Abstract
Computational methods have led two groups to predict the endogenous presence of a highly conserved, amidated, 14-aa neuropeptide called either spexin or NPQ. NPQ/spexin is part of a larger prohormone that contains 3 sets of RR residues, suggesting that it could yield more than one bioactive peptide; however, no in vivo activity has been demonstrated for any peptide processed from this precursor. Here we demonstrate biological activity for two peptides present within proNPQ/spexin. NPQ/spexin (NWTPQAMLYLKGAQ-NH2) and NPQ 53-70 (FISDQSRRKDLSDRPLPE) have differing renal and cardiovascular effects when administered intracerebroventricularly or intravenously into rats. Intracerebroventricular injection of NPQ/spexin produced a 13 ± 2 mmHg increase in mean arterial pressure, a 38 ± 8 bpm decrease in heart rate, and a profound decrease in urine flow rate. Intracerebroventricular administration of NPQ 53-70 produced a 26 ± 9 bpm decrease in heart rate with no change in mean arterial pressure, and a marked increase in urine flow rate. Intraventricular NPQ/spexin and NPQ 53-70 also produced antinociceptive activity in the warm water tail withdrawal assay in mice (ED50<30 and 10 nmol for NPQ/spexin and NPQ 53-70, respectively). We conclude that newly identified peptides derived from the NPQ/spexin precursor contribute to CNS-mediated control of arterial blood pressure and salt and water balance and modulate nociceptive responses.—Toll, L., Khroyan, T. V., Sonmez, K., Ozawa, A., Lindberg, I., McLaughlin, J. P., Eans, S. O., Shahien, A. A., Kapusta, D. R. Peptides derived from the prohormone proNPQ/spexin are potent central modulators of cardiovascular and renal function and nociception.
Keywords: antinociception, neuropeptide
Computational methods have joined classic pharmacology, reverse pharmacology (1–3), and chemical methods (4) for the identification of neuropeptides. These computational methods have been attempted mostly on the basis of the characteristics of the prohormones from which active neuropeptides are processed. Particularly important are the presence and location of the basic residues used for recognition by the prohormone convertases, which are required to produce the mature and biologically active peptides (reviewed in ref. 5). Using different hidden Markov model (HMM)-based computational methods, two independent groups identified a prohormone containing an amidated 14-aa peptide that was, respectively, called spexin (6) and NPQ (7, 8). This peptide, NWTPQAMLYLKGAQ-NH2, is identical in humans and mice and is highly conserved in vertebrate evolution (6). It is, however, not conserved in rats, in which the carboxyl-terminal Gly-Arg-Arg, which represents the putative processing and amidation site, is mutated to Gly-His-Arg.
Locations of both the prohormone and this peptide have recently been determined. The distribution of preproNPQ/spexin mRNA was examined in human tissues by Northern blot analysis by Sonmez et al. (7), who showed expression in several tissues, including brain and pancreas, with the highest expression in kidney, suggesting a possible role in salt and water balance. In situ hybridization studies showed that in the brain, preproNPQ/spexin mRNA is highly localized in Barrington's nucleus, with lesser amounts in the ventrolateral periaqueductal gray. In situ hybridization studies by Mirabeau et al. (6) demonstrated preproNPQ/spexin mRNA in the stomach. Mirabeau et al. (7) also demonstrated NPQ/spexin-induced contractions of the rat stomach fundus smooth muscle, providing the first known biological activity for this peptide. Comprehensive immunohistochemical studies have recently been performed to determine the localization of NPQ/spexin immunoreactivity in rat brain and other tissues, using antibodies to the amidated human/mouse peptide (9, 10). These studies showed considerable NPQ/spexin staining in skin, respiratory, digestive, urinary, and reproductive systems, retina, adrenal gland, and various brain regions.
Because of its high conservation among species and evidence for biological activity, the NPQ/spexin sequence is the most likely peptide derived from the prohormone. However, the precursor protein contains four RR sequences that could represent potential prohormone convertase processing sites. Therefore, after cleavage at the end of the signal sequence, proNPQ/spexin has the potential to generate at least three additional biologically active peptides (Fig. 1), all of which are predicted by NeuroPred software (cleavage probability 0.88 for each RR; http://neuroproteomics.scs.illinois.edu/cgi-bin/neuropred.py). In this study, we show that both NPQ/spexin and a second novel 18-aa peptide, NPQ 53-70 (FISDQSRRKDLSDRPLPE), each identified by our computational HMM, have biological activity after administration into the CNS and periphery. Both peptides evoke marked, but differential, effects on cardiovascular and renal function in conscious rats, and both peptides demonstrate antinociceptive activity in mice.
Figure 1.
Amino acid sequence of ppNPQ/spexin from human, mouse, and rat. Bold letters show potential processing sites. Areas shaded in gray show NPQ/spexin and NPQ 53-70. NPQ 53-70 includes an unprocessed RRK.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 275–300 g and C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) or ICR mice (Charles River Laboratories, Hollister, CA, USA) weighing 20–25 g at the start of the experiments were maintained on a 12-h light-dark cycle (lights on at 7:00 AM) with free access to a normal sodium diet and tap water ad libitum. Rats were housed 2/group, whereas mice were housed 4/group. Rats were used for cardiovascular and renal function studies, and mice were used for behavioral assessments. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal care and use committees at SRI International, Louisiana State University, and Torrey Pines Institute for Molecular Studies.
