time-resolved FRET study has demonstrated that oxytocin receptor dimers exist in vivo (6).
Although monomeric class A GPCRs are able to activate G proteins (7–9), accumulating evidence suggests that class A GPCR dimers exhibit functional properties that are different from those of their monomeric counterparts. For instance, it has been shown that class A GPCR dimerization can modulate the efficiency of agonist-induced G-protein activation (9), the extent of receptor phosphorylation (10), or G-protein coupling preference (11).
At present, the molecular mechanisms underlying the dimerization (oligomerization) of class A GPCRs are not well understood. However, considerable evidence suggests that residues located on the “outer” (lipid-facing) surface of the transmembrane helical bundle (TM1–7) play a key role in this process (4, 12, 13). Consistent with this notion, we recently demonstrated, by using a combined mutagenesis/bioluminescence resonance energy transfer (BRET) approach, that multiple TM subsegments located on different TM helices contribute to the formation of M3R dimers (oligomers) (14). Guided by these experimental data, we proposed, based on molecular modeling studies, the existence of multiple M3R dimeric complexes. However, one of these M3R dimer models, characterized by a TM5-TM5 interface, was energetically most favorable and was consistent with most of the experimental BRET data (14).
Studies with the M3R and many other class A GPCRs have shown that the C-terminal portion of TM5 (C-TM5) and the adjacent N-terminal segment of the third intracellular loop (N-i3 region) play critical roles in determining the efficiency and selectivity of receptor/G protein coupling and activation (15, 16). At present, it remains unclear whether this functionally critical receptor region is involved in receptor-receptor interactions. Thus, the goal of the present study was to investigate whether the C-TM5/N-i3 region is involved in M3R-M3R interactions and, if so, whether M3R activation has any effect on the relative orientation of the residues located at the M3R-M3R interface. Specifically, we tested the ability of a series of mutant M3Rs containing Cys substitutions within the C-TM5/N-i3 region to undergo disulfide cross-linking under oxidizing conditions, in either the absence or presence of the agonist, carbachol. Analysis of membranes prepared from transfected COS-7 cells led to the identification of several mutant M3Rs that almost exclusively existed as cross-linked dimers under oxidizing conditions, consistent with the existence of a low-energy TM5-TM5 M3R dimer.
Various experimental approaches, including X-ray crystallographic studies (17–20), have revealed the conformational changes that accompany class A GPCR activation in considerable detail. In contrast, little is known about the nature of the structural changes that agonists induce in GPCR dimers and the potential relevance of these changes for efficient receptor/G protein coupling. To address this question, we studied the ability of cross-linked M3R dimers to productively interact with heterotrimeric G proteins, using a [35S]GTPγS binding/Gαq/11 protein antibody-capture assay. We found that an M3R dimer that was cross-linked within the N-i3 region was severely impaired in its ability to activate G proteins, indicating that effective M3R/G protein coupling requires that the N-i3 region is conformationally flexible.
These findings shed new light on the structural basis of M3R function. Since all family A GPCRs share a high degree of structural homology, our data are likely to be of broad general relevance.
MATERIALS AND METHODS
Materials
Carbamylcholine chloride (carbachol), atropine sulfate, cupric sulfate (CuSO4), 1,10-phenanthroline, and N-ethylmaleimide were purchased from Sigma (St. Louis, MO, USA). BMOE (1.2-bis-maleimidoethane) and sulfo-NHS-S-S-biotin were obtained from Thermo Scientific (Waltham, MA, USA). [3H]N-methylscopolamine ([3H]NMS; 70.0 Ci/mmol) and myo-[3H]inositol (20 Ci/mmol) were obtained from PerkinElmer Life Sciences (Downers Grove, IL, USA). CuSO4 was mixed with 1,10-phenanthroline at a molar ratio of 1:3. The concentrations indicated in the text for the Cu(II)-(1,10-phenanthroline)3 complex (Cu-Phen) refer to molar copper concentrations. [35S]GTPγS (1000–1200 Ci/mmol) was purchased from PerkinElmer (Cambridge, UK). Protein-A-Sepharose CL-4B was obtained from GE Healthcare (Chalfont St. Giles, UK). The rabbit Gαq/11 antiserum (no. 2689) was generated against the C-terminal Gαq/11 sequence (C)LQLNLKEYNLV, as reported previously (21). The rabbit anti-β-actin polyclonal antibody was from Cell Signaling Technology (Danvers, MA, USA). The mouse anti-sodium potassium ATPase α 1 monoclonal antibody was purchased from Abcam (Cambridge, MA, USA).
Construction of Cys-substituted mutant M3Rs
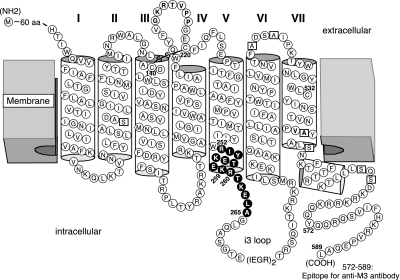
Cys substitutions were introduced into a pCD-based expression plasmid coding for a modified version of the rat M3R referred to as M3′(3C)-Xa (see Fig. 1 and ref. 22). This receptor construct lacks most endogenous Cys residues, except for C140, C220, and C532, which are required for proper M3R function, and all 5 N-glycosylation sites contained within the extracellular N-terminal domain, as well as the central portion of the i3 loop (A274–K469; this region was replaced with 2 adjacent factor Xa cleavage sites). Cys residues were substituted into the M3′(3C)-Xa construct by using the QuikChange site-directed mutagenesis kit (Stratagene, Foster City, CA) according to the manufacturer's instructions. The identity of all mutant M3R constructs was confirmed by sequencing the entire coding sequences.
Figure 1.
Introduction of Cys substitutions into a modified version of the rat M3R (M3′(3C)-Xa). All Cys substitutions were introduced into the M3′(3C)-Xa construct that lacked most endogenous Cys residues (see Materials and Methods for details). Endogenous Cys residues were substituted with serine or alanine (open squares), except for C140, C220, and C532, which were shown to be essential for proper M3R folding and activity (22). Solid circles indicate amino acids that were replaced with Cys residues (residues 2525.60-2655.73).
Transient expression of mutant M3R constructs in COS-7 cells and preparation of cell membranes
For all experiments, receptors were transiently expressed in COS-7 cells, as described previously (23). To increase receptor expression levels, transfected cells were incubated with 1 μM atropine for the last 24 h of culture (23, 24). For disulfide cross-linking experiments, COS-7 cells were transfected with receptor plasmid DNA (4 μg DNA/100-mm dish), as described previously (23). To reduce receptor expression levels, COS-7 cells were cotransfected with 0.05 μg of receptor DNA and 3.95 μg vector DNA (per 100-mm dish). COS-7 cells were harvested ∼48 h after transfections, and membranes were prepared for radioligand binding and disulfide cross-linking studies as described in detail previously (23).
Urea treatment of receptor-expressing cell membranes
For urea-stripping experiments, we followed a protocol similar to that described by Jian et al. (25). In brief, receptor-expressing COS-7 cell membranes were resuspended in binding buffer (25 mM sodium phosphate and 5 mM MgCl2, pH 7.4) containing 7 M urea and incubated for 30 min on ice. Subsequently, an equal volume of binding buffer was added, and membranes were centrifuged at 20,000 g for 15 min. Membrane pellets were washed 3 times with binding buffer and then resuspended in the same buffer for disulfide cross-linking studies.
Radioligand binding studies
Radioligand binding studies were carried out using membranes prepared from transfected COS-7 cells, as described in detail previously (23).
