Abstract
The vascular type of the Ehlers-Danlos syndrome (vEDS) is caused by dominant-negative mutations in the procollagen type III (COL3A1) gene. Patients with this autosomal dominant disorder have a shortened life expectancy due to complications from ruptured vessels or hollow organs. We tested the effectiveness of allele-specific RNA interference (RNAi) to reduce the mutated phenotype in fibroblasts. Small-interfering RNAs (siRNAs) discriminating between wild-type and mutant COL3A1 allele were identified by a luciferase reporter gene assay and in primary fibroblasts from a normal donor and a patient with vEDS. The best discriminative siRNA with the mutation at position 10 resulted in >90% silencing of the mutant allele without affecting the wild-type allele. Transmission and immunogold electron microscopy of extracted extracellular matrices from untreated fibroblasts of the patient with vEDS revealed structurally abnormal fibrils. After siRNA treatment, collagen fibrils became similar to fibrils from fibroblasts of normal and COL3A1 haploinsufficient donors. In addition, it was shown that expression of mutated COL3A1 activates the unfolded protein response and that reduction of the amount of mutated protein by siRNA reduces cellular stress. Taken together, the results provide evidence that allele-specific siRNAs are able to reduce negative effects of mutated COL3A1 proteins. Thus, the application of allele-specific RNAi may be a promising direction for future personalized therapies to reduce the severity of vEDS.—Müller, G. A., Hansen, U., Xu, Z., Griswold, B., Talan, M. I., McDonnell, N. B., Briest, W. Allele-specific siRNA knockdown as a personalized treatment strategy for vascular Ehlers-Danlos syndrome in human fibroblasts.
Keywords: extracellular matrix, collagen, RNA interference
Patients diagnosed with disorders caused by dominant-negative mutations in one allele of a single gene, such as the vascular type of Ehlers-Danlos syndrome (vEDS), currently have very few treatment options, limited to nonspecific symptomatic treatment, genetic counseling, and emergency intervention. Such choices are less than optimal and do not alleviate much of the suffering associated with monogenic diseases.
EDS is a heterogeneous group of heritable disorders characterized by production of abnormal collagen. The disease has been divided into 6 major subtypes based on clinical, genetic, and other criteria (1, 2). vEDS is a severe form that can lead to debilitating conditions or to sudden death. Although the exact prevalence of vEDS is still debatable, the currently accepted estimate varies between 1:100,000 and 1:250,000 people (3). However, growing awareness among medical professionals and wider availability of molecular genetic diagnosis might lead to higher prevalence estimation. vEDS is an autosomal dominant disorder in which the instability of joints and dermal abnormalities characteristic of other forms of EDS are exacerbated by susceptibility to spontaneous and often catastrophic rupture of large arteries and hollow organs, such as the bowel and uterus (4). Patients with vEDS often have a first major complication in the early 20s, and >80% have at least one complication by the age of 40, reducing the average life expectancy to 48 yr (5). Pregnant women with vEDS are at additional risk of rupture of uterus. The only evidence-based treatment strategy to date for vEDS is the recently published report of a multicenter-randomized trial with celiprolol, a long-acting β1 adrenergic receptor (AR) antagonist with partial β2 AR agonist properties, which decreased the incidence of arterial rupture in 47 mo of treatment (6). While the patients seem to show symptomatic improvement, the effect of celiprolol treatment on the extracellular matrix composition in affected tissues, as well as mutation-specific clinical outcomes, are yet unknown. Moreover, the study had some randomization problems, including an unequal distribution of mutations in the treatment and untreated groups that compromise the conclusion. Therefore, there is still a need for personalized treatment strategies that abolish the dominant-negative effect of mutated procollagen III.
A defect in the gene for the procollagen type III (COL3A1) is the usual genetic basis for vEDS. COL3A1 is a homotrimer of 3 identical α chains. The majority of the patients are heterozygous for a mutation in one copy of the COL3A1 gene. Because one of the two copies of the gene that encodes the monomers is mutated, only 1/8 of the trimers are not defective (7). Patients that are haploinsufficient for COL3A1 have a reduced amount of nonmutated COL3A1 and seem to exhibit less severe symptoms and subsequent complications (8–11). Some persons who are carriers of the haploinsufficiency-type mutations have, in fact, no phenotypic features of the disorder, and so do not develop vascular rupture (11).
Therefore, one approach to a targeted treatment of vEDS is the elimination of the mRNA of the mutated form of the COL3A1 gene to transform the more severe phenotype to the less severe haploinsufficient type. We designed our approach on the basis of previously reported genetic methodology using siRNAs (12), which allowed knocking down the specific mRNA of an allele with a single-point mutation. Therefore, when using classic siRNAs with a length of 21 nt (19 nt that are complementary to the mRNA plus a dTdT overhang), 19 different siRNAs should be tested for discriminative knockdown (12). In addition, to date, >200 different mutations in the COL3A1 gene are known to lead to vEDS (13). Therefore, as a basis for a personalized therapy, allele-specific siRNAs have to be developed for each specific mutation. Obviously, the identification of a selective siRNA for a certain mutation is a complex process.
The direct approach to select an appropriate, i.e., strongest and most selective, siRNA is to test the effectiveness of all 19 siRNAs by treating primary fibroblasts derived from a patient with vEDS with siRNA in cell culture. The assay allows direct measurement of the allele-specific knockdown of endogenous mRNA/protein. This approach is very complex and time-consuming. Here, we attempt to simplify the process and to investigate whether it is possible to test siRNAs using a luciferase reporter assay. Applying this method, siRNAs could be screened quickly in a high-throughput manner without the need of using cell culture samples from the patient. To date, there is only very limited information regarding the comparison of allele-specific RNAi using reporter assays or measuring endogenous gene products. To test the feasibility of both approaches, we used fibroblasts from a patient heterozygous for COL3A1 allele encoding a glycine substitution, G252V (p.Gly252Val). Glycine mutations are the most common class of mutation causing vEDS. The G252V mutation is caused by the exchange from a guanine to a thymidine at position 755 in the coding region of COL3A1 cDNA (c.755G>T). We created 19 different siRNAs targeting the mutation to compare their effectiveness in luciferase reporter assays and in fibroblasts derived from patient samples. In addition, we applied the siRNA with the best potential to silence the mutated allele without affecting the wild-type allele to analyze the effect on the unfolded protein response (UPR) and on the extracellular matrix. We were able to reduce the phenotype caused by mutated COL3A1 and conclude that a personalized therapy based on allele specific RNAi could be a promising approach to reduce the severity of vEDS.
