Abstract
Cigarette smoke (CS) exposure induces mucus obstruction and the development of chronic bronchitis (CB). While many of these responses are determined genetically, little is known about the effects CS can exert on pulmonary epithelia at the protein level. We, therefore, tested the hypothesis that CS exerts direct effects on the CFTR protein, which could impair airway hydration, leading to the mucus stasis characteristic of both cystic fibrosis and CB. In vivo and in vitro studies demonstrated that CS rapidly decreased CFTR activity, leading to airway surface liquid (ASL) volume depletion (i.e., dehydration). Further studies revealed that CS induced internalization of CFTR. Surprisingly, CS-internalized CFTR did not colocalize with lysosomal proteins. Instead, the bulk of CFTR shifted to a detergent-resistant fraction within the cell and colocalized with the intermediate filament vimentin, suggesting that CS induced CFTR movement into an aggresome-like, perinuclear compartment. To test whether airway dehydration could be reversed, we used hypertonic saline (HS) as an osmolyte to rehydrate ASL. HS restored ASL height in CS-exposed, dehydrated airway cultures. Similarly, inhaled HS restored mucus transport and increased clearance in patients with CB. Thus, we propose that CS exposure rapidly impairs CFTR function by internalizing CFTR, leading to ASL dehydration, which promotes mucus stasis and a failure of mucus clearance, leaving smokers at risk for developing CB. Furthermore, our data suggest that strategies to rehydrate airway surfaces may provide a novel form of therapy for patients with CB.—Clunes, L. A., Davies, C. M., Coakley, R. D., Aleksandrov, A. A., Henderson, A. G., Zeman, K. L., Worthington, E. N., Gentzsch, M., Kreda, S. M., Cholon, D., Bennett, W. D., Riordan, J. R., Boucher, R. C., Tarran, R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration.
Keywords: aggresome, chronic bronchitis, ion channel, lung disease
Chronic obstructive pulmonary disease (COPD), the fourth leading cause of death in the United States (1), is a syndrome associated with chronic airflow obstruction that is most often caused by cigarette smoke (CS). COPD encompasses two major phenotypes, emphysema and chronic bronchitis (CB), with CB being the more common (2). CB is characterized by mucus hypersecretion, chronic cough, sputum production, and mucus plugging, and it is punctuated by intermittent acute exacerbations. The pathogenesis of CB is at present unclear, but it is thought to represent an inflammatory reaction of the airways to CS, and consequently, current therapies are focused on anti-inflammatory agents and bronchodilators (3, 4). However, it has recently been suggested that CS affects the expression of the CFTR Cl− channel, which may predispose airway surfaces to chronic dehydration and mucus stasis/accumulation (5).
Cystic fibrosis (CF) is a mendelian genetic disease that exhibits as a major phenotype a severe CB. There are many similarities between CF, particularly in its early phase, and CS-induced CB. These similarities include a common spectrum of bacterial infections in young patients with CF and patients with CB, e.g., Haemophilus influenzae and Staphylococcus aureus (6, 7); persistent airway inflammation (8, 9); and intermittent acute exacerbations (10, 11). In contrast to CS-induced CB, the pathogenesis of CF bronchitis is becoming more clear. Recent data suggest that mutations in the CFTR gene lead to dehydration of airway surfaces that ultimately produces mucus stasis, mucus adhesion, inflammation, and infection (10, 12). The common phenotypic features of CB and CF raise the question of whether CS may adversely perturb CFTR gene/protein function, producing an exogenous, toxin-induced dehydration of airway surfaces that contributes to the pathogenesis of CS-induced CB.
The effects of CS exposure on airway epithelial gene expression have been well described and include changes in mucin, cytokine, and metalloprotease expession (13, 14). CFTR expression is sensitive to cell redox levels and oxidative stress (15). CS is a strong oxidant and has been reported to decrease CFTR expression transcriptionally (5). However, these data do not preclude the possibility that CS can also affect CFTR protein levels directly. Normally, CFTR is efficiently processed, and excess nascent CFTR is degraded by the 26S proteasome, while membrane CFTR can be internalized and degraded via the lysosomal route (16–18). In addition, some CFTR proteins instead traffic into aggresomal complexes. Aggresomes harbor insoluble proteins and are delineated by a cage of vimentin filaments (19). Aggresomes typically form when the cellular degradation machinery is overwhelmed, and they have been described in relation to many diseases, including CF, Parkinson's disease, and alcoholic liver disease (19–21).
In this study, we tested the hypothesis that CS alters CFTR function at the protein level. To this end, we measured CFTR activity in vivo and in vitro after acute CS exposures and then used biochemical and immunocytochemistry-based approaches to determine the fate of CFTR after CS exposure. We also tested the hypothesis that hypertonic saline (HS) could restore CS-induced airway surface liquid (ASL) depletion in vitro and reduce mucocilliary clearance (MCC) in subjects with CB in vivo.
MATERIALS AND METHODS
Nasal potential differences (NPDs) and mucus percentage solid determinations
Written, informed consent was received prior to experimentation, and studies were approved by the University of North Carolina (UNC) Institutional Committee on the Protection of the Rights of Human Subjects. Healthy smokers were recruited from UNC Smoking Cessation Clinics who had no prior history of lung disease, including asthma. Mucus wet-dry ratios were obtained to calculate the percentage solids, as described previously (22). NPDs were measured using a perfused recording electrode positioned under the inferior turbinate, with a reference electrode positioned subcutaneously (23).
Cell culture
Baby hamster kidney (BHK) cells stably expressing CFTR (BHKCFTR) were cultured on glass-bottomed Petri dishes (MatTek, Ashland, MA, USA) for imaging or standard plastic Petri dishes for biochemical experiments (23). Primary human bronchial epithelial cells (HBECs) and CALU3 cells were plated on 12-mm Transwell permeable supports (Corning-Costar, Corning, NY, USA) under air-liquid interface conditions (23).
CS exposure
BHKCFTR cells, HBECs, and CALU3 cells were exposed to whole CS with a LM1 smoke engine (Borgwaldt, Hamburg, Germany) calibrated to deliver a volume/surface area of CS that approximates in vivo exposure (25). The standard protocol was to expose subjects and cultures to 10 × 35-ml puffs of CS over 10 min, which is equivalent to 1 whole cigarette.
Under protocols approved by the UNC Institutional Committee on the Protection of the Rights of Human Subjects, 9 healthy subjects, without evidence of respiratory or nasal disorders and lacking a history of recent active or passive CS exposure, were studied. CS was mechanically generated from a research-grade cigarette (Kentucky 2R4F) and 10 × 35-ml puffs (equivalent to 1 cigarette) were delivered to the nasal epithelium through an olive placed snugly in the nostril. During CS administration, the subject exhaled through a resistor to close the soft palette and isolate the nasopharynx (26). With this maneuver, smoke did not enter the lower airways, instead exiting via the contralateral nostril, in which another olive had been placed to deliver smoke to a fume-hood exhaust. In separate control experiments, the protocol was repeated in an identical fashion, but CS was replaced with room air.
Bioelectric and ASL measurements
Transepithelial resistance and transepithelial electric potential (Vt) were measured by EVOM (World Precision Instruments, Sarasota, FL, USA) (22). ASL height was measured by XZ confocal microscopy (22).
