Abstract
The avian paramyxoviruses (APMVs) belong to the genus Avulavirus of family Paramyxoviridae. The APMVs are classified into nine serotypes on the basis of hemagglutination inhibition (HI) and neuraminidase inhibition (NI) assays, although some serologic cross-reaction exists. Newcastle disease virus (NDV), which constitutes serotype 1 (APMV-1), is an important pathogen of poultry, but the pathogenic potential of the other APMV serotypes is poorly understood. Although antibodies to APMV -2 to -9 are prevalent in chickens, the effect of prior exposure to these serotypes on susceptibility to NDV infection and disease was not known. In the present study, chickens were immunized with APMV-2 to -9 by the oculo-nasal route and later were challenged by the same route with a highly virulent strain of NDV. Among APMV-2 to -9, only APMV-3 induced serum antibodies that cross-reacted significantly with NDV and had significant NDV-neutralizing activity in vitro. In mock-immunized chickens, challenge NDV replicated throughout the respiratory tract as well as in the brain, spleen, and enteric tract. In contrast, in APMV-3-immunized chickens, challenge NDV replication was restricted to the upper respiratory tract and trachea. Some of the other APMVs also induced partial restriction of challenge NDV replication: for example, challenge NDV was not detected in the brains of APMV-9-immunized chickens, and shedding from the respiratory tract was reduced in chickens immunized with APMV-8 and -9. All of the chickens immunized with APMV-3 survived the NDV challenge; with APMV-2, -7, -8, and -9 the percentage survival was 30 %, 20%, 20%, and 52.5%, respectively; whereas none of the chickens immunized with APMV-4, -5, or -6 survived. These results show that prior infection of chickens with APMV-3 induced substantial protection against NDV challenge, whereas prior infection with APMV-2, -7, -8, and -9 can alter subsequent NDV infection. The induction of NDV-neutralizing antibodies was a marker for efficient protection, but partial protection also was observed in their absence.
Introduction
Newcastle disease (ND) is one of the most important diseases of poultry worldwide. It causes large economic loss to the poultry industry in many parts of the world. The causative agent, Newcastle disease virus (NDV), belongs to genus Avulavirus in the family of Paramyxoviridae [1]. The genus Avulavirus consists of the avian paramyxoviruses (APMVs), for which nine serotypes have been identified based on hemagglutination inhibition (HI) and neuraminidase inhibition (NI) assays [2]; more recently, evidence was reported for a potential tenth APMV serotype [3]. APMV-1 consists of all natural occurring strains of NDV and is the only well-characterized serotype due to its economic importance. Complete genome sequences have been determined for a number of NDV strains, and there is extensive information available on NDV molecular biology and pathogenesis. NDV strains have been classified into three pathotypes; lentogenic, mesogenic and velogenic. Lentogenic strains cause subclinical infections and are considered non-virulent. Mesogenic strains are of intermediate virulence causing low to moderate mortality, while velogenic strains are highly virulent causing high mortality [4]. Although NDV infects over 240 species of birds, the disease is most severe in chickens [5].
In contrast, little is known about the other eight APMV serotypes. As an initial step towards their characterization, complete genome sequences of APMV-2 to -9 were determined [6-13]. However, their biological characteristics and pathogenicity remain poorly understood. APMV-2 has been associated with mild respiratory disease and drop in egg production, and infertility in turkeys [14] [15]. APMV-3 has been associated with encephalitis and high mortality in caged birds, respiratory diseases in turkeys and stunted growth in young chickens [16] [17]. APMV-4 strains have been isolated from chickens, ducks and geese [17]. Experimental infection of chickens with APMV-4 resulted in mild interstitial pneumonia and catarrhal tracheitis [16]. APMV-5 strains are responsible for disease in budgerigars (Melopsittacus undulatus), causing depression, dyspnoea, diarrhea, torticollis, and acute fatal enteritis in immature budgerigars, leading to very high mortality [18]. APMV-6 was first isolated from a domestic duck and was found to cause mild respiratory disease and drop in egg production in turkeys, but was avirulent in chickens [16, 19, 20]. APMV-7 was first isolated from a hunter-killed dove and has also been isolated from a natural outbreak of respiratory disease in turkeys. APMV-7 infection in turkeys caused respiratory disease, mild multifocal nodular lymphocytic air sacculitis, and decreased egg production [21]. APMV-8 was isolated from a goose and a feral pintail duck [22]. APMV-9 strains have been isolated from ducks around the world [23]. APMV types -2, -3, and -7 have been associated with mild respiratory disease and egg production problems in domestic chickens [21]. There are no reports of isolation of APMV-5, -8 and -9 from poultry [20]. However, recent serosurveillance of commercial poultry farms in USA indicated the possible prevalence of all of the APMV serotypes except APMV-5 in chickens [24].
Chickens worldwide are routinely vaccinated against NDV using naturally occurring lentogenic and mesogenic strains as live vaccines. In general, lentogenic strains are used widely for initial vaccination and mesogenic strains are used for revaccination in countries where virulent NDV strains are prevalent. Recently, with the advent of NDV reverse genetics systems, recombinant NDV has been evaluated as a vaccine vector for other avian pathogens [25-27]. However, the efficacy of live NDV vaccines or NDV vectored vaccines potentially can be severely compromised by preexisting NDV antibodies or heterologous antibodies that can cross-react with NDV. Although the established APMV serotypes are quite distinct based both on HI and NI tests as well as deduced nucleotide and amino acid sequences, low-level cross reactions between serotypes have been observed by HI and NI tests (Discussion) [28]. Whether prior immunity to APMV-2 to -9 can restrict NDV replication and disease in chickens is not well studied. A single report published more than 30 years ago indicated that prior infection of chickens with APMV-2, -3, or -4 yielded varying levels of protection against virulent NDV challenge by the non-natural intramuscular route [29]. Therefore, the objective of the present study was to determine the degree of protection of chickens to NDV challenge induced by prior infection with APMV-2 to -9, with both immunization and challenge performed by a natural route.