Phosphoinositide hydrolysis assays
To study receptor-mediated phosphoinositide hydrolysis, transfected COS-7 cells were split into 12-well plates ∼24 h after transfection and labeled with myo-[3H]inositol. To increase receptor expression levels, cells were incubated with 1 μM atropine for the last 24 h of culture. Assays were carried out by incubating receptor-expressing cells with increasing concentrations of carbachol, essentially as described (23). Phosphoinositide data were analyzed using the nonlinear curve-fitting program Prism 4.0 (GraphPad Software, San Diego, CA, USA).
Disulfide cross-linking studies
Disulfide cross-linking studies were carried out as described in detail by Li et al. (23). In brief, receptor-containing COS-7 cell membranes were incubated either with the oxidizing agent Cu-Phen (100 μM) or with the irreversible chemical cross-linker BMOE (0.5 mM) in the absence or presence of the agonist carbachol. Reactions were carried out for 10 min at 22°C and then terminated by the addition of either EDTA plus N-ethylmaleimide (10 mM each; Cu-Phen-induced cross-linking) or 10 mM DTT (BMOE-mediated cross-linking), followed by a 10-min incubation on ice. Membrane proteins were then solubilized by incubation of samples with 1.2% digitonin (Roche Applied Science, Foster City, CA, USA) and used either directly for SDS-PAGE or stored at −70°C.
Western blotting studies
SDS-PAGE was performed as described previously (23). Samples containing 20 μg of solubilized membrane protein were incubated with Laemmli loading buffer for 30 min at 37°C, either in the absence of reducing agents (Cu-Phen-induced cross-linking) or in the presence of 10% β-mercaptoethanol (BMOE-mediated cross-linking), respectively. Samples were then loaded onto 10% Tris-glycine polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and run at 135 V in the presence of 0.1% SDS. Western blotting studies were performed essentially as described previously (23). Blots were probed with a rabbit anti-M3R polyclonal antibody directed against the C-terminal 18 aa of the rat M3R (24). Immunoreactive proteins were visualized by using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA) and autoradiography.
Isolation of cross-linked mutant M3Rs present on the cell surface
Intact COS-7 cells expressing different Cys-substituted mutant M3Rs were labeled with the membrane-impermeable biotinlyation agent, sulfo-NHS-S-S-biotin (Thermo Scientific), as described in detail by Hu et al. (26). Membranes prepared from these cells were incubated with 0.5 mM of the chemical cross-linker BMOE, followed by isolation of biotinylated proteins by streptavidin-agarose and SDS-PAGE (reducing conditions), and immunoblotting with the polyclonal anti-M3 antibody (for experimental details, see refs. 23, 26).
Coimmunoprecipitation studies
For coimmunoprecipitation experiments, we generated modified versions of Cys-substituted mutant M3Rs in which the C-terminal recognition sequence for the anti-M3R antibody (see Fig. 1) was replaced with a FLAG tag (DYKDDDDK). Membrane samples from COS-7 cells coexpressing both the nontagged and the FLAG-tagged versions of representative mutant receptors were treated with Cu-Phen (100 μM) and then solubilized as described above. Solubilized proteins (∼600 μg/sample) were then incubated for 1 h at 4°C with the anti-M3R antibody or an anti-FLAG monoclonal antibody (GenScript, Piscataway, NJ, USA). Subsequently, 30 μl of protein A-agarose (anti-M3R antibody) or protein A/G agarose (anti-FLAG antibody) was added, and the incubation was continued for an additional 1 h at 4°C. The protein A-agarose was washed 3 times with 0.2% digitonin in PBS buffer, and bound immunoreactive proteins were then eluted at 37°C for 15 min with 40 μl of 1× Laemmli loading buffer. Twenty microliters of sample was loaded per lane, and immunoblotting studies were performed with the monoclonal anti-FLAG antibody or the polyclonal anti-M3R antibody.
[35S]GTPγS binding/Gαq/11 immunoprecipitation assay
To compare the G-protein-coupling properties of non-cross-linked and cross-linked (Cu-Phen-treated) mutant M3Rs, receptor-containing COS-7 cell membranes (75 μg protein/sample) were treated with the agonist carbachol (1 mM; 2 min at 30°C) in the presence of 1 nM [35S]-GTPγS, followed by the selective immunoprecipitation of Gαq/11 proteins with an anti-Gαq/11-specific antiserum. The protocol that we followed has been described in detail previously (21). Nonspecific binding was determined in the presence of 10 μM GTPγS.
Generation of molecular models of rat M3R dimers
Guided by the high-resolution structure of the turkey β1-adrenergic receptor solved in its inactive state (β1-AR; ref. 27) and experimental BRET data obtained with mutant M3Rs, we recently generated various molecular models of human M3R dimers (14). These models putatively reflect the inactive state of the receptor (14). The lowest-energy M3R dimer that was compatible with most of the experimental data was characterized by a TM5-TM5 interface (14). For the present study, this model was modified by substituting the human M3R sequence with the corresponding rat sequence. Notably, the human and rat M3Rs show 98% sequence identity in the modeled regions (human M3R residues contained in the modeled regions: 65–205, 220–261, 484–560). In particular, the amino acids lining the TM5-TM5 interface in the lowest-energy M3R dimer are 100% conserved between the 2 receptors. Moreover, we further modified our M3R dimer model by superimposing on each of the 2 inactive protomers putative models of the activated M3R. The new model of the activated M3R was obtained via homology modeling, guided by the recently solved crystal structure of the activated β2-AR crystallized in complex with a full agonist and a Gs heterotrimer (ref. 17; see next section).
Construction of a homology model of the rat M3R based on the active state of the β2-AR
We constructed a new homology model of the rat M3R (containing residues 64–205, 220–270, and 482–559) based on the structure of the agonist-occupied human β2-AR cocrystallized with the nucleotide-free Gs heterotrimer (PDB ID: 3SN6; ref. 17). The 2 receptors show 35% sequence identity at the amino acid level in the modeled regions. This level of sequence identity is considered sufficient to generate reasonably accurate GPCR homology models (28–30). The homology modeling procedures were conducted with MOE, using the CHARMM22 force field (31). An initial automatic sequence alignment was obtained using the Blosum62 substitution matrix with penalties for gap openings and extensions of 7 and 1, respectively. No gaps were allowed in the regions with defined secondary structure. Gaps in the unstructured loops were consolidated into a single gap per loop, and positioned where insertions or deletions seemed compatible with the structure of the template. The proper alignment of the conserved motifs that characterize each of the 7 TM helices was also checked. The final sequence alignment used for the construction of the new M3R model (active state) is shown in Supplemental Fig. S1. Due to the lack of structural conservation in the second extracellular loop, only a segment of 7 residues, including the conserved Cys residue that connects this loop with TM3 via a disulfide bridge and the 6 following residues, was maintained in the final model for this domain. The rest of this loop was deleted. The final model of the rat M3R did not include most of the i3 loop because this domain was not solved in the 3SN6 structure (17).
RESULTS
Generation of Cys-substituted mutant M3Rs
We used a modified version of the rat M3R lacking most native Cys residues (M3′(3C)-Xa) as a template for Cys substitution mutagenesis (Fig. 1 and ref. 22). We previously demonstrated that the M3′(3C)-Xa receptor and the wild-type M3R exhibit similar ligand binding and G-protein-coupling properties (22). To test the hypothesis that residues contained within the C-TM5/N-i3 region are located at an M3R-M3R dimer interface, we generated 14 Cys-substituted mutant M3Rs. In these mutant receptors, residues 2525.60–2655.73 were replaced, one at a time, by Cys residues (superscripts refer to amino acid positions according to the Ballesteros-Weinstein GPCR numbering system; Fig. 1).