Lipid bilayer experiments
Single-channel CFTR experiments were performed as described previously (27). To expose CFTR-containing vesicles to CS, single puffs were blown through the transcompartment filled with 6 ml buffer solution. After 10 puffs, the chamber was covered with paraffin to isolate the gas phase above the chamber from air. Final recordings were started ∼5 min after the last puff was blown through.
Immunolocalization
Polarized HBECs were quickly embedded in OCT (Sakura Finetek, Torrance, CA, USA) and cryopreserved at −80°C. Thin frozen tissue sections (8 μm) were processed as described previously, then imaged by confocal microscope (28).
For surface labeling HA-CFTR, intact cells were exposed to CS or room air and fixed for 10 min in 4% paraformaldehyde before staining as described previously (29). To prelabel surface CFTR, BHKCFTR cultures were incubated with rabbit anti-HA polyclonal antibody (Abcam, Cambridge, MA, USA) for 1 h at 4°C. Cultures were then warmed to room temperature and exposed to air or CS, and then fixed at timed intervals in methanol before staining. To label total cellular CFTR, cells were exposed to CS or room air, then either fixed and permeabilized with methanol or paraformaldehyde followed by Triton-X, and stained.
Western blots
BHKCFTR cultures were exposed to either 10 min of CS or of room air puffed through the smoke engine before being lysed with either Nonidet P-40 or SDS buffer, and Western blotting was performed as described previously (29). To quantify the amount of cell surface CFTR, BHKCFTR cells were grown in 96-well black Isoplates (PerkinElmer, Waltham, MA, USA). Cells were exposed to CS or room air in a modified smoking chamber and were fixed for 10 min in 4% paraformaldehyde. Supersignal ELISA Pico chemiluminescence substrate (Pierce Chemical, Rockford, IL, USA) was added, and the luminescence was quantified using a Victor2 1420 multilabel plate reader (PerkinElmer; ref. 29).
Mucus clearance
Subjects were recruited from the UNC COPD database. Subjects were included if they had a ratio of FEV1/FVC < 0.7 with FEV1 > 40% of predicted, a minimum 20-pack year smoking history (current or former), an age of 40 yr or older, and a history of CB based on the clinical definition. They were excluded if they had radiation exposure that would exceed the recommended limits in the past year, a history of asthma, or a 15% change in FEV1 after a test dose of HS, intolerance to albuterol, chronic oxygen use, or the use of antibiotics in the past 4 wk prior to study.
The methods for measuring mucus clearance by serial γ camera imaging of inhaled, radiolabeled (Tc99m) sulfur colloid particles have been described in detail previously (24). In a randomized, crossover design, mucus clearance was measured twice in each patient: baseline and immediately after the inhalation of aerosolized 7% HS (5 ml in Pari LC Star nebulizer; Pari Respiratory Equipment, Inc., Midlothian, VA, USA). During a 30-min period of scanning, right lung activity over time was determined for each day of study. During this period, patients were encouraged to suppress spontaneous coughing, and cough frequency was recorded for comparison between the 2 study days. The 30-min rate of clearance from the right lung, calculated from the average of measurements at 2-min intervals, served as the primary index of mucus clearance. Baseline mucus clearance was also measured in a group of healthy nonsmoking adults for comparison to baseline measures in the COPD patients.
Real-time RT-PCR
Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA, USA) and reverse transcribed into cDNA using Superscript II (Invitrogen, Carlsbad, CA, USA). Quantification of CFTR mRNA expression was performed using SYBR Green detection in a LightCycler PCR machine (Roche Applied Science, Basel, Switzerland), according to the manufacturer's instructions, and using the following custom-made CFTR primers (MWG Biotech, High Point, NC, USA): 5-ACCACAGGCATAATCATGG-3′ (sense) and 5-AGCAGAATGAAACTCTTCCAC-3′ (antisense). Relative fold changes in target gene expression were calculated from the efficiency of the PCR and the crossing point deviation between samples from experimental groups and determined by normalization to expression of the reference gene (18 S).
Statistical analyses
Unless otherwise noted, all data are presented as means ± se for n experiments. Differences between means were tested for statistical significance using paired or unpaired t tests when the variances were homogeneously distributed, or the case of nonhomogeneity of variance, the Wilcoxon rank-sum or Mann-Whitney U tests were used as appropriate. From such comparisons, differences yielding values of P ≤ 0.05 were judged to be significant. HBECs derived from ≥3 donors were used per experiment, and experiments using cell lines were repeated on 3 separate occasions. All analyses were conducted using Instat software (GraphPad, San Diego, CA, USA).
RESULTS
Mucus dehydration and altered ion transport in chronic smokers
Mucin hypersecretion frequently occurs with chronic CS exposure and chronic inflammation (30). However, little is known about the hydration state of mucus following chronic smoke exposure. Accordingly, mucus samples were collected from 2- to 3-mm-diameter airways from smokers' lungs that were harvested at the time of surgery, and wet-dry ratios were obtained immediately after extraction of mucus from the lungs. From these samples, percentage solids values of 10.0 ± 0.7% were measured (n=8 donors; see Table 1). Mucus was also sampled from the trachea of these lungs, and this was similarly dehydrated (11.3±0.5%; n=8 donors). Mucus plugging was not observed in the lungs of nonsmokers. However, during postsurgery transplantation of these lungs to our research center, the proximal end of the trachea was sealed, and because of normal mucociliary transport, mucus accumulated at this site. Direct sampling revealed that this mucus was significantly less dehydrated than that from smokers (4.6±1.0%; n=5 donors; P = 0.003).
Table 1.
Subject demographics for smokers vs. control participants
| Subject group | Age | Sex | Smoke history |
|---|---|---|---|
| Controls for chronic smoker NPDs | 32 ± 7.1 | 3 M/5 F | Never smoked |
| Chronic smoker NPDs | 40.3 ± 6.8 | 2 M/2 F | 15 pack years |
| Controls for acute smoke exposure NPDs | 29.7 ± 5.6 | 3 M/2 F | Never smoked |
| Acute smoke exposure NPDs | 31.8 ± 4.4 | 2 M/2 F | Ex-smokers: >2 yr smoke free |
| Control lung donors: mucus | 32.2 ± 17.5 | 3 M/2 F | Nonsmokers |
| Smoker lung donors: mucus | 55 ± 11 | 4 M/4 F | 32 pack years |
Age, gender, and smoking history are listed as mean ± sd for all subjects used to study mucus percentage solids and NPDs.
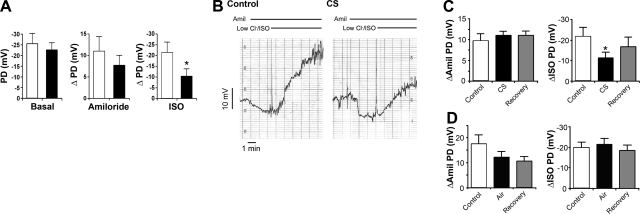
Adequate mucus hydration requires regulated ion transport activity, including sufficient CFTR function. However, little is known about ion transport rates in chronic smokers. Cantin et al. (5) recently reported that chronic smokers display abnormal ion transport characteristics that mimic aspects of CF airways. In particular, the researchers reported that chronic smokers lacked CFTR-dependent Cl− transport. In agreement with these studies, we found that the low Cl−/isoproterenol (ISO) transepithelial NPD response, which corresponds to activation of CFTR, was significantly decreased in the nasal epithelia of chronic smokers compared to nonsmoking controls (Fig. 1A).
