Abstract
Objective
The study of posttraumatic growth (PTG) has burgeoned over the last decade, particularly in the area of oncology. The aims of the study were to: (1) describe PTG in patients with hepatobiliary carcinoma, (2) examine agreement between the patient and caregiver measures of patient PTG, and (3) test the associations between PTG and other better established psychological factors and clinically relevant outcomes.
Methods
Two hundred and two patients with hepatobiliary carcinoma completed a battery of standardized questionnaires that measured posttraumatic growth, depressive symptoms, optimism, expressed emotion, and quality of life. A subsample of family caregivers also completed ratings of patient PTG, using the Posttraumatic Growth Inventory (PTGI, as well as their own PTG.
Results
No significant increase in PTG was observed between diagnosis and 6-months follow-up with the exception of the Relating to Others subscale of the PTGI. PTG was not found to be associated with quality of life or depressive symptoms. At diagnosis, the agreement between the patients' PTG and family caregivers' rating of PTG was found to be high (ICC= 0.34-0.74, p=0.001-0.05). Posttraumatic growth was found to be significantly associated with optimism [r=0.20 p=0.02-.0.05] and traumatic life events reported in the past three years including recent losses [F(1,52)=6.0, p=0.02] and severe physical injury [F(1,52)=5.5, p=0.02]. Caregivers reported PTG as a result of their loved one's diagnosis of cancer.
Conclusion
Preliminary results suggest that PTG is relatively stable over the first 6-months after diagnosis and results in changes as a result of a diagnosis of cancer were reported, and possibly observed, by others. Family caregivers also experience PTG as a result of their loved one's diagnosis of advanced cancer.
Introduction
Individuals facing potentially life-threatening events, such as a cancer diagnosis, or other major life events may experience alterations in their world-views and modify their thoughts and actions to include more activities and relationships that are personally fulfilling (Martin and Kleiber 2005). Recent literature exploring the implications of experiencing such traumatic events has increasingly centered on the phenomenon of positive changes or posttraumatic growth (PTG) following such challenges (Tedeschi and Calhoun 1996). Posttraumatic growth, also called benefit finding, has been found in individuals confronting a number of major life events, including motor vehicle accidents, bereavement, sexual abuse, severe burn injuries and head injuries (McMillen, Zuravin et al. 1995; Polatinsky and Esprey 2000; Salter and Stallard 2004; Barakat, Alderfer et al. 2006; Powell, Ekin-Wood et al. 2007; Rosenbach and Renneberg 2008). Recent research has increasingly focused on posttraumatic growth as it relates to one's physical and mental health (Park 2008).
Individuals diagnosed with cancer and their family members have been found in a number of studies to experience PTG (Schulz and Mohamed 2004; Widows, Jacobsen et al. 2005; Barakat, Alderfer et al. 2006; Mosher, Danoff-Burg et al. 2006; Mystakidou, Parpa et al. 2007). Cancer patients finding initial benefit in their diagnosis have been found to exhibit less emotional distress (Cordova, Cunningham et al. 2001; Carver and Antoni 2004; Schwarzer, Luszczynska et al. 2006). Studies have also found continued reports of benefit finding up to 7 years after the diagnosis of cancer (Stanton, Danoff-Burg et al. 2002; Carver and Antoni 2004; Schwarzer, Luszczynska et al. 2006). However, prospective studies of PTG in cancer patients have been rare, and little is known about the time course of PTG in relationship to clinical milestones including diagnosis and treatment.
Posttraumatic growth may be important in people with chronic illnesses for a number of reasons. It has been found to be associated with quality of life after adjusting for disease severity, race, and socioeconomic status (Tomich and Helgeson 2004). Posttraumatic growth has also been found to be a moderator of the relationship between symptoms of posttraumatic stress and quality of life; that is, PTG appears to play a protective role when present, while having a negative impact on patient quality of life and mood when absent (Morrill, Brewer et al. 2008). Posttraumatic growth has also been found to be associated with mood in several studies of patients diagnosed with cancer (Cordova, Cunningham et al. 2001; Lieberman, Golant et al. 2003; Ho, Chan et al. 2004; Duncan, Gidron et al. 2007; Jorngarden, Mattsson et al. 2007; Salsman, Segerstrom et al. 2009).