Materials and Methods
Viruses and cells
Chicken embryo fibroblast (DF-1) and African green monkey kidney (Vero) cell lines, obtained from the American Type Culture Collection (ATCC, Manassas, VA), were grown in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum. The prototype strains of all nine APMV serotypes used for immunization in this study were: APMV-1 strain LaSota/46, APMV-2 strain chicken/Yucaipa/California/56, APMV-3 strain parakeet/Netherlands/449/75, APMV-4 strain duck/Hong Kong/D3/75, APMV-5 strain budgerigar/Kunitachi/74, APMV-6 strain duck/Hong Kong/18/199/77, APMV-7 strain dove/Tennessee/4/75, APMV-8 strain goose/Delaware/1053/76, and APMV-9 strain duck/New York/22/78. The NDV challenge virus was the velogenic strain Texas-GB. All of the strains except for serotype 5 were obtained from National Veterinary Services Laboratory, Ames, Iowa. APMV-5 strain budgerigar/Kunitachi/74 was kindly provided by Dr. Ian Brown, Veterinary Laboratories Agency, Weybridge, Surrey, UK. All of the APMV serotypes except serotype 5 were grown in the allantoic cavity of 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs. APMV serotype 5 was grown in Vero cells.
Virus titration
All APMVs used for immunization were titrated by hemagglutination (HA) assay with 0.5% chicken RBC [30] except for APMV-5. In the case of APMV-5, the titer was determined by plaque assay on Vero cells. The APMV-5 samples were inoculated in triplicates onto 24-well plates of Vero cells at 80% confluency, incubated for 1 h, washed with PBS, overlaid with 0.8% methylcellulose, and observed for plaque production until 7 days post inoculation (dpi). The cells were fixed with methanol and stained with 1% crystal violet [9]. The value for each sample was based on the average plaque count from three wells. In the case of NDV Texas-GB, used as the challenge virus, the 50%-chicken-lethal-dose (CLD50) was determined by infecting 5-week-old chickens in groups of 3 with an 10-fold dilution series, and the CLD50 was determined by the method of Reed and Muench [31]. Challenge NDV titers in tissue homogenates were determined by a limiting end point dilution in DF-1 cell monolayers. The presence of the virus in individual wells was confirmed by HA assay. Virus titers were calculated by the method of Reed and Muench [31] and expressed as 50% tissue culture infectious dose (TCID50) units.
Immunization of chickens with the various APMV serotypes
Two-week-old SPF chickens (Charles River Laboratories International, Inc., Wilmington, MA, USA) in groups of 8 were immunized with each of the nine APMV serotypes, while one additional group remained as uninfected control. For APMV-1, immunization was with lentogenic NDV strain LaSota. Each chicken was immunized by the oculo-nasal route (50 μl in each eye and nostril), mimicking natural infection with a single dose of 200 μl of freshly harvested APMV-infected allantoic fluid containing 26 HAU of each APMV, except for APMV-5, which contained 104 PFU/ml. Serum samples were collected on day 21 post-immunization for analysis of antibody responses. All APMV immunizations in chickens were carried out in BSL 2 plus containment facility.
Challenge of APMV immunized chickens with virulent NDV strain Texas-GB
Three-weeks post immunization; chickens were transferred to a USDA-certified enhanced BSL3 containment facility for NDV lethal challenge. The challenge experiments were carried out with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland and were compliant with Animal Welfare Association (AWA) regulations. Each of the birds in each group was challenged with 200 μl (200 CLD50) of virulent NDV strain Texas-GB through the oculo-nasal route using the same procedure as for the immunizations described above. Oral and cloacal swabs were collected on day 4 post-infection to monitor shedding of the challenge virus. Three chickens from each group were sacrificed on day 4 post-challenge to evaluate challenge virus replication in different organs. Tissue samples were collected from the respiratory tract, including the nasal turbinates (upper respiratory tract), trachea, and lungs (lower respiratory tract), as well as from the lymphoid system (spleen) and nervous system (brain). The tissue samples were homogenized in cell culture medium (1 g/10 ml) and clarified by centrifugation. The challenge virus titers in homogenized tissue samples or clarified swab samples were determined by a limiting end point dilution assay as noted above.
The remaining five chickens in each group were observed daily for 14 days for disease symptoms and mortality following challenge. Resistance of APMV-1 to -9 immunized chickens against virulent NDV Texas-GB challenge was assessed by protection against both clinical disease and death. Clinical disease was scored using a weighted point system based on neurologic disease signs, as follows: 0 points, healthy chickens; 1 point, uncoordinated movement and twitching with no prostration; 2 points, paralysis with prostration; 3 points, death. The final score per group was calculated by a ratio of the cumulative daily scores to the cumulative number of daily surviving chickens. In addition, percentage survival was calculated daily. Post-challenge sera were collected from surviving chickens 14 days post-challenge.
Serological assays
Sero-conversion of chickens to the initial APMV immunization was evaluated by hemagglutination inhibition (HI) assay using the homologous virus as antigen, except in the case of APMV-5. The antibody titers in APMV-5 infected chickens were assessed by plaque reduction assay using Vero cells. Cross-reactivity of APMV-immunized chicken sera with NDV was measured by three assays: (i) enzyme linked immunoassay (ELISA), using a commercially available kit based on whole NDV antigen (Synbiotics Corporation, San Diego, CA), (ii) HI assay, using NDV Texas-GB as antigen, and (iii) neutralization assay using NDV Texas-GB in DF-1 cells. Comparison of pre- and post-challenge antibody responses in survivor chickens was performed by HI assay.
Statistical analysis
The serological antibody titer data from different chicken groups were evaluated statistically by one-way analysis of variance (ANOVA) for significant differences in variation. Statistical analysis for mean, standard deviation of data, one-way ANOVA and survival plot were analyzed by using computer software Prism 5.0 (GraphPad Software Inc., San Diego, CA).
The survival curves of APMV immunized chicken groups to NDV challenge were generated by Prism Graphpad software. The plotted survival curves represent survival as a function of time, using the method of Kaplan and Meier [32]. This method uses censored data that indicate a certain length of survival time during the protocol. Multiple comparisons of survival curves for statistical significance were carried out by using the logrank test, which compares the cumulative probability of survival at any specific time with the assumption that proportion of deaths per time, is same at all time points.