MATERIALS AND METHODS
COL3A1 mutations and siRNA design
The mutation of interest was a glycine substitution, G252V (p.Gly252Val) as a result of a c.755G>T mutation at position 755 downstream of ATG (A=+1; GenBank no. BC028178). As controls, cells from a patient with a different glycine substitution (c.1502G>C, p.G501A), as well as cells from a patient haploinsufficient for COL3A1 (c.2569C>T, p.Q857X) were applied. Sequencing for mutation identification in affected patients was conducted using ABI Big Dye Terminator V3.1 chemistry on the ABI 3100 Genetic Analyzer (Life Technologies, Carlsbad, CA, USA), and alignment was performed by Sequencer 4.10.1 (Gene Codes Corp., Ann Arbor, MI, USA) and Vector NTI Advance 11.0 (Life Technologies). The reference sequence of COL3A1 is from Ensembl Genome Browser (http://uswest.ensembl.org/index.html), on which nucleotide nomenclature at the cDNA level is based. For verification of the c.755G>T mutation in COL3A1 mRNA from the patient's fibroblasts, RNA was extracted (RNeasy Mini Kit; Qiagen, Valencia, CA, USA), cDNA was synthesized (Superscript III Reverse Transcriptase; Invitrogen, Carlsbad, CA, USA), and COL3A1 fragments were amplified using the primers COL3A1-for: 5′-ggaggactcgcaggctatc-3′ and COL3A1-rev: 5′-gcctggttgaccatcactg-3′. Sequencing was performed with the primer Seq-COL3A1-rev: 5′-gaaggagctgactgggttg-3′.
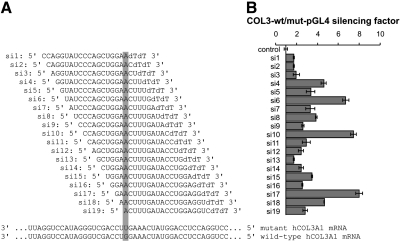
siRNAs (see Fig. 2A) ordered (desalted) from Invitrogen (Carlsbad, CA, USA) were diluted in H2O to get a 20 μM solution. The third nucleotide from the 3′ end of the sense strand of the siRNA was mismatched with the guide strand, creating an unpaired 5′ end to facilitate entry of the guide strand into the RNA-induced silencing complex (RISC), as recommended by Schwarz et al. (12). As a control, we used a nonsilencing siRNA (siC: 5′-GCUGGAGAUAGACUGCAUAdTdT-3′).
Figure 2.
Reporter gene assay of a tiled set of siRNAs targeting COL3A1G252V/+. A) Sequences of the guide siRNA strands used, indicating the site of the U:G mismatch. The mutant (matched) and wild-type (mismatched) COL3A1 mRNA sequences targeted by the siRNAs are shown at bottom. B) Silencing factor reflects the degree by which the siRNA was able to silence the mutated form of the COL3A1 sequence as compared to the wild-type sequence. It is based on the relative firefly luciferase expression for siRNAs cotransfected with a reporter plasmid containing the mutant (matched) or wild-type (mismatched) COL3A1 sequence fused to the luciferase 3′ untranslated region in HCT116 cells. Each experiment was performed in triplicate; data were normalized to Renilla luciferase activity and luciferase activity of cells transfected with a control siRNA. Data are shown as averages ± se.
Testing of siRNAs in a luciferase reporter assay
To test whether the siRNAs are able to silence the mutant COL3A1 mRNA without affecting the wild-type mRNA, luciferase reporter vectors with short inserts (136 bp) of either the wild-type or the mutant COL3A1 cDNA were prepared. The COL3A1 fragments were ligated into the 3′ untranslated region of the luciferase. Fragments were obtained by PCR (forward primer with XbaI site: AAAAATCTAGACCTGCTGGAAAAGATGGAG, reverse primer with FseI site: AAAAAGGCCGGCCGTCCATCGAAGCCTCTGTG; restriction sites underlined) and cloned into pGEMT (Promega, Madison, WI, USA). The mutation (position 80 of 136) was introduced by QuikChange PCR with 2 U Pfu turbo polymerase (Stratagene-Agilent Technologies, Santa Clara, CA, USA) and the provided buffer, 0.2 mM dNTPs, 125 ng primers (mutation underlined): forward CCTCCAGGTATCAAAGTTCCAGCTGGGATACCTGG, reverse CCAGGTATCCCAGCTGGAACTTTGATACCTGGAGG, in a 50-μl reaction. The template plasmid was digested after PCR by addition of 10 U DpnI (NEB, Ipswich, MA, USA). Plasmids were sequenced, cut by FseI and XbaI (NEB), and the inserts were ligated in the luciferase vector pGL4.10[luc2] (Promega).
Transfections were carried out in human colon carcinoma (HCT116) and human embryonic kidney (HEK293) cells grown in 10% DMEM (Invitrogen) with 1% penicillin/streptomycin (Invitrogen) in 24-well plates. Cells (40,000/well) were transfected with reporter plasmids (25 ng), siRNA (100 nM), Renilla control vector (25 ng; pGL4.70; Promega;) and Dharmafect Duo (1 μl; Dharmacon, Lafayette, CO, USA). Cells were seeded 24 h before transfection in antibiotic-free medium.
At 48 h after transfection, cells were lysed with 100 μl Passiv Lysis Buffer 1× (Promega). Luciferase expression was measured with the dual luciferase kit (Promega) and a luminometer. Firefly luciferase substrate (50 μl) was added to 40 μl lysate, and firefly luciferase activity was measured for 10 s. Renilla luciferase activity was measured for 10 s after addition of 50 μl substrate for Renilla. Each experiment was performed in triplicate, and the data were normalized to Renilla activity and luciferase activity of cells cotransfected with control siRNA.
Primary skin fibroblasts
The primary human skin fibroblast culture of cells with the COL3A1 mutation p.G252V was established from a 3-mm biopsy taken from the forearm of a 25-yr-old male matient with vEDS. A biopsy taken from an age- and sex-matched healthy volunteer served as a control. Primary human skin fibroblast culture of a patient with a different glycine substitution (p.G501A) and from a patient haploinsufficient for COL3A1 (p.Q857X) were utilized as additional controls. All samples were taken from the forearm. The study was approved by MedStar Institutional Review Board (2003-086). Skin biopsies were incubated in collagen I-coated 6-well plates (BD BioCoat; BD Biosciences, Bedford, MA, USA). Cells were cultured in DMEM supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 mg/ml), glucose (4.5 mg/ml), and l-glutamine (2.7 mM), and maintained under 5% CO2 at 37°C. For all experiments, cells from passages 2 to 6 were used.