Posttraumatic growth has been found to be associated with other psychological factors such as optimism and expressed emotion in cross-sectional studies, however prospective studies have not been performed and the direction of the relationship remains unclear. Optimism is one of the factors that has most consistently been found to be associated with PTG in patients diagnosed with cancer (Harrington, McGurk et al. 2008). In studies of early-stage and post-surgery breast cancer patients, optimistic patients reported higher levels of benefit finding, greater use of positive reframing and enhanced quality of life (Antoni, Lehman et al. 2001; Lechner, Carver et al. 2006). Some researchers believe that PTG may be the result of a cognitive process such as positive reappraisal or reframing (Park 2008). If so, it may be possible for others to demonstrate emotional and/or behavioral indicators of PTG; a possibility that is explored in the current study.
The possible relationship between expressed emotion and PTG has only begun to receive attention in the PTG literature. Cancer survivors who use prayer as an emotional outlet have also been reported to experience higher benefit finding and spiritual well-being than those who do not pray (Levine, Aviv et al. 2008). Caregivers of patients with chronic illness have also begun to be studied. Caregivers of those diagnosed with life threatening illnesses such as cancer and HIV report PTG in response to the diagnosis of their loved ones (Manne, Ostroff et al. 2004; Weiss 2004; Cadell 2007; Kim, Schulz et al. 2007). Only one study, to the authors' knowledge, has reported on the agreement between ratings of patient and caregiver PTG (Thornton and Perez 2006). Thornton and colleagues found that patient and caregiver PTG were modestly correlated when assessed one year following surgery for prostate cancer. We are not aware of any study that has investigated the ratings of patient PTG by a family caregiver and the patient's self-reported PTG. Agreement between these ratings would suggest that PTG is an observable change and may support the phenomenon of PTG.
To date, studies of PTG in cancer patients have been conducted in people diagnosed with types of cancer that have a relatively good prognosis (e.g., breast, prostate). Previous research has also been limited in regard to variability in socioeconomic status, gender, race, and education. Additionally, studies exploring the relationship between specific diseases and quality of life have only employed general measures of quality of life rather than disease-specific instruments.
Posttraumatic growth in people diagnosed with hepatobiliary carcinoma, which poses a significant threat to life has yet to be investigated. Patients with advanced cancer may be a unique population to study PTG secondary to the life threatening nature and high level of stress associated with this diagnosis. Hepatocellular carcinoma, which is the primary diagnosis in the present study, has a 3 year survival rate of approximately 15% [34]. The aims of the current study were to: (1) describe posttraumatic growth in patients with hepatobiliary carcinoma, (2) examine agreement between the patient and caregiver on measures of patient PTG, and (3) test the associations between PTG and other better established psychological factors (e.g., optimism, trauma, expressed emotion) and clinically relevant outcomes (quality of life and depressive symptoms).
Methods
Participants
Between June of 2003 and March of 2006, a total of 232 patients were approached for the study and 202 (87%) consented to participate. The primary reason for declining to participate was (1) being overwhelmed by the amount of information received (80%), (2) not interested (10%), (3) not having enough time (7%), and (4) distress (3%). Of the 202 patients assessed at diagnosis, 53 and 30 had complete follow-up assessments at 3- and 6-months, respectively. The large number of patients at baseline was based on changes in the design of the study in January of 2004 (cross-section to prospective study). Furthermore, due to the advanced nature of the cancer, a high rate of attrition was observed secondary to worsening of illness and death. Caregivers of 83 patients were recruited and administered the Posttraumatic Growth Inventory (PTGI) with regard to their assessment of patients' PTG. Of the 83 caregivers, the last consecutive family caregivers (n=42) who were recruited also rated their own PTG.