Figure 1.
In vivo assessments of whole CS action on CFTR function. A) In vivo NPD measured in nonsmoking control subjects (open bars; n=8) and chronic smokers (solid bars; n=4). Bar graphs show basal NPD and the responses to cumulative mucosal superfusion with amiloride (10−4 M) followed by a low Cl−/isoproterenol solution (ISO; 10−5 M). B) Typical paired NPD traces from a nonsmoking subject before (control, left panel) and 10 min after (right panel) acute 10 min CS exposure. Responses of basal PD to cumulative mucosal superfusion with amiloride (10−4 M) and a low-Cl−/isoproterenol (10−5 M) are shown (horizontal bars denote drug additions). C) Summary NPD data taken from B. Left panel: change in NPD in response to amiloride (ΔAmil PD) before (control), immediately after CS, and 45 min after CS exposure (recovery). Right panel: change in NPD in response to superfusion with low-Cl−/ISO solution for CS-exposed nostrils. n = 5/group. D) NPD data for air-exposed control subjects; n = 4/group. *P < 0.05 vs. control.
Acute CS exposure rapidly diminishes CFTR activity in vivo
To determine whether a reduced NPD response to ISO could be produced in healthy ex-smokers in a timeframe inconsistent with genomic changes in CFTR, we measured the NPD responses immediately after exposure to mainstream CS (10 puffs from 1 cigarette over 10 min) or an equivalent number of puffs of room air (control). CS exposure had no effect on the basal PD (pre-CS PD was 21.8±1.8 mV, post-CS PD was 21.9±2.8 mV; P=0.98). The amiloride-sensitive NPD was not significantly raised (Fig. 1B, C). However, the change in NPD in response to a low Cl−/ISO superfusion, as an index of CFTR-mediated Cl− secretory capacity, was reduced by ∼60% after CS, as compared to room air (Fig. 1B, C). This finding suggests that the CFTR-mediated Cl− secretory capacity had been diminished by CS in vivo. Some recovery in the ΔISO NPD occurred after 45 min post-CS. Room air exposure had no significant effect on any in vivo bioelectric property over time (Fig. 1D). Thus, we conclude that CS exposure rapidly inhibits CFTR activity in vivo.
Effect of CS on ASL hydration in vitro
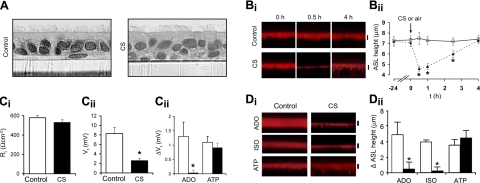
Normal airway epithelia balance Na+ absorption and Cl− secretion to maintain a volume of ASL sufficient to hydrate mucus for effective clearance (12). HBECs were exposed to CS from 1 cigarette or room air (control) to study the acute effects of CS on the regulation of ASL volume/height. No effect of CS on HBEC morphology was observed (Fig. 2A). However, the CS from 1 cigarette caused an acute reduction in ASL volume/height that lasted for >2.5 h (Fig. 2B).
Figure 2.
Effect of CS on airway epithelial CFTR function and airway hydration. A) Light micrographs of paraformaldehyde-fixed HBECs after 10 min air (control) or CS exposure. Bi) Confocal images of ASL height (red) in air-exposed (control) or CS-exposed HBECs. Bii) Graph showing mean ASL height with time after control (solid triangles) or CS (open squares) (both n=6). Ci) Transepithelial resistance Rt before and after CS exposure, measured by EVOM (13). Cii) EVOM electric potential difference Vt measurement of HBECs immediately after air or CS exposure. Ciii) ΔVt of air-exposed (control) or CS-exposed cultures in response to lumenally added adenosine (ADO; 10−5 M) or adenosine triphosphate (ATP; 10−4 M). CTL, all n = 7; CS, all n = 11. Di) Confocal images of ASL height of air-exposed (control) or CS-exposed (smoke) HBECs 10 min after addition of isoproterenol (ISO; 10−5 M, luminal), ADO (10−5 M, luminal), or ATP (10−4 M, luminal). Dii) Mean changes in ASL height for control and CS-exposed HBECs cultures in response to agonists. Control, all n = 6; CS, all n = 6. *P < 0.05 vs. control.
To elucidate the mechanisms of CS-induced ASL volume depletion, we first measured the transepithelial bioelectric correlates of ion transport. CS did not reduce transepithelial resistance Rt, suggesting that the ASL volume reduction did not reflect nonspecific effects on the paracellular path (Fig. 2Ci). In contrast, CS decreased the transepithelial electric potential difference Vt, suggesting that, in the absence of a change in Rt, a reduction in the rate of active ion transport had occurred (Fig. 2Cii). Because a reduction of Na+ transport would increase ASL volume rather than decrease it, we investigated whether CS inhibited the Cl− secretory paths that support Cl− secretion and ASL volume expansion. CS blunted the ΔVt response to ADO, but not ATP, suggesting that CS blocked the ADO-A2b-cAMP-CFTR-, but not ATP-P2Y2-R-Ca2+-activated Cl− channel secretion pathway (Fig. 2Ciii).
We next investigated directly the regulation of ASL height/volume by Cl− secretagogues after exposure to CS (Fig. 2D). ADO increased ASL volume in room air but not CS-exposed airway cultures (Fig. 2D). To test whether the CS-induced failure of ADO-induced ASL volume secretion reflected, in part, a defect in CFTR function, and not other elements of the ADO receptor signaling system (A2b-cAMP), 2 additional secretagogues were tested (Fig. 2D). ISO, which raises cellular cAMP levels and activates CFTR via β-adrenergic receptor activation, was also ineffective at increasing ASL volume compared to air-exposed controls (Fig. 2D). In contrast, ATP was effective in raising ASL volume via Ca2+-activated Cl− secretion following CS (Fig. 2D). Collectively, these data suggest that in vitro, CS diminished CFTR Cl− channel function.
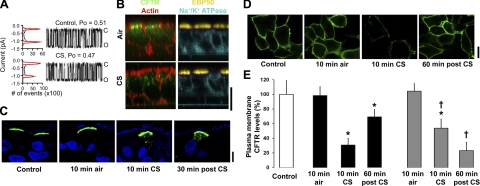
The effect of CS on CFTR channel number and open probability
The magnitude of the CFTR contribution to transepithelial Cl− secretion reflects both the number of CFTR Cl− channels at the apical cell membrane and the activation state of these channels (open probability, Po). We, therefore, tested the effects of CS on each component. Studies of CFTR channels reconstituted in bilayers revealed that CS induced no change in channel Po (Fig. 3A). However, using immunofluorescence techniques, we found that virtually all immunodetectable native CFTR was lost from the apical membrane of HBECs after CS exposure (Fig. 3B). In contrast, ezrin-binding protein 50 (EPB50), which helps anchor CFTR to the cytoskeleton, remained in the apical membrane after CS exposure (31), and the position of the basolateral Na+/K+ ATPase was not altered (Fig. 3B).
