Abstract
Conductive core-sheath TiO2-PEDOT nanocables were prepared using electrospun TiO2 nanofibers as template, followed by vapor phase polymerization of EDOT. Various techniques were employed to characterize the sample. The results reveal that the TiO2 core has an average diameter of ∼78 nm while the PEDOT sheath has a uniform thickness of ∼6 nm. The as-prepared TiO2-PEDOT nanocables display a fast and reversible response to gaseous NO2 and NH3 with a limit of detection as low as 7 ppb and 675 ppb (S/N=3), respectively. This study provides a route for the synthesis of conductive nanostructures which show excellent performance for sensing applications.
Keywords: poly(3,4-ethylenedioxythiophene); nanocables; gas sensor; vapor phase polymerization; electrospinning
1. Introduction
Controllable synthesis of conducting polymer nanostructures has been intensively investigated in the past decades due to their unique optical and electronic properties. Among various methods in the synthesis of conducting polymer nanostructures, the template method is the most efficient and controllable one [1,2]. Various templates such as carbon nanotubes and anodic alumina oxides have been used to provide scaffold/support or spatial confinement for the growth of desired conducting polymers [3–5]. Recently, electrospun nanofibers have emerged as ideal supporting templates in the fabrication of core-sheath nanostructures with a narrow diameter distribution because the deposition of conducting polymers on electrospun nanofibrous template could potentially yield a class of nanomaterials with a large surface to volume ratio, highly porous structure, and an ultrathin conducting layer, which favor the gas adsorption/desorption process and thus make such materials attractive as potential sensing elements [6,7].
It has been well documented that conducting polymers are sensitive to environmental compositions [8]. Among the commonly used conducting polymers, poly(3,4-ethylenedioxythiophene) (PEDOT) has attracted a lot of attention in recent years due to its excellent properties such as long-term stability, high conductivity, optical transparency in its doped state, low band gap, and moderate redox potential [9–12]. PEDOT is commonly accepted to be more environmentally stable than other conducting polymers such as polypyrrole and polyaniline [13,14]. These features make PEDOT and its derivatives appealing in gas sensing. To date, numerous PEDOT based gas sensors have been proposed to detect gaseous analytes such as NH3 [15–19], HCl [15,16], alcohol [20,21], and NO [22]. To synthesize PEDOT for such applications, electrochemical polymerization and chemical polymerization are widely used. Compared with electrochemical polymerization, chemical polymerization of EDOT can provide uniform coating on both conducting and non-conducting surface, requires relatively simple equipment, and adapts a flexible procedure; therefore, it has been widely employed to deposit PEDOT on different substrate [1,23].
Recently, a conducting polymer such as polypyrrole has been successfully coated on nanofbers and its application for ultrasensitive NH3 detection has also been demonstrated [6,24]. Herein, we report a facile method to fabricate conductive core-sheath TiO2-PEDOT nanocables by vapor phase polymerization of EDOT. Electrospun TiO2 nanofibers were used as the template. Various characterization techniques were applied to characterize the as-prepared samples and the results reveal that the TiO2 core has an average diameter of ∼78 nm, while the PEDOT sheath has a uniform thickness of ∼6 nm. We further demonstrated the application of the TiO2-PEDOT nanocables for NO2 and NH3 detection. The as-fabricated sensor exhibits fast response and good recovery upon exposure of gaseous analytes and also shows excellent limit of detection. This study provides a simple strategy to fabricate conductive nanomaterial, which is promising in various sensing applications.