Transfection of primary skin fibroblasts
To measure allele-specific mRNA knockdown, cells were seeded at their 4th or 5th passage 24 h before transfection in antibiotic-free medium. Transfections were carried out in T25 flasks. Cells (250,000) were transfected with 100 nM siRNA and Dharmafect1 (Dharmacon, Lafayette, CO, USA; 4 μl/flask). At 48 h after the transfections, cells were collected for RNA extraction.
For electron microscopic analysis of the extracellular matrix and the endoplasmic reticulum (ER), 106 cells were seeded in 10-cm plates at their 4th or 5th passage 24 h before transfection in antibiotic-free medium. Cells were transfected with 100 nM siRNA and Dharmafect1 (15 μl/plate). Medium was changed after 2 d to DMEM containing 1 mM ascorbate. Cells were incubated 10 d before they were collected for analysis of the extracellular matrix. For electron microscopic analysis of the ER, cells were collected 72 h after transfection.
Quantitative RT-PCR
Total RNA was extracted with the RNeasy kit (Qiagen), and the cDNA was synthesized with a random primer with the MultiScribe reverse transcriptase kit (Applied Biosystems, Foster City, CA, USA). The SYBR green QuantiTect mix (Qiagen) was applied for real time PCR detection of wild-type (forward primer: TGGACCTCCAGGTATCAAAGG) or mutated [c.755G>T; p.G252V; forward primer (mutation underlined): CTGGACCTCCAGGTATCAAAGT; reverse primer was used for both: GTCATTACCCCGAGCACCT] COL3A1. The reactions were run in 3 biological and 4 technical replicates in an ABI PRISM 7900HT with an annealing temperature of 60°C. The result was analyzed by the 2−ΔΔCT method normalized to GAPDH (forward primer: TGCACCACCAACTGCTTAG; reverse primer: GGATGCAGGGATGATGTTC). For the evaluation of the effect of the reduced amount of mutated COL3A1 in the fibroblasts, the expression of DDIT3 (forward primer: AGCAGAGGTCACAAGCACCT; reverse primer: CTGGGGAATGACCACTCTGT) was analyzed.
Extraction of the extracellular matrix of cultured primary skin fibroblasts
Cells growing in 10-cm plates were washed twice with PBS, collected using a cell scratcher, and resuspended in 1 ml extraction buffer [150 mM NaCl, 2 mM NaH2PO4, 20 mM EDTA, and Complete Protease Inhibitor Cocktail Tablets (Roche, Basel, Switzerland), pH 7.4]. An aliquot (200 μl) of the cell suspension was removed for RNA extraction to secure a successful mRNA knockdown of mutated COL3A1. The extracellular matrix of the remaining cells was extracted using an Ultra Turrax (IKA, Staufen, Germany) for 3 × 10 s. The obtained lysate was centrifuged for 10 min at 4°C at maximum speed, and the supernatant containing suprastructural fragments was used for electron microscopy analysis.
Immunoblot analysis
Extracellular matrix extracts (15 μg) were separated on a 8% SDS gel under reducing conditions and transferred to a PVDF membrane according to standard procedures. Western blot analysis applying a 1:1000 dilution of a rabbit polyclonal anti-COL3A1 antibody targeting a region between aa 75 and 125 (AP06517PU-N; Acris Antibodies GmbH, Herford, Germany) and a mouse monoclonal anti-β-actin antibody (AC-15; Sigma-Aldrich, Taufkirchen, Germany) targeting an N-terminal peptide were performed and analyzed with the Super Signal West Dura kit (Pierce, Rockford, IL, USA).
Electron microscopy
Aliquots of supramolecular fragments (see above) were spotted onto sheets of Parafilm, and nickel grids covered with Formvar/carbon were floated on the drops for 10 min to allow adsorption of matrix suprastructures. Afterward, grids were washed with PBS and treated for 30 min with 2% (w/v) dried skim milk in PBS. Next, the adsorbed material was allowed to react for 2 h with a rabbit polyclonal antibody against full-length collagen type I (BP 8028; Acris) and a mouse monoclonal antibody against an epitope near the N terminus of collagen type III (MAB 3392; Chemicon, Billerica, MA, USA) diluted 1:100 in PBS containing 0.2% dry milk. After washing several times with PBS, the grids were put on drops of 0.2% (w/v) milk solution containing colloidal gold particles coated with antibodies to rabbit and mouse immunoglobulins (12- and 18-nm gold particles, respectively; Jackson Immuno Research, Suffolk, UK) diluted 1:30. Finally, the grids were washed with distilled water and negatively stained with 2% uranyl acetate for 7 min. Grids were examined by a single specialist blinded to genotype. Control experiments were undertaken with the first antibody omitted. Electron micrographs were taken at 80 kV with a Philips EM 410 electron microscope (Philips, Amsterdam, The Netherlands).
For analysis of the ER by transmission electron microscopy, cells of primary skin fibroblast cultures were collected by a trypsin/EDTA treatment. After centrifugation, the obtained cell pellets were embedded in low-melting agarose and afterward fixed in 4% paraformaldehyde and 0.25% glutaraldehyde in 100 mM cacodylate buffer (pH 7.4) at 4°C. After postfixation in 2% osmium tetroxide, samples were rinsed in PBS, dehydrated in an ascending series of ethanol up to 70%, and embedded in LR white (London Resin Company, London, UK). Ultrathin sections were cut with an ultramicrotome and collected on Formvar-carbon-coated nickel grids for transmission electron microscopy. Finally, sections were negatively stained with 2% uranyl acetate for 10 min. Electron micrographs were taken at 80 kV with an EM-410 electron microscope (Philips).
Statistical analysis
All data are presented as means ± se. A multiple-sample comparison (ANOVA and the multiple range test as post hoc test using the criterion of the least significant differences) was applied to test the differences between the group different modes and time intervals of treatment for significance. A value of P < 0.05 was considered to be significant.