Patient inclusion criteria were: (1) a diagnosis of biopsy proven primary hepatobiliary carcinoma or colorectal carcinoma with metastases to the liver, (2) age 18 years or older, and (3) no report of suicidal ideation, thought disorder, or psychosis. Family caregivers were those persons who were involved in the care of the patient. The individual may or may not be related biologically or by marriage. The family caregiver often lived with the patient but in some cases the family caregiver lived outside the patient's home but had frequent contact with the patient.
Instruments
The Posttraumatic Growth Inventory (PTGI) was used to assess the extent to which participants experienced a positive outcome as a result of a traumatic event. The PTGI is a 21 item questionnaire that measures the extent to which participants experienced change on a six point scale (I did not experience change, to a very small degree, to a small degree, to a moderate degree, to a great degree, to a very great degree) (Tedeschi and Calhoun 1996). The PTGI is a validated measure of stress-related growth as it relates to cancer and other traumatic life events and has demonstrated satisfactory internal consistency and test-retest reliability (Weinrib, Rothrock et al. 2006; Mystakidou, Tsilika et al. 2008; Shakespeare-Finch and Enders 2008).
Caregivers were also given a modified version of PTGI, where the items were changed to query them about their perception of the patients' PTG. Caregivers also surveyed their own PTG, which was seen as a result of caring for a loved one diagnosed with cancer. The instructions were only modified to reflect the caregivers' request to rate the change of the patient's PTG; during an interview (approximately 30 minutes later), the caregiver was requested to rate their own PTG as a result of their loved one's diagnosis.
The Center for Epidemiological Studies Depression Scale (CES-D) was used to assess depressive symptoms. The CES-D includes 20 items that inquire about depressive symptoms during the seven days prior to administration. Patients respond using a four-point Likert Scale (0=rarely or none of the time—less than one day, 1=some or a little of the time—one or two days, 2=occasionally or a moderate amount of time—two or three days, and 3=most or all of the time—five to seven days)(Radloff 1977). A score of 16 or above suggests that the patient is clinically depressed. The CES-D has been shown to be reliable in measuring depression in breast cancer patients (Hann, Winter et al. 1999). The CES-D has also been shown to have good content validity (Okun, Stein et al. 1996).
The Life Orientation Test-Revised (LOT-R) was used to measure dispositional optimism. The LOT-R is a 10-item self-report questionnaire adapted from the original LOT 13-item questionnaire that measures outcome expectancies on a 5-point scale (strongly agree, disagree, neutral, agree, strongly agree) (Scheier and Carver 1985; Scheier, Carver et al. 1994). The LOT-R has been found to demonstrate good predictive and discriminant validity (Scheier, Carver et al. 1994) and acceptable internal consistency (Allan and Giles 2008).
The Courtland Emotional Control Scale (CECS) was used to assess the extent to which participants reported control of their emotions of anger, anxiety, and depression. The self-report questionnaire consists of 21 items and contains three subsections including angry/very annoyed, unhappy/miserable, and afraid/worried. A six point scale (almost never, sometimes, often, almost always, don't know, refused) is used to measure how the participants generally react to a specific situation (Watson and Greer 1983). The CECS was found to be reliable and valid in studies involving cancer patients (Ho, Chan et al. 2004).
The Traumatic Events Survey (TES) is a 30-item inventory that inquires about trauma in childhood (10-items) and adulthood (20-items). The format of the questions is “yes/no,” requiring that the patient disclose whether a certain event has occurred. If indicated that “yes” the event has taken place, an open-ended response is warranted (Elliot 1992).