Figure 3.
CFTR is lost from the plasma membrane during CS exposure. A) Single CFTR channel lipid bilayer recordings. All points in the histogram shown on the left and 2 min of CFTR single-channel recording on the right. Open (O) and closed (C) states are indicated. Top recording shows control conditions; bottom recording depicts the same single channel after CS exposure, blown through the buffer in the trans side. n = 9/group. B) Immunofluorescence analysis of native CFTR (green), EPB50 (yellow), Na+/K+ ATPase (cyan) and actin-phalloidin (red) in HBECs exposed to air or CS. C) Immunofluorescence of HBECs virally infected with CFTRHA (green), counterstained with DAPI (blue), and exposed to 10 min air or CS and fixed either 10 min or 30 min later. D) Surface labeling of HA-CFTR (green) stably expressed in nonpermeabilized BHK cells before exposure, 10 min after air or CS exposure, and 60 min after CS exposure. E) Mean surface CFTR levels in BHKCFTR cultures measured by ELISA. Open bar denotes control. Solid bars denote air and smoke exposure. Shaded bars denote air and CS exposure following 1 h preincubation with 100 nM brefeldin A. Cells were fixed 10 min after air or CS exposure or 60 min after CS exposure (post-CS). n = 16/group. Scale bars = 25 μm. *P < 0.05 vs. control.
To examine the effects of CS on surface CFTR in more detail, we performed 2 studies with heterologously expressed CFTR. First, we overexpressed HA-tagged CFTR in HBECs with an adenoviral vector to better localize CFTR internalization after CS exposure (28). In adenoviral/CFTR-transduced HBECs, CFTR internalization was increased post-CS, as compared to air exposure (Fig. 3C). Second, to study CFTR removal from the plasma membrane, we used BHKCFTR cells with an extracellular HA tag (29). Intact cells were treated with CS or air and then probed with an anti-HA antibody to image and quantify surface CFTR levels. Disappearance of CFTR from the plasma membrane was visible by confocal microscopy after CS exposure (Fig. 3D), and cell surface CFTR levels, as measured by cell surface ELISA, were also decreased (Fig. 3E). As with our studies of airway epithelia, this diminution was reversible, and by 60 min after the start of CS exposure (1 h total), surface CFTR levels had returned toward normal levels (Fig. 3D, E). Pretreatment with brefeldin A, which inhibits trafficking from the ER to the Golgi apparatus, prevented CFTR recovery (Fig. 3E). Thus, we conclude that CS contains materials that promote the acute loss of CFTR channels from plasma membranes.
CS diminishes CFTR levels but does not induce protein degradation
To investigate whether the smoke-induced internalization of CFTR was accompanied by a decrease in total cellular CFTR protein levels, we performed Western blotting on HBECs and BHKCFTR cells after CS or air exposure, utilizing an antibody directed against an epitope in the second nucleotide binding domain (designated antibody 596; ref. 29 and Fig. 4). CFTR protein levels rapidly diminished in HBECs (Fig. 4A), unlike the α subunit of the epithelial Na+ channel (αENaC), which was unaffected by CS exposure (Fig. 4B). CFTR protein levels also diminished 15 min post-CS exposure in BHK cells (Fig. 4C). This diminution was dose dependent and was visible with a minimum of 3 CS puffs (Fig. 4D). To investigate the mechanism of this apparent diminution of CFTR protein, we inhibited the proteosome, a known pathway for CFTR degradation, with ALLN and MG132 (17, 18). Neither compound had any effect on the reduction of CFTR levels (Fig. 4E, F). In contrast, prechilling the cells to 4°C fully inhibited CS-mediated reduction in CFTR levels (Fig. 4G), suggesting that this was a process that required metabolically active cells. To further explore this hypothesis, we prepared BHKCFTR-containing membrane vesicles. In the absence of an intact cell, CFTR was insensitive to CS exposure, and protein levels were not decreased (Fig. 4H).
Figure 4.
CFTR protein levels are diminished with CS exposure. A, B) Typical Western blots and densitometric analysis for native CFTR (A) and ENaC (B) in HBECs exposed to 10 min air or CS. C) Typical Western blot of CFTR and actin after exposure to varying puffs of CS during 10-min exposures. Lanes were run on the same gel but were noncontiguous. D) Mean densitometric analysis for CFTR following varying doses of air or CS (n=6/group). E) Western blot of CFTR and actin following the standard 10-min air or CS exposure after 2 h preexposure to vehicle or ALLN. n = 6/group. F) Mean CFTR densitometry after cultures were pretreated with vehicle, ALLN, or MG132 for 2 h prior to CS or air exposure. G) Western blot and mean densitometry of CFTR after air or CS exposure at 21 vs. 4°C. H) Western blot and mean densitometry of CFTR after air vs. CS exposure to intact cultures or to CFTR-containing membrane vesicles. BHK cells were lysed in Nonidet P-40 buffer, and CFTR was probed using the 596 antibody. *P < 0.05 vs. control; †P < 0.05 vs. standard CS exposure.
In addition to blocking proteosomal degradation of CFTR, MG132 also prevents CFTR from being internalized and trafficked to lysosomes (28, 29). Despite these reported effects of MG132 on CFTR trafficking, CS still induced diminution of CFTR protein levels following MG132 pretreatment (Fig. 4E, F), suggesting that CFTR did not traffic to lysosomes after CS exposure. However, to confirm that CFTR did not end up in lysosomes, we also directly looked for colocalization between CFTR and a lysosomal marker (LAMP1) following CS exposure. No colocalization was detected between CFTR and LAMP1 after CS exposure or vehicle (Fig. 5).
Figure 5.
Internalized CFTR does not enter lysosomes after CS exposure. Immunofluorescence analysis of HA-CFTR (red) with time vs. the lysosomal marker LAMP1 (green). Surface HA-CFTR was prelabeled at 4°C, and 10 min air or CS exposure was performed at room temperature. Cultures were fixed in MeOH 20 min after CS exposure. Images are representative of experiments performed on 3 separate occasions. Scale bars = 25 μm. Cellular outline was obtained from the transmitted light images and traced over the confocal micrographs in white.
If CS triggers removal of CFTR from the plasma membrane and sorting to lysosomes, degradation of CFTR would be expected. To search for degradation products of CFTR post-CS exposure, we initially probed for CFTR using 4–8% tris-acetate gels (Fig. 4), which have a minimum resolution of 31 kDa. However, we also probed for CFTR degradation products using 12% bis-tris gels that can resolve up to 2.5-kDa fragments. Furthermore, in addition to the 596 antibody, which is directed against an epitope in NBD2, we also probed with antibodies against the CFTR N terminus (designated 13-4), NBD1, (#660), R-domain (#570) and an anti-HA antibody against the HA tag on the second extracellular loop. In all cases, despite detecting a CS-induced decrease in full-size CFTR, we failed to detect CFTR fragments post-CS using 12% bis-tris gels (all n=3; data not shown).