RESULTS
Luciferase reporter assay as a potential tool for the identification of siRNAs applicable for specific knockdown of mutated COL3A1
To identify a siRNA that effectively discriminates a single-nucleotide mismatch between the mutated and the wild-type COL3A1 mRNA (Fig. 1), 19 different siRNAs were designed with the mutation on every possible position (Fig. 2A). These siRNAs were tested on established cell lines in a reporter gene assay using either the wild-type or the mutated COL3A1 sequence cloned in the 3′ untranslated region of the luciferase. To rule out cell-type specific artifacts, two different human cell lines were applied: HEK293 (human embryonic kidney) and HCT116 (human colon carcinoma) cells. Out of 19 tested siRNAs, three groups were identified. Group I siRNAs led to a knockdown of both wild-type and mutated COL3A1-luciferase reporters; group II siRNAs had no effect on both reporter constructs; group III siRNAs mediated a knockdown of mutated COL3A1-luciferase and had no, or only a marginal, effect on wild-type COL3A1-luciferase. By calculating the silencing factor of mutated COL3A1 vs. wild-type COL3A1, we selected siRNAs that specifically and most efficiently knocked down mutated COL3A1. Comparing the silencing factors between the HEK293 and HCT116 cell systems, we noticed that the differences between transfected siRNAs were more distinct in HCT116 cells. However, the three best-working siRNAs with the highest silencing factor could be identified in both cell systems as si6, si10, and si17 (Fig. 2B and Supplemental Fig. S1).
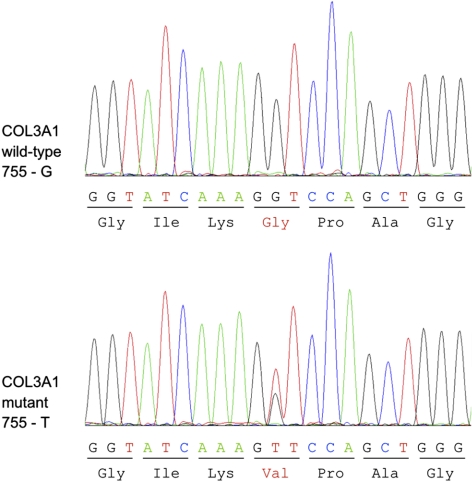
Figure 1.
Sequences of wild-type (755-G) and mutant (755-T) COL3A1 cDNA derived from skin fibroblasts. Because the patient with vEDS patient is heterozygous for COL3A1 c.755G>T mutation, sequencing of the region harboring the mutation results in detection of both guanine and thymidine at position 755. The G>T mutation leads to an exchange from valine to glycine on the protein level.
Knockdown of mutated COL3A1 in skin fibroblasts derived from a patient with vEDS
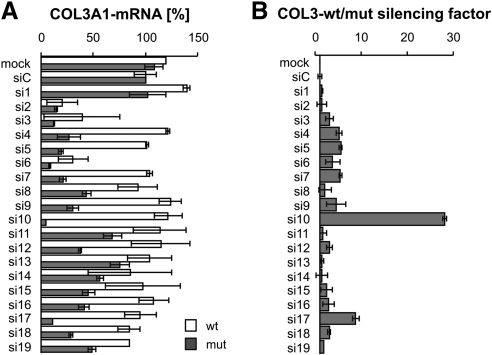
Next, we tested whether the same siRNAs (with the mutation at positions 6, 10, and 17), which showed specific knockdown properties in the reporter assay, would also be specific to mutated mRNA in primary skin fibroblasts from a patient with vEDS. For this purpose, all 19 siRNAs were tested in both primary fibroblasts from a patient heterozygous for COL3A1 allele encoding a glycine substitution, COL3A1G252V/+, and in wild-type control fibroblasts. These two types of fibroblasts did not differ morphologically. At 2 d after transfection, mRNA was extracted, the amounts of mutated and wild-type COL3A1 mRNAs were measured by quantitative PCR, and the silencing factor between wild-type COL3A1 mRNA and mutant mRNA was calculated (Fig. 3).
Figure 3.
Analysis of wild-type and mutated COL3A1 mRNA to measure the silencing effect of different siRNAs in human fibroblast tissue culture. A) Amounts of mutated (COL3A1G252V/+) and wild-type COL3A1 mRNA were analyzed by real-time RT-PCR in untransfected cells (mock), after transfection of a nonsilencing siRNA (siC) and a set of 19 siRNAs targeting mutated COL3A1 (si1–si19). Data are averages ± se of 2 biological replicates. Results were analyzed by the 2−ΔΔCT method normalized to GAPDH. Result of the nonsilencing control was set as 100%. B) Silencing factor was the result of the proportion of wild-type/mutated form of COL3A1 mRNA.
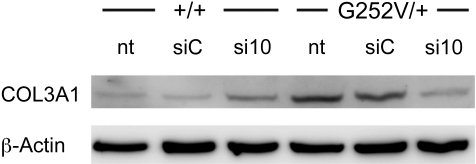
Interestingly, there was a noticeable difference to the results obtained from the luciferase reporter assay: siRNA si6 reduced the concentrations of both wild-type and mutant COL3A1 mRNA (Fig. 3). siRNA si17, which worked in the reporter assay slightly better than si10, did not significantly influence the expression of the wild-type mRNA and led to a moderate reduction of the mutant COL3A1 mRNA (silencing factor 8.8, Fig. 3). However, si10 was specific to mutated COL3A1 mRNA (Fig. 3) and did not affect the wild-type form of COL3A1; the silencing factor obtained after transfection of si10 was 28, resulting in dramatic reduction of the mutated form of COL3A1 (Fig. 3). All other tested siRNAs had only a weak potential to discriminate between mutated and wild-type COL3A1 mRNA, as already observed in the reporter assays (Fig. 2B and Supplemental Fig. S1). In addition, the knockdown of mutated COL3A1 mRNA by si10 resulted in a reduction of the amount of total collagen III protein in cells derived from a patient with vEDS, but not in wild-type control cells (Fig. 4).
Figure 4.
Western blot analysis of COL3A1 in extracts of human fibroblast tissue culture. Extracts (15 μg) of normal donor (+/+) and patient with vEDS (G252V/+) fibroblasts treated with 100 nM siC (control) or si10 siRNA for 12 d were compared with nontreated fibroblasts. COL3A1 was detected with a rabbit polyclonal antibody. As loading control, β-actin was detected with a mouse monoclonal antibody.