Peptides
NPQ/spexin and NPQ 53-70 were synthesized by Gen/Script Corp. (Piscataway, NY, USA).
Cardiovascular and renal function studies
Surgical and experimental methods
For studies in which peptides or vehicle were administered into the brain, a stainless steel cannula was stereotaxically implanted into the right lateral cerebral ventricle of rats anesthetized with ketamine in combination with xylazine, 5–7 d before experimentation, as described previously (11, 12). On the day of the study, rats were anesthetized with sodium methohexital (75 mg/kg i.p., supplemented with 10 mg/kg i.v. as needed; JHP Pharmaceuticals, LLC, Rochester, MI, USA) and implanted with left femoral artery (blood pressure measurement), vein (isotonic saline infusion), and urinary bladder (urine collection) catheters using standard techniques described previously (10, 11). After surgical preparation, rats were placed in a rat holder, a chamber with Plexiglas ends connected by stainless steel rods where the metal rods formed an inverted U shape and a flat base in which the rat sits. This rat holder permits forward and backward movement of the rat while minimizing movement during surgical recovery and allows for collection of urine. An intravenous infusion of isotonic saline (55 μl/min) was started and continued for the duration of the experiment. The experiment commenced after the animal regained full consciousness and cardiovascular and renal excretory functions stabilized (4–6 h). Mean arterial pressure and heart rate were continuously recorded using computer-driven Biopac data acquisition software (MP100 and AcqKnowledge 3.8.2; Biopac, Goleta, CA, USA). Urine volume was determined gravimetrically. Urine sodium concentration was measured by flame photometry (model 943; Instrumentation Laboratories, Lexington, MA, USA) and expressed as urinary sodium excretion.
Intracerebroventricular (i.c.v.) studies in rats
Studies were performed in conscious rats to determine the cardiovascular and renal responses produced by the administration of NPQ/spexin or NPQ 53-70 into the brain. After stabilization of parameters, systemic cardiovascular function and urine flow rate were measured during a 20-min baseline control period. After collection of baseline control measurements, NPQ/spexin (30 nmol), NPQ 53-70 (30 nmol), angiotensin II (200 ng), or isotonic saline (vehicle; 5 μl) was then administered by i.c.v. injection. Immediately after i.c.v. drug/vehicle administration, experimental urine samples were collected every 10 min over a 90-min session.
Intravenous (i.v.) bolus studies in rats
These studies examined the changes in cardiovascular and renal excretory function produced by the i.v. bolus injection of NPQ/spexin or NPQ 53-70 in conscious rats. Cardiovascular parameters and urine flow rate were initially measured during a 20-min baseline period. Next, NPQ/spexin (30, 100, and 300 nmol/kg), NPQ 53-70 (30, 100, and 300 nmol/kg) or isotonic saline vehicle (200 μl) was injected as an i.v. bolus to conscious rats (n=6/group). Immediately after administration of the i.v. bolus, experimental urine samples were collected every 10 min over a 60-min period.
Locomotor activity and antinociceptive studies in mice
Injection techniques
I.c.v. injections into mice were given according to the modified method of Haley and McCormick (13). Mice were anesthetized with isoflurane, and an incision was made to expose the scalp. Peptides were then injected directly into the ventricle with a 10-μl Hamilton microsyringe. The coordinates for i.c.v. injection (5 μl) were 2 mm lateral and 2 mm caudal with respect to bregma and −3 mm ventral from the skull surface.
Antinociceptive testing: the 55°C warm water tail withdrawal assay
The nociceptive stimulus was 55°C water, with the latency to withdraw the tail taken as the end point (14, 15). After determination of baseline latencies (1.40±0.03 s), mice (n=7–8/group) received an i.c.v. dose of vehicle [30% DMSO/70% sterile saline (0.9% NaCl)] or peptide ligand (0.1–30 nmol NPQ/spexin or 0.1–10 nmol NPQ 53-70), with or without a 20-min naloxone pretreatment (10 mg/kg s.c.). Mice were then tested for antinociception every 10–20 min up to 150 min postinjection. A cutoff time of 15 s was used in this study; if the mouse failed to display a tail withdrawal response during that time, the tail was removed from the water, and the animal was assigned a maximal antinociceptive score of 100%. At each time point, antinociception was calculated according to the following formula: % antinociception = 100 × (test latency−control latency)/(15−control latency).
Comprehensive Lab Animal Monitoring System (CLAMS) measurement of locomotor activity
Locomotor activity was recorded using the automated, computer-controlled CLAMS apparatus (Columbus Instruments, Columbus, OH, USA; ref. 16). The cages were 23.5 cm (length) × 11.5 cm (width) × 13 cm (height) and equipped with infrared emitters along the longitudinal axis. Locomotor activity was calculated as consecutive beam breakages. Mice were placed in the cages and habituated for a 45-min period. Animals (n=7–8/group) then received i.c.v. injections of vehicle (30% DMSO/70% sterile saline, 0.9%), NPQ/spexin (30 nmol i.c.v.), or NPQ 53-70 (10 nmol i.c.v.). Doses of NPQ/spexin and NPQ 53-70 were selected for testing because these doses produced maximal effects in antinociceptive testing in mice. After administration of the compounds, mice were returned to the test cages for 90 min, and locomotor activity was measured in 60-s intervals.