CS exposure reduces CFTR solubility and induces CFTR relocation to a perinuclear compartment
We performed the Western blots shown in Fig. 4 using Nonidet P-40, since this detergent has been found to be superior for extraction of membrane proteins, such as CFTR (28). However, to ensure that we had fully solubilized CFTR after smoke exposure, we also utilized a higher concentration of ionic detergent. Surprisingly, on extraction with a high concentration of SDS (i.e., 10%), the CS-induced reduction in the CFTR signal was no longer observed (Fig. 6A, B), suggesting that CS exposure resulted in CFTR becoming significantly less detergent soluble without inducing CFTR degradation. The experiments shown in Fig. 6A, B were performed in BHKCFTR cells. To test whether this effect was also observable in airway epithelia, we also exposed polarized CALU3 cells, grown at an air-liquid interface to CS, since these cells endogenously express CFTR and have previously been used for CS-exposure experiments (5, 32). Following CS exposure, a reduction in CFTR levels could be detected in Nonidet P-40- but not SDS-lysed cultures (Fig. 6C).
Figure 6.
CFTR solubility is altered after CS exposure. A) Western blot showing CFTR from BHKCFTR cells lysed following standard exposure to air or CS using Nonidet P-40, or lysis buffer containing 10% SDS. B) Mean densitometry for CFTR taken from A. n = 6/group. C) Western blot showing CFTR from polarized CALU3 airway epithelial cultures lysed using Nonidet P-40 vs. 10% SDS before (control) and after the standard single CS exposure. D) Representative immunofluorescence of CFTR stably expressed in BHK cells following exposure to air or acute CS and either fixation in paraformaldehyde (PFA) followed by permeablization with Triton-X (Tx) or by fixation/permeablization with methanol (MeOH). In all cases, cells were then probed with the 596 CFTR antibody; confocal microscope laser/gain settings were identical for all images. E) CFTR expression levels, normalized to 18 S, in polarized CALU3 cultures chronically exposed to CS (see Materials and Methods). F, G) Western blot (F) and mean densitometry (G) for polarized CALU3 cultures chronically exposed to CS and lysed in Nonidet P-40 or 10% SDS. Scale bar = 25 μm. Open bars denote air exposure. Solid bars denote CS exposure. *P < 0.05 vs. air; †P < 0.05 vs. CFTR in Nonidet P-40 buffer.
To further investigate the change in solubility of CFTR, we compared immunostaining of CFTR after CS exposure with either paraformaldehyde followed by permeabilization with 1% Triton-X or following fixation/permeabilization with methanol using the 596 antibody against CFTR's NBD2. While CFTR could be detected equally well in air-exposed BHK cultures using either paraformaldehyde or methanol, CFTR could only be detected following methanol fixation post-CS exposure (Fig. 6D). Thus, the pool of CFTR that exhibits reduced solubility post-CS may become accessible to the antibody only following harsher permeabilization with methanol.
Chronic smoke exposure has previously been shown to decrease CFTR expression levels in CALU3 cells (5). To investigate whether CFTR remained insoluble to Nonidet P-40 after chronic smoke exposure, we utilized the protocol employed by Cantin et al. (5) and exposed polarized CALU3 cells to smoke from 1 cigarette every 2 h for 8 h. After this time, CFTR gene expression was significantly reduced (Fig. 6E), and CFTR protein levels declined following lysis in Nonidet P-40 (Fig. 6F, G). However, CFTR protein could be recovered after lysis in 10% SDS, suggesting that CFTR remained in a detergent-resistant fraction after 8 h of chronic CS exposure (Fig. 6F, G).
Since CFTR was significantly less soluble after CS exposure and did not colocalize with LAMP1, we investigated whether CS exposure could induce CFTR trafficking to aggresomes. Accordingly, we investigated whether CFTR localized with vimentin, an intermediate filament that plays an active role in aggresome formation (19). Surface-labeled CFTR was internalized with time after air exposure, but it could no longer be detected after 24-h air exposure (Fig. 7). However, post-CS exposure, both CFTR and vimentin were clearly drawn into a perinuclear compartment where they colocalized for up to 24 h, suggesting that CFTR trafficked to aggresome-like compartment (Fig. 7).
Figure 7.
CFTR associates with vimentin after CS exposure. Immunofluorescence analysis of HA-CFTR (red) with time vs. the intermediate filament vimentin (green). Surface HA-CFTR was prelabeled at 4°C, 10 min air or CS exposures were performed at room temperature, and cultures were fixed in MeOH at timed intervals thereafter. Transmitted light images were simultaneously obtained, and the outline of the cells is traced (white) over the CFTR and vimentin images. Images are representative of experiments performed on 3 separate occasions. Scale bars = 10 μm.
Rehydration therapy with HS restores ASL volume in vitro and mucus clearance in vivo. Our data suggest that CS exposure internalizes CFTR, leading to ASL volume depletion and mucus dehydration. In CF, this dehydration can be reversed by the acute addition of HS (24, 33). We, therefore, tested the effects of HS on CS-exposed HBECs. Despite starting from a significantly lower ASL height of ∼4.5 μm, compared to the ∼7.5 μm seen in controls, CS-exposed HBECs responded to HS exposure with an increase in ASL height that was significantly greater than control cultures (Fig. 8A, B), likely reflecting the reduced ability to absorb HS in the absence of CFTR (24).
Figure 8.
HS reverses CS-induced ASL dehydration and restores mucus clearance in patients with CB. A) Confocal micrographs of ASL height (red) after CS or air (control) exposure. B) Mean data taken from A. Control, solid squares; CS, solid triangles; n = 6/group. C) Whole-lung radioparticle retention (indexed as fraction of total deposited radiotracer remaining in the lung with time) as a function of vehicle (solid circles) or 7% HS (solid squares) pretreatment in CB subjects. D) Mean rate of clearance of radiotracer over 30 min for control healthy subjects (open bars; n=20) and CB subjects pretreated with vehicle (baseline; solid bars; n=7) or 7% HS (shaded bars; n=7). *P < 0.05 vs. control or healthy baseline; †P ≤ 0.05 vs. CB baseline.
To test the efficacy of HS in vivo, we measured mucus clearance in patients with CB (Table 2) under basal conditions and after exposure to HS (7%, 5 ml). The clearance of radiotracer particles by patients with CB was slow under basal conditions (Fig. 8C, D) compared to healthy controls (Fig. 8D), as previously reported (34). HS significantly accelerated mucus clearance in CB subjects into the normal range (Fig. 8C, D). Note, this early measurement period was not associated with increased coughing due to the antecedent HS administration (n=7).
Table 2.
Subject demographics for mucus clearance assays
| Parameter | Control | CB |
|---|---|---|
| Sex | 10 M/10 F | 7 M |
| Age | 26.4 ± 5 | 55.4 ± 4.3* |
| FVC (% predicted) | 106 ± 14 | 96 ± 24 |
| FEV1 (% predicted) | 103 ± 12 | 68 ± 16* |
| FEF25–75 (% predicted) | 95 ± 24 | 41.1 ± 16.4* |
Age, sex, and lung function assays are listed as mean ± sd for all subjects used to study mucus clearance.
P < 0.05 vs. control.