Pharmacological properties of Cys-substituted mutant M3Rs
Initially, we carried out radioligand binding studies to examine whether the 14 mutant M3Rs retained the ability to bind muscarinic ligands with high affinity. [3H]NMS saturation binding studies with membranes prepared from receptor-expressing COS-7 cells showed that all mutant M3Rs were able to bind the muscarinic antagonist [3H]NMS with high affinity (range of [3H]NMS KD values: ∼0.8–1.8 nM; Table 1). Moreover, all mutant M3Rs showed high expression levels (range of [3H]NMS Bmax values: ∼3.5–9.6 pmol/mg; Table 1). [3H]NMS/carbachol inhibition binding studies demonstrated that the 14 mutant M3Rs retained the ability to bind the agonist carbachol, with affinities that differed from its affinity for the M3′(3C)-Xa receptor by < 2.5-fold (range of carbachol Ki values: ∼12–74 μM; Table 1).
Table 1.
Pharmacological properties of Cys-substituted mutant M3Rs
| Receptor | Ballesteros- Weinstein number | [3H]-NMS binding |
Carbachol binding, Ki (μM) | Carbachol-induced inositol monophosphate production |
|||
|---|---|---|---|---|---|---|---|
| KD (nM) | Bmax (pmol/mg protein) | EC50 (nM) | Emax (fold above basal) | Basal activity (%) | |||
| M3′(3C)-Xa | 1.45 ± 0.17 | 9.29 ± 0.35 | 34.3 ± 4.8 | 47 ± 14 | 4.5 ± 0.7 | 100 | |
| R252C | 5.60 | 1.68 ± 0.33 | 3.51 ± 0.35 | 67.5 ± 14.5 | 127 ± 28 | 3.6 ± 1.5 | 92 ± 13 |
| I253C | 5.61 | 1.84 ± 0.53 | 7.63 ± 2.21 | 74.4 ± 22.7 | 177 ± 56 | 4.8 ± 2.6 | 91 ± 9 |
| Y254C | 5.62 | 1.50 ± 0.51 | 4.83 ± 1.47 | 48.8 ± 5.9 | 338 ± 101 | 4.0 ± 2.6 | 112 ± 34 |
| K255C | 5.63 | 0.91 ± 0.26 | 9.58 ± 1.13 | 41.3 ± 0.2 | 56 ± 13 | 3.9 ± 2.0 | 78 ± 14 |
| E256C | 5.64 | 1.59 ± 0.54 | 6.26 ± 0.64 | 12.2 ± 1.6 | 9.7 ± 2.2 | 4.5 ± 1.6 | 132 ± 42 |
| T257C | 5.65 | 1.05 ± 0.48 | 7.96 ± 1.14 | 33.5 ± 5.7 | 16 ± 6.9 | 2.9 ± 0.7 | 103 ± 9 |
| E258C | 5.66 | 1.02 ± 0.65 | 8.73 ± 1.06 | 22.2 ± 7.9 | 5.3 ± 2.0 | 4.4 ± 0.1 | 203 ± 40 |
| K259C | 5.67 | 0.98 ± 0.47 | 8.25 ± 0.70 | 25.2 ± 3.0 | 24 ± 6.8 | 3.1 ± 0.6 | 86 ± 20 |
| R260C | 5.68 | 0.91 ± 0.48 | 6.57 ± 0.98 | 36.9 ± 6.3 | 436 ± 180 | 2.2 ± 1.0 | 73 ± 17 |
| T261C | 5.69 | 0.86 ± 0.33 | 7.83 ± 0.91 | 33.7 ± 6.6 | 62 ± 13 | 2.9 ± 0.4 | 83 ± 24 |
| K262C | 5.70 | 0.87 ± 0.15 | 5.41 ± 0.16 | 62.4 ± 16.9 | 11 ± 2.6 | 2.8 ± 1.1 | 70 ± 4 |
| E263C | 5.71 | 1.16 ± 0.31 | 6.16 ± 0.54 | 26.8 ± 1.0 | 10 ± 5.6 | 2.7 ± 0.7 | 78 ± 13 |
| L264C | 5.72 | 1.24 ± 0.23 | 6.64 ± 0.22 | 20.9 ± 0.8 | 17 ± 4.6 | 2.8 ± 0.5 | 70 ± 13 |
| A265C | 5.73 | 1.38 ± 0.28 | 8.24 ± 0.58 | 48.2 ± 3.8 | 11 ± 6.5 | 3.1 ± 0.2 | 64 ± 2 |
COS-7 cells were transiently transfected with the indicated M3′(3C)-Xa-derived Cys-substituted mutant M3R (rat) constructs. Radioligand binding and PI assays were performed and analyzed as described in Materials and Methods. Basal inositol monophosphate levels for the M3′ (3C)-Xa construct were 11,043 ± 1891 dpm (=100%). Data are shown as means ± se of 2–4 independent experiments.
To study the ability of the different mutant M3Rs to activate G proteins of the Gq family, we measured carbachol-induced increases in inositol phosphate production using transfected COS-7 cells. Carbachol stimulation of all 14 mutant receptors caused significant increases in inositol phosphate accumulation (Table 1). However, several mutant receptors, including I253C5.61, Y254C5.62, and R260C5.68, showed pronounced reductions in carbachol potencies (increases in EC50 values) and/or Emax values (R260C5.68–A265C5.73) (Table 1). These functional data are consistent with previous findings that the C-TM5/N-i3 region contains amino acids that are critical for productive M3R/G protein coupling (15, 16).
Identification of intermolecular M3R-M3R contact sites
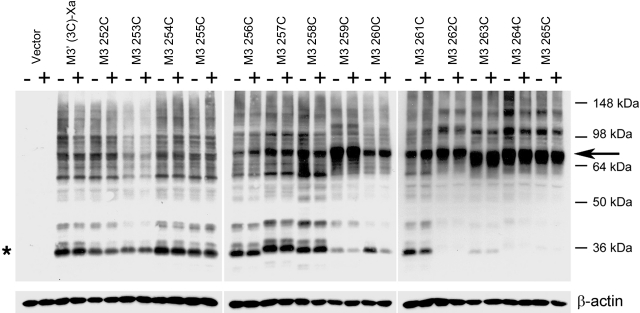
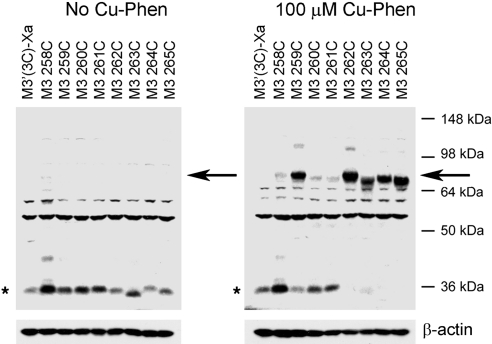
To detect specific intermolecular M3R-M3R contact sites, we incubated membranes prepared from COS-7 cells expressing the different mutant receptors with the oxidizing agent Cu(II)-(1,10-phenanthroline)3 (Cu-Phen; 100 μM), in order to promote the formation of disulfide bonds between vicinal Cys residues. The successful formation of disulfide bonds was monitored by visualizing cross-linked receptor dimers via Western blotting analysis (nonreducing conditions). Receptor proteins were detected by using a polyclonal antibody directed against the last 18 aa of the rat M3R (Fig. 1 and ref. 24). Since the M3′(3C)-Xa-based constructs have a molecular mass of ∼40 kDa, the cross-linked receptor dimers are predicted to migrate at ∼80 kDa. To examine the effect of receptor activation on disulfide cross-link formation, cross-linking reactions were carried out in the absence or presence of carbachol (1 mM).
The results of a representative cross-linking experiment are shown in Fig. 2. As expected, membranes prepared from vector-transfected COS-7 cells did not yield any immunoreactive bands (Fig. 2, first lane). In contrast, we observed multiple immunoreactive species using membranes obtained from COS-7 cells expressing the M3′(3C)-Xa construct (Fig. 2, second lane). The most intense immunoreactive band had the size of a putative receptor monomer (∼40 kDa; Fig. 2, asterisk). Previous studies have demonstrated that this band corresponds to functional cell surface M3Rs (24). M3′(3C)-Xa-containing samples yielded several additional immunoreactive bands, perhaps due to nonspecific aggregation/cross-linking of the M3′(3C)-Xa protein or M3′(3C)-Xa-derived fragments generated by proteolysis with other cellular proteins.