Taken together, we identified a single siRNA that is able to knock down mutated COL3A1 mRNA (c.755G>T) with a high efficiency without affecting the wild-type mRNA. Moreover, our data suggest that a reporter assay may serve as simplified preliminary screening, narrowing the number of siRNAs that could work well in patient cells. However, experiments for the final identification of siRNAs that selectively and efficiently knock down a mutated mRNA should be performed in cell culture cells derived from patient samples by measuring changes in the amount of COL3A1 mRNA in response to siRNA treatment.
Attenuation of the UPR by allele-specific RNAi
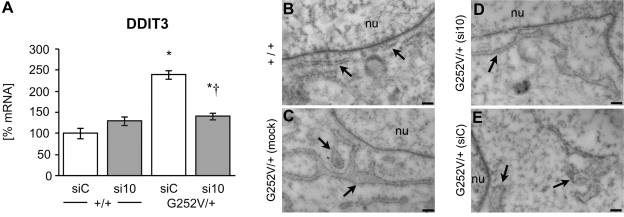
The expression of the mutated form of COL3A1 may cause retention in the ER by misfolded proteins, as was seen for fibrillin-1 mutations (14), and may induce a UPR (15). DNA damage-inducible transcript 3 (DDIT3) is expressed ubiquitously at low levels and elevated by cellular stress. DDIT3 expression is elevated during the ER UPR. Therefore, we analyzed the mRNA expression of DDIT3 in fibroblasts treated with the most effective silencing siRNA, si10. Indeed, the expression of DDIT3 was elevated in COL3A1G252V/+ fibroblasts in comparison to the expression measured in wild-type cells. However, treatment with siRNA significantly reduced DDIT3 expression to a level similar to that of wild-type fibroblasts (Fig. 5A). These results indicate that expression of mutated COL3A1 activates the UPR and that reduction of the amount of mutated protein by siRNA reduces cellular stress.
Figure 5.
Effect of allele-specific RNAi on the ER UPR in human fibroblast tissue culture. A) Amount of DDIT3 mRNA was analyzed in wild-type and COL3A1G252V/+ fibroblasts by real-time RT PCR. Fibroblasts were treated with 100 nM siC (control) or si10 siRNA for 2 d. Data are averagse ± se of 3 biological and 4 technical replicates. Results were analyzed by the 2−ΔΔCT method normalized to GAPDH. Result of a nonsilencing control was set as 100%. *P < 0.05 vs. WT control; †P < 0.05 vs. COL3A1G252V/+ control. B–E) Analysis of the morphology of the ER of cultured primary fibroblasts by transmission electron microscopy. Fibroblasts from cell cultures were collected after trypsin/EDTA treatment and embedded in LRwhite for ultrathin sectioning. In normal fibroblasts (B), the ER close to the nucleus appeared as long and regular tubes, whereas the ER of fibroblasts with the Col3A1G252V/+ mutation (C) showed a broadening of the tubes with several expansions. Treatment of the mutated cells with siRNA 10 (D) resulted in changes of the ER structure toward the typical morphology of normal fibroblasts. In contrast, the morphology of the ER was not affected when a nonsilencing siRNA was applied (E). Tubes of the ER were still enlarged. Arrows indicate ER. nu, nucleus. Scale bars = 200 nm.
It has been previously reported that as a part of the UPR, factors that induce ER membrane production are activated, causing expansion of the ER (16–18). Such changes in the structure of the ER have also been shown in response to the expression of mutated Col4a3 and Col4a4 (19).
To analyze the morphology of the ER as a further marker for ER stress caused by the expression of mutated COL3A1, primary skin fibroblasts obtained from cell cultures were embedded, and ultrathin sections thereof were analyzed by transmission electron microscopy. The ER close to the nucleus of normal fibroblasts displayed a typical morphology with small and long tubes, whereas the ER of fibroblasts with the G252V/+ mutation revealed a broadening of these ER tubes with several expansions. In contrast, the morphology of the ER of fibroblasts treated with si10 was changed and very similar to the morphology of the ER of the normal fibroblasts. Moreover, the morphology of the ER of fibroblasts treated with nonsilencing RNA was not affected, visible by the broadening of the ER tubes (Fig. 5B–E).
vEDS skin fibroblasts form collagen fibers after allele-specific siRNA treatment
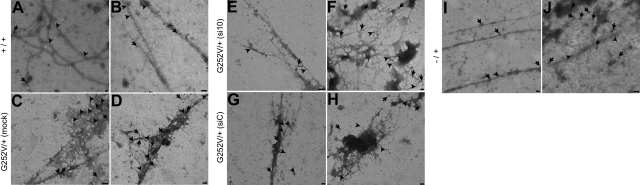
Patients with vEDS are not able to form highly organized extracellular matrices. Alterations in collagen structures in the tissue of patients were clearly visible by electron microscopy (20). We were eager to test whether the altered collagen structure in cell cultures from a patient with vEDS would be affected by treatment with si10, and whether the morphology of the extracellular matrix and especially of the collagen fibrils would improve. Typically thin and weakly banded collagen fibrils were formed by COL3A1 wild-type fibroblasts. A labeling with antibodies against collagen types I and III revealed the presence of these collagens in the formed fibrils (Fig. 6A, B). In contrast, amorphous material and disorganized bundles of collagen fibrils were formed by fibroblasts of the patient with COL3A1G252V/+. The contours of the formed collagen fibrils were irregular in shape and often embedded in amorphous material, which was absent in the normal fibroblast cell cultures. Immunogold electron microscopy demonstrated the presence of collagen types I and III in the amorphous network-like structures (Fig. 6C, D, G, H). After treatment of the mutated cells with si10, the morphology of the extracellular matrix was changed and became similar to the morphology in healthy control fibroblasts: the formed collagen fibrils became more regular in shape and formed typical networks of collagen fibrils with less amorphous material bound to the surface of the collagen fibrils (Fig. 6E, F). Treatment of the cells with a nonsilencing control siRNA did not alter the morphology of the extracellular matrix (Fig. 6G, H). We were, therefore, able to demonstrate that it is possible to reconstitute the extracellular matrix produced by primary skin fibroblasts derived from a patient with vEDS after selective knockdown of mutated COL3A1. The analysis of the extracellular matrix of primary skin fibroblast cell cultures derived from a patient with a haploinsufficient mutation (COL3A1−/+) revealed that this matrix is very similar to the extracellular matrix formed by normal fibroblasts, which is consistent with the observation that haploinsufficient mutations result in a milder phenotype than amino acid exchanges in the COL3A1 protein (refs. 8–11; compare Fig. 6A, B; I, J). To prove the specificity of si10 to the c.755G>T mutation, fibroblast cultures derived from a patient with a different COL3A1 mutation (c.1502G>C, p.G501A) were treated in the same manner as described previously and subsequently analyzed by electron microscopy (Supplemental Fig. S2). Bundles of thin collagen fibrils and amorphous electron dense material were formed by fibroblasts with the G501A mutation. However, in contrast to fibroblasts with the G252V/+ mutation, the appearance of the extracellular matrix was not affected by the treatment with si10, demonstrating that the allele-specific silencing with si10 is highly specific for the G252V cells.