Results
Evaluation of APMV serotype-specific serum antibody responses following immunization of chickens with the various APMV serotypes
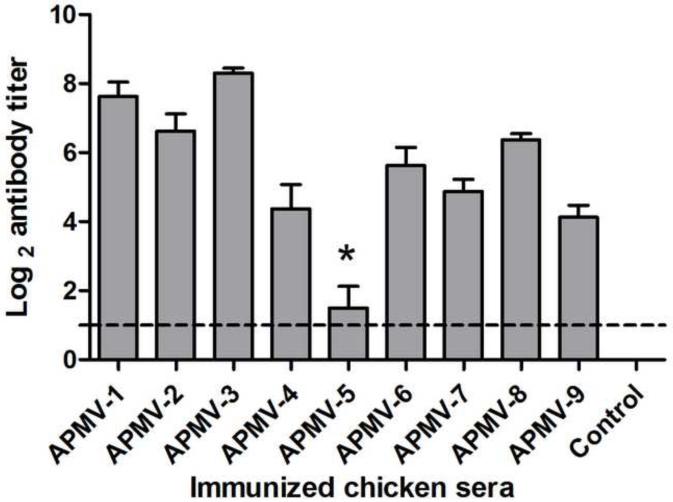
To evaluate the immunogenicity of the APMV serotypes, groups of chickens were inoculated oculo-nasally with each APMV serotype, mimicking natural infection, or were mock-infected. APMV-1 was represented by the lentogenic LaSota strain. Each chicken was inoculated with 200 μl (26 HA unit) of APMV-2 to-9 except APMV-5. Since APMV-5 does not have HA activity, 200 μl of APMV-5 (104 pfu/ml) was used for inoculation. AMPV-5 did not grow well in chicken eggs, so higher titered virus stocks were not available. We have also observed the clinical signs of chickens inoculated with APMV-2 to-9. But none of the chickens in any of the groups exhibited clinical signs. Based on the scoring system that was used for NDV, the clinical score of chicken inoculated with APMV-1 to -9 were zero (0.00). Serum antibody responses to the infecting viruses were determined 21 days post-immunization by serotype-specific HI assay using chicken erythrocytes except in the case of APMV-5, for which antibody responses were assayed by plaque reduction neutralization assay (Figure 1). Each of the APMV serotypes, with the exception of APMV-5, induced a substantial serum antibody response. However, the magnitude of the response varied, which may reflect differences in the natural susceptibility of chickens to replication of the various APMV serotypes. The highest mean HI antibody titers (Log2) were observed in chickens with APMV-3 (8.3 ± 0.48) and APMV-1 (7.62 ± 1.18), moderate mean HI antibody titers were observed with APMV-2 (6.62 ± 1.4), APMV-8 (6.37 ± 0.51) and APMV-6 (5.62 ± 1.5), and the lowest mean HI antibody titers were observed with APMV-7 (4.87 ± 0.99), APMV-4 (4.37 ± 1.99) and APMV-9 (4.12 ± 0.99). In the case of APMV-5, only four of the eight birds seroconverted, of which two had mean antibody titers of log2 4 and the other two had mean antibody titers of log2 2. This suggests that chickens are either less susceptible to APMV-5 infection or a lower dose compared other APMVs was used for inoculation. All of the birds in the other groups seroconverted.
Figure 1. Homologous serum antibody responses in chickens inoculated with APMV serotypes 1-9.
Chickens in groups of 8 were inoculated via the oculo-nasal route with prototype strains of APMV serotypes 1-9, using the lentogenic LaSota strain in the case of APMV-1. A negative control group was mock-inoculated. Sero-conversion to the infecting APMV serotype was evaluated by HI assay except in the case of APMV-5 (*), which was evaluated by 50% plaque reduction assay. Antibody titers are expressed as mean reciprocal log2 titer with SEM (standard error of the mean) indicated, and titers above the dotted line were considered positive. Statistical differences were calculated by one-way ANOVA based on non-parametric test with a P value < 0.0001.
Cross-reaction of APMV serotype-specific chicken antisera with NDV
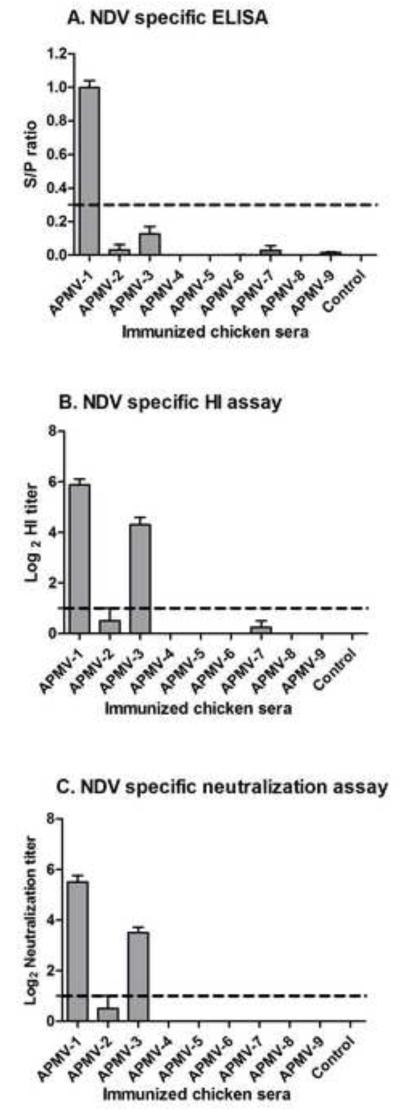
The antigenic cross-reactivity of each APMV with NDV was evaluated by analyzing serum collected 21 days post-immunization by an NDV-specific ELISA using whole NDV antigen (Figure 2A), and by HI assay (log2) against NDV challenge virus strain Texas-GB (Figure 2B). Using these two tests, antisera raised against NDV strain LaSota exhibited high ELISA and HI titers (ELISA-0.99 ± 0.11, HI-5.87 ± 0.64) against NDV strain Texas-GB, which was expected for viruses from the same serotype. Among antisera raised against APMV -2 to -9, the highest cross-reaction against NDV was found with antisera to APMV-3 (ELISA-0.13 ± 0.11, HI-4.3 ± 0.94), which cross-reacted relatively weakly in ELISA against whole NDV but exhibited a substantial level of cross-reactivity by the HI assay. Very low cross-reactivity against NDV was observed by either test with antisera to APMV-2 (ELISA-0.03 ± 0.08, HI-0.5 ± 1.4) and APMV-7 (ELISA-0.02 ± 0.07, HI-0.25 ± 0.70). APMV-9 antisera also exhibited very low cross-reactivity (0.01± 0.01) with NDV by ELISA but not by HI assay. Antisera raised against APMV-4, -5, -6, or -8 did not exhibit detectable cross-reactivity against NDV by these two tests.