Figure 2.
Detection of disulfide cross-linked mutant M3R homodimers via Western blotting. Membranes prepared from COS-7 cells expressing the indicated Cys-substituted mutant M3Rs were incubated with 100 μM Cu-Phen in the absence (−) or presence (+) of 1 mM carbachol. Membrane extracts were then subjected to SDS-PAGE under nonreducing conditions. Receptor proteins were visualized via Western blotting analysis using the polyclonal anti-M3R antibody. Putative receptor monomers (asterisk) corresponding to functional cell surface receptors can be detected at ∼40 kDa (24). The ∼80 kDa bands (arrow) are predicted to correspond to cross-linked receptor dimers. Similar results were obtained in 2 to 4 independent Western blotting experiments.
Most M3′(3C)-Xa-derived mutant receptors yielded a pattern of immunoreactive bands similar to that observed with the M3′(3C)-Xa construct (Fig. 2). However, 5 of the 14 analyzed mutant receptors (259C5.67, 262C5.70, 263C5.71, 264C5.72, and 265C5.73) showed a clearly different pattern. In the case of these mutant M3Rs, the monomeric receptor species (∼40 kDa) was no longer or only barely detectable by the M3R antibody. Instead, all 5 mutant receptors yielded an intense high-molecular-mass band, corresponding in size to an M3R dimer (∼80 kDa).
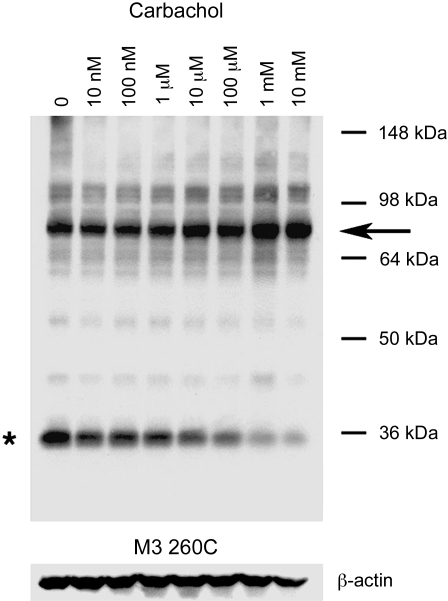
Except for the 260C5.68 mutant receptor, incubation of receptor-expressing COS-7 cell membranes with carbachol (1 mM; oxidizing conditions) had no significant effect on the pattern of bands observed in Western blotting experiments (Fig. 2). In the case of the 260C construct, agonist treatment promoted the formation of the ∼80-kDa (dimeric) receptor species, consistent with the agonist-dependent loss in the intensity of the ∼40-kDa monomer band (Fig. 2). Figure 3 shows a representative Western blot illustrating that carbachol treatment of 260C-containing membranes promoted cross-link formation in a concentration-dependent fashion (oxidizing conditions). Quantitative analysis of the intensities of immunoreactive bands showed that carbachol (1 mM) increased the dimer/monomer ratio from 1.44 ± 0.40 (no carbachol) to 7.30 ± 1.76 (arbitrary units; n=3).
Figure 3.
Agonist-dependent formation of disulfide cross-linked homodimers between mutant M3Rs containing the R260C point mutation. Membranes prepared from COS-7 cells expressing the R260C mutant M3R were incubated with 100 μM Cu-Phen in the absence or presence of the indicated concentrations of carbachol. Membrane extracts were then subjected to SDS-PAGE under nonreducing conditions, and receptors were visualized via Western blotting analysis using the polyclonal anti-M3R antibody. Asterisk and arrow indicate putative receptor monomers and dimers, respectively. Two additional blots gave similar results.
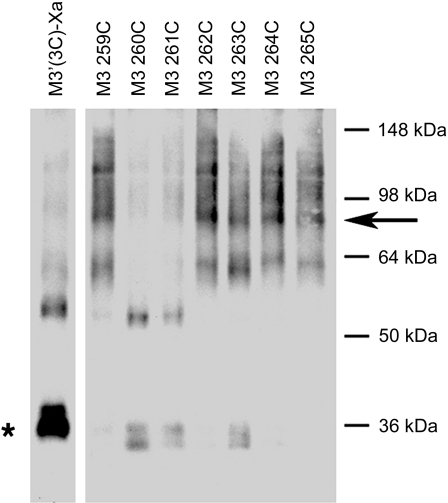
Coimmunoprecipitation experiments confirm the identity of M3R dimers
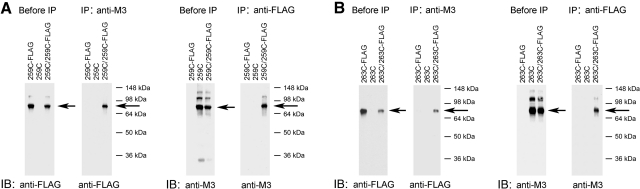
As described above, several of the analyzed mutant M3Rs yielded intense ∼80-kDa immunoreactive species following Cu-Phen treatment of receptor-expressing COS-7 cell membranes. To provide additional evidence that these bands represented M3R dimers, we carried out coimmunoprecipitation experiments with 2 representative mutant M3Rs, 259C5.67, and 263C5.71. Initially, we generated FLAG-tagged versions of these receptors in which the C-terminal recognition sequence for the anti-M3R antibody was replaced with a FLAG tag.
Membranes prepared from COS-7 cells coexpressing 259C and 259C-FLAG (or 263C and 263C-FLAG, respectively) were treated with Cu-Phen (100 μM) to induce receptor cross-linking. Following membrane lysis, we immunoprecipitated M3R-associated proteins with the polyclonal anti-M3 antibody, followed by Western blotting using a monoclonal anti-FLAG antibody. In a reciprocal fashion, we immunoprecipitated M3R-associated proteins with the monoclonal anti-FLAG antibody, followed by Western blotting using the polyclonal anti-M3 antibody. In these experiments, the ∼80-kDa band was the only immunoreactive species that was consistently detectable by both anti-M3 and anti-FLAG antibodies (Fig. 4). Under the same experimental conditions, this band was not observed with samples derived from cells that had been transfected with the single-mutant-receptor constructs alone (Fig. 4). Taken together, these data strongly support the concept that the ∼80-kDa immunoreactive species observed in the cross-linking experiments represent receptor dimers.
Figure 4.
Coimmunoprecipitation confirming the formation of mutant M3R homodimers. Data are shown for 2 representative mutant M3Rs, 259C and 263C. A) Coimmunoprecipitation of cross-linked 259C and 259C-FLAG mutant M3Rs. B) Coimmunoprecipitation of cross-linked 263C and 263C-FLAG mutant M3Rs. For these experiments, we first generated a modified version of the 259C and 263C mutant M3Rs in which the C-terminal recognition sequence for the anti-M3R antibody (Fig. 1) was replaced with a FLAG tag, resulting in the 259C-FLAG and 263C-FLAG constructs, respectively. Membrane samples prepared from COS-7 cells coexpressing 259C and 259C-FLAG (A) or 263C and 263C-FLAG (B) were subjected to the cross-linking procedure using 100 μM Cu-Phen as described in Materials and Methods. Solubilized membrane proteins were then incubated with the anti-M3R polyclonal antibody [immunoprecipitation (IP): anti-M3] or a monoclonal anti-FLAG antibody (IP: anti-FLAG). Subsequently, protein A-agarose was added, and the bound immunoreactive proteins were eluted and blotted with a monoclonal anti-FLAG antibody [immunoblot (IB): anti-FLAG] or the anti-M3R polyclonal antibody (IB: anti-M3) under nonreducing conditions. Western blots of membrane proteins prior to immunoprecipitation are shown in the left panels for comparison (before IP). Arrows indicate ∼80-kDa bands corresponding to the cross-linked M3R dimers. Blots shown are representative of 2 to 4 independent experiments.