Statistical analysis
All data are expressed as means ± se. Differences occurring between treatment groups (e.g., NPQ/spexin peptide vs. vehicle) were assessed by a 2-way repeated-measures ANOVA [SigmaStat statistical software (Systat Software, Inc., Point Richmond, CA, USA) for cardiovascular and renal experiments and Prism 5.0 (GraphPad Software, La Jolla, CA, USA) for behavioral assessments] with treatment group as the between-group variable and test time as the repeated measure. The magnitude of the changes in cardiovascular and renal excretory parameters at different time points after i.c.v. or i.v. bolus injection of NPQ/spexin, NPQ 53-70, or vehicle was compared by a 1-way repeated-measures ANOVA with a subsequent Dunnett's test. Post hoc analysis was performed using Holm-Sidak tests and, where appropriate, a Student's t test was also used. Statistical significance was defined as P < 0.05.
In vitro proteolysis reactions using proprotein and prohormone convertases
The preparation of mouse prohormone convertase (PC) 1/3, PC2, and soluble human furin from Chinese hamster ovary cell-conditioned medium has been described previously (17–19). The purity of recombinant enzymes was estimated as >99% using SDS-PAGE stained with Coomassie Brilliant Blue. Recombinant His-tagged human proNPQ/spexin (2 μg) was incubated with 2 U of either PC1/3, PC2, or furin in 50-μl reactions containing 100 mM sodium acetate (pH 5.5) for PC1/3 (for PC2, pH 5.0; for furin, 100 mM Hepes, pH 7.0) and 5 mM CaCl2 at 37°C for the time periods indicated. The reactions were then subjected to SDS-PAGE using 18% Tris-HCl acrylamide gels, and the gels were stained with Coomassie Brilliant Blue. One unit of PC activity is equal to the amount of the enzyme that is required to cleave 1 pmol/min of the pRTKR-aminomethyl coumarin (Peptides International, Inc., Lexington, KY, USA) fluorogenic substrate. To determine the cleavage products generated by PC2, 2 μg of His-tagged human proNPQ/spexin was cleaved by PC2 for 6 h at 37°C in 1-ml reactions using the reaction conditions described above. The cleavage products were applied to a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) and eluted with 60% isopropanol containing 0.1% trifluoroacetic acid, and the eluent was lyophilized. The lyophilized sample was resuspended in 2% acetonitrile and 0.1% trifluoroacetic acid at a concentration of 0.13 mg/ml and subjected to matrix-assisted laser desorption ionization/time of flight mass spectrometry.
RESULTS
Cardiovascular and renal effects
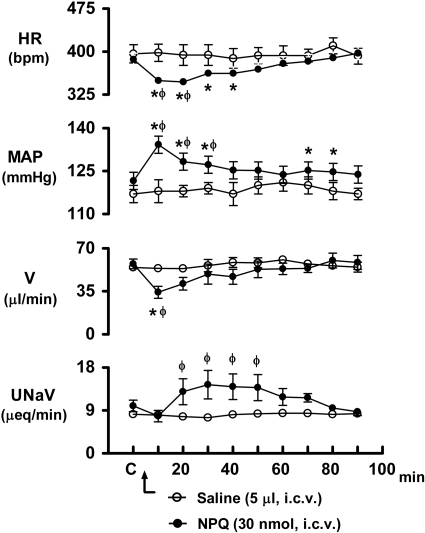
As shown in Fig. 2, i.c.v. injection of NPQ/spexin (30 nmol) produced an immediate increase in mean arterial pressure (control, 121±3 mmHg; NPQ/spexin 10 min, 134±3 mmHg) and decrease in heart rate (control, 386±11 bpm; NPQ/spexin 10 min, 349±11 bpm) in rats (see Supplemental Fig. S1A for original blood pressure/heart rate tracing). In a comparison between treatment groups, there was a significant interaction of drug and time for both mean arterial pressure (F9,90=5.179, P<0.001) and heart rate (F9,90=6.20, P<0.001). Compared with the respective predrug control levels, the pressor and bradycardic responses produced by central NPQ/spexin remained significantly elevated for 30 and 40 min after drug injection, respectively. Concurrent with these cardiovascular responses, i.c.v. NPQ/spexin also produced a profound and immediate decrease in urine flow rate (control, 57±2 μl/min; NPQ/spexin 10 min, 33±6 μl/min) and a delayed increase in urinary sodium excretion (control, 9.9±1.2 μEq/min; NPQ/spexin 30 min, 14.4±2.9 μEq/min) (Fig. 2). Differences in mean values showed a significant interaction of drug and time for urine flow rate (F9,90=2.21, P=0.028) and urinary sodium excretion (F9,90=3.42, P=0.001). For comparative purposes, changes in systemic cardiovascular function and urine flow rate were examined in rats that were administered the neuropeptide, angiotensin II (200 ng) i.c.v. In these studies, i.c.v. angiotensin II produced significant increases in arterial blood pressure (control, 120±2 mmHg; angiotensin II 10 min, 137±3 mmHg) and decreases in heart rate (control, 402±17 bpm; angiotensin II 10 min, 360±15 bpm) and urine flow rate (control, 53±2 μl/min; angiotensin II 10 min, 28±4 μl/min).