DISCUSSION
COPD is defined by persistent obstruction of the airways that occurs phenotypically as CB, emphysema, or a mixed syndrome. CB is defined by chronic cough, not caused by another condition, that produces sputum for ≥3 mo during each of 2 consecutive years. In CB, the mucous glands hypertrophy and goblet cell metaplasia occurs throughout the conducting airways. The airways also become inflamed, and the bronchial walls thicken (35). The vast majority of COPD cases are caused by tobacco usage and how CS exposure causes COPD is not fully understood.
We found that mucus from chronic smokers was ∼10% solids, which is intermediate between normal (≤4%) and CF mucus (>17%) (22). Previous reports have also indicated that CB sputum is dehydrated, as indexed by the increased percentage of solids, and that mucus clearance is reduced (34, 36, 37). Whereas we cannot exclude the possibility that the increase in mucus percentage solids in our study was influenced by age differences between control participants and smokers (see Table 1), this decrease in mucus hydration in CB mirrors the decrease in CFTR activity in the nasal epithelium of chronic smokers (Fig. 1A). Combined, these findings suggest that a lack of CFTR-mediated ASL hydration, in part, contributed to CS-induced mucus dehydration. Interestingly, the CS-induced inhibition of CFTR was not absolute, as typically seen in patients with severe CF, and chronic smokers retained ∼45% of normal CFTR function (Fig. 1A). The residual CFTR function may explain why chronic smoke exposure takes >20 yr to produce lung disease, unlike severe CF, where lung disease is apparent much earlier in life and progresses more rapidly.
To determine whether smoke exposure acutely affected CFTR activity in vivo, a protocol was utilized that delivered a dose of CS to the nasal cavity that was representative of lower lung exposure (25). For this study, we chose ex-smokers who had not smoked for ≥2 yr and who exhibited normal CFTR function, compared to previous reports (38, 39). Using this approach, we observed an acute, large, CS-induced inhibition of the low Cl−/ISO NPD response that was consistent with the inhibition of CFTR Cl− secretion and, consequently, the capacity to hydrate airway surfaces (Fig. 1B, C). Thus, the collective data suggest that CS perturbs CFTR and its hydrating activity both acutely and chronically in the airways in smokers.
Our data provided evidence for a direct and rapid effect of CS on airway epithelial hydration (Fig. 2). HBECs exposed to CS could not adequately hydrate their mucosal surfaces in vitro (Fig. 2B), and this function was not restored by the addition of secretagogues that raise cell cAMP levels and stimulate CFTR function, e.g., ADO or ISO (Fig. 2C, D). These data, coupled with the maintained efficacy of ATP in restoring volume in CS-exposed airway epithelia via activation of Ca2+-activated Cl− channels, suggested that CS has adverse effects on CFTR itself without affecting Ca2+-activated Cl− secretion.
On the basis of the hypothesis that CS impairs CFTR-regulated hydration of secretions, we searched for a mechanism of acute CS-induced CFTR dysregulation/dysfunction. While the open probability of CFTR was unaffected by CS (Fig. 3A), we observed a rapid decrease in the number of CFTR channels in the apical membrane of both airway epithelia and BHKCFTR cells (Fig. 3B–E), leading us to conclude that CS exposure induced a rapid internalization of the CFTR protein. Following CS exposure, a rapid reduction in CFTR protein levels was observed in HBECs (Fig. 4A) while ENaC was unaffected by CS exposure (Fig. 4B). This finding was mirrored in BHKCFTR cells (Fig. 4C, D). This observation was not an artifact induced by CS exposure, such as CS interfering with CFTR-antibody binding, since CFTR was detected equally well after CS exposure at 4°C and following exposure to plasma membrane vesicles containing CFTR (Fig. 4G, H). The apparent reduction in CFTR solubility was not affected by inhibition of CFTR internalization (MG132) or by proteosomal inhibition (ALLN and MG132; Fig. 4E, F), and CFTR did not appear to associate with lysosomes (Fig. 5).
We also observed a significant decrease in detergent solubility of CFTR following CS exposure, with a high concentration of SDS being required for CFTR solubilization in both BHKCFTR cells and CALU3 airway epithelia, (Fig. 6). Interestingly, prelabeled CFTR internalized to a perinuclear compartment post-CS exposure, where it remained for 24 h (Fig. 7). CFTR appeared to closely associate with vimentin, an intermediate filament protein that has previously been shown to play a role in, and be an indicator of, aggresome formation (19).
After acute CS exposure, the inhibition of CFTR waned with time, and new CFTR was identified in the plasma membrane within ∼60 min after CS exposure (Fig. 3E). To investigate the source of this recovered CFTR, we pretreated cells with the protein trafficking inhibitor brefeldin A. This compound prevented the recovery of plasma membrane CFTR after a single CS exposure (Fig. 3). Since CS-internalized CFTR remained in the perinuclear aggresomal compartment for 24 h (Fig. 7), these data suggest that newly synthesized CFTRs are inserted in the plasma membrane, while CS-exposed CFTRs remain in the perinuclear region for extended periods. Note, CFTR has previously been shown to have a much higher turnover rate in BHK cells than in airway epithelia (28), and it took ASL height ∼4 h to return to normal levels after CS exposure from 1 cigarette, suggesting that recovery is slower in airway epithelia (Fig. 2).
In its normal cellular itinerary, CFTR is synthesized on endoplasmic reticulum-associated ribosomes and moves through the secretory pathway to the plasma membrane, where its anion channel function is required. CFTR molecules that fail to traffic from the ER to the Golgi are targeted for degradation via the proteasome (16–18). Cell surface CFTR may be endocytosed and either recycled to the plasma membrane or routed to the lysosome for degradation (40, 41). Complex quality control machineries determine the fates of both the ER and endosomal CFTR pools, and it has recently been found that these machineries share many molecular components, including chaperones, cochaperones, and elements of the ubiquitin/proteasome system (40).
In addition to lysosomal and proteosomal degradation, a third disposal pathway exists for proteins, including CFTR, which can accumulate in aggresomes (42). Such aggregation is thought to occur when normal degradative pathways are overwhelmed by excess protein. However, we speculate that aggregation may also occur when a protein is rapidly shunted to a new compartment and may be driven by the sudden emergence of hydrophobic regions of proteins that would not normally be exposed to the cytosol. On the basis of the decrease in protein solubility and the association with vimentin (Figs. 6 and 7), it was tempting to speculate that CFTR was targeted to aggresomes post-CS exposure. However, important differences exist between the fate of CS-exposed CFTR and CFTR that has entered the classic aggresome described by Kopito and colleagues (discussed in ref. 42). First, aggresomal CFTR is thought to be derived from immature, ER-localized CFTR that cannot be translocated to the Golgi (42). In contrast, we have tracked mature, surface CFTR inward, to a perinuclear compartment (Fig. 7). Second, classic aggresomal CFTR concentrates in a centriolar compartment. Conversely, CFTR post-CS appears to encircle the nucleus and has a distinctly different appearance. Thus, on the basis of these dissimilarities, it is likely that CS-exposed CFTR is not relocated to classic aggresomes.