Figure 2. Cross-reaction with NDV by antisera raised against the other APMV serotypes.
Cross-reaction with NDV by sera raised against the other APMV serotypes, from the experiment in Figure 1, was determined by NDV-specific ELISA (A), HI (B), and neutralization (C) assays. Antibody titers are expressed as means with SEM (standard error of the mean) indicated, and titers above the dotted lines were considered positive. The NDV ELISA (A) was a commercial kit involving whole virus antigen, and antibody titers are expressed as a S/P (sample/positive) ratio. The NDV-specific HI (B) and neutralization assays (C) were determined using the NDV strain Texas-GB, and titers are expressed as reciprocal log2. Statistical differences by one-way ANOVA non-parametric test had shown a significant P value of P< 0.0001 for these three tests.
Neutralization of NDV by APMV serotype-specific chicken antisera
The ability of the day-21 antisera raised against the various APMV serotypes to neutralize APMV-1 was assessed by a micro-neutralization assay against NDV strain Texas-GB (Figure 2C). The antisera from birds immunized with NDV strain LaSota had the highest neutralizing antibody titers to NDV strain Texas GB, log2 5.5 ± 0.75, as would be expected for two viruses from the same serotype. The next-highest titers were observed with antisera from chickens immunized with APMV-3, log2 3.5 ± 0.7, indicating a close antigenic relationship within APMV-1. Antiserum from only one out of eight chickens immunized with APMV-2 had NDV-neutralizing activity with a titer of log2 4. The antisera from chickens infected with APMV-4, -5, -6, -7, -8, or -9 did not have any detectable NDV-neutralizing activity.
NDV replication and shedding following NDV challenge of chickens previously immunized with APMV-1-9
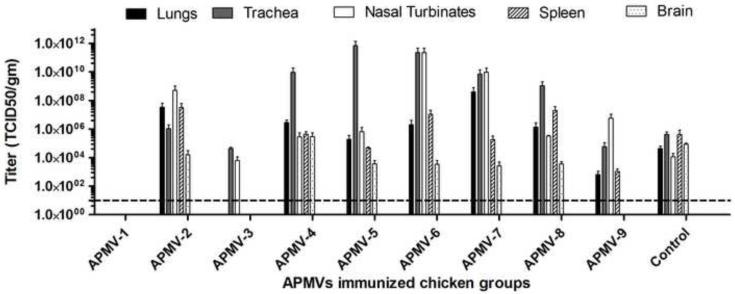
The groups of chickens that had been inoculated with APMV-1-9 or mock-inoculated in the experiment described above were challenged 21 days post-inoculation with an otherwise highly lethal dose (200 CLD50) of virulent NDV strain Texas-GB by the oculo-nasal route. Three birds from each group were sacrificed on day 4 post-challenge and tissue samples were collected from the respiratory tract (nasal turbinates, trachea, and lungs), lymphoid system (spleen), and nervous system (brain) of each chicken, and the NDV challenge virus titers were determined by limiting dilution assay of tissue homogenates (Figure 3).
Figure 3. Replication of NDV challenge virus in various organs of chickens previously immunized with the other APMV serotypes.
The groups of chickens that had been immunized with the various APMV serotypes, or mock-immunized, in the experiment shown in Figure 1 were challenged three weeks post-immunization with 200 50%-chicken-lethal-dose (CLD50) units of virulent NDV strain Texas-GB by the oculo-nasal route. Three chickens from each group were sacrificed on day 4 post-challenge, and the indicated organs were harvested and the titers of NDV challenge virus were determined by limiting dilution assay on DF-1 cells. Titers are expressed in log10 TCID50 per gram of tissue with SEM indicated. Titers above the dotted line (10 TCID50/ml) were considered positive.
There was some variability in the titers of NDV challenge virus in the chickens prior inoculated with APMV -2 to -9. It was clear that no NDV challenge virus was detected in chickens that had previously been immunized with NDV strain LaSota, demonstrating complete cross-protection against challenge virus replication within APMV-1. No NDV challenge virus was detected in the lungs, spleen, and brains of chickens previously immunized with APMV-3, indicating substantial cross-protection that limited challenge virus replication to the nasal turbinates (upper respiratory tract) and trachea. Similarly, with APMV-9, no challenge virus was detected in the brain, and the titers in the lungs and spleen were reduced, suggesting that spread of the virus from the upper respiratory tract was partly restricted. NDV challenge virus was detected in all of the sampled organs of chickens that had previously been inoculated with the other APMV serotypes, namely APMV-2, -4, -5, -6, -7, or -8, although in a number of cases the titers in the brains were reduced, suggesting some restriction of spread. Interestingly, it was observed that the titers of NDV challenge virus in the trachea of the APMV-4, -5, -6, -7, and -8 groups were substantially higher than in the mock-immunized group, and the same was true for the nasal turbinates of the APMV-6 and -7 groups. This could be either due to lack of cross-reacting local immune response or any possible antibody enhancement of viral replication.
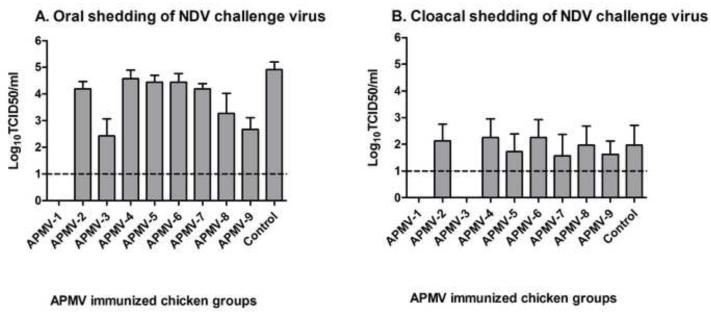
Shedding of NDV challenge virus in this same experiment was assessed by collecting oral and cloacal swab samples on day 4 post-challenge from all eight chickens in each group. The magnitude of shedding was determined by virus titration of the swab samples on DF-1 cells by limiting dilution (Figure 4). The oral shedding virus titers (Figure 4A) demonstrated complete restriction of shedding in the case of chickens previously immunized with APMV-1 and substantial restriction with APMV-3 and APMV-9. Cloacal shedding of NDV challenge virus (Figure 4B) was substantially lower compared oral shedding. No cloacal shedding was observed for birds previously immunized with APMV-1, consistent with the complete restriction of challenge virus replication noted above. Also, no cloacal shedding was observed in any of the chickens that had been previously immunized with APMV-3, indicating that, although there was shedding from the respiratory tract, there was no spread of challenge virus to the gastrointestinal tract. There was no significant reduction in cloacal shedding in any of the other APMV-immunized groups.