The Patient Relationship Questionnaire (PRQ) was used to assess the relationship between the patient and caregiver. The PRQ is a 34-item questionnaire that contains three questions that requests disclosure of the patient-caregiver relationship and information relevant to their relationship, such as duration and residential proximity. The remaining questions inquire about the emotional contact and contribution of the relationship between the patient and caregiver. The questions are in Likert Scale format, including “strongly disagree, disagree, agree and disagree, agree, and strongly agree.”
The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) was used to assess changes in symptoms and side-effects of treatment and quality of life. The FACT-Hep includes both the FACT-General (a 27-item instrument that measures four dimensions of quality of life) and a module with 18 items specific to hepatobiliary disease (Cella, Tulsky et al. 1993; Heffernan, Cella et al. 2002). The FACT-G has four subscales of well-being including physical (PWB), social and family (SFWB), emotional (EWB), and functional (FWB) (Cella, Tulsky et al. 1993). The hepatobiliary module includes items that pertain to symptoms of liver disease as well as side effects of treatment (Heffernan, Cella et al. 2002). The FACT is one of the most widely utilized quality of life questionnaires in clinical trials for new cancer treatments, and both the FACT-G as well as the FACT-Hep have been demonstrated to be valid and reliable instruments (Lee, Chun et al. 2004; Ashing-Giwa, Kim et al. 2008; Zhu, Lang et al. 2008). The internal consistency was between 0.72 and 0.94 for the FACT-Hep and test-retest ranged between 0.84 to 0.91. Convergent and divergent validity were demonstrated by examining the FACT subscales with scales measuring mood, social support, and social desirability.
Procedure
The study was approved by the University of Pittsburgh Institutional Review Board prior to the commencement of the study. All patients who met the inclusion criteria were approached by their treating healthcare provider to determine their interest in learning more about the purpose, risk and benefits of the study. Patients agreeing to learn more about the study were seen by a research assistant trained in psychology or a clinical psychologist to explain the study in detail and ask for the patients' informed consent. Upon receipt of written informed consent the patient and his/her caregiver were asked to complete a battery of questionnaires by interview within 1-4 weeks of diagnosis of liver lesion. The patients and caregivers were interviewed separately. Patients completed these interviews at baseline, and participants who were alive and able to continue to complete questionnaires were assessed again at 3- and 6-months after diagnosis. The participants who received a CES-D score greater than 16 (clinical range) were referred for treatment of their depressive symptoms. Participants were given information on two evidenced-based treatments (e.g., cognitive-behavioral therapy and pharmacological treatment) and were provided local referrals for psychologists and/or psychiatrists. There was no remuneration for participation in the study.
Data Analysis
Data were entered and verified in SPSS v16. Descriptive statistics were performed on patient and caregiver characteristics. Internal consistency of the PTGI was assessed using Cronbach's alpha. Agreements between the patient and caregiver ratings of PTG were analyzed using one-way random interclass correlations. Pearson correlation coefficients were employed to test associations between select variables. The distribution of the data was normal and therefore Analysis of Variance (ANOVA) was performed to test differences between groups.
Results
Participant Characteristics
The patient demographic and disease-specific information can be found in Table 1. The majority of patients were male (73%) with a mean age of 63 years (range of 30-94 years). The majority of the patients were diagnosed with hepatocellular or cholangio carcinoma (72%, n=134). The remaining 28% (n=34) of patients had a diagnosis of another primary cancer (e.g., gallbladder) or recurrence of another primary cancer (e.g., colorectal) with metastases to the liver. Seventy-eight percent of patients had cirrhosis, and patients had an average tumor size of 6 cm with a range from 0 to 20 cm. The number of lesions ranged from 1 to 6 lesions, with a mean of 4 lesions. Thirty-eight percent of the sample experienced vascular invasion and 63% had hypervascular lesions. With regard to treatment, most patients received chemotherapy alone (70%, n=124), surgery (14%, n=25), or a combination of chemotherapy and surgery (8%, n=13), while 8% (n=14) of patients received no treatment. No significant relationships between sociodemographic or disease specific factors and PTG were observed.