Receptor cross-linking patterns are retained at low receptor densities
Table 1 shows that all receptor constructs were expressed at relatively highly levels (∼3.5–9.6 pmol/mg). To examine whether the observed cross-linking patterns could also be obtained at lower (more physiological) receptor levels, we expressed the 258C–265C mutant M3Rs at ∼0.2 pmol/mg by reducing the amount of transfected receptor DNA. Following oxidation of receptor-expressing COS-7-cell membranes with Cu-Phen (100 μM), the 259C, 262C, 263C, 264C, and 265C mutant receptors showed complete or nearly complete cross-linking (disappearance of the monomer band and appearance of the ∼80-kDa dimeric species; Fig. 5), similar to the pattern seen after expression of these receptors at considerably higher levels (Fig. 2).
Figure 5.
Detection of disulfide cross-linked M3R dimers following expression of Cys-substituted mutant M3Rs at low levels. COS-7 cells were transfected with reduced amounts of plasmid DNA coding for the indicated receptor constructs (see Materials and Methods for details), in order to achieve relatively low receptor densities (∼2 pmol/mg membrane protein). Membranes prepared from these cells were then incubated in the absence (left panel) or presence of 100 μM Cu-Phen (right panel). Subsequently, membrane extracts were subjected to SDS-PAGE under nonreducing conditions, and receptor proteins were visualized via Western blotting using the polyclonal anti-M3R antibody. Asterisks and arrows indicate putative receptor monomers and dimers, respectively. Blots shown are representative of 3 independent experiments.
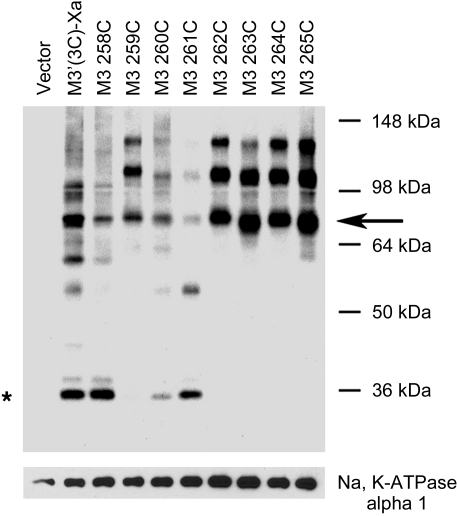
Cross-linked M3R dimers are present at the cell surface
To confirm that the cross-linked mutant M3R dimers were present at the cell surface, we incubated intact COS-7 cells expressing the 259C–265C mutant receptors with the membrane-impermeant biotinylation reagent, sulfo-NHS-S-S-biotin. After treatment of cell membranes with the Cys-specific bifunctional cross-linker BMOE (0.5 mM), membranes were lysed, and biotinylated cell surface proteins were isolated using agarose-conjugated streptavidin. Biotinylated proteins were then analyzed by Western blotting using the anti-M3R antibody (Fig. 6). These experiments confirmed the presence of cross-linked 259C, 262C, 263C, 264C, and 265C dimers at the cell surface.
Figure 6.
Detection of cross-linked mutant M3R homodimers present on the cell surface. Intact COS-7 cells expressing the indicated Cys-substituted mutant M3Rs were labeled with the membrane-impermeable biotinlyation agent, sulfo-NHS-S-S-biotin. Membranes prepared from these cells were incubated with 0.5 mM of the chemical cross-linker, BMOE, followed by isolation of biotinylated proteins by streptavidin-agarose, SDS-PAGE (reducing conditions), and immunoblotting with the polyclonal anti-M3 antibody. Asterisk and arrow indicate putative receptor monomers and dimers, respectively. Blot shown is representative of 2 independent experiments.
Receptor cross-linking patterns are unaffected by urea treatment of mutant M3R-expressing membranes
To test the potential effect of heterotrimeric G proteins on the formation of disulfide cross-linked M3R dimers, we treated mutant M3R-containing membranes with a high concentration (7 M) of the chaotropic agent urea, a procedure known to result in the removal (or inactivation) of heterotrimeric G proteins and other peripheral membrane proteins. Western blotting studies showed that urea-treated membrane samples yielded disulfide cross-linking patterns that were similar to those observed with membrane preparations that had not been exposed to urea (Fig. 7). These data indicate that the formation of disulfide cross-linked M3R dimers did not require the presence of heterotrimeric G proteins.
Figure 7.
Detection of disulfide cross-linked mutant M3R homodimers using urea-stripped membranes. Membranes prepared from COS-7 cells transfected with vector DNA or the indicated mutant M3R constructs were subjected to urea stripping (treatment with 7 M urea) prior to incubation with 100 μM Cu-Phen. Membrane extracts were then subjected to SDS-PAGE under nonreducing conditions. Receptor proteins were detected via Western blotting using the polyclonal anti-M3R antibody. Asterisk and arrow indicate putative receptor monomers and dimers, respectively. For control purposes, blots were also probed with a mouse anti-α 1 sodium potassium ATPase monoclonal antibody (predicted molecular mass: 112 kDa). Blot shown is representative of 2 independent experiments.
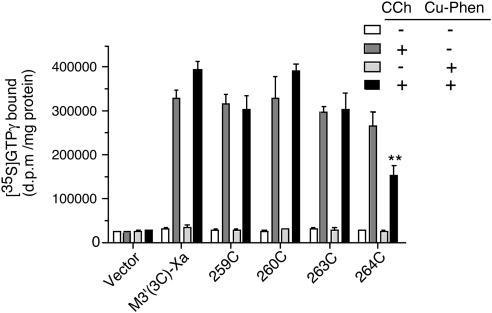
G-protein-coupling properties of cross-linked mutant M3R dimers
Since several of the analyzed mutant M3Rs almost exclusively existed as cross-linked receptor dimers in COS-7 cell membranes, we were able to study what effect the cross-linking of M3R dimers at defined positions had on receptor function. For this analysis, we selected 4 representative mutant M3Rs (259C5.67, 260C5.68, 263C5.71, and 264C5.72). We studied receptor-mediated G-protein activation by treating nonoxidized or oxidized (Cu-Phen-treated) membranes with carbachol (1 mM) and then monitored G protein activation using a [35S]GTPγS binding/Gαq/11 immunoprecipitation assay (21). Following Cu-Phen treatment, all analyzed receptor constructs retained the ability to bind [3H]NMS and the agonist carbachol with high affinity (Table 2). Moreover, all tested mutant M3Rs were expressed at comparable levels (similar [3H]NMS Bmax values; Table 2).
Table 2.
Ligand binding properties of representative Cys-substituted mutant M3Rs following Cu-Phen treatment
| Receptor | [3H]-NMS binding |
Carbachol binding, Ki (μM) | |
|---|---|---|---|
| KD pM | Bmax (pmol/mg protein) | ||
| M3′(3C)-Xa | 524 ± 98 | 5.82 ± 1.39 | 45.7 ± 8.9 |
| K259C | 877 ± 365 | 5.00 ± 2.41 | 32.7 ± 4.1 |
| R260C | 733 ± 137 | 3.99 ± 1.71 | 46.1 ± 0.8 |
| E263C | 421 ± 210 | 4.42 ± 0.08 | 26.0 ± 9.6 |
| L264C | 719 ± 96 | 4.85 ± 0.45 | 26.2 ± 9.7 |
Membranes prepared from COS-7 cells expressing the indicated M3′(3C)-Xa-derived Cys-substituted mutant M3R (rat) constructs were incubated with 100 μM Cu-Phen (10 min at 22°C), and radioligand binding assays were performed and analyzed as described in Materials and Methods. Data are shown as means ± se of 2 or 3 independent experiments.