Figure 4. Shedding of NDV challenge virus in chickens previously immunized with the other APMV serotypes.
From the challenge experiment in Figure 3, oral (A) and cloacal (B) swabs were collected from the 8 birds in each group on day 4 post challenge and virus titers were determined by a limiting dilution assay on DF-1 cells. Titers are expressed as means with the SEM indicated, and titers above the dotted line (1.0 log10) were considered positive. Statistical differences by one-way ANOVA nonparametric test had shown a significant P value (P< 0.0001).
Protective immunity against virulent NDV challenge in chickens immunized with APMV1-9
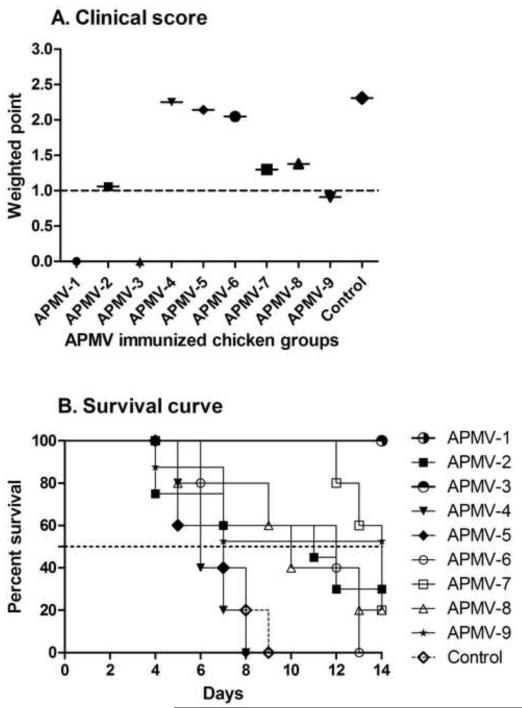
From the experiment described above, the remaining chickens (5 chickens per group) that had been immunized with the various APMV serotypes and challenged with the virulent NDV strain Texas-GB were observed daily for clinical signs and mortality out to 14 days post-challenge. Clinical signs were scored daily for signs of neurologic disease (scores of 0-3; the scoring system is described in the Materials and Methods and the legend to Figure 5A), and a cumulative weighted score was calculated each group (Figure 5A). Scores of ≥1 were indicative of disease. Chickens that had been previously immunized with APMV-1or APMV-3 had scores of 0, indicative of a high degree of protection against disease, whereas the APMV-2 and APMV-9 groups had scores of approximately 1.0, indicative of clinical disease. The severity of disease was somewhat higher in chickens that had been immunized with APMV-7 and -8, and was even higher in chickens that had been immunized with APMV-4, -5 and -6 (Figure 5A).
Figure 5. Protective immunity against virulent NDV challenge in chickens previously immunized with the other APMV serotypes.
The five remaining chickens in each of the APMV-immunized, NDV-challenged groups from Figures 3 and 4 were observed daily from days 4 to 14 post-challenge and were assigned daily clinical scores of 0 for healthy chickens, 1 point for uncoordinated movement and twitching with no prostration, 2 points for paralysis with prostration, and 3 points for death. Weighted clinical scores were calculated by the ratio of the cumulative clinical scores to the cumulative number of chickens (A). A clinical score ≥1 (dotted line) was considered to indicate clinical disease. (B) Percent survival of APMV-immunized chickens following NDV challenge. Statistical differences between the groups were determined by the log-rank test with a P value of< 0.0001, which compare the group with highest survival (APMV-1, or -3). The dotted line represents median survival of APMV immunized groups following NDV challenge. This indicates the time at which half the animals are dead.
The percentage of chickens remaining alive on each day post-challenge is summarized in Figure 5B. No deaths occurred among the chickens previously immunized with APMV-1 and -3. In contrast, chickens that had been mock-immunized or immunized with APMV-4 or -5 all died, and with similar kinetics. All of the chickens in the APMV-6 group also died, but did so less rapidly. Some of the chickens survived in each of the other groups: AMPV-7 (1/5), -8 (1/5), -2 (2/5), and -9 (3/5). The percent survival rate was calculated by Kaplan-Meier method [32] using censored survival data. The percent survival was measured 20% for APMV-7 and -8, 30% for APMV-2 and 52.5% for APMV-9. The median survival is the time at which half of the animals have died. The median survival to NDV challenge in APMV-2, -4, -5, -6, -7 and -8 immunized birds were predicted to 11, 6, 7, 12, 14, and 10 days, respectively and not predicted for APMV-1, -3 and -9 where survival was more than fifty percent.
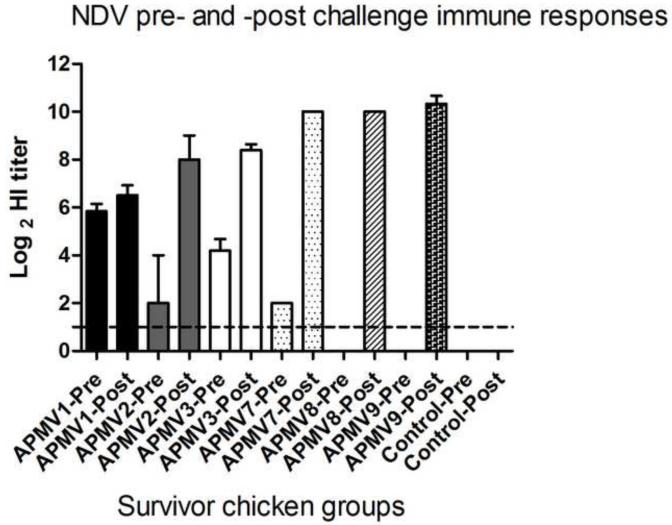
Comparison of pre- and post-challenge NDV-specific serum antibody responses in survivor chickens
Sera were collected from the surviving chickens 14 days post challenge (day 35 after the initial immunization). Antibody titers to NDV in pre-challenge (day 21) and post-challenge (day 35) serum samples were measured by HI assay using NDV strain Texas-GB. There was little or no change in NDV-specific HI antibody titers following challenge in chickens that had been immunized with APMV-1 (Figure 6), consistent with complete restriction of challenge NDV replication. The APMV-3-immunized group, in which all of the chickens survived the lethal challenge, had an increase in HI titer of >4.0 log2 following challenge, consistent with replication of challenge NDV. The APMV-2 group, in which 2 of 5 chickens survived had an increase of 6 log2, compared to pre-challenge sera. The lone survivor chicken of APMV-7 group had an increase in HI titer of 8 log2. The sole survivor in the APMV-8 group had no detectable NDV-specific titer pre-challenge and had a post-challenge titer of 10 log2. In the APMV-9 group, 3 out of 5 chickens survived challenge and one was paralyzed: the HI titers in these birds increased 10 log2 following challenge. The variation in the magnitude of the post-challenge increase in NDV-specific HI antibody titer may reflect differences in the level of challenge virus replication in the various APMV-immunized groups.