Figure 6.
Ultrastructural analysis of extracellular matrices derived from fibroblast cell cultures by electron microscopy. Extracellular matrices of cultured primary skin fibroblasts were extracted and spotted on EM grids for immunogold labeling. Two representative examples for each cultured fibroblast type are shown. A, B) Extracellular matrix derived from normal fibroblasts showed typical thin and weakly banded collagen fibrils. C, D) Extracellular matrix of COL3A1G252V/+ fibroblasts formed disorganized bundles of collagen fibrils with irregular contours. In addition, amorphous and electron dense material was present, which is often strongly labeled with antibodies against collagen type III (C, arrowheads). E, F) Extracellular matrix of COL3A1G252V/+ fibroblasts formed typical thin collagen fibrils with regular contours after treatment with siRNA 10. G, H) Extracellular matrix of COL3A1G252V/+ fibroblasts was not affected by treatment with a nonsilencing control siRNA (siC) showing the same phenotype as untreated COL3A1G252V/+ fibroblasts. I, J) Extracellular matrix of fibroblasts with a haploinsufficient mutation showed long and thin collagen fibrils very similar to the matrix formed by normal fibroblasts. Immunogold labeling: large gold particles (18 nm, arrows) correspond to collagen type I labeling; small gold particles (12 nm, arrowheads) correspond to collagen type III labeling. Scale bars = 100 nm.
DISCUSSION
vEDS is a severe disease resulting from mutations in the COL3A1 gene leading to life-threatening complications associated with a rupture of major vessels or hollow organs. Using fibroblasts from a patient with vEDS and a healthy donor, we showed the possibility of selectively silencing the mutated form of the COL3A1 gene (without affecting the wild-type allele of COL3A1), resulting in restoration of the structural integrity of affected fibroblasts and in a reduction of the UPR. We also suggested the optimal way of identifying the best siRNA for silencing a mutation. We used a combined approach in which a luciferase reporting assay served as a preliminary tool, narrowing the number of candidate siRNAs, and in which the final selection was refined by a direct assay conducted on the fibroblast from the particular patient.
siRNA selection
Allele-specific silencing of several mutant targets has been studied for diseases including osteogenesis imperfecta (21), spinocerebellar ataxia (22), pachyonychia congenital (23), Huntington′s disease (24), and sialuria (25). In most of these studies, reporter systems like green fluorescent protein (GFP) or luciferase fusion proteins were applied to test siRNAs for discriminative knockdown of point-mutated mRNAs rather than cells expressing endogenous mutated RNAs. Reporter assays are easier to perform. Moreover, in downstream assays, it is often impossible to discriminate between wild-type and mutant proteins. However, reporter gene assays have some problems, such as cell type-dependent phenomena, artifacts of overexpression, and changes in RNA structure originating from fusing target sequences with reporter RNAs. To date, there is very little information available with respect to comparison of a reporter gene assay and the effect of siRNA on the function of the targeted protein. In the present study, we have been able to compare results of reporter assays with result of an assay using patient-derived cells.
In original approaches, mismatches are placed around central positions 10 and 11 in the siRNA duplex, corresponding to nucleotides in the target mRNA cleaved by the EIF2C2 (previously known as Ago2) component of the RISC (26, 27). It is now clear that both location and type of nucleotide mismatch influence the allele-specific discrimination. Du et al. (28) reported significant tolerance for mismatches at positions 1–4 and 12–19; i.e., mismatches at these positions offer poor discrimination. Schwarz et al. (12) confirmed some of these findings, reporting that mismatches in the 5′ seed region (i.e., positions 2–7) are weakly selective but that mismatches at other positions, notably centrally and also at position 16, are powerfully discriminative. Recently, Huang et al. (29) tried to create a general model for mismatch discrimination using several hundreds of different reporter plasmids and various siRNAs. In our report, we confirmed that mutations in central positions are most suitable for allele-specific siRNA design by demonstrating that the mismatch at the centered position 10 was powerfully discriminative for mutated COL3A1 (c.755G>T). However, combining our results with published data, we conclude that for each mutation, a set of different siRNAs has to be tested.
Evaluation of siRNAs in fibroblasts with mutated COL3A1
Interestingly, the attempt to predict the best acting siRNA by cotransfecting a vector with the target sequence cloned in the 3′ untranslated region of the luciferase was only partially successful. In the reporter assay, siRNAs with the mutation at positions 6, 10, and 17 repressed the expression of the mutated reporter variant to the same extent. This selective knockdown could be observed in both HCT116 and HEK293 cell lines (Fig. 2 and Supplemental Fig. S1). This observation could not be entirely verified by testing fibroblasts from a patient with vEDS and measuring endogenous COL3A1 mRNA after siRNA treatment. Of 3 candidate siRNAs identified in reporter assays, only si10 was highly discriminative, whereas si6 and si17 had only limited potential for a strong and discriminative knockdown (Fig. 3). Since we used two different cell systems for reporter assays, we would propose that these differences are not cell type specific. The reason might more likely be found in the lack of a consistent and reliable interaction of the siRNA with the expression system for the luciferase in transfected cells. An improvement of the artificial system could be the use of different reporter enzymes for the target sequence and the wild-type sequence as used by Ohnishi et al. (30, 31), who examined the effect of siRNA duplexes against the mutant allele in allele-specific gene silencing and off-target silencing against the wild-type allele under heterozygous conditions, which were generated by cotransfecting reporter alleles and siRNA duplexes into cultured human cells. Two reporter alleles were used, encoding the firefly and Renilla luciferase genes carrying mutant and wild-type allelic sequences in their 3′-untranslated regions (30). However, Ohnishi et al. (30) did not compare the results in the artificial reporter gene system with the effect of their siRNA in a tissue culture of cells heterozygous for their target sequence. Therefore, we conclude that the identification of allele-specific siRNAs with a maximal silencing activity for the mutated allele and with no or little effect on the wild-type allele should always go through final testing in a tissue culture of the particular patient.
