Abstract
Obesity, a major health concern, results from an imbalance between energy intake and expenditure. Leptin-deficient ob/ob mice are paradigmatic of obesity, resulting from excess energy intake and storage. Mice lacking acyl-CoA oxidase 1 (Acox1), the first enzyme of the peroxisomal fatty acid β-oxidation system, are characterized by increased energy expenditure and a lean body phenotype caused by sustained activation of peroxisome proliferator-activated receptor α (PPARα) by endogenous ligands in liver that remain unmetabolized in the absence of Acox1. We generated ob/ob mice deficient in Acox1 (Acox1−/−) to determine how the activation of PPARα by endogenous ligands might affect the obesity of ob/ob mice. In contrast to Acox1−/− (14.3±1.2 g at 6 mo) and the Acox1-deficient (ob/ob) double-mutant mice (23.8±4.6 g at 6 mo), the ob/ob mice are severely obese (54.3±3.2 g at 6 mo) and had significantly more (P<0.01) epididymal fat content. The resistance of Acox1−/−/ob/ob mice to obesity is due to increased PPARα-mediated up-regulation of genes involved in fatty acid oxidation in liver. Activation of PPARα in Acox1-deficient ob/ob mice also reduces serum glucose and insulin (P<0.05) and improves glucose tolerance and insulin sensitivity. Further, PPARα activation reduces hepatic steatosis and increases hepatocellular regenerative response in Acox1−/−/ob/ob mice at a more accelerated pace than in mice lacking only Acox1. However, Acox1−/−/ob/ob mice manifest hepatic endoplasmic reticulum (ER) stress and also develop hepatocellular carcinomas (8 of 8 mice) similar to those observed in Acox1−/− mice (10 of 10 mice), but unlike in ob/ob (0 of 14 mice) and OB/OB (0 of 6 mice) mice, suggesting that superimposed ER stress and PPARα activation contribute to carcinogenesis in a fatty liver. Finally, absence of Acox1 in ob/ob mice can impart resistance to high-fat diet (60% fat)-induced obesity, and their liver had significantly (P<0.01) more cell proliferation. These studies with Acox1−/−/ob/ob mice indicate that sustained activation of lipid-sensing nuclear receptor PPARα attenuates obesity and restores glucose homeostasis by ameliorating insulin resistance but increases the risk for liver cancer development, in part related to excess energy combustion.—Huang, J., Jia, Y., Fu, T., Viswakarma, N., Bai, L., Sambasiva Rao, M., Zhu, Y., Borensztajn, J., Reddy, J. K. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice.
Keywords: fatty acyl-CoA oxidase-1, Acox1 deficiency, endoplasmic reticulum stress, liver tumors
Obesity, a disorder of energy imbalance between caloric intake and expenditure, is a global health problem in adults, as well as in children (1, 2). When the consumption of energy exceeds the combustion of calories, the unburnt energy is stored as triglycerides (TAGs) in adipose tissues and subsequently in liver and other organs, resulting in the development of obesity over time (2). Obesity contributes to the development of metabolic syndrome, comprising type 2 diabetes mellitus, atherogenic dyslipidemia, hypertension, and hepatic steatosis (fatty liver), as well as cancer and other disturbances (1, 2).
Among animal models of obesity, mice deficient in leptin, referred to as ob/ob, are considered paradigmatic of this condition (3, 4). Leptin is a satiety-controlling cytokine, secreted by adipocytes, that suppresses appetite and stimulates energy metabolism (3, 4). Leptin-deficient (ob/ob) obese mice are in perpetual starvation mode, resulting in constant hunger and hyperphagia, with the consequent accumulation of energy in the form of TAGs stored in adipocytes that become increasingly larger and more numerous (1, 4). Obesity also leads to progressive accumulation of TAGs in liver, giving rise to hepatic steatosis (5). Leptin-deficient ob/ob mice also display hyperglycemia and elevated plasma insulin levels (4).
Prevention or reversal of obesity can be achieved by controlling appetite and limiting food intake or, alternatively, by manipulating critical pathways to enhance energy expenditure (1, 4). During the past 2 decades, “lipid-sensing” peroxisome proliferator-activated receptors (PPARα, PPARγ, and PPARβ/δ; refs. 6, 7), have received special attention in the maintenance of overall energy balance (1, 8, 9). PPARα is expressed predominantly in liver and to a lesser extent in heart, kidney, skeletal muscle, intestine, and brown fat, where it controls fatty acid oxidation (6–8). PPARγ is critical for conserving energy, as it contributes to adipogenesis, whereas both PPARα and PPARβ/δ participate in energy burning (7–11). PPARβ/δ is expressed ubiquitously and appears to be a powerful regulator of fatty acid catabolism and energy homeostasis (1, 11). PPARα activation by synthetic ligands exerts beneficial effects on obesity and in the management of hepatic steatosis (12–14). These effects can be attributed to PPARα-induced transcriptional activation of many genes that are involved in peroxisomal and mitochondrial β-oxidation and microsomal ω-oxidation of fatty acids, predominantly in liver (9, 15). Work from our laboratory has further shown that profound and sustained activation of PPARα occurs in mice with disruption of the acyl-CoA oxidase 1 (Acox1) gene (16, 17). This activation is mediated by endogenous ligands of PPARα that remain unmetabolized in the absence of Acox1 (8, 16–19). PPARα activation in Acox1−/− mice leads to induction of PPARα target genes, especially genes controlling fatty acid oxidation, resulting in increased energy burning and lean body phenotype (16, 17).
In the present study, we set out to examine how the activation of PPARα by its endogenous ligands affects the obesity of leptin-deficient mice. For this purpose, we generated ob/ob mice deficient in Acox1 and showed that these Acox1−/−/ob/ob double mutants are resistant to obesity because of increased energy expenditure associated with PPARα-mediated up-regulation of genes involved in fatty acid oxidation in liver. Furthermore, we noted that lack of Acox1 attenuates high-fat-diet-induced obesity. These studies also show that activation of PPARα in Acox1-deficient ob/ob mice improves glucose tolerance and insulin sensitivity. In addition, PPARα activation decreases hepatic steatosis and increases hepatocellular regenerative response in Acox1−/−/ob/ob mice at a more accelerated pace than in mice lacking only Acox1. Thus, Acox1−/−/ob/ob mice, unlike ob/ob mice, manifest hepatic endoplasmic reticulum (ER) stress along with hepatocellular regeneration and develop hepatocellular carcinomas similar to those observed in Acox1-null mice.