Figure 6. Comparison of pre-challenge (day 21) and post-challenge (day 35) NDV-specific serum antibody responses in chickens that had been previously immunized with the other APMV serotypes.
The NDV-specific serum antibody titers were measured by HI assay from birds surviving NDV challenge. The antibody titers were expressed as the mean reciprocal log2 titer with SEM indicated. Titers above 1.0 log2 were considered positive. Statistical differences by one-way ANOVA nonparametric test had shown a significant P value (P< 0.0001).
Discussion
The APMVs of genus Avulavirus are separated into nine subtypes (APMV-1 to -9) based on HI and NI assays [20], with a preliminary report of a possible tenth serotype [3]. Although the established serotypes are quite distinct, some cross-reactivity between serotypes have been reported by HI tests [28]. Among the APMV serotypes, virulent strains of APMV-1 (NDV) cause severe disease in chickens. Virulent NDV is widely prevalent in the chicken populations of Asia, Africa and South America. APMV-2, -3, and -7 also have been reported in chickens and turkeys in association with respiratory disease or decrease in egg production [17], and APMV-4, -6, and -7 also have been reported in chickens or turkeys. There are no reports of isolation of APMV-5, -8, and -9 from chickens [20], but recent sero-surveillance of commercial poultry farms in USA indicated the possible presence of all APMV serotypes except APMV-5 in chickens [24]. All the APMV inoculated chickens survived and did not show any apparent clinical sign. Therefore these viruses may not have any economic impact on poultry production. The effect of prior infection of chickens with APMV-2 to -9 on subsequent infection and disease by NDV was largely unknown. A single report from 30 years ago indicated that prior immunization of chickens with APMV-2 induced protection against intramuscular challenge with virulent NDV, while APMV-3 and APMV-4 induced, respectively, little and no protection [29]. Therefore, the present study was undertaken to evaluate the resistance of chickens to NDV infection induced by prior infection with each of the established APMV serotypes, using a natural route of infection for both immunization and challenge.
In the present study, infection of two-week-old chickens by the oculo-nasal route with prototype strains of the various APMV serotypes resulted in substantial serum HI antibody responses against the infecting virus in every case except APMV-5, for which only half of the chickens seroconverted, and only with low antibody responses. Thus, with the exception of APMV-5, all of the APMV serotypes replicated in the chickens. However, no clinical signs were observed in any chickens. This suggests that APMV-2 to -9 cause mild or inapparent infections in chickens, which is consistent with previous results measuring minimum death time test in embryonated chicken eggs [6-8, 10-13, 33]. To date, APMV-5 has never been isolated from a species of bird other than the Neophema species of budgerigar, in which it causes high mortality. These results indicate that this virus probably is strongly host-restricted and that chickens are a poorly susceptible host. The next-lowest homologous serum antibody responses were observed with APMV-4 and -9, suggesting that chickens might have reduced susceptibility to infection with these APMV serotypes.
Previously, on the basis of HI and NI assays, a complex pattern of asymmetric and symmetric serological cross-reactions were observed that divided the APMVs into two subgroups, one containing NDV (APMV-1) and APMV-3, -4, -7, -8, -9, and the other containing APMV-2 and -6 [28]. Among these viruses, NDV was most closely related to APMV-3 and -9. APMV-5 was not included in that study [28]. The present study showed that NDV has high cross-reactivity with APMV-3; very low cross-reactivity with APMV-2, and -7; and no detectable cross-reactivity with APMV-4, -5, -6, -8, and -9. This difference in results likely reflects experimental differences: the previous study included antisera that had been raised by multiple injections in various animal species by various investigators, and relied on tests (HI and NI) that inherently are based mainly on the HN protein. In the present study, we used whole-virus ELISA, HI, and neutralization assays, and used sera that had been raised following a single oculo-nasal infection that mimics natural infection. Traditionally, serologic groups for viruses in general are defined with convalescent antisera, as was used in the present study. One limitation in the present study was that cross-reactivity involving APMV-5 remains uncertain given its poor infectivity and immunogenicity.
Assessment of the clinical score and percentage survival post-challenge showed that prior immunization with APMV-3 resulted in no disease and 100% survival. In comparison, disease and survival of chickens immunized with APMV-2, -7, -8, and -9 were intermediate, whereas the chickens immunized with APMV-4, -5, and -6 exhibited a high level of disease and no survival. Consistent with this, replication of challenge NDV in the APMV-3-immunized animals was restricted to the upper respiratory tract and trachea, and the virus did not spread to the lungs, spleen, brain, and enteric tract. In addition, shedding of challenge NDV from the respiratory tract was reduced for chickens immunized with APMV-3, -8, and -9, and spread to the brain was prevented with APMV-9. Taken together, these results indicated that prior infection with APMV-3 was highly protective against NDV challenge, that APMV-2, -3, -7, -8, and -9 conferred some protection, and that APMV-4, -5, and -6 conferred little or no protection.
Alexander et al [29] previously investigated cross-protection of APMV-2, -3, and -4 against NDV, and observed little survival associated with prior immunization with APMV-3, and no survival with APMV, -2 and -4. The present results differ in that we observed partial survival with APMV-2 and complete survival with APMV-3; the two studies agree on the lack of survival associated with immunization with APMV-4. There were a number of other differences between the two studies. Alexander et al [29] immunized the chickens by either one or two combined intranasal and intramuscular infections, whereas in the present study the chickens were immunized by a single oculo-nasal infection, which more closely mimics natural infection. In the former study, the chickens were challenged with virulent NDV by intramuscular injection, which is an unnatural route, whereas in the present study the challenge was by the oculo-nasal route. Also, Alexander et al [29] measured only serology and mortality, whereas the present study measured in addition challenge virus replication in a number of organs, shedding from the respiratory and enteric tracts, and clinical signs. As noted, the present study detected NDV-neutralizing activity only in sera from chickens immunized with APMV-3 (and the NDV LaSota positive control), which correlated with complete survival, whereas the previous study did not measure neutralizing antibodies. In addition, the present study extended analysis to the other APMV serotypes.