Cu-Phen treatment had no significant effect on the ability of the M3′(3C)-Xa background receptor to mediate agonist-induced G-protein activation (carbachol-stimulated 12.6±1.1- and 13.6±1.5-fold increases in Gαq/11-[35S]GTPγS binding in the respective absence and presence of membrane pretreatment with Cu-Phen; Fig. 8). Similar findings were obtained with the 259C, 260C, and 263C constructs (Fig. 8). However, following Cu-Phen treatment, the 264C construct was functionally severely impaired, as indicated by a ∼50% reduction in functional activity (carbachol-stimulated 10.5±1.3- and 5.3±0.9-fold increases in Gαq/11-[35S]GTPγS binding in the respective absence and presence of membrane pretreatment with Cu-Phen; Fig. 8).
Figure 8.
G-protein-coupling properties of cross-linked mutant M3R dimers. COS-7 cells were transfected with the indicated receptor constructs or vector DNA. Subsequently, cell membranes were incubated in the absence or presence of Cu-Phen (100 μM). To compare the G-protein-coupling properties of the non-cross-linked and cross-linked (Cu-Phen-treated) receptors, cell membranes were treated with the agonist carbachol (CCh; 1 mM) in the presence of [35S]-GTPγS for 2 min at 30°C. Agonist-stimulated [35S]-GTPγS binding was assessed by selective immunoprecipitation of [35S]-GTPγS-labeled Gαq/11, using an anti-Gαq/11 antiserum. Data are shown as means ± se of 3 separate experiments, each carried out in duplicate. **P < 0.01 vs. absence of Cu-Phen pretreatment on CCh response of 264C M3R.
Molecular modeling studies
The cross-linking data described above are in very good agreement with a low-energy model of an M3R dimer characterized by a TM5-TM5 interface (Fig. 9; ref. 14). This model predicts that the α-carbons of K2595.67 are located very close to each other (∼8.5 Å) at the dimer interface (Supplemental Table S1). Consistent with this observation, the 259C construct underwent efficient disulfide cross-linking under oxidizing conditions.
Figure 9.
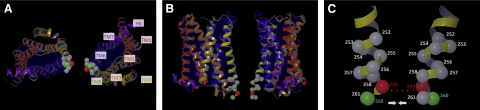
Molecular model of the inactive state of the low-energy rat M3R dimer characterized by a TM5-TM5 interface. A) View from the cytosol. B) Side view. C) Detailed view of the C-terminal portion of TM5. TM1–TM7 and helix 8 (H8) are indicated by different colors (red, TM1; orange, TM2; light orange, TM3; dark yellow, TM4; light yellow, TM5; blue, TM6; dark purple, TM7; purple, H8). The α-carbons of residues 252–261 are shown in space-filling mode. The α-carbons of residues 2595.67 and 2605.68 are shown in red and green, respectively; all other α-carbons are in gray. Note that residue 259 in one M3R protomer is located in the vicinity of residue 259 in the second M3R protomer (stippled line). In contrast, residue 260 in one M3R protomer is predicted to project away from residue 260 in the other M3R protomer in the inactive state of the receptor dimer. Molecular modeling studies predict that the C-terminal portions of TM5 in the M3R dimer move somewhat closer toward each other following M3R activation (indicated by the arrows; see Fig. 10 and text for details). N-i3 region containing residues 262–265 was not included in this model because of the lack of high-resolution structures of biogenic amine GPCRs (inactive state) containing this structural element. Figure generated with Maestro (31).
As described in the previous paragraphs, the 262C5.70, 263C5.71, 264C5.72, and 265C5.73 mutant M3Rs were also able to form dimers with high efficiency (under oxidizing conditions). These residues are located within a cytoplasmic region that links TM5 and TM6 (N-i3). In X-ray crystallographic studies reflecting the inactive structure of the β-adrenergic receptors, this region was either removed to promote the formation of suitable receptor crystals or failed to give a distinct refraction pattern, indicative of considerable conformational flexibility (27, 32, 33). For this reason, we did not include the region containing the 262–265 sequence in the model of the inactive M3R dimer state shown in Fig. 9.
Very recently, the active state of the β2-adrenergic receptor (β2-AR), in a complex of the agonist-occupied receptor with the nucleotide-free Gs heterotrimer, has been solved by X-ray crystallography (PDB ID: 3SN6; ref. 17). Using this published structure as a template, we constructed a molecular model of the active form of the M3R dimer characterized by a TM5-TM5 interface (see Materials and Methods for details). This model suggests that the α-carbons of residues 2605.68 at the cytoplasmic end of TM5 are ∼2–3 Å closer to each other in the active M3R dimer model, as compared to the inactive one (10.9 vs. 13.3 Å, respectively; Figs. 9C, arrows; and 10), providing a possible explanation for the observation that agonist treatment promoted the formation of cross-linked 260C dimers (Fig. 3). Interestingly, the structure of the activated β2-AR showed an intracellular α-helical extension of TM5 by 7 residues (17), allowing us to model the N-i3 regions of the active M3R dimer until residue 2705.78 (Fig. 10).
Figure 10.
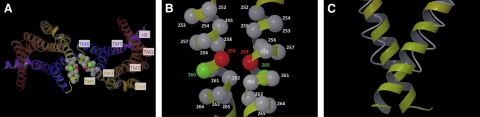
Molecular model of the active state of the low-energy rat M3R dimer characterized by a TM5-TM5 interface. A) View from the cytosol. B) Detailed view of the C-terminal portion of TM5. TM1–TM7 and helix 8 (H8) are highlighted by different colors (red, TM1; orange, TM2; light orange, TM3; dark yellow, TM4; light yellow, TM5; blue, TM6; dark purple, TM7; purple, H8). The α-carbons of residues 252–265 are shown in space-filling mode. The α-carbons of residues 2595.67 and 2605.68 are shown in red and green, respectively; all other α-carbons are in gray. C) Simultaneous view of the C-terminal portion of TM5 in the active (thick ribbon) and inactive (thin tube) rat M3R dimer models. Our molecular modeling studies predict that the C-terminal portions of TM5 in the M3R dimer move somewhat closer toward each other following M3R activation (see text for details). Figure generated with Maestro (31).
DISCUSSION
Studies with the M3R and many other GPCRs have shown that the C-TM5/N-i3 region contains structural elements that are critical for agonist-induced G-protein recognition and activation (15, 16). Using the M3R as a model system, we tested the hypothesis that this receptor segment is located at the interface of an M3R-M3R dimer. Specifically, we subjected M3R residues 2525.60–2655.73 to Cys substitution mutagenesis and examined whether the resulting mutant M3Rs containing single Cys residues within the C-TM5/N-i3 region were able to form disulfide cross-linked dimers under oxidizing conditions. Cross-linking reactions were carried out with receptors being present in their native membrane environment (membranes were prepared from receptor-expressing COS-7 cells).
In the absence of the agonist carbachol, 5 of the 14 analyzed mutant receptors (259C5.67 and 262C5.70–265C5.73) almost exclusively existed as cross-linked receptor dimers, as demonstrated by the appearance of a ∼80-kDa band on Western blots (Fig. 2). Coimmunoprecipitation studies demonstrated that the ∼80-kDa bands did in fact represent cross-linked dimeric M3R species (Fig. 4). Biotinlyation assays showed that all cross-linked mutant M3R dimers could be detected at the cell surface (Fig. 6). Moreover, the observed disulfide cross-linking pattern remained unaffected by >10-fold reduction of receptor expression levels (to achieve M3R densities similar to those found physiologically in tissues; <1 pmol/mg protein; Fig. 5) or by the removal of heterotrimeric G proteins via urea stripping of receptor-containing membranes (Fig. 7). Taken together, these observations exclude the possibility that the cross-linking data that we obtained represent artifacts caused by unphysiologically high receptor expression levels or require the presence of G proteins.