Figure 2.
Effect of i.c.v. NPQ/spexin (30 nmol) and isotonic saline vehicle on heart rate (HR), mean arterial pressure (MAP), urine flow rate (V), and urinary sodium excretion (UNaV) in rats. n = 6/group; means ± se. *P < 0.05 vs. the corresponding control (C); φP < 0.05 vs. saline value at same time point.
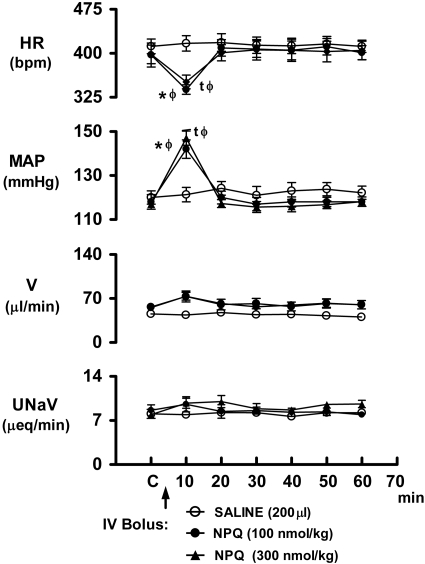
The i.v. bolus injection of NPQ/spexin (100 and 300 nmol/kg) to conscious rats produced marked pressor and bradycardic responses that were immediate in onset but of relatively short duration (20 min; Fig. 3 and Supplemental Fig. S1B for original tracing). The ANOVA indicated that there was a significant drug × time interaction for both mean arterial pressure (F12,108=23.522,) and heart rate (F12,108=9.214). Potentially related to their short duration of action in the periphery, the NPQ/spexin-evoked cardiovascular responses occurred over time (i.e., interaction) without significantly altering urine flow rate (F12,108=1.645) or urinary sodium excretion (F12,108=1.124) (Fig. 3). As depicted in Supplemental Fig. S2A, the pressor and bradycardic effects produced by i.v. bolus NPQ were dose-dependent (mean arterial pressure, F3,20=53.65; heart rate, F3,20=15.226).
Figure 3.
Time-course responses in cardiovascular and renal excretory function produced by i.v. bolus NPQ/spexin in conscious Sprague-Dawley rats. n = 6/group; means ± se. *,tP < 0.05 vs. respective control (C); φP < 0.05 vs. saline value at same time point. HR, heart rate; MAP, mean arterial pressure; V, urine flow rate; UNaV, urinary sodium excretion.
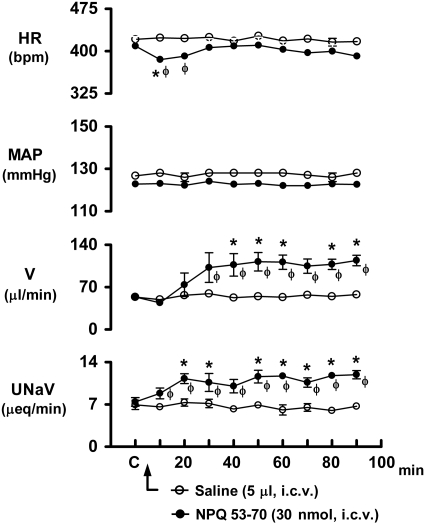
The cardiovascular and renal excretory responses produced by the i.c.v. administration of NPQ 53-70 in conscious rats are shown in Fig. 4. After drug injection, NPQ 53-70 failed to alter mean arterial pressure over the 90-min protocol. However, this peptide produced a significant decrease in heart rate (control, 409±17 bpm; NPQ/spexin 10 min, 385±11 bpm), which persisted for 20 min (F9,90=1.993) (see Supplemental Fig. S1C for original tracing). Central NPQ 53-70 also produced a slow onset but marked increase in urine flow rate, which was significantly elevated (F9,90=3.197, P=0.002) above the respective control value 40 min after drug administration and remained elevated for the remainder of the 90-min protocol (control, 54±2 μl/min; NPQ 53-70 90 min, 114±9 μl/min). Concurrent with the diuretic response, central NPQ 53-70 also produced a significant (F9,90=3.174, P=0.002) and sustained increase in urinary sodium excretion (control, 7.43±0.72 μEq/min; NPQ 53-70 90 min, 11.92±0.69 μEq/min). In contrast to central administration, the i.v. bolus injection of NPQ 53-70 did not alter any cardiovascular or renal excretory parameter when tested at doses of 30, 100, or 300 nmol/kg (Supplemental Fig. S2B).
Figure 4.
Effect of i.c.v. NPQ 53-70 (30 nmol) and isotonic saline vehicle on heart rate (HR), mean arterial pressure (MAP), urine flow rate (V), and urinary sodium excretion (UNaV) in rats. n = 6/group; means ± se. *P < 0.05 vs. respective control (C); φP < 0.05 vs. saline value at same time point.
Behavioral effects
Both NPQ/spexin and NPQ 53-70 were tested for behavioral activity in mice. I.c.v. administration of NPQ/spexin (30 nmol) or NPQ 53-70 (10 nmol) resulted in an equivalent number of average ambulations over a 90-min period (6.56±0.92 and 6.63±0.71, respectively). These ambulations were not statistically different from those observed after administration of vehicle (6.89±0.91; 1-way ANOVA, F2,21=0.04, P=0.96). Likewise, the overall ANOVA indicated that there was no significant interaction effect (F34,357=1.37) between treatment with saline, NPQ/spexin, or NPQ-53-70 (Supplemental Fig. S3A).