Successful attenuation of negative effects of unfolded proteins
The ER is responsible for protein folding within each cell and is highly sensitive to alterations in its homeostasis. Disruption of this homeostasis leads to accumulation of unfolded proteins. The imbalance between unfolded proteins and the capacity of the ER to handle this load is referred to as ER stress (32, 33). To cope with ER stress, the ER has evolved a signaling network, the UPR. The intent of the UPR is to adapt to the changing environment and reestablish normal ER function. This involves the reduction in protein synthesis and translocation into the ER, followed by the transcriptional activation of UPR target genes, including ER chaperones. If these adaptive responses cannot compensate for the ER stress, then apoptosis is triggered. This presumably protects the organism from cells that display misfolded proteins. A UPR-induced cell death mediator, such as DDIT3 also known as CHOP, is involved in this third step (34, 35). Expression of DDIT3 was indeed elevated in fibroblasts from the patient with vEDS, indicating that the mutated COL3A1 induced an UPR. Effectiveness of allele-specific RNAi in our study was shown by attenuation of increased expression of DDIT3 mRNA (Fig. 5A).
In addition to changes in the expression pattern of certain genes involved in the regulation of the UPR, cells with an activated UPR show a typical expansion of the ER (16–18). Interestingly, expanded ER structures have been detected in cells expressing mutated Col4a3 and Col4a4, respectively (19). We also observed expansions of the ER in skin fibroblasts from the patient with the COL3A1G252V/+ mutation in comparison to the ER in wild-type cells. The specific reduction of the mutated protein resulted in a morphology of the ER similar to wild-type fibroblasts (Fig. 5B). These results provide further evidence that the activation of the UPR by mutated COL3A1 can be attenuated by allele-specific RNAi.
Haploinsufficient phenotype
There is increasing evidence that haploinsufficient patients with vEDS develop a less severe phenotype (8–11). Although the phenotypes of some haploinsufficient patients with vEDS partially overlap with the phenotype of patients with amino acid substitutions in COL3A1 (36), allele-specific silencing of mutant targets could be a valuable addition to nonspecific therapies, such as treatment with the β-blocker celiprolol (6). In addition, we were able to prove that it is possible to pharmacologically boost the collagen production and thus to increase the strength of the extracellular matrix in a mouse model haploinsufficient for Col3a1 (37). Therefore, a personalized siRNA therapy to replace mutated collagen condition with reduced normal collagen condition with consequent pharmacological stimulation of total collagen production could be a promising therapeutic approach.
Future challenges toward a personalized therapy of vEDS
Although RNAi offers novel therapeutic applications and can obviate some of the shortcomings of conventional gene therapy, it has its own limitations. Delivery is a major obstacle to the clinical use of RNAi therapies. The delivery of synthetic siRNAs requires improvement via chemical stabilization and the development of targeting methods, but in many respects, these molecules can be treated and optimized as conventional pharmaceutical agents. In contrast, virally expressed siRNAs retain the advantages but also many of the limitations of standard gene therapy vector delivery systems.
CONCLUSIONS
We have demonstrated for the first time that it is possible to selectively reduce the expression of the disease-causing allele of the COL3A1 gene without altering the expression of the wild-type allele in human fibroblasts. This treatment was able to reduce the negative effects of mutated proteins by reducing the UPR of the ER. Furthermore, knockdown of mutated COL3A1 results in the formation of proper collagen fibers. Thus, after developing an efficient and reliable method of siRNA delivery, the use of allele-specific RNAi appears to be a promising option for a personalized treatment of vEDS.
Supplementary Material
Acknowledgments
The work was fully funded by the Intramural Research Program of the U.S. National Institutes of Health, National Institute on Aging (NIA; Bethesda, MD, USA). The authors are indebted to Shannon Marshall (NIA), Gerburg Hoelscher (University Hospital of Münster), and Barbara Schedding (University Hospital of Münster) for expert technical assistance. The authors owe special thanks to Kenneth R. Boheler (NIA) for helpful discussions.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Beighton P., De Paepe A., Steinmann B., Tsipouras P., Wenstrup R. J. (1998) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am. J. Med. Genet. 77, 31–37 [DOI] [PubMed] [Google Scholar]
- 2. Callewaert B., Malfait F., Loeys B., De Paepe A. (2008) Ehlers-Danlos syndromes and Marfan syndrome. Best Pract. Res. 22, 165–189 [DOI] [PubMed] [Google Scholar]
- 3. Germain D. P. (2007) Ehlers-Danlos syndrome type IV. Orphanet J. Rare Dis. 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watanabe A., Shimada T. (2008) Vascular type of Ehlers-Danlos syndrome. J. Nippon Med. School 75, 254–261 [DOI] [PubMed] [Google Scholar]
- 5. Pepin M., Schwarze U., Superti-Furga A., Byers P. H. (2000) Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N. Engl. J. Med. 342, 673–680 [DOI] [PubMed] [Google Scholar]
- 6. Ong K. T., Perdu J., De Backer J., Bozec E., Collignon P., Emmerich J., Fauret A. L., Fiessinger J. N., Germain D. P., Georgesco G., Hulot J. S., De Paepe A., Plauchu H., Jeunemaitre X., Laurent S., Boutouyrie P. (2010) Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet 376, 1476–1484 [DOI] [PubMed] [Google Scholar]
- 7. Pyeritz R. E. (2000) Ehlers-Danlos syndrome. N. Engl. J. Med. 342, 730–732 [DOI] [PubMed] [Google Scholar]
- 8. Plancke A., Holder-Espinasse M., Rigau V., Manouvrier S., Claustres M., Van Kien P. K. (2009) Homozygosity for a null allele of COL3A1 results in recessive Ehlers-Danlos syndrome. Eur. J. Hum. Genet. 17, 1411–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khalique Z., Lyons O. T., Clough R. E., Bell R. E., Reidy J. F., Schwarze U., Byers P. H., Taylor P. R. (2009) Successful endovascular repair of acute type B aortic dissection in undiagnosed Ehlers-Danlos syndrome type IV. Eur. J. Vasc. Endovasc. Surg. 38, 608–609 [DOI] [PubMed] [Google Scholar]