259C5.67 is located at the bottom of TM5 (Figs. 1 and 9), as suggested by molecular modeling studies using the high-resolution structure of the β1-AR (27) as a template. Guided by the results of a BRET study analyzing a large number of mutant M3Rs, we recently proposed the existence of a low-energy M3R dimer characterized by a TM5-TM5 interface (14). According to this model, 259C on one M3R protomer directly faces 259C on the other M3R protomer (Fig. 9). Assuming that the C-terminal portion of TM5 is endowed with a certain degree of conformational flexibility, the short distance (8.5 Å) between the two 259C α-carbons in the M3R dimer shown in Fig. 9 should greatly facilitate disulfide cross-linking. Supplemental Table S1 shows that the distances between all other pairs of Cα atoms (residues 252–261) are predicted to be >9.4 Å, providing a possible explanation for the finding that the 259C receptor was the only construct containing a TM5 Cys substitution that underwent highly efficient disulfide cross-linking.
Besides the 259C construct, 4 additional mutant receptors, 262C5.70–265C5.73, also showed nearly complete disulfide cross-linking when receptor-containing COS-7 cell membranes were subjected to oxidizing conditions. Since these 4 residues are located within the N-i3 region, which represents a direct extension of TM5, these data provide additional support for the existence of a TM5-TM5 M3R dimer. X-ray crystallographic studies suggest that the region in which residues 262–265 are located is characterized by a lack of secondary structure and a high degree of conformational flexibility (27, 32, 33). This observation provides a possible explanation for our finding that Cys substitution of these 4 consecutive N-i3 residues (262–265) resulted in mutant M3Rs that almost exclusively existed as cross-linked dimers.
With only one exception (260C5.68), agonist (carbachol) treatment of receptor-expressing COS-7 cell membranes had no significant effect on the cross-linking pattern observed with the analyzed mutant M3Rs. This finding supports the concept that TM5-TM5 M3R dimers form in a constitutive fashion and that the overall arrangement of the TM5-TM5 interface remains unchanged following M3R activation. Consistent with this notion, previous BRET studies suggested that agonist treatment had little effect on the extent of M3R dimerization (34).
Using a disulfide cross-linking approach similar to the one described here, Guo et al. (35, 36) demonstrated the existence of D2 dopamine receptor dimers characterized by a TM4-TM4 interface. Biochemical analysis of Cys-substituted mutant D2 receptors (TM4) revealed striking changes in disulfide cross-linking patterns following agonist treatment (36), suggesting that structural changes at the TM4-TM4 interface are part of the D2 receptor activation mechanism. In contrast, in the present study, we observed similar cross-linking patterns in the absence or presence of agonist. It is therefore likely that the extent of the agonist-induced structural changes at TM-TM dimer interfaces depends on the nature of the TM region and receptor subtype under investigation.
Interestingly, agonist treatment of membranes containing the R260C5.68 greatly facilitated the formation of cross-linked M3R dimers (Fig. 3), suggesting that the C-terminal ends of TM5 in the M3R dimer move closer toward each other following receptor activation (also see Fig. 10). Inositol phosphate accumulation assays showed that the R260C point mutation severely interfered with agonist-induced receptor activation (∼10-fold decrease in carbachol potency, combined with a ∼50% reduction in Emax; Table 1), consistent with the outcome of a previous site-directed mutagenesis study carried out with the structurally closely related M5 muscarinic receptor (37). Given the important functional role of R260 in productive receptor/G-protein coupling, it is likely that the activity-dependent structural reorientation of R260 plays a critical role in the M3R activation process.
Very recently, Rasmussen et al. (17) succeeded in solving the crystal structure of an active state of the β2-AR consisting of the agonist-occupied receptor and the nucleotide-free Gs heterotrimer. The most pronounced conformational change in the β2-AR, as compared to the inactive state of the receptor, is a 14 Å outward movement of the cytoplasmic end of TM6. A more subtle outward movement (by ∼3 Å) of the cytoplasmic end of TM5 as well as an inward movement of the intracellular end of TM7 are also visible. Moreover, an intracellular α-helical extension of TM5 by 7 residues can be observed in the crystal structure of the activated β2-AR (17). As a result, TM5 extends until residue S2365.75 in the active state of the β2-AR, corresponding to L2675.75 in the rat M3R. Given the high structural homology between the 2 receptors, it is likely that agonist activation of the M3R results in similar conformational changes as described for the β2-AR.
Guided by the coordinates of the active state of the β2-AR (17) and our molecular model of the TM5/TM5 M3R dimer in its inactive state, we generated a putative model of the agonist-activated state of this receptor dimer (Fig. 10). This model, particularly the interface of the cytoplasmic ends of TM5, provided a useful structural context for interpreting the results of [35S]GTPγS binding studies that we performed with 4 representative mutant M3Rs (259C5.67, 260C5.68, 263C5.71, and 264C5.72). Specifically, we examined the ability of carbachol to induce receptor-mediated activation of Gq/11 using a [35S]GTPγS binding/Gαq/11 protein antibody-capture assay. These functional studies were carried out with nonoxidized and oxidized (Cu-Phen-treated) membrane preparations. We found that the cross-linked 259C and 263C M3R dimers retained full functional activity (Fig. 8), consistent with the observation that residues 259 and 263 on one M3R protomer directly face the corresponding residues (259 and 263, respectively) on the second M3R protomer in the active state of the receptor (Fig. 10). These data indicate that cross-linking of the TM5-TM5 M3R dimer at positions 259 or 263 does not interfere with the pronounced outward movement of the cytoplasmic end of TM6 that is thought to be required for receptor activation (17).
Similar to the 259C and 263C receptors, the 260C construct retained full functional activity following Cu-Phen treatment of receptor-expressing membranes (Fig. 8). However, the 260C receptor did not undergo efficient disulfide cross-linking under these experimental conditions (Cu-Phen treatment in the absence of agonist; Fig. 3). While the 260C mutant receptor showed a ∼50% reduction in Emax in inositol monophosphate accumulation assays (Table 1), it displayed similar efficacy as the control construct (M3′(3C)-Xa) in the [35S]GTPγS binding assays (Fig. 8). One possible explanation for this discrepancy is that the [35S]GTPγS binding assay represents a short-term assay (stimulation of receptor-expressing membranes with carbachol for 2 min), the inositol monophosphate accumulation assay involves intact cells stimulated with carbachol for 1 h (23). It is therefore likely that the latter assay is more sensitive to differences in receptor signaling caused by changes in receptor desensitization, sequestration, or down-regulation.
In contrast to all other analyzed mutant M3Rs, the cross-linked 264C receptor dimer was severely impaired in its ability to facilitate GTP-for-GDP exchange on Gq/11 (Fig. 8). Figure 10 suggests that residue 264 on one M3R protomer projects away from residue 264 on the second M3R protomer in the active state of the receptor. It is therefore likely that the 264C-264C cross-link that we induced in the absence of agonist prevents the cytoplasmic end of TM5 to adopt a conformation that is critical for efficient receptor/G protein coupling. Consistent with this notion, the structure of the active state of the β2-AR suggests that the corresponding residue in the β2-AR (I2335.72) is located directly at the receptor/Gαs interface, making contacts with 2 functionally important regions of Gαs (β6 strand and α5 helix; ref. 17).