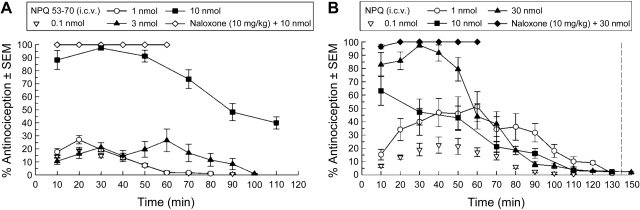
I.c.v. administration of both NPQ/spexin and NPQ 53-70 into mice induced dose-dependent antinociceptive activity using the warm water tail withdrawal assay. A dose of 10 nmol of NPQ 53-70 produced a maximal antinociceptive response at 30 min with an effect that lasted up to 2 h (Fig. 5A). An overall ANOVA indicated a significant interaction effect (F12,105=4.05) after treatment with NPQ 53-70. Likewise, NPQ/spexin treatment produced dose-dependent antinociception, with an overall ANOVA demonstrating an interaction effect (F20,165=13.3). NPQ/spexin was not maximally effective at 10 nmol, but at 30 nmol produced an antinociceptive response that lasted for longer than 1 h and a dose of 1 nmol produced 50% of the maximal effect at 60 min (Fig. 5B). These robust effects were not opioid receptor-mediated, because antinociception produced by either peptide was not inhibited by 10 mg/kg naloxone (s.c.).
Figure 5.
Antinociceptive activity ± se of NPQ 53-70 (A) and NPQ/spexin (B) after i.c.v. administration (n=7–8/group) to mice in the warm water tail withdrawal procedure.
Processing of preproNPQ/spexin
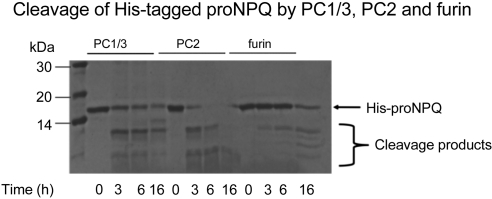
We investigated processing of recombinant proNPQ/spexin by incubating the bacterially derived purified human His-tagged precursor with purified recombinant convertases. A Coomassie-stained gel is shown in Fig. 6. This figure shows that PC2 and to a lesser extent PC1 and furin were able to cleave this precursor into 3 to 4 Coomassie-stained fragments. To determine the major products of prohormone convertase cleavage, mass spectroscopic analysis of parallel reactions was performed. These data show that fragments corresponding to C-terminally extended NPQ/spexin, residue N (aa 36) to R (aa 51) and N (aa 36) to R (aa 52) (predicted mass of 1849.9377 and 2006.0388, respectively), and fragments corresponding to C-terminally extended NPQ 53-70, residue F (aa 53) to R (aa 71) and F (aa 53) to R (aa 72) (predicted masses of 2315.2214 and 2471.3325), were recovered from PC1/3 and PC2 digests. These data confirm that prohormone convertases can cleave proNPQ/spexin at residues R (aa 35), R (aa 52), and R (aa 72). A high probability of cleavage is also indicated by the cleavage prediction program NeuroPred. Mass spectroscopy data, although supporting the existence of the particular cleavages that generate the peptides studied here, do not rule out the occurrence of other cleavages, because larger and smaller peptides may be difficult to detect; in fact, larger potential intermediates are evident in Supplemental Fig. S4.
Figure 6.
Coomassie-stained gel of proNPQ/spexin produced by incubating the bacterially derived purified recombinant proNPQ/spexin precursor with purified recombinant convertase.
DISCUSSION
Neuropeptides bind to G-protein-coupled receptors on neurons distributed throughout the brain and periphery, as well as throughout target organs, and in so doing modulate virtually all physiological processes. Although many neuropeptides have been studied extensively and their physiological actions are well known, the functions of others have yet to be revealed. In particular, a newly discovered neuropeptide, called both spexin and NPQ by its respective discoverers, is a peptide with biological activities that have yet to be identified (6, 7). Moreover, the prohormone from which NPQ/spexin is derived has several putative processing sites and therefore has the potential of generating additional biologically active peptides. Here we report that both NPQ/spexin and a second newly discovered peptide that we have called NPQ 53-70 have novel cardiovascular-, renal-, and CNS-mediated behavioral actions.