2. Results and Discussion
2.1. Characterization of Conductive Core-sheath TiO2-PEDOT Nanocables
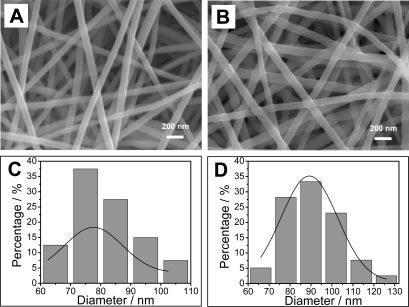
Electrospinning is a straightforward and versatile technique to produce continuous fibers with nanoscale diameter and a large surface to volume ratio [25]. In this research, TiO2 is chosen as template in the preparation of conductive TiO2-PEDOT nanocables due to its excellent chemical stability in oxidizing environments. Figure 1A shows the typical SEM image of the as-prepared TiO2 nanofibers. It is clearly seen that the sample consists of numerous randomly-oriented nanofibers with relatively uniform morphology and size in the area examined. When the white TiO2 nanofibers were dipped into FeCl3-ethanol solution and dried in air, their color became yellowish due to the adsorption of FeCl3. After the sample was exposed to EDOT vapor at 95 °C [26], the adsorbed FeCl3 triggered the vapor phase polymerization of EDOT. One obvious phenomenon is that the sample became dark blue, indicating the formation of PEDOT on the surface of TiO2 nanofibers. Figure 1B reveals that the morphology of the as-prepared conductive TiO2-PEDOT nanocables is similar to that of TiO2 nanofibers and only the diameter is slightly bigger than that of corresponding TiO2 nanofibers. Diameter distributions for the samples before and after PEDOT coating were further obtained using 40 randomly chosen TiO2 nanofibers and TiO2-PEDOT nanocables in corresponding SEM images and then analyzed by Gaussian fitting. One can see from Figures 1C-D that more than 85% of the TiO2-PEDOT nanocables have diameters ranging from 72 to 108 nm with a Gaussian mean diameter of 89.2 nm, while the diameter of TiO2 nanofiber template has a Gaussian mean value of 77.6 nm. As the difference of the two Gaussian mean diameters is 11.6 nm, which is equal to twice of the thickness of PEDOT sheath, the thickness of the coated PEDOT layer is calculated to be ∼5.8 nm.
Figure 1.
(A) SEM image of electrospun TiO2 nanofibers. (B) SEM image of TiO2-PEDOT nanocables after the vapor phase polymerization of PEDOT on TiO2 nanofibers. (C) and (D) Histograms showing the size distribution of TiO2 nanofibers and TiO2-PEDOT nanocables, respectively.
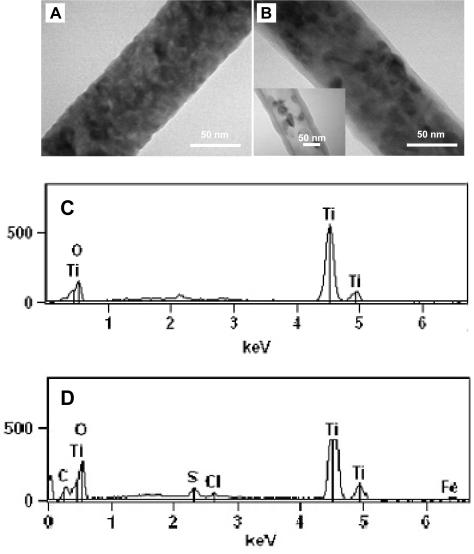
The detailed morphology of the samples were further characterized and analyzed by TEM. Figure 2A and B show typical TEM images of the TiO2 nanofiber before and after PEDOT-coating. Clearly seen from the TEM images, TiO2 nanofiber has a rough surface and consists of many nanoparticles (Figure 2A), while TiO2-PEDOT nanocable displays a core-sheath structure with an extremely thin (∼6 nm) layer of PEDOT (Figure 2B), which is in good agreement with the analysis of Gaussian fitting previously. The TEM images also clearly indicate the uniformity of TiO2 core as well as the uniformity and continuity of PEDOT sheath. In addition, the vapor polymerization of EDOT using FeCl3 as an oxidant would not transform the morphology of the electrospun TiO2 nanofibers. It has been reported that TiO2 could be dissolved in HF solution [27]. In order to better reveal the core-sheath structure after PEDOT-coating, the as-prepared TiO2-PEDOT nanocables were soaked into a 5 mol/L HF solution under overnight-sonication to selectively remove TiO2 cores. It can be seen that a tubular structure was indeed observed after the dissolution of TiO2 cores. However, the PEDOT layer after HF treatment is thicker than that before treatment (inset of Figure 2B). A similar phenomenon has been observed by other group [28] and it may be attributed to the irreversible swelling-effect of PEDOT in solution. Compared to conventional PEDOT films prepared by chemical polymerization [29,30], the as-prepared PEDOT layer is much thinner, which may be attributed to the limited adsorption of oxidant FeCl3 on TiO2 surface. Such ultrathin PEDOT layer makes it promising in various sensor applications. The composition change during the PEDOT-coating process could also be revealed by EDX analysis. Figure 2C reveals that the TiO2 nanofibers are only composed of elements of titanium and oxygen. No other peak was observed, indicating that the PVP used in the electrospinning has been completely removed by calcination. The EDX peaks were further fitted by Chi-squared filter applying the Proza (Phi-Rho-Z) correction method, and the calculated ratio of Ti to O was an approximate 1:2, in good agreement with the stoichiometric proportion of TiO2. In contrast, peaks associated with carbon and sulfur were observed for the sample after PEDOT-coating (Figure 2D), indicating the formation of PEDOT. The iron and chlorine were also identified in the PEDOT-coated sample, which was reasonable as FeCl3 was used as the oxidant and Fe and Cl were left in the PEDOT layer after the vapor phase polymerization.