While the presence of NDV-neutralizing serum antibody was a marker for efficient protection against NDV replication and complete protection against NDV disease and death, we also observed reductions in clinical signs, mortality, and sometimes replication in the absence of a significant NDV-neutralizing response. This suggests that other factors, such as cell-mediated immunity, also contributed to protection. This would be consistent with the previous observation that human CD8+ cytotoxic T cells enriched for reactivity to human parainfluenza virus serotype 1 also exhibited reactivity with human parainfluenza virus serotype 3 [34]. We did not investigate the durability of the protective response in the present study. However, previous work in other animal models suggests that protection that is associated with neutralizing antibodies is durable, whereas protection due to cellular immunity can wane within a period of several months [35, 36]. Evaluation of this in chickens would be of interest since, unlike the previous studies; this would involve a permissive host.
Somewhat unexpectedly, the extent of sequence relatedness between NDV and a different APMV serotype did not predict the level of cross-protection. For example, NDV is most closely related to APMV-9 by genome nucleotide sequence (60%) as well as the amino acid sequences of the protective and neutralization antigens F (54.4%) and HN (62%) [11, 13, 33], but APMV-9 provided only a moderate level of protection against challenge NDV replication as well as disease and death. As compare to APMV-9, percent identity of F and HN proteins between APMV-3 and APMV-1 is 30.8% and 32.6% respectively, but level of protection by APMV-3 is more than APMV-9. In another example, APMV-3 and -4 are closely related phylogenetically to each other among the APMVs and yet differed widely in protection against NDV: APMV-3 provided a high level of resistance to NDV replication, disease, and death, whereas APMV-4 provided essentially no resistance. This suggests that protection depended on specific B-cell and T-cell epitopes whose conservation cannot be predicted from the extent of overall sequence identity.
In summary, we have evaluated the effect of prior infection of chickens with APMV-2 to -9 on the replication and pathogenicity of virulent NDV challenge. The present results using convalescent antisera indicated that most of serotypes 2-9 have little or no serologic cross-reactivity with APMV-1. There was significant cross-reactivity with APMV-1 only in the case of APMV-3, and only by HI and neutralizing assays. There also was trace reactivity in one or more assays with APMV-1 versus APMV-2, -7, and -9, but this was well below the test thresholds. Nonetheless, prior infection with a number of APMV serotypes afforded some protection against NDV challenge, at least in the short term. Furthermore, the extent of protection could not be predicted from published cross-reactivity patterns using hyperimmune sera [28] or by amino acid sequence relatedness. These results will have practical implications on NDV live vaccination programs, for example pre-existing antibody to other APMVs can affect NDV vaccination.
Highlights.
We have evaluated cross-protection of APMV-2 to-9 immunized chickens against NDV
APMV-3 induced cross reacting antibodies and provided significant protection to NDV
APMV-2,-9 prior infection reduced mortality and clinical signs of NDV
APMV-4 to -8 prior infection did not provide significant protection to NDV
These results will have practical implications on NDV and APMV vaccination strategies
Acknowledgements
We thank Daniel Rockemann for his excellent technical assistance and Lashae Green for proofreading the manuscript. “This research was supported by funding from NIAID contract no. N01A060009 (85% support) and NIAID-NIH Intramural Research Program (15% support). The views expressed herein do not necessarily reflect the official policies of the Department of Health and Human Services; nor does the mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Lamb RA, Parks GD. Paramyxoviridae: The Viruses and Their Replication. In: Knipe PMH DM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5th edition Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 1449–96. [Google Scholar]
- [2].Alexander DJ. Newcastle disease and other avian paramyxoviruses. Rev Sci Tech. 2000 Aug;19(2):443–62. doi: 10.20506/rst.19.2.1231. [DOI] [PubMed] [Google Scholar]
- [3].Miller PJ, Afonso CL, Spackman E, Scott MA, Pedersen JC, Senne DA, et al. Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J Virol. 2010 Nov;84(21):11496–504. doi: 10.1128/JVI.00822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beard CW, Hanson RP, Hofstad MS, Barnes HJ, Calnek BW, Reid WM, Yoder HW, editors. Diseases of Poultry. 8th Edition Iowa State University Press; Ames: 1984. Newcastle disease; pp. 452–70. [Google Scholar]
- [5].Kaleta EF, Baldauf C. Newcastle disease in free-living and pet birds. In: Alexander DJ, editor. Newcastle disease. Kluwer Academic Publishers; Boston: 1988. pp. 197–246. [Google Scholar]
- [6].Subbiah M, Xiao S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 2 (strain Yucaipa) and comparison with other paramyxoviruses. Virus Res. 2008 Oct;137(1):40–8. doi: 10.1016/j.virusres.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kumar S, Nayak B, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 3 reveals an unusually long trailer region. Virus Res. 2008 Nov;137(2):189–97. doi: 10.1016/j.virusres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nayak B, Kumar S, Collins PL, Samal SK. Molecular characterization and complete genome sequence of avian paramyxovirus type 4 prototype strain duck/Hong Kong/D3/75. Virol J. 2008;5:124. doi: 10.1186/1743-422X-5-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Samuel AS, Paldurai A, Kumar S, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus (APMV) serotype 5 completes the analysis of nine APMV serotypes and reveals the longest APMV genome. PLoS One. 5(2):e9269. doi: 10.1371/journal.pone.0009269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiao S, Subbiah M, Kumar S, De Nardi R, Terregino C, Collins PL, et al. Complete genome sequences of avian paramyxovirus serotype 6 prototype strain Hong Kong and a recent novel strain from Italy: evidence for the existence of subgroups within the serotype. Virus Res. 2010 Jun;150(1-2):61–72. doi: 10.1016/j.virusres.