Both NPQ/spexin and NPQ 53-70 demonstrated potent biological activities in rodents. When administered by i.c.v. injection to conscious rats, NPQ/spexin produced significant cardiovascular and renal actions. I.c.v. administration of NPQ/spexin produced a marked increase in mean arterial pressure and decrease in heart rate in rats immediately after drug injection. Concurrent with these cardiovascular responses, central NPQ/spexin also altered renal excretory function, producing an immediate decrease in urine output and a delayed natriuresis. The cardiovascular and renal responses to NPQ/spexin were similar to those found after i.c.v. injection of angiotensin II. The similarity in responses produced by NPQ/spexin and angiotensin II is of considerable interest, because angiotensin II is a highly potent and efficacious endogenous vasoconstrictor peptide known to participate in the etiology of neurogenic hypertension (20). In other studies, we have shown that the inhibitory neuropeptide nociceptin/orphanin FQ also produces centrally mediated cardiovascular and renal responses in conscious rats, with i.c.v. dose ranges between 5 and 20 nmol (12). In addition to a CNS site of action, the i.v. bolus injection of NPQ/spexin (30, 100, and 300 nmol/kg) also produced immediate but short-lived dose-related pressor and bradycardic responses in conscious rats. When administered as an i.v. bolus, the relatively short duration of action of NPQ/spexin is most likely due to rapid metabolism of the peptide, which might explain its lack of effect on renal excretory function.
The novel neuropeptide NPQ 53-70 also evoked significant changes in systemic cardiovascular and renal excretory function when administered into the CNS of rats. However, the pattern of responses produced by i.c.v. administration of NPQ 53-70 did not mimic the responses produced by centrally administered NPQ/spexin. Central NPQ 53-70 (30 nmol) evoked a transient decrease in heart rate but failed to alter mean arterial blood pressure. Alternatively, the bradycardia produced by i.c.v. NPQ/spexin was associated with a concurrent increase in mean arterial pressure. Further evidence that these two peptides are likely to have distinct physiological roles in cardiovascular biology is the finding that central NPQ 53-70 also produced a marked and sustained increase in urine flow rate and urinary sodium excretion. This result is in contrast to the effect of central NPQ/spexin, which instead evoked a pronounced antidiuretic response and transient natriuresis. Finally, unlike the i.v. bolus administration of NPQ/spexin, which caused pronounced changes in systemic cardiovascular function, the i.v. bolus injection of NPQ 53-70 did not alter systemic cardiovascular (or renal excretory) function.
Our studies provide clear evidence that NPQ/spexin and NPQ 53-70 profoundly affect cardiovascular and renal function in the rat; however, the particular responses generated by each peptide are different and most likely depend on the locus of action of the ligand (e.g., brain or periphery). Further studies are required to determine the neural and/or humoral mechanisms by which NPQ/spexin and NPQ 53-70 peptides influence blood pressure and the handling of water/sodium and to determine the brain site(s) mediating the systemic cardiovascular and renal responses.
Further studies are also required to identify the NPQ/spexin peptide that is active in the rat. Even if species as distant as fish are included, rat is the only species that has a change in the cleavage site, with a sequence of Gly-His-Arg rather than of Gly-Arg-Arg. Although not as common as protein convertase cleavage of Arg-Arg or Lys-Arg, single basic cleavages are well known, so PC cleavage of Gly-His-Arg is possible (21). Amidation of this peptide is also in question. C-terminal histidines are hydrolyzed by carboxypeptidase E, but extremely slowly, with a reaction rate several orders of magnitude lower than that of substrates with C-terminal lysines or arginines (22, 23). However, because carboxypeptidase E has an enzymatic rate several orders of magnitude greater than that of protein convertases (24), His removal and subsequent amidation to produce NPQ/spexin should theoretically be possible in vivo. Alternatively, the active NPQ/spexin peptide in rats could be NWTPQAMLYLKGAQGH. If so, this would imply that the NPQ/spexin receptor may recognize the amino terminus of the peptide rather than the carboxyl terminus.
Cardiovascular and renal actions of NPQ/spexin and related peptides are not surprising considering the demonstrated location of preproNPQ/spexin mRNA in rat brain. In situ hybridization studies demonstrated intense staining in Barrington's nucleus (the pontine micturition center; ref. 7). These studies indicated that proNPQ/spexin-containing cells overlap in their distribution with cells that express corticotrophin-releasing factor (CRF) in Barrington's nucleus as well as serotonergic and dopaminergic cells in the ventrolateral periaquiductal gray. Barrington's nucleus contains neurons that send polysynaptic projections to the bladder, colon, spleen, and kidney (25, 26). Activation of CRF-containing neurons in Barrington's nucleus have been proposed to inhibit bladder contraction (i.e., inhibit micturition; ref. 27) and play a role in mediating stress-induced colonic alterations (28). Barrington's nucleus also receives neuronal inputs from the forebrain, including the paraventricular nucleus (29). Thus, by acting at multiple brain sites, NPQ/spexin and/or NPQ 53-70 may play an interactive role with CRF in co-coordinating gastrointestinal and urinary function with fluid and cardiovascular homeostasis under basal conditions and during stress/pathology.
When NPQ/spexin and NPQ 53-70 were injected i.c.v. into mice at doses up to 10 nmol, there were no overt changes in behavior compared with that of control animals. The mice show no apparent distress, and there is no effect on locomotor activity (Supplemental Fig. S3A). Indeed, no differences were observed in other normal behaviors such as grooming, rearing, and sniffing after NPQ/spexin administration compared with those in control mice (Supplemental Fig. S3B). However, there is a very significant effect on response to noxious stimuli. Both NPQ/spexin and NPQ 53-70 exhibit antinociceptive activity lasting up to 1–2 h after injection. In the warm water tail withdrawal paradigm, NPQ 53-70 is longer lasting and more potent than NPQ/spexin and produces a maximal antinociceptive response between 30 and 50 min, at a 10-nmol dose (Fig. 4). Naloxone did not block the antinociceptive activity of either peptide, indicating that this response is not opioid receptor-mediated. Thus, although NPQ/spexin and NPQ 53-70 showed differential effects on cardiovascular and renal function, these two peptides were similar in their CNS ability to evoke antinociception. Taken together these antinociceptive, systemic cardiovascular, and renal excretory responses produced by NPQ 53-70 are the first demonstration of biological activity of this peptide, a second peptide product of the proNPQ/spexin precursor.