Figure 2.
(A) TEM image of an individual TiO2 nanofiber. (B) TEM image of a core-sheath TiO2-PEDOT nanocable. The inset shows PEDOT nanotube obtained by selectively removing TiO2 core in 5 mol/L HF aqueous solution. (C) EDX element analysis result for TiO2 nanofibers. (D) EDX element analysis result for TiO2-PEDOT nanocables.
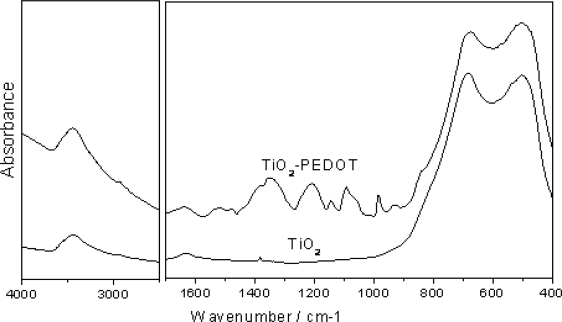
The formation of PEDOT on TiO2 was further confirmed by the FTIR spectra (Figure 3). The TiO2 nanofibers have the main bands between 400 and 700 cm−1, which are attributed to Ti–O stretching and Ti–O–Ti bridging stretching modes [31]. In addition, no band related to CH stretching of hydrocarbon was observed, indicating that PVP has been removed by calcination at 500 °C. After PEDOT-coating, the characteristic bands corresponding to PEDOT were observed in the range of 900–1520 cm−1. Specifically, the peaks at around 1,520 and 1,344 cm−1 can be assigned to C=C and C–C stretching mode of thiophene ring, respectively, while the peaks centered at 1,208, 1,145, and 1,093 cm−1 are ascribed to C–O–C bond stretching in the ethylene dioxy group. The peaks at 985 and 834 cm−1 can be assigned to C–S bond in the thiophene ring. It has also been reported that C–S bond usually have another peak at ∼682 cm−1. However, this peak was not observed in FTIR spectrum of TiO2-PEDOT, and it may be concealed by the intense TiO2 peak in the same region. The presence of water and hydroxy groups was also manifested, as the existence of a bending mode of H–O–H at 1,635 cm−1 and a strong stretching vibration of O–H at 3,440 cm−1, which can be attributed to the moisture bound to the samples and/or the possible presence of reduced oxidant salt species FeCl2 in a hydrate form [6]. Both IR spectra of TiO2-PEDOT nanocables and TiO2 nanofibers were in good agreement with the literature [26,31–33]. It is noteworthy that the strong TiO2 peaks in the region between 400–700 cm−1 are observed for both samples and the PEDOT coating cannot conceal their presence, indicating that PEDOT sheath is ultra-thin, in good agreement with TEM results.
Figure 3.
FTIR spectra of the electrospun TiO2 nanofibers and TiO2-PEDOT nanocables.