MATERIALS AND METHODS
Animals
Acox1-deficient mice were generated and maintained on C57BL/6J background (16, 20). The heterozygous leptin-deficient OB/ob mice (The Jackson Laboratory, Bar Harbor, ME, USA) were crossed with Acox1+/− mice (16) to generate Acox1+/−/OB/ob mice, which were further bred to produce Acox1−/−/ob/ob double-mutant mice and their littermates ob/ob, Acox1−/−, and wild-type OB/OB. For genotyping ob/ob mice, the primers used were forward 5′-TGTCCAAGATGGACCAGACTC-3′ and reverse 5′- ACTGGTCTGAGGCAGGGAGCA-3′, and for genotyping Acox1 mice, the primers used were forward 5′-TATTCGGCTATGACTGGGCACA-3′ and reverse 5′-GATGGATACTTTCTCGGCAGGA-3′. Age-matched, 1- to 18-mo-old, male and female mice were used in this study. Each experimental group consisted of 4–14 mice. Mice were housed in a temperature-controlled (23°C) environment, using a 12-h light-dark cycle, and were fed standard rodent chow (Harlan-Teklad, Indianapolis, IN, USA) and water ad libitum. For food intake measurement, 3 mice were housed individually, and the daily caloric intake was determined over a 3-d period and normalized against the initial body weight (kcal/g body wt/d). These measurements were repeated 4 times using different batches of mice. The weight of liver and fat depots was measured and normalized against body weight. For studying the effect of a high-fat diet, 2-mo-old mice were fed a diet containing 60% kcal fat (Research Diets, New Brunswick, NJ, USA; cat. no. D12492) for 3 mo. All animal procedures employed in this study were reviewed and approved by the Northwestern University Institutional Animal Care and Use Committee.
Serum chemistry and fast protein liquid chromatography (FPLC)
Blood glucose levels were determined by a glucose meter (One Touch, LifeScan, Milpitas, CA). Enzymatic assay kits were used for the determination of serum insulin (enzyme-linked immunosorbent assay; Crystal Chem, Downers Grove, IL, USA), TAGs (Thermo Scientific, Rockford, IL, USA), and total cholesterol (Wako Chemicals, Richmond, VA, USA), as described previously (20). Plasma lipoprotein profile was analyzed by FPLC, as described previously (21). Cholesterol level was measured in the eluted fractions using the above commercial kit.
Glucose tolerance test and insulin tolerance test
Acox1−/−/ob/ob mice and littermates were used for determining glucose tolerance and insulin tolerance, as described previously (22, 23). Briefly, after 16 h without food, glucose (1.5 mg/g body wt in PBS) was administered intraperitoneally. Blood glucose levels were measured using tail blood obtained at 0, 30, 60, and 120 min after glucose injection (22). For insulin tolerance testing, after 4 h without food, mice were injected with insulin (0.75 mU/g body wt, in PBS; Sigma, St. Louis, MO, USA) intraperitoneally. Blood glucose levels were determined at 0, 15, 30, and 60 min after insulin injection (22).
Morphology
For histological analysis, tissues were fixed in 4% paraformaldehyde and embedded in paraffin; 4-μm-thick sections were cut and stained with hematoxylin and eosin (H&E) or Sirius Red, as described previously (20, 24). White adipocyte size was quantified by using the ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA), as described previously (25). For Oil Red O staining, frozen sections of liver were stained in 0.5% Oil Red O solution for 2 h in a 50°C oven and then in 85% propylene glycol solution for 5 min. Sections were rinsed in distilled water, stained in Gill's hematoxylin for 2 s, washed, and mounted with aqueous mounting medium. Tissues were processed for localization of catalase, enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase L-bifunctional enzyme (L-PBE), and bromodeoxyuridine (BrdUrd), as described elsewhere (16).
Gene expression analysis
Total RNA was extracted from different tissues using TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA), and Northern blotting was performed using 20 μg of total RNA as described previously (24). Western blotting procedure used was as described elsewhere (26, 27). For real-time quantitative PCR (qPCR), cDNAs were synthesized as described previously (20, 23, 24, 27). qPCR assays were performed using SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA, USA). 18S rRNA was used for the normalization of qPCR data. The primer sequences used are listed in Supplemental Table S1.
Statistics
Data were analyzed using SPSS 1-way ANOVA (SPSS, Chicago, IL, USA); values of P < 0.05 were considered significant.
RESULTS
Acox1 deficiency prevents ob/ob mice from becoming obese
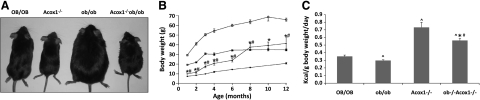
In agreement with previous observations (16), mice lacking the Acox1 gene are leaner than wild-type mice as a result of the activation of PPARα by endogenous ligands and the consequent increased energy expenditure associated with the up-regulation of genes involved in fatty acid oxidation in liver (Fig. 1A, B). Striking effects on body size are also observed in ob/ob mice lacking the Acox1 gene (Acox1−/−/ob/ob). These animals, up to 6 mo of age, are also significantly smaller and leaner than wild-type (OB/OB) as well as ob/ob mice (Fig. 1A, B). By 8 mo, the body weights of double mutants were similar to that of wild-type OB/OB mice but remained significantly lower than ob/ob mice, and these differences persisted in older mice (Fig. 1B). The lower body weights of Acox1−/− and Acox1−/−/ob/ob mice cannot be attributed to lower food consumption, since these mice consumed more food per gram of body weight than OB/OB and ob/ob mice, suggesting an increase in energy expenditure associated with Acox1 deficiency (Fig. 1C).
Figure 1.
Acox1-deficient ob/ob mice (Acox1−/−/ob/ob) do not become obese. A) Physical appearance of chow-fed 3-mo-old wild-type (OB/OB), Acox1−/−, ob/ob, and Acox1−/−/ob/ob mice. B) Body weight of mice from 1 to 12 mo of age. C) Food consumption. Three mice in each group were housed in separate cages, and daily food intake (kcal) per body weight was determined by measuring 3-d consumption. ⋀P < 0.05 vs. OB/OB; *P < 0.05 vs. ob/ob; #P < 0.05 vs. Acox1−/−.
Acox1 deficiency reverses hyperglycemia and hyperlipidemia of ob/ob mice
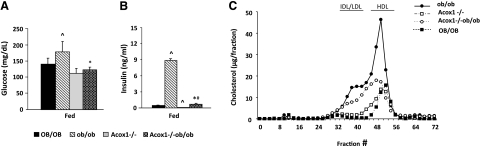
Leptin-deficient ob/ob mice are obese and diabetic, with hyperglycemia, hyperinsulinemia, and greatly increased plasma HDL cholesterol levels (4, 28). Under both fed and unfed states, ob/ob mice had higher blood glucose levels compared to OB/OB (WT), Acox1−/−, and Acox1−/−/ob/ob mice at both age 3 and 8 mo (Fig. 2A and data not shown). Reduction of blood glucose content in unfed Acox1−/−/ob/ob mice suggests that these double mutants are resistant to diabetes. Serum insulin level in ob/ob mice is higher than that seen in wild-type mice (Fig. 2B). Low blood glucose and normal serum insulin in Acox1−/−/ob/ob mice, as compared to ob/ob mice, suggest that Acox1 deficiency increases insulin sensitivity in ob/ob mice. Acox1−/−/ob/ob mice further revealed an increase in glucose tolerance and insulin sensitivity compared to ob/ob mice (Supplemental Fig. S1A, B). Acox1 ablation in ob/ob mice also partially reverses the HDL-associated increase in plasma cholesterol (Fig. 2C).
Figure 2.