Table 1. Patient and Caregiver Characteristics.
| PATIENT (n=202) | |
| Gender (%) | |
| Male | 73 |
| Female | 27 |
| Age | |
| Mean | 63 |
| Range | 30-94 |
| Diagnosis (%) | |
| Hepatocelluar carcinoma | 67 |
| Other Primary with liver metastases | 28 |
| Cholangiocarcinoma | 5 |
| Treatment (%) | |
| Chemotherapy | 70 |
| Surgery | 14 |
| Combination | 8 |
| No Treatment | 8 |
| Cirrhosis (%) | |
| Yes | 78 |
| No | 22 |
| Tumor Size (cm) | |
| Mean | 6 |
| Range | 1-20 |
| Lesions | |
| Mean | 4 |
| Range | 1-6 |
| Vascular Invasion (%) | |
| Hypervascular | 63 |
| Hypovascular | 32 |
| Mixed vascularity | 5 |
| CAREGIVER (n=83) | |
| Relationship to Patient (%) | |
| Spouse | 63 |
| Aunt/Uncle | 17 |
| Sibling | 7 |
| Parent | 6 |
| Son/Daughter | 3 |
| Significant Other | 2 |
| Relationship Duration (years) | |
| Mean | 35 |
| Range | 0.25 – 71 |
| Patient/Caregiver Proximity (%) | |
| Same home | 78 |
| Same city | 17 |
| More than 50 miles away | 3 |
| Less than 50 miles away | 2 |
The majority of caregivers were identified as spouses, followed by aunts/uncles, siblings, parents, son/daughter, and significant other (Table 1). Patients and caregivers were reported to have known each other for a mean of 35 years, with a range of 3 months to 71 years. The majority of patients/caregivers lived in the same home or the same city.
Descriptive statistics of PTGI
To assess the internal consistency of patient and caregiver (self and patient rating) PTGI scores, Cronbach alphas can be found in Table 2. All scales were found to have excellent reliability. The subscales of the patient PTGI ranged from α= 0.76-0.95; caregivers' report of patients' PTGI α= 0.78-0.95; and the caregivers' PTGI α= 0.81-0.95.
Table 2. Cronbach Alphas of Patient and Caregiver (self and patient rating) PTGI subscales.
| Patient PTG (n=202) | |
| Relating to Others | 0.88 |
| Appreciation of Life | 0.85 |
| New Possibilities | 0.78 |
| Spiritual Change | 0.79 |
| Personal Strength | 0.78 |
| Total | 0.95 |
| Caregivers' rating of Patient PTG (n=83) | |
| Relating to Others | 0.92 |
| Appreciation of Life | 0.81 |
| New Possibilities | 0.86 |
| Spiritual Change | 0.91 |
| Personal Strength | 0.78 |
| Total | 0.95 |
| Caregivers' PTG (n=42) | |
| Relating to Others | 0.88 |
| Appreciation of Life | 0.84 |
| New Possibilities | 0.81 |
| Spiritual Change | 0.95 |
| Personal Strength | 0.84 |
| Total | 0.95 |
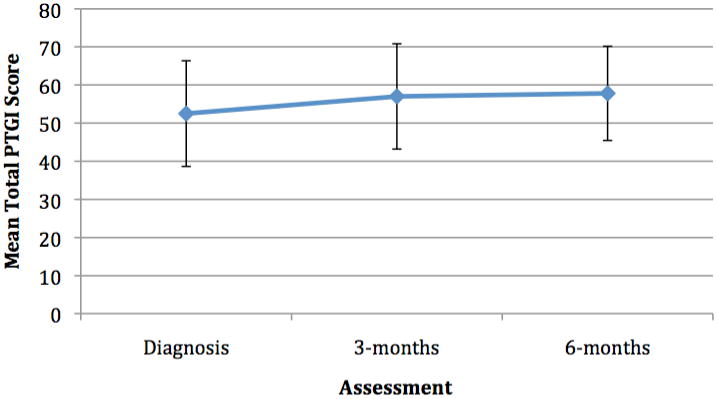
Changes Over Time in PTG
Changes in PTG over time were assessed among patients who survived for 3-months and 6-months (n=30) (see Figure 2). Repeated measures ANOVA was employed and significant changes were observed on the Relating to Others subscale [F(2,15)=4.94, p=0.02] of the PTGI from diagnosis to 6-months with increases over time. No significant differences over time were observed for any of the other subscales of the PTGI or for the total PTGI. See Figure 1.