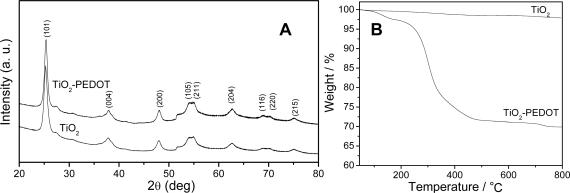
Figure 4A shows the XRD patterns of the TiO2 nanofibers and the core-sheath TiO2-PEDOT nanocables. It was found that TiO2 nanofibers are crystalline and all diffraction peaks can be assigned to the anatase phase of titania, in good agreement with other reports [6,25]. The similar XRD pattern was also obtained for TiO2-PEDOT nanocables, implying that PEDOT layer is ultra-thin and likely to be in an amorphous state. In order to study the thermal stability of PEDOT coated on TiO2 template, the thermogravimetric (TG) analysis was also carried out before and after PEDOT-coating and presented in Figure 4B. The TG of TiO2-PEDOT nanocables shows an initial weight loss of 2.6% is observed up to 180 °C, which may be attributed to the evaporation of the physically adsorbed water and the leaching of the dopant from the polymer composite matrix. The largest weight loss (25.8%) occurs from 180 °C to 505 °C, which is due to the degradation of PEDOT. A total weight loss of 28.4% has been observed up to 800 °C. As a comparison, the thermal behavior of the TiO2 nanofibers is very stable. There is no significant weight loss observed in the temperature range from room temperature to 800 °C, which is likely due to the highly thermal stability of TiO2.
Figure 4.
(A) XRD patterns of the electrospun TiO2 nanofibers and TiO2-PEDOT nanocables. (B) TGA in the oxygen atmosphere of TiO2 nanofibers and TiO2-PEDOT nanocables.
2.2. Gas Sensing Performance
TiO2 nanofibers are very fragile. However, the PEDOT coating on TiO2 nanofibers enhances the mechanical property of the as-prepared TiO2-PEDOT nanocables. The TiO2-PEDOT nancables possesses an ultra-thin layer of PEDOT, porous mesh structure, and highly specific surface to volume ratio, which favor the adsorption and desorption of gas molecules. In addition, SEM and TEM images show that the ultra-thin PEDOT layer is homogeneous and uniform, thus it can greatly suppress the noises generated from structural defects and then have the potential to improve the limit of detection [3]. Moreover, the PEDOT layer on the TiO2 nanofibers has a longer conjugation length which can facilitate the charge transport in the TiO2-PEDOT nanocables [3]. All these features make the as-prepared core-sheath TiO2-PEDOT nanocables an excellent sensing material.
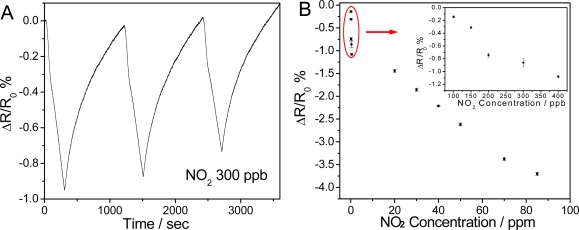
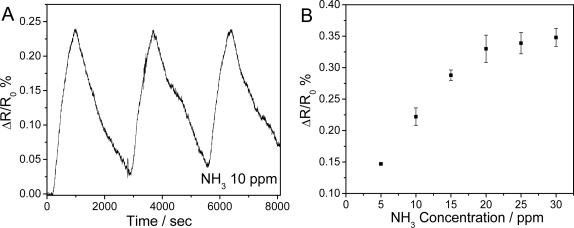
Figures 5A and 6A represent typical electrical responses of the TiO2-PEDOT nanocables as a function of time upon periodic exposure to gaseous NO2 (300 ppb) and NH3 (10 ppm), respectively. Air was chosen as the carrier gas in both experiments in order to simulate the most common sensing environment. Upon the exposure of TiO2-PEDOT nanocables to 300 ppb NO2, the normalized resistance of TiO2-PEDOT nanocables decreases rapidly by 0.861%. On the contrary, exposure of the same device to 10 ppm NH3 efficiently increases the resistance by 0.222%. For three “on-off” cycles of NO2 or NH3, the TiO2-PEDOT nanocables show fast, reproducible, and sensitive responses for all three cycles, suggesting that TiO2-PEDOT nanocable is a good sensing element in the detection of NO2 and NH3. By purging with dry air, the response of TiO2-PEDOT nanocables can be near-completely recovered, indicating the quick desorption of gas molecules from the ultra-thin PEDOT layer and the good reproducibility of TiO2-PEDOT nanocables based sensor. The good performance and fast response/recovery can be attributed to the highly porous structure and ultrathin PEDOT-coating layer (∼6 nm), in which porous structure allows the free access of analyte molecules to PEDOT while ultrathin PEDOT layer greatly reduces the diffusion resistance.