Plasma glucose, insulin, and lipoprotein profiles. A, B) Plasma glucose (A) and insulin levels (B) were measured in 3-mo-old mice fed chow ad libitum. C) Lipoprotein profiles were measured in mice after 16 h without food. IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein. Solid squares, OB/OB; solid circles, ob/ob; open squares, Acox1−/−; open circles, Acox1−/−/ob/ob. ⋀P < 0.05 vs. OB/OB; *P < 0.05 vs. ob/ob; #P < 0.05 vs. Acox1−/−.
Acox1 deficiency reduces hepatic steatosis and adipose tissue mass of ob/ob mice
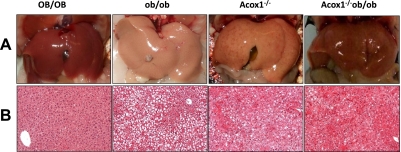
The obesity in ob/ob mice is associated with severe hepatic steatosis and enlarged retroperitoneal, reproductive (i.e., epididymal in males), scapular, and inguinal fat pads (28). Examination of epididymal white adipose tissue (WAT) depots (Supplemental Fig. S1C, D) revealed that Acox1−/− ob/ob mice had significantly less fat at these sites than OB/OB and ob/ob mice. Overall, epididymal WAT measurements are considered as reliable indicators of body fat mass (29–31). Histological examination of epididymal WAT revealed that fat cells in ob/ob mice are larger than those of OB/OB, Acox1−/−, and Acox1−/−/ob/ob mice (Supplemental Fig. S1D). These observations were confirmed by measurement of adipocyte size using ImageJ (Supplemental Fig. S1E). In Acox1−/−/ob/ob mice, the white adipocyte size appeared smaller than that noted in ob/ob mice but larger than that of OB/OB and Acox1−/− mice. Lipid accumulation was markedly diminished in the liver of Acox1−/− ob/ob mice at 3 mo of age (Fig. 3A). Liver weight/body weight ratios were higher in Acox1−/− and Acox1−/−/ob/ob mice (Supplemental Fig. S2A). As expected, ob/ob liver displayed bland macrovesicular steatosis (Fig. 3B), and Acox1-deficient mice, <3 mo old, displayed microvesicular steatosis with evidence of hepatocellular regeneration that increased with age. In Acox1−/−/ob/ob mice, the fatty change in liver was markedly attenuated as compared to that observed in ob/ob liver. Oil Red O staining data confirmed the changes in hepatic steatosis (data not shown). In addition, hepatocellular regeneration in Acox1−/−/ob/ob mice appeared more advanced than that seen in age-matched Acox1−/− mice.
Figure 3.
Hepatic lipid accumulation. Gross (A) and histological (B) appearance of liver of in 3-mo-old OB/OB, ob/ob, Acox1−/−, and Acox1−/−/ob/ob mice maintained on a chow diet.
PPARα activation increases fat oxidation and contributes to obesity resistance in Acox1−/−/ob/ob mice
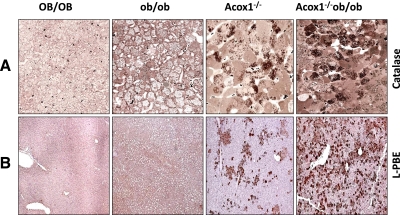
Progressive changes in morphology occurring in Acox1−/− mouse liver have been well documented (16, 17, 20). The initial manifestation of microvesicular hepatic steatosis in Acox1−/− mice leads to regenerative proliferation of liver cells that progressively replace steatotic hepatocytes. Regenerated Acox1−/− hepatocytes reveal peroxisome proliferation and other features that are consistent with PPARα activation (17). We now show that disruption of the Acox1 gene in ob/ob mice results in rapid and progressive increase in regenerative liver cell population, as compared to that noted in Acox1−/− mice (Fig. 4). These Acox1-null hepatocytes are devoid of fat and display massive peroxisome proliferation (Fig. 4), as well as increased expression of PPARα-regulated fatty acid oxidation system genes, such as L-PBE (Fig. 4). It would appear that since Acox1-deficient mice, as well as leptin-deficient mice, develop severe fatty change in liver, the combined deficiency of these two genes accelerates hepatocellular regenerative process with augmentation of PPARα function, resulting in increased fatty acid oxidation (Fig. 4).
Figure 4.
Regenerating hepatocytes in liver of 3-mo-old Acox1−/− and Acox1−/−/ob/ob mice reveal morphological evidence of PPARα activation. A) Semithin (0.5 μm) sections of liver processed for peroxisomal marker enzyme catalase. Dark brown granules in the cytoplasm are catalase-containing peroxisomes. B) Immunohistochemical localization of peroxisomal bifunctional enzyme (L-PBE), the second enzyme of the peroxisomal β-oxidation system. In Acox1−/−/ob/ob liver, many regenerated hepatocytes reveal intense L-PBE staining, indicating peroxisome proliferation and robust PPARα activation. In Acox1−/− liver, periportal hepatocytes show intense L-PBE staining, but cells with steatosis do not. Wild-type (OB/OB) and ob/ob mice do not show increase in L-PBE staining.
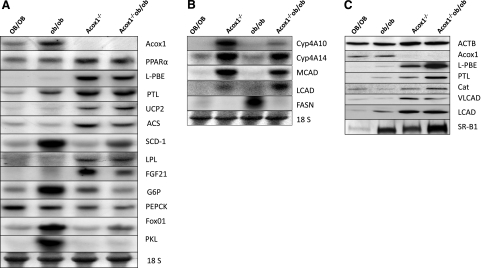
We investigated the molecular basis for the observed phenotypic changes in Acox1−/−/ob/ob mice by analyzing the key regulatory genes involved in hepatic lipid and energy metabolism. On Northern blot analysis, hepatic PPARα mRNA expression was increased nearly 2-fold in both Acox1−/− and Acox1−/−/ob/ob mice (Fig. 5A). PPARα mRNA expression levels in OB/OB and ob/ob mouse liver was comparable (Fig. 5A). Various reports suggest that hepatic PPARα expression in ob/ob mouse liver is either similar or slightly increased when compared to normal (OB/OB) mice (32, 33). An increase in peroxisome population and induction of L-PBE enzyme in Acox1−/−/ob/ob mouse liver indicates transcriptional activation of PPARα-regulated genes involved in energy utilization (Figs. 5 and 6). Northern blot results revealed the induction of genes involved in peroxisomal, mitochondrial, and microsomal fatty acid oxidation systems in Acox1−/−/ob/ob mouse liver (Fig. 5A, B). First, there is a prominent increase in hepatic mRNA levels of L-PBE and peroxisomal 3-ketoacyl-CoA thiolase A (PTL), the second and third enzymes of the peroxisomal β-oxidation system, similar to that observed in Acox1−/− mice (Fig. 5A). The mitochondrial fatty acid β-oxidation genes, namely, long- and medium- chain acyl-CoA dehydrogenases (LCADs and MCADs) are significantly up-regulated in Acox1−/− and Acox1−/−/ob/ob mice (Fig. 5B). Also illustrated are the increases in mRNA levels of Cyp4A10 and Cyp4A14 involved in microsomal fatty acid ω-oxidation (Fig. 5B). It is noteworthy that both Cyp4a10 and Cyp4A14 show reduced expression in ob/ob mice. Genes involved in fatty acid oxidation have been shown to be directly up-regulated by PPARα, which is increased in Acox1−/− and Acox1−/−/ob/ob mouse liver (Fig. 5A). Western blot results further confirmed the increases in hepatic L-PBE, PTL, VLCAD, and LCAD protein content in Acox1−/− background (Fig. 5C). Uncoupling protein 2 (UCP2), a gene involved in energy expenditure and mitochondrial oxidation is also up-regulated (Fig. 5A).