Misfolded surface CFTR is also subject to a peripheral quality control system (41). It is possible that CS exposure causes surface CFTR to be misfolded and subjected to peripheral quality control, leading to its internalization to an aggresome-like compartment. Alternatively, rapid, CS-induced CFTR internalization may overwhelm normal CFTR trafficking pathways, leading to its aggregation. Importantly, this is the first demonstration that CFTR can traffic from the plasma membrane to a low-solubility, perinuclear compartment without showing significant signs of lysosomal degradation. We do not see a large amount of insoluble CFTR under basal conditions in either BHKCFTR cells or CALU3s, suggesting that CFTR is does not normally exist in this compartment and that CS exposure drives this relocation.
CS-induced airway remodeling is driven, in part, by changes in gene expression (13, 14). In this context, Cantin et al. (5) reported that CS exposure inhibited the transcription of the CFTR gene via oxidative stress mechanisms, which, over a long-term basis, would also be predicted to reduce CFTR protein in the apical membrane. However, these data do not preclude the possibility that cigarette smoke exposure also exerts acute but persistent effects at the protein level (Figs. 3, 4, 6), and these two mechanisms may be additive. Following an 8-h chronic smoke exposure protocol that decreased CFTR gene expression, we observed CS-induced changes in CFTR protein solubility, indicating simultaneous changes in CFTR gene and CFTR cell biological effects following CS exposure (Fig. 6). However, CFTR protein could be fully recovered with 10% SDS, suggesting that altered CFTR trafficking dominated after 8 h of chronic smoke exposure. However, over longer time frames (i.e., days, weeks, and months), it is likely that decreased CFTR gene expression will begin to have a greater effect on CFTR plasma membrane levels. Thus, every new smoke exposure is predicted to send membrane CFTR toward the aggresome-like compartment which, coupled with diminished CFTR gene expression, would serve to lower surface CFTR levels for extended periods in chronic smokers.
With respect to the therapeutic implication of these findings, the effect of HS on ASL height restoration in vitro was significantly greater after CS exposure than after air exposure (Fig. 8A, B). This potentiation of the HS response has previously been observed in CF and likely reflects the absence of Cl− absorption through CFTR down its concentration gradient when ASL NaCl is unphysiologically high. However, both the in vitro and the in vivo effects of HS were shorter in duration and magnitude than observed in CF (22, 24). This difference likely reflects there being essentially no active CFTR in HBECs derived from patients with CF, vs. only 50–75% of CFTR being inhibited by CS exposure (Figs. 1 and 2).
With respect to the in vivo experiments, it is important to point out that the controls were not age matched. Accordingly, some slowing of mucus clearance in vivo in subjects with CB may have been associated with age in addition to the adverse effects of CB (Fig. 8C). However, few previous studies have investigated the effect of aging on MCC. Svartengren et al. (43) observed a significant age-dependent decrease in clearance from both the large and small airways, consistent with two earlier studies that also found evidence of decreased MCC in elderly patients (44, 45). In contrast, a study by Mortensen et al. (46) reported no effect of age on MCC. Thus, the effect of aging on MCC in our CB cohort is not clear. Regardless, our data suggest that rehydration of CB airways with HS may be useful in mobilizing and clearing mucus and that therapeutic maneuvers should be designed to extend the duration of efficacy of hydrating agents so that clinical benefit can be fully realized.
In summary, we have demonstrated that CS rapidly induces the removal of CFTR from the plasma membrane, leading to a reduction in CFTR function, which leads to dehydration of airway surfaces. In keeping with an interaction between CS and CFTR, it has been recently reported that passive CS exposure worsens the outcome for subjects with CF, particularly those with mild mutations that are associated with residual CFTR function (47). Furthermore, these data suggest that therapies designed to restore the hydration of airway surfaces may constitute an important new strategy to reduce the infections and acute exacerbations that are associated with the CS-CB syndrome, as recently observed in CF (24, 33). Thus, HS exposure to directly rehydrate airway surfaces (24, 33) or the use of small-molecule “correctors,” which increase trafficking of CFTR to the plasma membrane (48), may serve as novel therapies for the treatment of CB.
Acknowledgments
The authors thank the UNC CF Center Tissue and Histology Cores for isolating cells from donor lungs and for sectioning cultures, respectively. The assistance of L. A. Aleksandrov, J. E. Rasmussen, and M. J. Watson is gratefully acknowledged.
R.D.C., M.G., and S.K. were funded by the Cystic Fibrosis Foundation. A.A.A., M.G., and J.R. were funded by the U.S. National Institutes of Health (NIH; 5R01DK051870, 5R01DK051619). R.C.B. was funded by NIH HL084934 and HL34332. R.T. was funded by NIH HL084934, a British American Tobacco research grant, and a Novartis Institute biomedical research grant.
REFERENCES
- 1. Mannino D. M., Homa D. M., Akinbami L. J., Ford E. S., Redd S. C. (2002) Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill. Summ. 51, 1–16 [PubMed] [Google Scholar]
- 2. Gerald L. B., Bailey W. C. (2002) Global initiative for chronic obstructive lung disease. J. Cardiopulm. Rehabil. 22, 234–244 [DOI] [PubMed] [Google Scholar]
- 3. Barnes P. J. (2004) Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 56, 515–548 [DOI] [PubMed] [Google Scholar]
- 4. Calverley P. M., Anderson J. A., Celli B., Ferguson G. T., Jenkins C., Jones P. W., Yates J. C., Vestbo J. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 356, 775–789 [DOI] [PubMed] [Google Scholar]
- 5. Cantin A. M., Hanrahan J. W., Bilodeau G., Ellis L., Dupuis A., Liao J., Zielenski J., Durie P. (2006) Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care. Med. 173, 1139–1144 [DOI] [PubMed] [Google Scholar]
- 6. Sethi S. (2000) Bacterial infection and the pathogenesis of COPD. Chest 117, 286S–291S [DOI] [PubMed] [Google Scholar]
- 7. Taylor L., Corey M., Matlow A., Sweezey N. B., Ratjen F. (2006) Comparison of throat swabs and nasopharyngeal suction specimens in non-sputum-producing patients with cystic fibrosis. Pediatr. Pulmonol. 41, 839–843 [DOI] [PubMed] [Google Scholar]
- 8. Chmiel J. F., Konstan M. W. (2007) Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin. Chest Med. 28, 331–346 [DOI] [PubMed] [Google Scholar]
- 9. Hill A. T., Campbell E. J., Hill S. L., Bayley D. L., Stockley R. A. (2000) Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 109, 288–295 [DOI] [PubMed] [Google Scholar]
- 10. Davis P. B. (2006) Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 173, 475–482 [DOI] [PubMed] [Google Scholar]
- 11. Murphy T. F. (2009) Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 15, 138–142 [DOI] [PubMed] [Google Scholar]
- 12. Schmid A., Clunes L. A., Salathe M., Verdugo P., Dietl P., Davis C. W., Tarran R. (2011) Nucleotide-mediated airway clearance. Subcell. Biochem. 55, 95–138 [DOI] [PubMed] [Google Scholar]