Figure 5.
(A) Typical response of TiO2-PEDOT nanocables upon periodic exposure of 300 ppb NO2. (B) The calibration plot for NO2 at an applied DC bias of 0.1 V and room temperature.
Figure 6.
(A) Typical response of TiO2-PEDOT nanocables upon periodic exposure of 10 ppm NH3. (B) The calibration plot for NH3 at an applied DC bias of 0.1 V and room temperature.
The gas sensing mechanism of TiO2-PEDOT nanocables can be illustrated based on the electrical properties of the sensing element and the chemical property of the analyte molecules. In this study, FeCl3 was used as oxidant and the as-prepared PEDOT is p-type. NO2 has an unpaired electron and is known as a strong oxidizer. Upon NO2 adsorption, charge transfer from PEDOT surface to NO2 is likely to occur because of the strong electron-withdrawing power of the NO2 molecules, which might generate more hole-carriers in PEDOT and thus result in the enhanced conductance of PEDOT. In contrast, NH3 is a Lewis base with a lone electron pair that can be donated to other species. The charge transfer between NH3 and p-type PEDOT leads to the formation of neutral polymer chains and results in the decrease of charge carrier density, which decreases the conductivity of PEDOT.
The response of TiO2-PEDOT nanocables as a function of NO2 and NH3 concentration was presented in Figures 5B and 6B, respectively. For both NO2 and NH3, the response of TiO2-PEDOT nanocables increases with the analyte concentration. At low NO2 concentrations (<400 ppb), the response of the developed TiO2-PEDOT nanocables exhibits linear behavior (Figure 5B inset). The normalized resistance change could reach 0.14% upon 100 ppb NO2 exposure. The detection limit was estimated to be 7 ppb NO2 at a signal-to-noise ratio of 3, which is lower than EPA current standard of 53 ppb NO2 for an annual arithmetic mean [34]. However, in the high range of NO2 concentration (0.4–85 ppm), another linear region was observed with less sensitivity (slope) in Figure 5B. There are only few reports using PEDOT as sensing materials in NO2 detection. Therefore, these results indicate that core-sheath TiO2-PEDOT nanocables are promising in sensitive NO2 detection. On the other hand, the normalized resistance change of TiO2-PEDOT nanofibers for NH3 shows a saturation behavior (Figure 6B). One can see that the response initially increases with the concentration of NH3 and then gradually levels off at a higher concentration. The detection limit was estimated to be 675 ppb NH3 (S/N = 3), which is significantly lower than the recommended threshold limit value of 25 ppm for human exposure [35] and better than most of reported values based on conducting polymers [19]. It is necessary to point out that the response magnitude of the TiO2-PEDOT nanocables for the same concentration of NH3 and/or NO2 is lower than those of PEDOT Langmuir-Blodgett (LB) film and PEDOT nanorods [15,16], however, the limits of detection for NO2 and NH3 achieved in this study is better than most reported values using PEDOT as the sensing element. As the limit of detection is dependent on response magnitude as well as the noise level, such good detection limit obtained in this study may be attributed to ultra-thin, homogeneous and uniform PEDOT layer, which can greatly suppress the noises generated from structural defects and thus improve the limit of detection.
3. Experimental Section
3.1. Materials
3,4-Ethylenedioxythiophene (EDOT), titanium isopropoxide (Ti(OiPr)4, 97%), and polyvinyl-pyrrolidone (PVP, MW = 1,300,000 g/mol) were obtained from Sigma–Aldrich. Acetic acid (99.8%) and ethanol were bought from Acros Organics and Fisher Scientific, respectively. For gas sensing studies, dry air, nitrogen dioxide (NO2, 1.036 × 104 ppm and 49.28 ppm in dry air), and ammonia (NH3, 915.3 ppm in dry air) were purchased from Airgas.
3.2. Fabrication of TiO2 Nanofibers
TiO2 nanofibers were prepared by electrospinning using a well-established procedure [25]. Briefly, a 1 mL solution containing 0.1 mL Ti(OiPr)4, 0.2 mL acetic acid, 0.03g PVP and 0.7 mL ethanol was prepared and electrospun using a 23 gauge needle with a flow rate of 0.3 mL/h at an applied potential of 7 kV and a collection distance of 5 cm. The collected nanofibers were left in air overnight, followed by calcination in a muffle furnace at 500 °C for 3 h to remove PVP and generate TiO2 nanofibers.