Figure 5.
Gene expression changes in liver. A) Northern blots depict the expression levels of PPARα, peroxisomal β-oxidation system genes (Acox1, L-PBE, and PTL), and other genes as indicated in Acox1−/− and Acox1−/−/ob/ob mice, as compared to OB/OB and ob/ob mice. B) Northern blots show increased expression levels of ω-oxidation system genes Cyp4A10 and Cyp4A14 and mitochondrial β-oxidation system genes MCAD and LCAD in Acox1−/− and Acox1−/−/ob/ob mouse liver. In contrast, FASN gene expression is increased only in ob/ob mouse liver. 18S was used as a loading control. C) Western blot analysis of liver homogenates. Increase in protein content of fatty acid oxidation genes L-PBE, PTL, VLCAD, and LCAD are evident. Peroxisomal marker protein catalase (Cat) is increased in ACOX1−/− and Acox1−/−/ob/ob mouse liver. SR-B1 levels are higher in ob/ob, ACOX1−/−, and Acox1−/−/ob/ob mouse liver as compared to OB/OB. β-Actin (ACTB) was used as a loading control.
Figure 6.
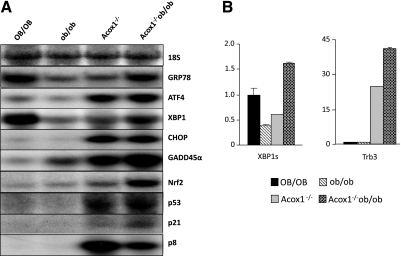
Enhanced ER stress response gene expression in Acox1−/−/ob/ob mouse liver. A) Northern blot analyses depict general up-regulation of unfolded protein response gene expression in Acox1−/−/ob/ob mice compared to ob/ob or Acox1−/− mice. B) Increased splicing of XBP1 (XBP1s) and Trb3 mRNA expression in Acox1−/−/ob/ob mice compared to ob/ob mice by qPCR. 18S was used as an internal control.
Reduction in lipogenesis in Acox1-deficient ob/ob mice
The de novo lipogenesis related gene acetyl-CoA synthetase (ACS) showed an increase in its expression in both Acox1−/− and Acox1−/−/ob/ob mouse liver. The stearoyl-CoA desaturase-1 (SCD-1) gene is up-regulated in ob/ob mice (32, 34), while a relatively lower level of expression was observed in Acox1−/−/ob/ob mouse liver (Fig. 5A). Lower levels of SCD-1 may contribute to decreased hepatic steatosis in Acox1−/−/ob/ob mice compared to ob/ob mice. Fatty acid synthase (FASN) is highly induced in ob/ob mice, as reported previously (35), but FASN mRNA levels were not increased in Acox1−/− and Acox1−/−/ob/ob mouse liver (Fig. 5B), suggesting that hepatic lipogenesis is not increased in Acox1−/−/ob/ob mice. Also of interest is a modest increase in the expression of lipoprotein lipase (LPL) and fibroblast growth factor 21 (FGF21) in both Acox1−/− and Acox1−/−/ob/ob mouse liver (Fig. 5A). Significant increases in microsomal triglyceride transfer protein (MTP) were found in Acox1−/−/ob/ob mouse liver (Supplemental Fig. S2B). MTP regulates hepatic lipid secretion and may play a role in the reduction of hepatic steatosis in these mice (22).
Glucose-6-phosphatase (G6P), which is involved in gluconeogenesis, is highly up-regulated in ob/ob mice, while another gluconeogenic response gene, PEPCK, showed no obvious change among the four groups (Fig. 5A). Forkhead box O1 (FoxO1), a negative regulator for insulin and a stimulator of gluconeogenesis (36), is also up-regulated in ob/ob mice, contributing to hyperglycemia (Fig. 5A). Liver pyruvate kinase (PKL) is up-regulated in ob/ob mice, indicating increased glycolysis, which may be secondary to hyperglycemia (Fig. 5A). Overall, glucose response gene expression is not changed in Acox1−/−/ob/ob mouse liver, unlike in ob/ob mouse liver.
Resistance to high-fat-diet-induced hepatic steatosis in Acox1−/−/ob/ob mice
Because high-fat diet can increase WAT content and induce hepatic steatosis in mice (37), we investigated whether the absence of Acox1 affects diet-induced obesity. We fed 2-mo-old OB/OB, ob/ob, Acox1−/−, and Acox1−/−/ob/ob mice a high-fat diet (60%) for 3 mo (Supplemental Fig. S2C). Acox1−/−/ob/ob double-mutant mice fed a high-fat diet gained body weight more than Acox1−/− mice, but the weight gain was lower than that noted in high-fat-diet-fed wild-type (OB/OB) and ob/ob mice (Supplemental Fig. S2C). Macrovesicular fatty change was prominent in high-fat-diet-fed OB/OB and ob/ob mouse liver when compared to Acox1−/− and Acox1−/− ob/ob mouse liver, which had variable degrees of microvesicular fatty change (Supplemental Fig. S2D). This is attributed to an increase in regenerating hepatocyte population resulting from Acox1 deficiency. In a 3-d BrdUrd incorporation study conducted at the end of high-fat-diet feeding, increased numbers of BrdUrd-labeled hepatocytes were found in Acox1−/− (∼15%) and Acox1−/−/ob/ob (∼26%) mice compared to ob/ob (∼3%) and OB/OB (∼2%) mice (Supplemental Fig. S2D).
Increased energy expenditure in Acox1−/−/ob/ob mice
The expression levels of some of the genes that regulate energy homeostasis in liver, skeletal muscle, heart, and adipose tissues are shown in Supplemental Fig. S3. Carnitine palmitoyl transferase I (CPT1α) and UCP3 expression levels increased in Acox1−/− and Acox1−/−/ob/ob mouse liver as compared to that noted in ob/ob mice (Supplemental Fig. S3A). UCP2 and PPARγ levels were higher in ob/ob mouse liver, but more in Acox1−/− and Acox1−/−/ob/ob liver when compared to wild-type OB/OB mice. PPARγ coactivator-1β (PGC1β) was generally up-regulated in Acox1−/− and Acox1−/−/ob/ob mouse liver, but not in ob/ob mice (Supplemental Fig. S3A). UCP1 expression in liver (38) is higher in Acox1−/−/ob/ob mice, which may be related to the lean phenotype in this ob/ob mouse with deficiency of Acox1 (Fig. 1).