Despite the demonstrated biological activity of chemically synthesized NPQ/spexin and NPQ 53-70, the endogenous presence of these particular peptide species in the brain or other organs has not yet been conclusively demonstrated. Experiments examining the in vitro hydrolysis of proNPQ/spexin with PC2 have shown that the brain-expressed enzyme PC2 can efficiently process this precursor and produce the expected basic residue extended versions of both NPQ/spexin and NPQ 53-70 (Supplemental Fig. S4), indicating probably endogenous production of NPQ/spexin and NPQ 53-70. Mirabeau et al. (6) have shown that NPQ/spexin immunoreactive peptides colocalize with insulin in secretory vesicles, suggesting that proNPQ/spexin-derived peptides possess the correct targeting signals to arrive at the secretory granule compartment, where PC2 is located.
A great deal remains to be learned about this neuropeptide precursor and its processed peptides. Experiments designed to identify the molecular forms of NPQ/spexin and NPQ 53-70 using immunological methods are currently underway. The receptors mediating the actions of these two peptides must also be identified. In addition, based on the proximity of preproNPQ/spexin mRNA to CRF in Barrington's nucleus and dopaminergic neurons in the ventrolateral periaqueductal gray, which project to the CRF-containing area of the bed nucleus of the stria terminalis, there is reason to believe that NPQ/spexin-derived peptides may have a significant role in the stress response and potential stress-mediated gastrointestinal disturbances (7, 30).
Our initial experiments provide significant CNS and peripheral actions for neuropeptides arising from a single precursor protein highly localized to very limited brain regions. This very discrete localization is similar to that of two other recently discovered peptides, NPS and hypocretin/orexin, both of which have a wide range of important physiological functions emanating from a very small population of neurons in a discrete brain region (31, 32). We anticipate a similar impact of proNPQ/spexin-derived peptides in health and disease.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant R21-DA016629 to L.T., American Heart Association grant 0855293E and NIH grant P20-RR018766 to D.R.K., and NIH grant DA027170 to I.L.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Meunier J. C., Mollereau C., Toll L., Suaudeau C., Moisand C., Alvinerie P., Butour J. L., Guillemot J. C., Ferrara P., Monsarrat B., Mazarguil H., Vassart G., Parmentier M., Costentin J. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535 [DOI] [PubMed] [Google Scholar]
- 2. Reinscheid R. K., Nothacker H. P., Bourson A., Ardati A., Henningsen R. A., Bunzow J. R., Grandy D. K., Langen H., Monsma F. J., Jr., Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794 [DOI] [PubMed] [Google Scholar]
- 3. Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., Williams S. C., Richardson J. A., Kozlowski G. P., Wilson S., Arch J. R., Buckingham R. E., Haynes A. C., Carr S. A., Annan R. S., McNulty D. E., Liu W. S., Terrett J. A., Elshourbagy N. A., Bergsma D. J., Yanagisawa M. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 [DOI] [PubMed] [Google Scholar]
- 4. Mutt V. (1980) Chemistry, isolation and purification of gastrointestinal hormones. Biochem. Soc. Trans. 8, 11–14 [DOI] [PubMed] [Google Scholar]
- 5. Steiner D. F. (1998) The proprotein convertases. Curr. Opin. Chem. Biol. 2, 31–39 [DOI] [PubMed] [Google Scholar]
- 6. Mirabeau O., Perlas E., Severini C., Audero E., Gascuel O., Possenti R., Birney E., Rosenthal N., Gross C. (2007) Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 17, 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonmez K., Zaveri N. T., Kerman I. A., Burke S., Neal C. R., Xie X., Watson S. J., Toll L. (2009) Evolutionary sequence modeling for discovery of peptide hormones. PLoS Comput. Biol. 5, e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonmez K. T., Toll L., Zaveri N. T. (2007) Evolutionary sequence modeling for discovery of peptide hormones. Proc. IEEE Int. Conf. Acoustics, Speech, Signal Process. (ICASSP 2007) 1, 377–380 [Google Scholar]
- 9. Porzionato A., Rucinski M., Macchi V., Stecco C., Malendowicz L. K., De Caro R. (2010) Spexin expression in normal rat tissues. J. Histochem. Cytochem. 58, 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rucinski M., Porzionato A., Ziolkowska A., Szyszka M., Macchi V., De Caro R., Malendowicz L. K. (2010) Expression of the spexin gene in the rat adrenal gland and evidences suggesting that spexin inhibits adrenocortical cell proliferation. Peptides 31, 676–682 [DOI] [PubMed] [Google Scholar]