Figure 2. Total Mean PTGI Score by Disease.
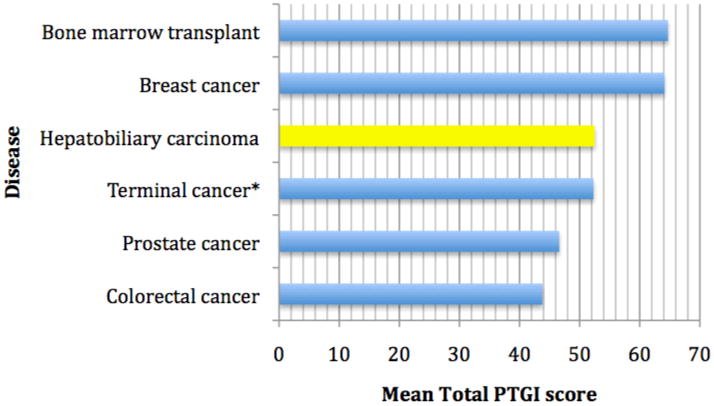
*Gastrointestinal, lung, urogenital, and breast cancer.
Figure 1. Patient PTG Ratings at Diagnosis, 3-months and 6-months(n=30).
Patient and Caregiver Agreement on the PTGI
Agreement was analyzed at the time of diagnosis using interclass correlations (one-way random) between the patient and family caregivers' ratings of PTG. The patient and caregivers' rating of the patients' PTG were in agreement on the Relating to Others (ICC=0.34, p=0.05), Spiritual Change (ICC=0.74, p=0.001), Personal Strength (ICC=0.48, p=0.005), and Total PTGI subscales (ICC=0.47, p=0.005). See Table 3. The only subscales in which the patient and family caregiver PTG did not reach significant agreement were the Appreciation of Life (ICC=0.28, p=0.10) and New Possibilities subscales (ICC=0.29, p=0.09).
Table 3. One-way random interclass correlation coefficients for baseline proxy ratings of patient PTGI.
| Proxies | Scale | Correlation Coefficient | P-value | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Patient PTG & Caregiver's Proxy Rating of Patient's PTG (n=83) | |||||
| Relating to Others | 0.34 | 0.05 | −0.80 | −0.60 | |
| Appreciation | 0.28 | 0.10 | −0.18 | 0.56 | |
| Possibilities | 0.29 | 0.09 | −0.16 | 0.57 | |
| Spirituality | 0.74 | 0.001 | 0.57 | 0.84 | |
| Strength | 0.48 | 0.005 | 0.15 | 0.68 | |
| Total | 0.47 | 0.005 | 0.14 | 0.68 | |
| Patient PTG & Caregiver PTG (n=42) | |||||
| Relating to Others | 0.22 | 0.22 | −0.46 | 0.58 | |
| Appreciation | 0.24 | 0.20 | −0.42 | 0.59 | |
| Possibilities | 0.18 | 0.27 | −0.54 | 0.56 | |
| Spirituality | 0.59 | 0.002 | 0.24 | 0.78 | |
| Strength | 0.61 | 0.002 | 0.26 | 0.79 | |
| Total | 0.41 | 0.05 | −0.10 | 0.68 | |
| Caregiver PTG & Caregiver's Proxy Rating of Patient's PTG (n=42) | |||||
| Relating to Others | 0.83 | 0.001 | 0.67 | 0.91 | |
| Appreciation | 0.81 | 0.001 | 0.64 | 0.91 | |
| Possibilities | 0.91 | 0.001 | 0.82 | 0.95 | |
| Spirituality | 0.85 | 0.001 | 0.71 | 0.92 | |
| Strength | 0.73 | 0.001 | 0.47 | 0.86 | |
| Total | 0.90 | 0.001 | 0.80 | 0.95 | |
A correlation between the patient and caregivers' ratings of their own PTG were found to be significant on the spirituality (r= 0.38, p=0.02) and the personal strength subscale (r=0.44, =0.004). A trend toward significance was also found on the overall PTGI subscale (r=0.30, p=0.06).