3.3. Synthesis of Conductive Core-sheath TiO2-PEDOT Nanocables
Vapor deposition polymerization was employed to synthesize the ultra-thin PEDOT coating layer. The collected TiO2 nanofibers serving as the template for PEDOT coating was carefully dipped into 0.1 mol/L FeCl3 ethanol solution for 30 min, and then taken out and dried in air for 10 min. After the TiO2 nanofibers with adsorbed FeCl3 were exposed to saturated EDOT vapor at 95 °C for 2 days, TiO2-PEDOT nanocables were produced with TiO2 as the core and PEDOT as the sheath.
3.4. Characterization of TiO2 Nanofibers and TiO2-PEDOT Nanocables
Field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) were employed to characterize the morphology and the size of the as-prepared TiO2 nanofibers and TiO2-PEDOT nanocables. An attached Oxford energy dispersive X-ray (EDX) detector in the FESEM was used for chemical composition analysis. Fourier Transform Infrared (FT-IR) spectra were recorded for KBr disks containing samples with a Nicolet Magna-IR 560 spectrometer. The IR spectra were analyzed using the software of Omnic 7.2a from Thermo Electron Corporation. X-ray diffraction patterns (XRD) were obtained with an Oxford diffraction XcaliburTM PX Ultra with ONYX detector to study the crystal structure of the samples. The thermogravimetry (TG) was performed using a TA instruments (TGA Q500) under an air flow of 60 mL/min and a heating rate of 10 °C/min from room temperature to 800 °C.
3.5. Gas Sensing
A simple TiO2-PEDOT nanocables-based resistor-type gas sensor was fabricated by placing two electrodes on the as-prepared nanocables with a gap of ∼1 mm. Gas sensing experiments were carried out in a homemade 5 cm3 sealed glass chamber with gas inlet and outlet ports. The sensor circuit was subjected to a fixed 0.1 V DC bias and the current was continuously measured by a CHI-601C electrochemical analyzer (CH Instruments Inc., Austin, TX, USA). Dry air was used as carrier gas to obtain the diluted analyte mixture. The mixing of the analyte and carrier gas was regulated by computer-controlled gas mixing system (S-4000, Environics Inc., Tolland, CT, USA). The total flow rate of the gas mixture was set to be 1.2 L/min for NO2 detection and 0.2 L/min for NH3 sensing. Prior to the measurement, a stable baseline was obtained by purging dry air through the sensor for one hour, and then NO2 and NH3 with various concentrations was introduced to the gas chamber. In a typical NO2 sensing experiment, the sensor was first exposed to NO2 (100 ppb to 85 ppm) for 5 min, followed by dry air for 15 min to recover the sensor, and then the procedure was repeated. For NH3 sensing (5 to 30 ppm), the similar procedure was applied except the exposure and recovery time is 15 min and 30 min, respectively. The electric resistance of the sensor was calculated by applying Ohm’s law (R = V/I) while the normalized resistance change is defined as ΔR/R0% = [(R – R0) / R0] × 100%, where R0 is the initial electrical resistance of the sensor in dry air and R is the measured real-time resistance. All experiments were conducted at ambient temperature.
4. Conclusions
In summary, we have demonstrated a simple and efficient approach to fabricate conductive core-sheath TiO2-PEDOT nanocables using the as-electrospun TiO2 nanofibers as template. The as-prepared TiO2-PEDOT nanocables possess highly porous structure, large surface to volume ratio, and ultra-thin PEDOT layer, providing an excellent material for gas sensing. When used in chemiresistive mode, the TiO2-PEDOT nanocables exhibited good, reversible, and reproducible concentration-dependent response to gaseous NH3 and NO2. The good limit of detection and fast response/recovery can be attributed to background noise reduction and free access of gaseous molecules to ultra-thin PEDOT. This study provides a promising route for the facile and cost-effective synthesis of conductive nanomaterial with ultrathin conducting PEDOT layer.