Muscle and brown adipose tissue (BAT) play an important role in energy expenditure in the mouse (39). As shown in Supplemental Fig. S3B, PPARγ expression in muscle is higher in ob/ob, Acox1−/−, and Acox1−/−/ob/ob mice similar to that observed in the liver. Increases in UCP2 and PGC1β (40) gene expression are noted in Acox1−/−/ob/ob mouse skeletal muscle when compared to ob/ob mice (Supplemental Fig. S3B). In the BAT of Acox1−/− mice, most of the genes involved in energy metabolism, such as PPARα, CPT1α, PGC1α, PGC1β, and UCP2 are up-regulated (Supplemental Fig. S3C). Similar patterns of expression of these genes were discerned in Acox1−/−/ob/ob mouse BAT but not in ob/ob mice. Among all these genes, UCP2 is up-regulated in ob/ob, Acox1−/−, and Acox1−/−/ob/ob mice compared to their wild-type controls, although a relatively lower level was observed in Acox1−/−/ob/ob mice. UCP1 in BAT is slightly down-regulated in ob/ob mice, which contributes to decrease in energy expenditure (34). Up-regulation of UCP1 in Acox1−/− mice may contribute to a reduction in WAT, but UCP1 expression did not increase in the double-mutant mice (Supplemental Fig. S3D). Lipolysis response gene AdipoQ expression is lower in ob/ob mice, and it is also down-regulated in Acox1−/−/ob/ob mice. In the heart, as in BAT, UCP2 is up-regulated in ob/ob, Acox1−/−, and Acox1−/−/ob/ob mice. UCP1 expression in heart is lower in Acox1−/− and Acox1−/−/ob/ob mice, while UCP3 expression is increased in ob/ob mice (Supplemental Fig. S3E).
Endoplasmic reticulum (ER) stress in Acox1−/−/ob/ob mouse liver
ER stress and unfolded protein response (UPR) signaling regulate many metabolic events, including lipid and glucose metabolism (41, 42). Previously, we have found progressively worsening ER stress in Acox1−/− mouse liver, which may contribute to the development of hepatic steatosis and liver tumors in this mutant mouse (20). Northern blot and qPCR data revealed that the expression levels of ER stress response genes, such as glucose-regulated protein 78 kDa (GRP78/Bip), total X box binding protein-1 (XBP1u) and splicing XBP1 (XBP1s), growth arrest and DNA damage-inducible gene 45α (GADD45α), and pseudokinase tribbles homologue 3 (Trb3) are generally higher in Acox1−/−/ob/ob mouse liver when compared to ob/ob mice (Fig. 6A, B). GRP78 and XBP1 expression levels are similar to wild-type mice, while GADD45α and Trb3 expression is elevated. Expression levels of p53 and p21 are also up-regulated in Acox1−/− and Acox1−/−/ob/ob mice. Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), a transcription factor that regulates the expression of many cytoprotective genes involved in endogenous and exogenous oxidative stress (43–46), is induced in Acox1−/− and Acox1−/−/ob/ob mice (Fig. 6). It is noteworthy that GRP78, XBP1u, and XBP1s genes are down-regulated in ob/ob mice (Fig. 6A, B). Increases in XBP1 and C/EBP homologous protein (CHOP/CHOP10) mRNA levels were noted in Acox1−/−/ob/ob mouse liver when compared to ob/ob mice (Fig. 6A; refs. 44, 45). Transcription factor p8 (also known as nuclear protein 1 or NUPR1), a stress response gene (46), is highly up-regulated in Acox1−/− mouse (20), and similar increases were seen in Acox1−/−/ob/ob mouse liver (Fig. 6A). It appears that p8 plays a critical role in the induction of ER stress in Acox1−/− and Acox1−/− ob/ob mice. Increase in p8 mRNA in Acox1−/−/ob/ob mouse liver further confirms that it is a novel PPARα and PPARγ target gene and is indicative of PPARα activation in these double-mutant mice (20).
Development of hepatocellular carcinomas in aged Acox1−/−/ob/ob mice
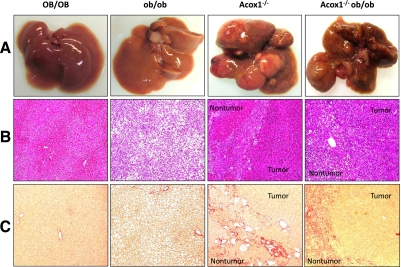
Previously, we have shown that there is a progressive age-related increase in pericellular and bridging fibrosis in the liver of Acox1−/− mice (20). Hepatic fibrogenesis in Acox-null mice appears to be a reflection of multifactorial perturbations, including ER stress, fatty acid toxicity leading to lipoapoptosis of liver cells, and stellate cell activation (20). Activation of PPARα by both synthetic and endogenous ligands results in the induction of hepatocellular proliferation and the development of hepatocellular carcinomas by nongenotoxic mechanism (8, 17, 18, 24, 47–49). Acox1-null mice develop hepatocellular carcinomas due to sustained activation of PPARα by unmetabolized endogenous ligands that are substrates of Acox1 (8, 17, 18). To assess the long-term effects of Acox1 deficiency in ob/ob mice, we examined liver for chronic changes, including fibrosis and development of tumors (Fig. 7). Multiple liver tumors were found in both Acox1−/− (10 of 10) and Acox1−/−/ob/ob (8 of 8) mice killed between 14 and 18 mo of age (Fig. 7A). No tumor was found in OB/OB (0 of 6) and ob/ob (0 of 14) mice. Histological examination (∼25 tumors from Acox1−/− and ∼33 tumors from Acox1−/−/ob/ob mice) revealed that all tumors were well to moderately differentiated hepatocellular carcinomas (Fig. 7B). OB/OB mouse liver displayed normal histological appearance, while ob/ob liver at 14 mo still had severe macrovesicular steatosis (Fig. 7B). Fibrosis was minimal in ob/ob liver but severe in nontumorous portions of liver in both Acox1−/− and Acox1−/−/ob/ob mice (Fig. 7C).
Figure 7.
Aggravated fibrotic changes and liver tumor development in Acox1−/− and Acox1−/−/ob/ob mice maintained on chow. A, B) No liver tumor is seen in OB/OB and ob/ob mice, whereas multiple liver tumors are present in both Acox1−/− and Acox1−/−/ob/ob mice, which are well to moderately differentiated hepatocellular carcinomas. C) Acox1−/− and Acox1−/−/ob/ob mice with liver tumors reveal extensive Sirius Red-positive fibrous septa in nontumor portions of liver.
DISCUSSION
We have previously reported that germ-line deletion of Acox1 results in the generation of a lean mouse with a complex phenotype, manifesting as steatohepatitis, hepatocellular regeneration with massive spontaneous peroxisome proliferation, and enhanced transcriptional activation in liver, of PPARα-regulated genes (16, 17). In the absence of Acox1, the unmetabolized substrates of this enzyme, such as the very long-chain fatty acyl-CoAs, function as endogenous ligands for the lipid-sensing nuclear receptor PPARα to up-regulate mitochondrial, peroxisomal, and microsomal fatty acid oxidation systems (8, 9, 16, 17). The sustained increase in fatty acid oxidation resulting from the absence of Acox1 contributes to the lean body phenotype of Acox1-mutant mice (17). Moreover, the perpetually heightened PPARα transcriptional mode in Acox1-deficient mice not only up-regulates the expression of genes involved in fatty acid oxidation but also contributes to increased lipid transport, inflammation, and carcinogenesis (7–9, 17). Recently, we have shown that reintroduction of the Acox1 gene by adenoviral infusion reversed the Acox1-null phenotype (50). Furthermore, we showed that the human Acox1 gene is functional in Acox1-null mice and effectively prevented the metabolic dysfunctions that lead to ER stress and hepatocarcinogenesis (20). These observations point to the critical role played by Acox1 in maintaining the lipid homeostasis by regulating PPARα function (17).