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xiao S, Paldurai A, Nayak B, Subbiah M, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 7 (strain Tennessee) and comparison with other paramyxoviruses. Virus Res. 2009 Oct;145(1):80–91. doi: 10.1016/j.virusres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paldurai A, Subbiah M, Kumar S, Collins PL, Samal SK. Complete genome sequences of avian paramyxovirus type 8 strains goose/Delaware/1053/76 and pintail/Wakuya/20/78. Virus Res. 2009 Jun;142(1-2):144–53. doi: 10.1016/j.virusres.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Samuel AS, Kumar S, Madhuri S, Collins PL, Samal SK. Complete sequence of the genome of avian paramyxovirus type 9 and comparison with other paramyxoviruses. Virus Res. 2009 Jun;142(1-2):10–8. doi: 10.1016/j.virusres.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bankowski RA, Almquist J, Dombrucki J. Effect of paramyxovirus yucaipa on fertility, hatchability, and poult yield of turkeys. Avian Dis. 1981 Apr-Jun;25(2):517–20. [PubMed] [Google Scholar]
- [15].Lipkind MA, Weisman Y, Shihmanter E, Shoham D, Aronovici A. The isolation of yucaipa-like paramyxoviruses from epizootics of a respiratory disease in turkey poultry farms in Israel. Vet Rec. 1979 Dec 22-29;105(25-26):577–8. [PubMed] [Google Scholar]
- [16].Alexander DJ, Pattison M, Macpherson I. Avian paramyxoviruses of PMV-3 serotype in British turkeys. Avian Pathol. 1983 Oct;12(4):469–82. doi: 10.1080/03079458308436192. [DOI] [PubMed] [Google Scholar]
- [17].Tumova B, Robinson JH, Easterday BC. A hitherto unreported paramyxovirus of turkeys. Res Vet Sci. 1979 Sep;27(2):135–40. [PubMed] [Google Scholar]
- [18].Nerome K, Nakayama M, Ishida M, Fukumi H. Isolation of a new avian paramyxovirus from budgerigar (Melopsittacus undulatus) J Gen Virol. 1978 Feb;38(2):293–301. doi: 10.1099/0022-1317-38-2-293. [DOI] [PubMed] [Google Scholar]
- [19].Chang PC, Hsieh ML, Shien JH, Graham DA, Lee MS, Shieh HK. Complete nucleotide sequence of avian paramyxovirus type 6 isolated from ducks. J Gen Virol. 2001 Sep;82(Pt 9):2157–68. doi: 10.1099/0022-1317-82-9-2157. [DOI] [PubMed] [Google Scholar]
- [20].Alexander D, Senne D. Avian Paramyxoviruses 2-9. Diseases of Poultry. 2008 [Google Scholar]
- [21].Saif YM, Mohan R, Ward L, Senne DA, Panigrahy B, Dearth RN. Natural and experimental infection of turkeys with avian paramyxovirus-7. Avian Dis. 1997 Apr-Jun;41(2):326–9. [PubMed] [Google Scholar]
- [22].Yamane N, Arikawa J, Odagiri T, Ishida N. Characterization of avian paramyxoviruses isolated from feral ducks in northern Japan: the presence of three distinct viruses in nature. Microbiol Immunol. 1982;26(7):557–68. doi: 10.1111/mim.1982.26.7.557. [DOI] [PubMed] [Google Scholar]
- [23].Capua I, De Nardi R, Beato MS, Terregino C, Scremin M, Guberti V. Isolation of an avian paramyxovirus type 9 from migratory waterfowl in Italy. Vet Rec. 2004 Jul 31;155(5):156. [PubMed] [Google Scholar]
- [24].Warke A, Appleby L, Mundt E. Prevalence of antibodies to different avian paramyxoviruses in commercial poultry in the United States. Avian Dis. 2008 Dec;52(4):694–7. doi: 10.1637/8390-070308-RESNOTE.1. [DOI] [PubMed] [Google Scholar]
- [25].Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc Natl Acad Sci U S A. 2006 May 23;103(21):8203–8. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nayak B, Rout SN, Kumar S, Khalil MS, Fouda MM, Ahmed LE, et al. Immunization of chickens with Newcastle disease virus expressing H5 hemagglutinin protects against highly pathogenic H5N1 avian influenza viruses. PLoS One. 2009;4(8):e6509. doi: 10.1371/journal.pone.0006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang Z, Elankumaran S, Yunus AS, Samal SK. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J Virol. 2004 Sep;78(18):10054–63. doi: 10.1128/JVI.78.18.10054-10063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lipkind M, Shihmanter E. Antigenic relationships between avian paramyxoviruses. I. Quantitative characteristics based on hemagglutination and neuraminidase inhibition tests. Arch Virol. 1986;89(1-4):89–111. doi: 10.1007/BF01309882. [DOI] [PubMed] [Google Scholar]
- [29].Alexander DJ, Chettle NJ, Parsons G. Resistance of chickens to challenge with the virulent Herts 33 strain of Newcastle disease virus induced by prior infection with serologically distinct avian paramyxoviruses. Res Vet Sci. 1979 Mar;26(2):198–201. [PubMed] [Google Scholar]
- [30].OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; NEWCASTLE DISEASE. CHAPTER 2.1.15. 2004 http://www.oie.int/eng/OIE/organisation/en_LR.htm.
- [31].Hierholzer JC, Killington RA. In: Virus isolation and quantitation. Mahy BW, Kangro HI, editors. Virology methods manual Academic Press; London, United Kingdom: 1996. pp. 25–46. [Google Scholar]
- [32].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- [33].Samuel AS, Paldurai A, Kumar S, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus (APMV) serotype 5 completes the analysis of nine APMV serotypes and reveals the longest APMV genome. PLoS One. 2010;5(2):e9269. doi: 10.1371/journal.pone.0009269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dave VP, Allan JE, Slobod KS, Smith FS, Ryan KW, Takimoto T, et al. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology. 1994 Mar;199(2):376–83. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- [35].Connors M, Collins PL, Firestone CY, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991 Mar;65(3):1634–7. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tao T, Davoodi F, Cho CJ, Skiadopoulos MH, Durbin AP, Collins PL, et al. A live attenuated recombinant chimeric parainfluenza virus (PIV) candidate vaccine containing the hemagglutinin-neuraminidase and fusion glycoproteins of PIV1 and the remaining proteins from PIV3 induces resistance to PIV1 even in animals immune to PIV3. Vaccine. 2000 Jan 31;18(14):1359–66. doi: 10.1016/s0264-410x(99)00406-5. [DOI] [PubMed] [Google Scholar]