Acknowledgments
We greatly appreciate the funding from NSF, USGS, and DHS. “Points of view in this document are those of the author(s) and do not necessarily represent the official position of the funding agencies.” YW also thanks the partial support from UConn Center for Environmental Science and Engineering.
References and Notes
- 1.Nair S., Hsiao E., Kim S.H. Fabrication of electrically-conducting nonwoven porous mats of polystyrene-polypyrrole core-shell nanofibers via electrospinning and vapor phase polymerization. J. Mater. Chem. 2008;18:5155–5161. [Google Scholar]
- 2.Cao H.Q., Xu Y., Hong J.M., Liu H.B., Yin G., Li B.L., Tie C.Y., Xu Z. Sol-gel template synthesis of an array of single crystal CdS nanowires on a porous alumina template. Adv. Mater. 2001;13:1393–1394. [Google Scholar]
- 3.Jang J., Bae J. Carbon nanofiber/polypyrrole nanocable as toxic gas sensor. Sens. Actua. B. 2007;122:7–13. [Google Scholar]
- 4.Rao C.N.R., Satishkumar B.C., Govindaraj A. Zirconia nanotubes. Chem. Commun. 1997;16:1581–1582. [Google Scholar]
- 5.Hoyer P. Formation of a titanium dioxide nanotube array. Langmuir. 1996;12:1411–1413. [Google Scholar]
- 6.Lu X.F., Zhao Q.D., Liu X.C., Wang D.J., Zhang W.J., Wang C., Wei Y. Preparation and characterization of polypyrrole/TiO2 coaxial nanocables. Macromol. Rapid Commun. 2006;27:430–434. [Google Scholar]
- 7.Greiner A., Wendorff J.H. Electrospinning: a fascinating method for the preparation of ultrathin fibres. Angew. Chem. Int. Ed. 2007;46:5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 8.Swager T.M. The molecular wire approach to sensory signal amplification. Acc. Chem. Res. 1998;31:201–207. [Google Scholar]
- 9.Kvarnstrom C., Neugebauer H., Blomquist S., Ahonen H.J., Kankare J., Ivaska A. In situ spectroelectrochemical characterization of poly(3,4-ethylenedioxythiophene) Electrochim. Acta. 1999;44:2739–2750. [Google Scholar]
- 10.Selvaganesh S.V., Mathiyarasu J., Phani K.L.N., Yegnaraman V. Chemical synthesis of PEDOT-Au nanocomposite. Nanoscale Res. Lett. 2007;2:546–549. [Google Scholar]
- 11.Roncali J., Blanchard P., Frere P. 3,4-Ethylenedioxythiophene (EDOT) as a versatile building block for advanced functional p-conjugated systems. J. Mater. Chem. 2005;15:1589–1610. [Google Scholar]
- 12.Groenendaal B.L., Jonas F., Freitag D., Pielartzik H., Reynolds J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv. Mater. 2000;12:481–494. [Google Scholar]
- 13.Yamato H., Ohwa M., Wernet W. Stability of polypyrrole and poly(3,4-ethylenedioxythiophene) for biosensor application. J. Electroanal. Chem. 1995;397:163–170. [Google Scholar]
- 14.Vazquez M., Bobacka J., Ivaska A., Lewenstam A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuat. B. 2002;82:7–13. [Google Scholar]
- 15.Jang J., Chang M., Yoon H. Chemical sensors based on highly conductive poly(3,4-ethylenedioxythiophene) nanorods. Adv. Mater. 2005;17:1616–1625. [Google Scholar]
- 16.Yang Y.J., Jiang Y.D., Xu J.H., Yu J.S. Preparation and properties of multilayer poly(3,4-ethylenedioxythiophene) Langmuir-Blodgett film. Thin Solid Films. 2008;516:2120–2124. [Google Scholar]
- 17.Xu J.H., Jiang Y.D., Yang Y.J., Yu J.S. Self-assembly of conducting polymer nanowires at air-water interface and its application for gas sensors. Mater. Sci. Eng. B. 2009;157:87–92. [Google Scholar]
- 18.Yang Y.J., Jiang Y.D., Xu J.H., Yu J.S. Conducting polymeric nanoparticles synthesized in reverse micelles and their gas sensitivity based on quartz crystal microbalance. Polymer. 2007;48:4459–4465. [Google Scholar]