To evaluate the genetic influence of Acox1 in obesity, we have now generated and characterized mice deficient in both Acox1 and leptin by breeding the mutant Acox1 allele onto the leptin-deficient (ob/ob) obese mice. Ablation of Acox1 function from ob/ob mice resulted in the generation of Acox1−/−/ob/ob double mutants that, unlike ob/ob mice, appeared resistant to leptin-deficient genetic obesity due to increased energy expenditure. Excess energy combustion in these double mutants is due, in most part, to marked PPARα-mediated up-regulation in the liver of genes involved in fatty acid oxidation. Energy expenditure mechanisms are also up-regulated in skeletal muscle and BAT of Acox1−/− and Acox1−/−/ob/ob double mutants. While leptin deficiency causes obesity development as a result of excess energy consumption (4), the deficiency of Acox1, on the other hand, enhances energy combustion (17) to counter the development of obesity. Resistance of Acox1−/−/ob/ob mice in developing obesity results in a significant reduction in the amount of their abdominal fat, compared to that of ob/ob mice. This reduction in fat content is also evident in mice lacking Acox1, reflecting that ablation of Acox1 in ob/ob mice imparts a dominant functional effect of enhanced energy expenditure by up-regulating PPARα function. When food intake is represented as kilocalories per mouse per day, the intake of ob/ob mice is more than that of OB/OB, Acox1−/−, and Acox1−/−/ob/ob mice. However, when expressed as kilocalories per gram of body weight per day, Acox1−/− as well as Acox1−/−/ob/ob mice consumed more food than OB/OB and ob/ob mice, suggesting that Acox1-deficient ob/ob mice expend more energy. Systemic Acox1 deficiency in mice may affect the metabolism of very long-chain fatty acids and fatty acyl-CoAs in the hypothalamus to influence PPARα function in regulating energy metabolism and body weight.
Genetic ablation of Acox1 activity in ob/ob mice also resulted in a reduction of hepatic steatosis as a result of augmented regeneration of hepatocytes, induction of peroxisome proliferation and dramatic up-regulation of PPARα-regulated hepatic fatty acid oxidation. Regenerated hepatocytes in Acox1−/−/ob/ob mice, like those observed in Acox1-deficient mouse liver (17, 20), are better equipped to burn more energy and less prone to lipid storage. Acox1-deficient ob/ob mice revealed dramatic increases in the hepatic expression of fatty acid oxidation system genes that include mitochondrial and peroxisomal β-oxidation and microsomal ω-oxidation (9). Thus, structurally and metabolically, the Acox1−/−/ob/ob liver differs from the predominantly fatty liver of ob/ob mice. Histological analysis of ob/ob mouse liver reveals the presence of numerous large lipid vacuoles. In contrast, a majority of hepatocytes in Acox1−/−/ob/ob mouse liver are rich in granular cytoplasm with numerous peroxisomes but generally devoid of lipid vacuoles. A few hepatocytes in these ob/ob mice lacking expression of Acox1 displayed the presence of smaller lipid vacuoles. Hepatic steatosis in ob/ob mice is related, in part, to increased hepatic lipogenesis, which is controlled by the transcription factor carbohydrate responsive element-binding protein (ChREBP; ref. 51). ChREBP regulates lipogenic genes, including acetyl-CoA carboxylase and fatty acid synthase. Liver-specific inhibition of ChREBP in ob/ob mice markedly improved hepatic steatosis by specifically decreasing lipogenic rates (51). Our data suggest that FASN gene expression is reduced in both Acox1−/− and Acox1−/−/ob/ob mice (Fig. 5B).
Mice lacking Acox1 activity manifest hepatic ER stress attributable to PPARα activation and up-regulation of the ER stress-related transcription factor p8 (20). p8 is a transcriptional regulator of ER stress-related effectors, such as Trb3, ATF4, CHOP, and other factors indicative of unfolded protein response. Increasing evidence points to the importance of ER stress in metabolic disorders and carcinogenesis (20). Of interest is that although the fatty liver of ob/ob mice revealed no overt increases in the expression levels of ER stress response genes, the Acox1−/−/ob/ob double-mutant liver revealed higher levels of expression of a series of ER stress genes, such as GRP78, XBP1u, Trb3, p8, and Nrf2 mRNAs, similar to that noted in Acox1−/− mouse liver (20). Furthermore, an increase in the expression of CHOP and ATF4 mRNA level was also evident in the Acox1−/−/ob/ob mouse liver but not in ob/ob liver. We have recently found that transcription factor p8, a key regulator of ER stress response gene, is regulated by lipid sensor PPARα (20). Thus, heightened activation of PPARα and p8 in Acox1-lacking ob/ob liver, in contrast to ob/ob mouse liver, may contribute to ER stress and tumorigenesis, as described in Acox1-deficient mice. There is also an increase in p53 and p21 gene expression in both Acox1−/− and Acox1−/−/ob/ob mice along with p8 (Fig. 6A). p8 is known to form a complex with p53 and p300, and this complex appears to contribute to the transcriptional induction of p21 (46). p21 is a potent cyclin-dependent kinase inhibitor, and when down-regulated, it could functionally induce a regenerative response.
Epidemiological studies have shown that obesity and diabetes are risk factors for nonalcoholic fatty liver disease and hepatocellular carcinoma (52). Our observations suggest that obesity-associated fatty liver may require additional insults to elicit steatohepatitis, ER stress and activation of lipid-sensing nuclear receptors, such as PPARα, to induce liver tumorigenesis and that Acox1−/−/ob/ob double-mutant mice offer a model to study the role of energy metabolism in liver carcinogenesis. Increased liver cell proliferation in these livers burning excess energy may augment genetic variation (49).
Of interest is that Acox1 deficiency, either alone or in combination with leptin deficiency, results in an increase in the expression of Nrf2 transcription factor, which regulates the expression of genes essential for protection against oxidative injury (43, 44). Recently, Nrf2 has been shown to regulate PPARγ through binding to antioxidant response elements (AREs) present in the 5′ upstream region of PPARγ (43). Since Nrf2 expression is increased in both Acox1−/− and Acox1−/−ob/ob mouse liver, we looked at the Nrf2 5′ upstream region for the presence of peroxisome proliferator response elements (PPREs). Several putative PPREs have been found in Nrf2 promoter, which remain to be analyzed for their functional competence. It is possible that PPARα and other PPAR isotypes could induce Nrf2, which, in turn, may up-regulate PPARγ and PPARα expression in these mice.