- 11. Kapusta D. R., Burmeister M. A., Calo G., Guerrini R., Gottlieb H. B., Kenigs V. A. (2005) Functional selectivity of nociceptin/orphanin FQ peptide receptor partial agonists on cardiovascular and renal function. J. Pharmacol. Exp. Ther. 314, 643–651 [DOI] [PubMed] [Google Scholar]
- 12. Kapusta D. R., Sezen S. F., Chang J. K., Lippton H., Kenigs V. A. (1997) Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci. 60, PL15–PL21 [DOI] [PubMed] [Google Scholar]
- 13. Haley T. J., McCormick W. G. (1957) Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br. J. Pharmacol. Chemother. 12, 12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McLaughlin J. P., Hill K. P., Jiang Q., Sebastian A., Archer S., Bidlack J. M. (1999) Nitrocinnamoyl and chlorocinnamoyl derivatives of dihydrocodeinone: in vivo and in vitro characterization of mu-selective agonist and antagonist activity. J. Pharmacol. Exp. Ther. 289, 304–311 [PubMed] [Google Scholar]
- 15. Vaught J. L., Takemori A. E. (1979) Differential effects of leucine and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J. Pharmacol. Exp. Ther. 208, 86–90 [PubMed] [Google Scholar]
- 16. Reilley K. J., Giulianotti M., Dooley C. T., Nefzi A., McLaughlin J. P., Houghten R. A. (2010) Identification of two novel, potent, low-liability antinociceptive compounds from the direct in vivo screening of a large mixture-based combinatorial library. AAPS J. 12, 318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kacprzak M. M., Peinado J. R., Than M. E., Appel J., Henrich S., Lipkind G., Houghten R. A., Bode W., Lindberg I. (2004) Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-d-arginine. J. Biol. Chem. 279, 36788–36794 [DOI] [PubMed] [Google Scholar]
- 18. Lamango N. S., Zhu X., Lindberg I. (1996) Purification and enzymatic characterization of recombinant prohormone convertase 2: stabilization of activity by 21 kDa 7B2. Arch. Biochem. Biophys. 330, 238–250 [DOI] [PubMed] [Google Scholar]
- 19. Zhou Y., Lindberg I. (1993) Purification and characterization of the prohormone convertase PC1(PC3). J. Biol. Chem. 268, 5615–5623 [PubMed] [Google Scholar]
- 20. DiBona G. F. (2001) Peripheral and central interactions between the renin-angiotensin system and the renal sympathetic nerves in control of renal function. Ann. N. Y. Acad. Sci. 940, 395–406 [DOI] [PubMed] [Google Scholar]
- 21. Devi L. (1991) Consensus sequence for processing of peptide precursors at monobasic sites. FEBS Lett. 280, 189–194 [DOI] [PubMed] [Google Scholar]
- 22. Smyth D. G., Maruthainar K., Darby N. J., Fricker L. D. (1989) Catalysis of slow C-terminal processing reactions by carboxypeptidase H. J. Neurochem. 53, 489–493 [DOI] [PubMed] [Google Scholar]
- 23. Fricker L. D. (1991) Peptide Processing Exopeptidases. In Peptide Biosynthesis and Processing (Fricker L. D., ed) pp. 199–229, CRC Press, Boca Raton, FL, USA [Google Scholar]
- 24. Cameron A., Apletalina E., Lindberg I. (2001) The enzymology of prohormone convertases PC1 and PC2. In The Enzymes (Dalbey R. E., ed), Academic Press, San Diego, CA, USA [Google Scholar]
- 25. Cano G., Card J. P., Rinaman L., Sved A. F. (2000) Connections of Barrington's nucleus to the sympathetic nervous system in rats. J. Auton. Nerv. Syst. 79, 117–128 [DOI] [PubMed] [Google Scholar]
- 26. Rouzade-Dominguez M. L., Pernar L., Beck S., Valentino R. J. (2003) Convergent responses of Barrington's nucleus neurons to pelvic visceral stimuli in the rat: a juxtacellular labelling study. Eur. J. Neurosci. 18, 3325–3334 [DOI] [PubMed] [Google Scholar]
- 27. Pavcovich L. A., Valentino R. J. (1995) Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington's nucleus. Neurosci. Lett. 196, 185–188 [DOI] [PubMed] [Google Scholar]
- 28. Valentino R. J., Kosboth M., Colflesh M., Miselis R. R. (2000) Transneuronal labeling from the rat distal colon: anatomic evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J. Comp. Neurol. 417, 399–414 [PubMed] [Google Scholar]
- 29. Valentino R. J., Page M. E., Luppi P. H., Zhu Y., Van Bockstaele E., Aston-Jones G. (1994) Evidence for widespread afferents to Barrington's nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience 62, 125–143 [DOI] [PubMed] [Google Scholar]
- 30. Sved A. F., Cano G., Passerin A. M., Rabin B. S. (2002) The locus coeruleus, Barrington's nucleus, and neural circuits of stress. Physiol. Behav. 77, 737–742 [DOI] [PubMed] [Google Scholar]
- 31. de Lecea L., Kilduff T. S., Peyron C., Gao X., Foye P. E., Danielson P. E., Fukuhara C., Battenberg E. L., Gautvik V. T., Bartlett F. S., 2nd, Frankel W. N., van den Pol A. N., Bloom F. E., Gautvik K. M., Sutcliffe J. G. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 95, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Y. L., Gall C. M., Jackson V. R., Civelli O., Reinscheid R. K. (2007) Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 500, 84–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.