- 10. Than Naing B., Watanabe A., Hatamochi A., Morisaki H., Shimada T. (2010) Nonsense mutations of COL3A1 gene causing nonsense-mediated mRNA decay in two Japanese patients with vascular type of Ehlers-Danlos syndrome. In 60th Annual Meeting of the American Society of Human Genetics (abstract/program 2370), American Society of Human Genetics, Bethesda, MD, USA [Google Scholar]
- 11. Leistritz D. F., Pepin M. G., Schwarze U., Byers P. H. (2011) COL3A1 haploinsufficiency results in a variety of Ehlers-Danlos syndrome type IV with delayed onset of complications and longer life expectancy. Genet. Med. 13, 717–722 [DOI] [PubMed] [Google Scholar]
- 12. Schwarz D. S., Ding H., Kennington L., Moore J. T., Schelter J., Burchard J., Linsley P. S., Aronin N., Xu Z., Zamore P. D. (2006) Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2, e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stenson P. D. (2009) The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff, Vol. 2010, Cardiff University, Cardiff, UK [Google Scholar]
- 14. Whiteman P., Willis A. C., Warner A., Brown J., Redfield C., Handford P. A. (2007) Cellular and molecular studies of Marfan syndrome mutations identify co-operative protein folding in the cbEGF12–13 region of fibrillin-1. Hum. Mol. Genet. 16, 907–918 [DOI] [PubMed] [Google Scholar]
- 15. Sorensen S., Ranheim T., Bakken K. S., Leren T. P., Kulseth M. A. (2006) Retention of mutant low density lipoprotein receptor in endoplasmic reticulum (ER) leads to ER stress. J. Biol. Chem. 281, 468–476 [DOI] [PubMed] [Google Scholar]
- 16. Sriburi R., Jackowski S., Mori K., Brewer J. W. (2004) XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee A. H., Chu G. C., Iwakoshi N. N., Glimcher L. H. (2005) XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bommiasamy H., Back S. H., Fagone P., Lee K., Meshinchi S., Vink E., Sriburi R., Frank M., Jackowski S., Kaufman R. J., Brewer J. W. (2009) ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 122, 1626–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Firtina Z., Danysh B. P., Bai X., Gould D. B., Kobayashi T., Duncan M. K. (2009) Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J. Biol. Chem. 284, 35872–35884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hausser I., Anton-Lamprecht I. (1994) Differential ultrastructural aberrations of collagen fibrils in Ehlers-Danlos syndrome types I-IV as a means of diagnostics and classification. Hum. Genet. 93, 394–407 [DOI] [PubMed] [Google Scholar]
- 21. Millington-Ward S., McMahon H. P., Allen D., Tuohy G., Kiang A. S., Palfi A., Kenna P. F., Humphries P., Farrar G. J. (2004) RNAi of COL1A1 in mesenchymal progenitor cells. Eur. J. Hum. Genet. 12, 864–866 [DOI] [PubMed] [Google Scholar]
- 22. Xia H., Mao Q., Eliason S. L., Harper S. Q., Martins I. H., Orr H. T., Paulson H. L., Yang L., Kotin R. M., Davidson B. L. (2004) RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med. 10, 816–820 [DOI] [PubMed] [Google Scholar]
- 23. Hickerson R. P., Smith F. J., Reeves R. E., Contag C. H., Leake D., Leachman S. A., Milstone L. M., McLean W. H., Kaspar R. L. (2008) Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. J. Invest. Dermatol. 128, 594–605 [DOI] [PubMed] [Google Scholar]
- 24. Pfister E. L., Kennington L., Straubhaar J., Wagh S., Liu W., DiFiglia M., Landwehrmeyer B., Vonsattel J. P., Zamore P. D., Aronin N. (2009) Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington's disease patients. Curr. Biol. 19, 774–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klootwijk R. D., Savelkoul P. J., Ciccone C., Manoli I., Caplen N. J., Krasnewich D. M., Gahl W. A., Huizing M. (2008) Allele-specific silencing of the dominant disease allele in sialuria by RNA interference. FASEB J. 22, 3846–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matranga C., Tomari Y., Shin C., Bartel D. P., Zamore P. D. (2005) Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell 123, 607–620 [DOI] [PubMed] [Google Scholar]
- 27. Schwarz D. S., Tomari Y., Zamore P. D. (2004) The RNA-induced silencing complex is a Mg2+-dependent endonuclease. Curr. Biol. 14, 787–791 [DOI] [PubMed] [Google Scholar]
- 28. Du Q., Thonberg H., Wang J., Wahlestedt C., Liang Z. (2005) A systematic analysis of the silencing effects of an active siRNA at all single-nucleotide mismatched target sites. Nucleic Acids Res. 33, 1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang H., Qiao R., Zhao D., Zhang T., Li Y., Yi F., Lai F., Hong J., Ding X., Yang Z., Zhang L., Du Q., Liang Z. (2009) Profiling of mismatch discrimination in RNAi enabled rational design of allele-specific siRNAs. Nucleic Acids Res. 37, 7560–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohnishi Y., Tokunaga K., Kaneko K., Hohjoh H. (2006) Assessment of allele-specific gene silencing by RNA interference with mutant and wild-type reporter alleles. J. RNAi. Gene Sil. 2, 154–160 [PMC free article] [PubMed] [Google Scholar]
- 31. Ohnishi Y., Tamura Y., Yoshida M., Tokunaga K., Hohjoh H. (2008) Enhancement of allele discrimination by introduction of nucleotide mismatches into siRNA in allele-specific gene silencing by RNAi. PLoS One 3, e2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroder M., Kaufman R. J. (2005) The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 [DOI] [PubMed] [Google Scholar]
- 33. Xu C., Bailly-Maitre B., Reed J. C. (2005) Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 115, 2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szegezdi E., Logue S. E., Gorman A. M., Samali A. (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7, 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwarze U., Schievink W. I., Petty E., Jaff M. R., Babovic-Vuksanovic D., Cherry K. J., Pepin M., Byers P. H. (2001) Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am. J. Hum. Genet. 69, 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Briest W., Cooper T. K., Tae H. J., Krawczyk M., McDonnell N. B., Talan M. I. (2011) Doxycycline ameliorates the susceptibility to aortic lesions in a mouse model for the vascular type of Ehlers-Danlos syndrome. J. Pharmacol. Exp. Ther. 337, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.