The relative resistance of mice lacking both Acox1 and leptin in developing obesity illustrates that Acox1 inhibition may be a novel therapeutic modality to manipulate obesity by interfering with the ability of this enzyme to inactivate endogenous ligands of PPARα (8, 16–18). Thus, measured inhibition of Acox1 by novel therapeutic agents may result in a failure of inactivation of putative endogenous PPARα ligands that, in turn, enhance the expression of PPARα-regulated genes to augment energy expenditure. It should be noted that chronic increase in hepatic fatty acid oxidation by hepatic gene transfer of CPT1α, using safe nonimmunoreactive adeno-associated viruses, improves high-fat-diet-induced and genetically obese phenotype (53). Of interest is that increase in hepatic fatty acid oxidation alone by hepatic CPT1α gene transfer was sufficient to exert a systemic effect on epididymal adipose tissue weight (53). Furthermore, continuous up-regulation of fat oxidation in acetyl-CoA carboxylase 2 (Acc2)-knockout mice has been shown to increase total energy expenditure, resulting in lean body mass due to the reduction in adipose tissue content (54). Acc2 ablation also improved insulin sensitivity, suggesting that Acc2 inhibition is a viable therapeutic option for the treatment of obesity and type 2 diabetes (54). Recent reports of genetic manipulations of ob/ob mice by breeding these mice with other genetically altered mouse lineages of interest, such as generating ob/ob mice lacking klotho (55), have the potential to yield new leads in understanding the molecular complexities involved in the maintenance of energy homeostasis and for developing therapeutic agents. Nonetheless, the data from both Acox1−/− and Acox1−/−/ob/ob mice raises issues regarding the liver tumors developing in both Acox1−/− and Acox1−/−/ob/ob mice, despite the beneficial effects of reducing obesity and improving certain metabolic aspects.
Supplementary Material
Acknowledgments
This research was supported, in part, by U.S. National Institutes of Health grants GM23750 and DK083163 to J.K.R. The authors thank Dr. Aurore Vluggens for her gracious and generous assistance in the analysis of liver tumors.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Evans R. M., Barish G. D., Wang Y. X. (2004) PPARs and the complex journey to obesity. Nat. Med. 10, 355–361 [DOI] [PubMed] [Google Scholar]
- 2. Gurevich-Panigrahi T., Panigrahi S., Wiechec E., Los M. (2009) Obesity: pathophysiology and clinical management. Curr. Med. Chem. 16, 506–521 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 [DOI] [PubMed] [Google Scholar]
- 4. Friedman J. M., Halaas J. L. (1998) Leptin and the regulation of body weight in mammals. Nature 395, 763–770 [DOI] [PubMed] [Google Scholar]
- 5. Cohen J. C., Horton J. D., Hobbs H. H. (2011) Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Issemann I., Green S. (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347, 645–650 [DOI] [PubMed] [Google Scholar]
- 7. Desvergne B., Wahli W. (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20, 649–688 [DOI] [PubMed] [Google Scholar]
- 8. Pyper S. R., Viswakarma N., Yu S., Reddy J. K. (2010) PPARα: energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 8, e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reddy J. K., Hashimoto T. (2001) Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor-α: an adaptive metabolic system. Annu. Rev. Nutr. 21, 193–230 [DOI] [PubMed] [Google Scholar]
- 10. Tontonoz P., Hu E., Spiegelman B. M. (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid activated transcription factor. Cell 79, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 11. Peters J. M., Lee S. S., Li W., Ward J. M., Gavrilova O., Everett C., Reitman M. L., Hudson L. D., Gonzalez F. J. (2000) Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β/δ. Mol. Cell. Biol. 20, 5119–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao M. S., Papreddy K., Musunuri S., Okonkwo A. (2002) Prevention/reversal of choline-deficiency-induced steatohepatitis by a peroxisome proliferator-activated receptor-α ligand in rats. In Vivo 16, 145–152 [PubMed] [Google Scholar]
- 13. Ip E., Hall P., Robertson G., Leclercq I. (2004) Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 39, 1286–1296 [DOI] [PubMed] [Google Scholar]
- 14. Chen H., Dardik B., Qiu L., Ren X., Caplan S. L., Burkey B., Boettcher B. R., Gromada J. (2010) Cevoglitazar, a novel peroxisome proliferator-activated receptor–α/γ dual agonist, potently reduces food intake and body weight in obese mice and cynomologus monkeys. Endocrinology 151, 3115–3124 [DOI] [PubMed] [Google Scholar]
- 15. Reddy J. K., Goel S. K., Nemali M. R., Carrino J. J., Laffler T. G., Reddy M. K., Sperbeck S. J., Osumi T., Hashimoto T., Lalwani N. D., Rao M. S. (1986) Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proc. Natl. Acad. Sci. U. S. A. 83, 1747–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan C. Y., Pan J., Chu R., Lee D., Kluckman K. D., Usuda N., Singh I., Yeldandi A. V., Rao M. S., Maeda N., Reddy J. K. (1996) Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J. Biol. Chem. 271, 24698–24710 [DOI] [PubMed] [Google Scholar]
- 17. Fan C. Y., Pan J., Usuda N., Yeldandi A. V., Rao M. S., Reddy J. K. (1998) Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J. Biol. Chem. 273, 15639–15645 [DOI] [PubMed] [Google Scholar]
- 18. Yeldandi A. V., Rao M. S., Reddy J. K. (2000) Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 448, 159–177 [DOI] [PubMed] [Google Scholar]
- 19. Chakravarthy M. V., Lodhi I. J., Yin L., Malapaka R. R., Xu H. E., Turk J. (2009) Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell 138, 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang J., Viswakarma N., Yu S., Jia Y., Bai L., Vluggens A., Cherkaoui-Malki M., Rao M. S., Borensztajn J., Reddy J. K. (2011) Progressive endoplasmic reticulum stress contributes to hepatocarcinogenesis in fatty acyl-CoA oxidase 1-deficient mice. Am. J. Pathol. 179, 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu T., Borensztajn J. (2006) Simvastatin causes the formation of cholesterol-rich remnants in mice lacking apoE. Biochem. Biophys. Res. Commun. 341, 1172–1176 [DOI] [PubMed] [Google Scholar]
- 22. Huang J., Iqbal J., Saha P. K., Liu J., Chan L., Hussain M. M., Moore D. D., Wang L. (2007) Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology 46, 147–157 [DOI] [PubMed] [Google Scholar]
- 23. Bai L., Jia Y., Viswakarma N., Huang J., Vluggens A., Wolins N. E., Jafari N., Rao M. S., Borensztajn J., Yang G., Reddy J. K. (2011) Transcription coactivator mediator subunit MED1 is required for the development of fatty liver in the mouse. Hepatology 53, 1164–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsumoto K., Yu S., Jia Y., Ahmed M. R., Viswakarma N., Sarkar J., Kashireddy P. V., Rao M. S., Karpus W., Gonzalez F. J., Reddy J. K. (2007) Critical role for transcription coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein/TRAP220 in liver regeneration and PPARα ligand-induced liver tumor development. J. Biol. Chem. 282, 17053–17060 [DOI] [PubMed] [Google Scholar]
