Abstract
We determined the effect of 3-methoxybenzamide (3-MB), a competitive inhibitor of poly(ADP-ribose) polymerase (E.C. 2.4.2.30), on intrachromosomal homologous recombination in mouse Ltk- cells. We used a cell line that contained in its genome two defective Herpes thymidine kinase (tk) genes as closely linked direct repeats. Intrachromosomal homologous recombination events were monitored by selecting for tk-positive segregants that arose during propagation of the cells and recombination rates were determined by fluctuation analysis. We found that growth of cells in the continuous presence of 2mM 3-MB increased intrachromosomal recombination between 3 and 4-fold. Growth of cells in the presence of 2mM m-anisic acid, a non-inhibitory analog of 3-MB, had no effect on intrachromosomal recombination rates. Additionally, we found that 3-MB increased both gene conversions and crossovers to similar extents, adding to the evidence that these two types of intrachromosomal rearrangements share a common pathway. These findings contrast with our previous studies [Waldman, B.C. and Waldman, A.S. (1990) Nucleic Acids Res., 18, 5981-5988] in which we determined that 3-MB inhibits illegitimate recombination and has no effect on extrachromosomal homologous recombination in mouse Ltk- cells. An hypothesis is offered that explains the influence of 3-MB on different recombination pathways in mammalian cells in terms of the role that poly(ADP-ribosylation) plays in DNA break-repair.
Full text
PDF


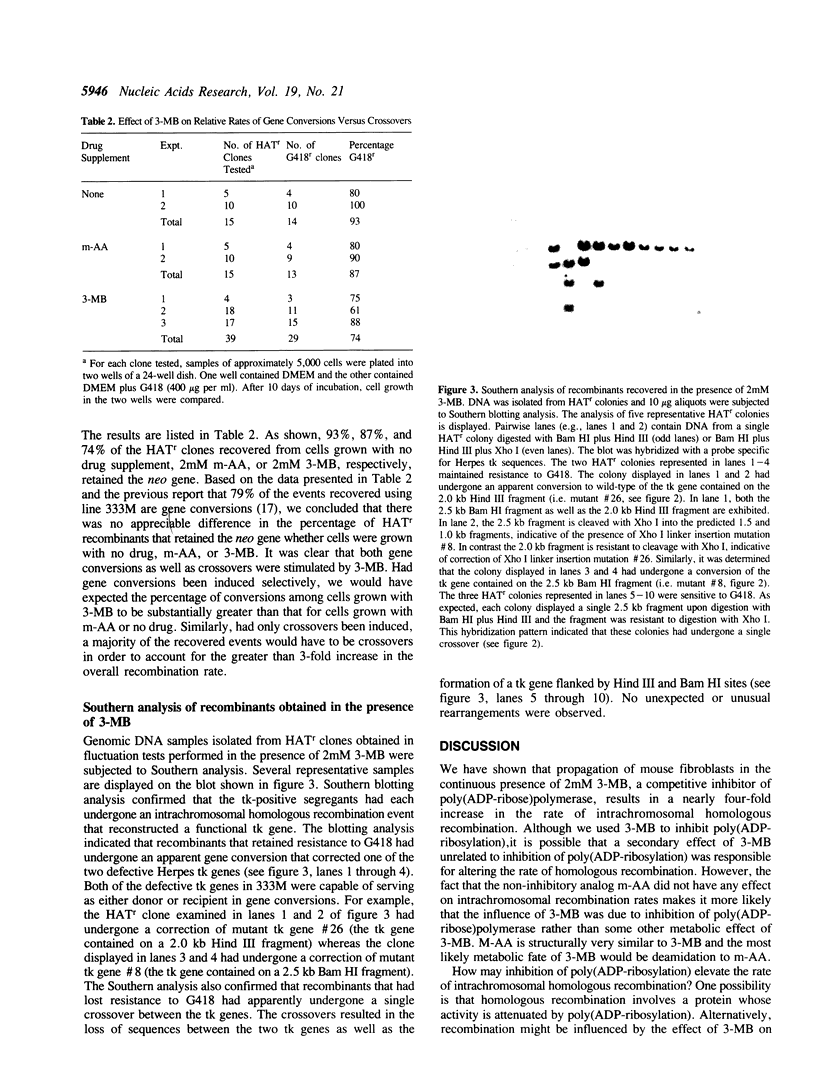

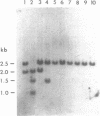
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlett C. F., Lehmann A. R. Human disorders showing increased sensitivity to the induction of genetic damage. Annu Rev Genet. 1978;12:95–115. doi: 10.1146/annurev.ge.12.120178.000523. [DOI] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Bollag R. J., Liskay R. M. Conservative intrachromosomal recombination between inverted repeats in mouse cells: association between reciprocal exchange and gene conversion. Genetics. 1988 May;119(1):161–169. doi: 10.1093/genetics/119.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Capizzi R. L., Jameson J. W. A table for the estimation of the spontaneous mutation rate of cells in culture. Mutat Res. 1973 Jan;17(1):147–148. doi: 10.1016/0027-5107(73)90265-0. [DOI] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Panayotou G. N., Bowler L. D., Hardas B. D., Broom T., Walther C., Shall S. ADP-ribosylation is involved in the integration of foreign DNA into the mammalian cell genome. Nucleic Acids Res. 1988 Dec 9;16(23):11319–11326. doi: 10.1093/nar/16.23.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Martin G. R. Cut, paste, and save: new approaches to altering specific genes in mice. Cell. 1989 Jan 27;56(2):145–147. doi: 10.1016/0092-8674(89)90887-8. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L., Letsou A. Homologous recombination between repeated chromosomal sequences in mouse cells. Cold Spring Harb Symp Quant Biol. 1984;49:183–189. doi: 10.1101/sqb.1984.049.01.021. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu M., Shima H., Aonuma S., Nakagama H., Nagao M., Sugimura T. Deletion of transfected oncogenes from NIH 3T3 transformants by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9066–9070. doi: 10.1073/pnas.85.23.9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Differential effects of base-pair mismatch on intrachromosomal versus extrachromosomal recombination in mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5340–5344. doi: 10.1073/pnas.84.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman B. C., Waldman A. S. Illegitimate and homologous recombination in mammalian cells: differential sensitivity to an inhibitor of poly(ADP-ribosylation). Nucleic Acids Res. 1990 Oct 25;18(20):5981–5988. doi: 10.1093/nar/18.20.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Y., Maher V. M., Liskay R. M., McCormick J. J. Carcinogens can induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mol Cell Biol. 1988 Jan;8(1):196–202. doi: 10.1128/mcb.8.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]