- 13. Brody J. S., Steiling K. (2011) Interaction of cigarette exposure and airway epithelial cell gene expression. Annu. Rev. Physiol. 73, 437–456 [DOI] [PubMed] [Google Scholar]
- 14. Zeskind J. E., Lenburg M. E., Spira A. (2008) Translating the COPD transcriptome: insights into pathogenesis and tools for clinical management. Proc. Am. Thorac. Soc. 5, 834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantin A. M., Bilodeau G., Ouellet C., Liao J., Hanrahan J. W. (2006) Oxidant stress suppresses CFTR expression. Am. J. Physiol. Cell Physiol. 290, C262–C270 [DOI] [PubMed] [Google Scholar]
- 16. Benharouga M., Haardt M., Kartner N., Lukacs G. L. (2001) COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J. Cell Biol. 153, 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen T. J., Loo M. A., Pind S., Williams D. B., Goldberg A. L., Riordan J. R. (1995) Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83, 129–135 [DOI] [PubMed] [Google Scholar]
- 18. Ward C. L., Omura S., Kopito R. R. (1995) Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121–127 [DOI] [PubMed] [Google Scholar]
- 19. Johnston J. A., Ward C. L., Kopito R. R. (1998) Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143, 1883–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. French B. A., van Leeuwen F., Riley N. E., Yuan Q. X., Bardag-Gorce F., Gaal K., Lue Y. H., Marceau N., French S. W. (2001) Aggresome formation in liver cells in response to different toxic mechanisms: role of the ubiquitin-proteasome pathway and the frameshift mutant of ubiquitin. Exp. Mol. Pathol. 71, 241–246 [DOI] [PubMed] [Google Scholar]
- 21. Olzmann J. A., Li L., Chin L. S. (2008) Aggresome formation and neurodegenerative diseases: therapeutic implications. Curr. Med. Chem. 15, 47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarran R., Grubb B. R., Parsons D., Picher M., Hirsh A. J., Davis C. W., Boucher R. C. (2001) The CF salt controversy: in vivo observations and therapeutic approaches. Mol. Cell 8, 149–158 [DOI] [PubMed] [Google Scholar]
- 23. Coakley R. D., Sun H., Clunes L. A., Rasmussen J. E., Stackhouse J. R., Okada S. F., Fricks I., Young S. L., Tarran R. (2008) 17β-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J. Clin. Invest. 118, 4025–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donaldson S. H., Bennett W. D., Zeman K. L., Knowles M. R., Tarran R., Boucher R. C. (2006) Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med. 354, 241–250 [DOI] [PubMed] [Google Scholar]
- 25. Clunes L. A., Bridges A., Alexis N., Tarran R. (2008) In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J. Anal. Toxicol. 32, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawyer K., Brown J. S., Hazucha M. J., Bennett W. D. (2007) The effect of exercise on nasal uptake of ozone in healthy human adults. J. Appl. Physiol. 102, 1380–1386 [DOI] [PubMed] [Google Scholar]
- 27. Aleksandrov A. A., Riordan J. R. (1998) Regulation of CFTR ion channel gating by MgATP. FEBS Lett. 431, 97–101 [DOI] [PubMed] [Google Scholar]
- 28. Cholon D. M., O'Neal W. K., Randell S. H., Riordan J. R., Gentzsch M. (2010) Modulation of endocytic trafficking and apical stability of CFTR in primary human airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L304–L314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gentzsch M., Chang X. B., Cui L., Wu Y., Ozols V. V., Choudhury A., Pagano R. E., Riordan J. R. (2004) Endocytic trafficking routes of wild-type and DeltaF508 cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell 15, 2684–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leikauf G. D., Borchers M. T., Prows D. R., Simpson L. G. (2002) Mucin apoprotein expression in COPD. Chest 121, 166S–182S [DOI] [PubMed] [Google Scholar]
- 31. Short D. B., Trotter K. W., Reczek D., Kreda S. M., Bretscher A., Boucher R. C., Stutts M. J., Milgram S. L. (1998) An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273, 19797–19801 [DOI] [PubMed] [Google Scholar]
- 32. Shen B. Q., Finkbeiner W. E., Wine J. J., Mrsny R. J., Widdicombe J. H. (1994) Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 266, L493–L501 [DOI] [PubMed] [Google Scholar]
- 33. Elkins M. R., Robinson M., Rose B. R., Harbour C., Moriarty C. P., Marks G. B., Belousova E. G., Xuan W., Bye P. T. (2006) A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 354, 229–240 [DOI] [PubMed] [Google Scholar]
- 34. Goodman R. M., Yergin B. M., Landa J. F., Golivanux M. H., Sackner M. A. (1978) Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers, and patients with chronic bronchitis. Am. Rev. Respir. Dis. 117, 205–214 [DOI] [PubMed] [Google Scholar]
- 35. Hogg J. C., Timens W. (2009) The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 4, 435–459 [DOI] [PubMed] [Google Scholar]
- 36. Barton A. D., Ryder K., Lourenco R. V., Dralle W., Weiss S. G. (1976) Inflammatory reaction and airway damage in cystic fibrosis. J. Lab. Clin. Med. 88, 423–426 [PubMed] [Google Scholar]
- 37. Rubin B. K., Ramirez O., Zayas J. G., Finegan B., King M. (1992) Respiratory mucus from asymptomatic smokers is better hydrated and more easily cleared by mucociliary action. Am. Rev. Respir. Dis. 145, 545–547 [DOI] [PubMed] [Google Scholar]
- 38. Knowles M., Gatzy J., Boucher R. (1981) Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N. Engl. J. Med. 305, 1489–1495 [DOI] [PubMed] [Google Scholar]
- 39. Rollins B. M., Burn M., Coakley R. D., Chambers L. A., Hirsh A. J., Clunes M. T., Lethem M. I., Donaldson S. H., Tarran R. (2008) A2B adenosine receptors regulate the mucus clearance component of the lung's innate defense system. Am. J. Respir. Cell Mol. Biol. 39, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ameen N., Silvis M., Bradbury N. A. (2007) Endocytic trafficking of CFTR in health and disease. J. Cyst. Fibros. 6, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Okiyoneda T., Barriere H., Bagdany M., Rabeh W. M., Du K., Hohfeld J., Young J. C., Lukacs G. L. (2010) Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329, 805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kopito R. R. (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10, 524–530 [DOI] [PubMed] [Google Scholar]
- 43. Svartengren M., Falk R., Philipson K. (2005) Long-term clearance from small airways decreases with age. Eur. Respir. J. 26, 609–615 [DOI] [PubMed] [Google Scholar]
- 44. Incalzi R. A., Maini C. L., Fuso L., Giordano A., Carbonin P. U., Galli G. (1989) Effects of aging on mucociliary clearance. Compr. Gerontol. A 3(Suppl.), 65–68 [PubMed] [Google Scholar]
- 45. Puchelle E., Zahm J. M., Bertrand A. (1979) Influence of age on bronchial mucociliary transport. Scand. J. Respir. Dis. 60, 307–313 [PubMed] [Google Scholar]
- 46. Mortensen J., Lange P., Nyboe J., Groth S. (1994) Lung mucociliary clearance. Eur. J. Nucl. Med. 21, 953–961 [DOI] [PubMed] [Google Scholar]
- 47. Collaco J. M., Vanscoy L., Bremer L., McDougal K., Blackman S. M., Bowers A., Naughton K., Jennings J., Ellen J., Cutting G. R. (2008) Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA 299, 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A., Zhou J., McCartney J., Arumugam V., Decker C., Yang J., Young C., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U. S. A. 106, 18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]