- 25. Abdelkarim M., Caron S., Duhem C., Prawitt I., Dumont J., Lucas A., Bouchaert E., Briand O., Brozek J., Kuipers F., Fievet C., Cariou B., Staels B. (2010) The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-γ and interfering with the Wnt/β-catenin pathway. J. Biol. Chem. 285, 36759–36767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jia Y., Qi C., Kashireddi P., Surapureddi S., Zhu Y. J., Rao M. S., Le Roith D., Chambon P., Gonzalez F. J., Reddy J. K. (2004) Transcription coactivator PBP, the peroxisome proliferator-activated receptor (PPAR)-binding protein, is required for PPARα-regulated gene expression in liver. J. Biol. Chem. 279, 24427–24434 [DOI] [PubMed] [Google Scholar]
- 27. Yu S., Matsusue K., Kashireddy P., Cao W. Q., Yeldandi V., Yeldandi A. V., Rao M. S., Gonzalez F. J., Reddy J. K. (2003) Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor-γ1 (PPAR-γ1) overexpression. J. Biol. Chem. 278, 498–505 [DOI] [PubMed] [Google Scholar]
- 28. Coleman D. L. (1978) Obese and Diabetes: two mutant genes causing diabetes-obsese syndromes in mice. Diabetologia 14, 141–168 [DOI] [PubMed] [Google Scholar]
- 29. Erickson J. C., Hollopeter G., Palmiter R. D. (1996) Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274, 1704–1707 [DOI] [PubMed] [Google Scholar]
- 30. Ntambi J. M., Miyazaki M., Stoehr J. P., Lan H., Kendziorski C. M., Yandell B. S., Song Y., Cohen P., Friedman J. M., Attie A. D. (2002) Loss of stearoyl-CoA desaturase-1function protects mice against adiposity. Proc. Natl. Acad. Sci. U. S. A. 99, 11482–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holt L. J., Lyons R. J., Ryan A. S., Beale S. M., Ward A., Cooney G. J., Daly R. J. (2009) Dual ablation of Grb10 and Grb14 in mice reveals their combined role in regulation of insulin signaling and glucose homeostasis. Mol. Endocrinol. 23, 1406–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsusue K., Haluzik M., Lamberr G., Yim S.-H., Gavrilova O., Ward J. M., Brewer B., Jr., Reitmen M. L., Gonzalez F. J. (2003) Liver-specific disruption of PPARγ in leptic-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest. 111, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Badman M. K., Kennedy A. R., Adams A. C., Pissios P., Maratos-Flier E. (2009) A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am. J. Physiol. Endocrinol. Metab. 297, E1197–E1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segal-Lieberman G., Bradley R. L., Kokkotou E., Carlson M., Trombly D. J., Wang X., Bates S., Myers M.G., Jr., Flier J. S., Maratos-Flier E. (2003) Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc. Natl. Acad. Sci. U. S. A. 100, 10085–10090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F. J. (2008) Hepatic steatosis in leptin-deficient mice is promoted by the PPARγ target gene Fsp27. Cell Metab. 7, 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qu S., Altomonte J., Perdomo G., He J., Fan Y., Kamagate A., Meseck M., Dong H. H. (2006) Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology 147, 5641–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zadravec D., Brolinson A., Fisher R. M., Carneheim C., Csikasz R. I., Bertrand-Michel J., Borén J., Guillou H., Rudling M., Jacobsson A. (2010) PPARα ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J. 24, 4366–4377 [DOI] [PubMed] [Google Scholar]
- 38. Han D. H., Nolte L. A., Ju J. S., Coleman T., Holloszy J. O., Semenkovich C. F. (2004) UCP-mediated energy depletion in skeletal muscle increases glucose transport despite lipid accumulation and mitochondrial dysfunction. Am. J. Physiol. Endocrinol. Metab. 286, E347–E353 [DOI] [PubMed] [Google Scholar]
- 39. Muoio D. M., Newgard C. B. (2006) Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 75, 367–401 [DOI] [PubMed] [Google Scholar]
- 40. Chavin K. D., Yang S., Lin H. Z., Chatham J., Chacko V. P., Hoek J. B., Walajtys-Rode E., Rashid A., Chen C. H., Huang C. C., Wu T. C., Lane M. D., Diehl A. M. (1999) Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J. Biol. Chem. 274, 5692–5700 [DOI] [PubMed] [Google Scholar]
- 41. Oyadomari S., Harding H. P., Zhang Y., Oyadomari M., Ron D. (2008) Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 7, 520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yanagitani K., Kimata Y., Kadokura H., Kohno K. (2011) Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science 331, 586–589 [DOI] [PubMed] [Google Scholar]
- 43. Cho H.-Y., Gladwell W., Wang X., Chorley B., Bell D., Reddy S. P., Kleeberger S. R. (2010) Nrf2-regulated PPAR expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care. Med. 182, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang J., Tabbi-Anneni I., Gunda V., Wang L. (2010) Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1211–G1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park S.-H., Choi H. J., Yang H., Do K. H., Kim J., Lee D. W., Moon Y. (2010) Endoplasmic reticulum stress-activated C/EBP homologous protein enhances nuclear factor NF-κB signals via repression of peroxisome proliferator-activated receptor. J. Biol. Chem. 285, 35330–35339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goruppi S., Iovanna J. L. (2010) The stress-inducible protein p8 is involved in several physiological and pathological processes. J. Biol. Chem. 285, 1577–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reddy J. K., Azarnoff D. L., Hignite C. E. (1980) Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature 283, 397–398 [DOI] [PubMed] [Google Scholar]
- 48. Shah Y. M., Morimura K., Yang Q., Tanabe T., Takagi M., Gonzalez F. J. (2007) Peroxisome proliferator-activated receptor regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol. Cell. Biol. 27, 4238–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duncan A. W., Taylor M. H., Hickey R. D., Hanlon Newell A. E., Lenzi M. L., Olson S. B., Finegold M. J., Grompe M. (2010) The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 467, 707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vluggens A., Andreoletti P., Viswakarma N., Jia Y., Matsumoto K., Kulik W., Khan M., Huang J., Guo D., Yu S., Sarkar J., Singh I., Rao M. S., Wanders R. J., Reddy J. K., Cherkaoui-Malki M. (2010) Functional significance of the two ACOX1 isoforms and their crosstalks with PPARα and RXRα. Lab. Invest. 90, 696–708 [DOI] [PubMed] [Google Scholar]
- 51. Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J. R. B., Girard J., Postic C. (2006) Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 55, 2159–2170 [DOI] [PubMed] [Google Scholar]
- 52. Caldwell S. H., Crespo D. M., Kang H. S., Al-Osaimi A. M. S. (2004) Obesity and hepatocellular carcinoma. Gastroenterology 127, S97–S103 [DOI] [PubMed] [Google Scholar]
- 53. Orellana-Gavalda J. M., Herrero L., Malandrino M. I., Paneda A., Rodriguez-Pena M. S., Petry H., Asins G., Deventer S. V., Hegardt F. G., Serra D. (2011) Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty acid oxidation. Hepatology 53, 821–832 [DOI] [PubMed] [Google Scholar]
- 54. Choi C. S., Savage D. B., Abu-Elheiga L., Liu Z. X., Kim S., Kulkarni A., Distefano A., Hwang Y. J., Reznick R. M., Codella R., Zhang D., Cline G. W., Wakil S. J., Shulman G. I. (2007) Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc. Natl. Acad. Sci. U. S. A. 104, 16480–16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ohnishi M., Kato S., Akiyoshi J., Atfi A., Razzaque M. S. (2011) Dietary and genetic evidence for enhancing glucose metabolism and reducing obesity by inhibiting klotho functions. FASEB J. 25, 2031–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.