- 19.Wang Y.J., Coti K.K., Jun W., Alam M.M., Shyue J.J., Lu W.X., Padture N.P., Tseng H.R. Individually addressable crystalline conducting polymer nanowires in a microelectrode sensor array. Nanotechnology. 2007;18:424021. doi: 10.1088/0957-4484/18/42/424021. [DOI] [PubMed] [Google Scholar]
- 20.Yoon H., Chang M., Jang J. Formation of 1D poly(3,4-ethylenedioxythiophene) nanomaterials in reverse microemulsions and their application to chemical sensors. Adv. Funct. Mater. 2007;17:431–436. [Google Scholar]
- 21.Mabrook M.F., Pearson C., Petty M.C. Inkjet-printed polymer films for the detection of organic vapors. IEEE Sensors J. 2006;6:1435–1444. [Google Scholar]
- 22.Lu H.H., Lin C.Y., Hsiao T.C., Fang Y.Y., Ho K.C., Yang D.F., Lee C.K., Hsu S.M., Lin C.W. Electrical properties of single and multiple poly(3,4-ethylenedioxythiophene) nanowires for sensing nitric oxide gas. Anal. Chim. Acta. 2009;640:68–74. doi: 10.1016/j.aca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Luo S.C., Yu H.H., Wan A.C.A., Han Y., Ying J.Y. A general synthesis for PEDOT-coated nonconductive materials and PEDOT hollow particles by aqueous chemical polymerization. Small. 2008;4:2051–2058. doi: 10.1002/smll.200800033. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Jia W.Z., Strout T., Schempf A., Zhang H., Li B.K., Cui J.H., Lei Y. Ammonia gas sensor using polypyrrole-coated TiO2/ZnO. Electroanalysis. 2009;21:1432–1438. [Google Scholar]
- 25.Li D., Xia Y.N. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003;3:555–560. [Google Scholar]
- 26.Im S.G., Gleason K.K. Systematic control of the electrical conductivity of poly(3,4-ethylenedioxythiophene) via oxidative chemical vapor deposition. Macromolecules. 2007;40:6552–6556. [Google Scholar]
- 27.Bright E., Readey D.W. Dissolution kinetics of TiO2 in HF-HCl solutions. J. Am. Ceram. Soc. 1987;70:900–906. [Google Scholar]
- 28.Hernandez S.C., Chaudhuri D., Chen W., Myung N.V., Mulchandani A. Single polypyrrole nanowire ammonia gas sensor. Electroanalysis. 2007;19:2125–2130. [Google Scholar]
- 29.Bobacka J., Lewenstam A., Ivaska A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J. Electroanal. Chem. 2000;489:17–27. [Google Scholar]
- 30.Pettersson L.A.A., Carlsson F., Inganas O., Arwin H. Spectroscopic ellipsometry studies of the optical properties of doped poly(3,4-ethylenedioxythiophene): an anisotropic metal. Thin Solid Films. 1998;313:356–361. [Google Scholar]
- 31.Yu J.G., Yu H.G., Cheng B., Zhao X.J., Yu J.C., Ho W.K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B. 2003;107:13871–13879. [Google Scholar]
- 32.Singh K., Ohlan A., Saini P., Dhawan S.K. Poly(3,4-ethylenedioxythiophene) gamma-Fe2O3 polymer composite-super paramagnetic behavior and variable range hopping 1D conduction mechanism-synthesis and characterization. Polym. Adv. Technol. 2008;19:229–236. [Google Scholar]
- 33.Meng H., Perepichka D.F., Bendikov M., Wudl F., Pan G.Z., Yu W.J., Dong W.J., Brown S. Solid-state synthesis of a conducting polythiophene via an unprecedented heterocyclic coupling reaction. J. Am. Chem. Soc. 2003;125:15151–15162. doi: 10.1021/ja037115y. [DOI] [PubMed] [Google Scholar]
- 34.Ram M.K., Yavuz O., Aldissi M. NO2 gas sensing based on ordered ultrathin films of conducting polymer and its nanocomposite. Synth. Met. 2005;151:77–84. [Google Scholar]
- 35.Liu H.Q., Kameoka J., Czaplewski D.A., Craighead H.G. Polymeric nanowire chemical sensor. Nano Lett. 2004;4:671–675. [Google Scholar]