It should be noted in this context that an CXCR4 receptor dimer has been identified via X-ray crystallography (38). Although this dimer is also endowed with a TM5-TM5 interface, it differs significantly from our putative model of the M3R dimer. In the CXCR4 receptor dimer, the 2 receptor protomers establish contacts through the extracellular ends of TM5. On the contrary, in our M3R model, receptor-receptor interactions involve residues at the intracellular ends of TM5.
We previously proposed, based on the outcome of systematic mutagenesis studies and BRET saturation experiments, that multiple dimeric M3R arrangements may exist in a dynamic equilibrium (14). The disulfide cross-linking experiments that we carried out in the present study were predicted to trap TM5-TM5 M3R dimers, continuously removing this specific dimer from the dynamic equilibrium of different M3R dimeric species. As a result of this process, all dimeric forms of the M3R will ultimately be trapped as TM5-TM5 M3R dimers under the chosen experimental conditions.
In summary, this study provides novel information about how M3Rs function at the molecular level. Since all family A GPCRs share a high degree of structural homology, our data are likely to be of broad general relevance.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors thank Ms. Vanivilasini Balachandran and Mr. Zifan Xiang (NIH, NIDDK) for excellent technical assistance.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 2. Lagerström M. C., Schiöth H. B. (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–357 [DOI] [PubMed] [Google Scholar]
- 3. Angers S., Salahpour A., Bouvier M. (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu. Rev. Pharmacol. Toxicol. 42, 409–435 [DOI] [PubMed] [Google Scholar]
- 4. Milligan G. (2007) G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim. Biophys. Acta 1768, 825–835 [DOI] [PubMed] [Google Scholar]
- 5. Palczewski K. (2010) Oligomeric forms of G protein-coupled receptors (GPCRs). Trends Biochem. Sci. 35, 595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albizu L., Cottet M., Kralikova M., Stoev S., Seyer R., Brabet I., Roux T., Bazin H., Bourrier E., Lamarque L., Breton C., Rives M. L., Newman A., Javitch J., Trinquet E., Manning M., Pin J. P., Mouillac B., Durroux T. (2010) Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 6, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U. S. A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whorton M. R., Jastrzebska B., Park P. S., Fotiadis D., Engel A., Palczewski K., Sunahara R. K. (2008) Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J. Biol. Chem. 283, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White J. F., Grodnitzky J., Louis J. M., Trinh L. B., Shiloach J., Gutierrez J., Northup J. K., Grisshammer R. (2007) Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc. Natl. Acad. Sci. U. S. A. 104, 12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song G. J., Jones B. W., Hinkle P. M. (2007) Dimerization of the thyrotropin-releasing hormone receptor potentiates hormone-dependent receptor phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 104, 18303–18308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rovira X., Pin J. P., Giraldo J. (2010) The asymmetric/symmetric activation of GPCR dimers as a possible mechanistic rationale for multiple signalling pathways. Trends Pharmacol. Sci. 31, 15–21 [DOI] [PubMed] [Google Scholar]
- 12. Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. (2004) The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 564, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Filizola M., Weinstein H. (2005) The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 272, 2926–2938 [DOI] [PubMed] [Google Scholar]
- 14. McMillin S. M., Heusel M., Liu T., Costanzi S., Wess J. (2011) Structural basis of M3 muscarinic receptor dimer/oligomer formation. J. Biol. Chem. 286, 28584–28598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wess J. (1996) Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 10, 69–99 [DOI] [PubMed] [Google Scholar]
- 16. Wess J. (1998) Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol. Ther. 80, 231–264 [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen S. G., Devree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choe H. W., Kim Y. J., Park J. H., Morizumi T., Pai E. F., Krauss N., Hofmann K. P., Scheerer P., Ernst O. P. (2011) Crystal structure of metarhodopsin II. Nature 471, 651–655 [DOI] [PubMed] [Google Scholar]
- 19. Standfuss J., Edwards P. C., D'Antona A., Fransen M., Xie G., Oprian D. D., Schertler G. F. (2011) The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature 471, 656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu F., Wu H., Katritch V., Han G. W., Jacobson K. A., Gao Z. G., Cherezov V., Stevens R. C. (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akam E. C., Challiss R. A. J., Nahorski S. R. (2001) Gq/11 and Gi/o activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: dependence on agonist as well as receptor-subtype. Br. J. Pharmacol. 132, 950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng F. Y., Hopp A., Soldner A., Wess J. (1999) Use of a disulfide cross-linking strategy to study muscarinic receptor structure and mechanisms of activation. J. Biol. Chem. 274, 16629–16640 [DOI] [PubMed] [Google Scholar]
- 23. Li J. H., Han S. J., Hamdan F. F., Kim S. K., Jacobson K. A., Bloodworth L. M., Zhang X., Wess J. (2007) Distinct structural changes in a G protein-coupled receptor caused by different classes of agonist ligands. J. Biol. Chem. 282, 26284–26293 [DOI] [PubMed] [Google Scholar]
- 24. Ward S. D., Hamdan F. F., Bloodworth L. M., Wess J. (2002) Conformational changes that occur during M3 muscarinic acetylcholine receptor activation probed by the use of an in situ disulfide cross-linking strategy. J. Biol. Chem. 277, 2247–2257 [DOI] [PubMed] [Google Scholar]
- 25. Jian X., Sainz E., Clark W. A., Jensen R. T., Battey J. F., Northup J. K. (1999) The bombesin receptor subtypes have distinct G protein specificities. J. Biol. Chem. 274, 11573–11581 [DOI] [PubMed] [Google Scholar]
- 26. Hu J., Wang Y., Zhang X., Lloyd J., Li J., Karpiak J., Costanzi S., Wess J. (2010) Structural basis of G protein-coupled receptor-G protein interactions. Nat. Chem. Biol. 6, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warne T., Serrano-Vega M., Baker J., Moukhametzianov R., Edwards P., Henderson R., Leslie A., Tate C., Schertler G. (2008) Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454, 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costanzi S. (2008) On the applicability of GPCR homology models to computer-aided drug discovery: a comparison between in silico and crystal structures of the β2-adrenergic receptor. J. Med. Chem. 51, 2907–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michino M., Abola E., 2008 Participants, G., Brooks C. R., Dixon J., Moult J., Stevens R. (2009) Community-wide assessment of GPCR structure modelling and ligand docking: GPCR Dock 2008. Nat. Rev. Drug Discov. 8, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costanzi S. (2010) Modelling G protein-coupled receptors: a concrete possibility. Chim. Oggi. 28, 26–30 [PMC free article] [PubMed] [Google Scholar]
- 31. Chemical Computing Group (2010) The molecular operating environment (MOE), version 2010.10. Chemical Computing Group, Montreal, QC, Canada, http://www.chemcomp.com [Google Scholar]
- 32. Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., Kobilka B. K. (2007) GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318, 1266–1273 [DOI] [PubMed] [Google Scholar]
- 34. Goin J. C., Nathanson N. M. (2006) Quantitative analysis of muscarinic acetylcholine receptor homo- and heterodimerization in live cells: regulation of receptor down-regulation by heterodimerization. J. Biol. Chem. 281, 5416–5425 [DOI] [PubMed] [Google Scholar]
- 35. Guo W., Shi L., Javitch J. A. (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 278, 4385–4388 [DOI] [PubMed] [Google Scholar]
- 36. Guo W., Shi L., Filizola M., Weinstein H., Javitch J. (2005) Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc. Natl. Acad. Sci. U. S. A. 102, 17495–17500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burstein E. S., Spalding T. A., Brann M. R. (1996) Amino acid side chains that define muscarinic receptor/G-protein coupling. Studies of the third intracellular loop. J. Biol. Chem. 271, 2882–2885 [DOI] [PubMed] [Google Scholar]
- 38. Wu B., Chien E. Y., Mol C. D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F. C., Hamel D. J., Kuhn P., Handel T. M., Cherezov V., Stevens R. C. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.