Past Trauma and PTG
In a subsample of patients (n=53) several additional outcomes were assessed and analyzed. In regard to recent major life events, patients who reported a recent loss [F(1,52)=6.0, p=0.02] or were seriously physically injured in the last three years [F(1,52)=5.5,p=0.02] were more likely to report PTG at the time of a diagnosis of cancer. No association was found between current PTG and other major life events such as divorce or separation, being a victim of violence, or sudden change in employment status was found to be associated with PTG; however, the number of traumatic events was limited.
Patient-Caregiver Relationship and Posttraumatic Growth
The quality of the patient and caregiver relationship was also examined and no significant association was found between the level of agreement in regard to PTG between the patient and caregiver ratings and the patient-caregiver relationship quality.
Optimism and Posttraumatic Growth
At diagnosis, PTG was found to be highly correlated with optimism. The Appreciation of Life (r=0.24, p=0.02), Personal Strength (r=0.02, p=0.04) and total PTGI (r=0.20, p=0.05) subscales were all found to be significantly associated with optimism as measured by the Life Orientation Test (LOT).
Expressed Emotion and Posttraumatic Growth
No significant relationships were observed between PTG and expressed emotion with the exception of the anxiety subscale of the Courtland Emotional Control Scale (CECS). Patients who were more likely to express anxiety were also more likely to have higher scores on the Appreciation of Life subscale of the PTGI (r=0.20, p=0.05).
Depression, Quality of Life, and Posttraumatic Growth
Associations between PTG and quality of life as well as depression were also examined; however, no relationships between these variables and overall PTG and quality of life or depression were found. At diagnosis, patients who scored a 16 or above on the CES-D (clinical range) were more likely to report a higher Appreciation of Life subscale score [F(1,52)=7.1, p=0.01; mean=12.5, SD=6)] versus those who had a CES-D score of less than 16 (mean=8.2, SD=5.0).
Discussion
This is the first study to examine posttraumatic growth in patients diagnosed with hepatobiliary carcinoma and their family caregivers. Using the Posttraumatic Growth Inventory, both the patient (self) and caregiver (patient rating and self rating), were shown to have adequate reliability in this population. Comparing the PTGI mean subscale scores, patients with hepatobiliary carcinoma are generally found to report lower PTG when compared to patients with other cancer types (see Figure 2). Breast cancer and bone marrow transplant patients generally report higher scores (Cordova, Cunningham et al. 2001; Weiss 2002; Sears, Stanton et al. 2003; Manne, Ostroff et al. 2004), whereas colorectal, prostate, and other patients at the end of life tend to report lower mean PTGI scores (Widows, Jacobsen et al. 2005; Thornton and Perez 2006; Mystakidou, Parpa et al. 2007). Differences in PTGI scores may explained by differences observed in cancer types, age of diagnosis, and severity of disease. The cancer types which had lower PTGI scores were predominantly male (e.g., prostate, colorectal, and hepatobiliary), which is consistent with the general literature concerning PTGI in which females tend to report higher levels of PTG.
A small sample of patients followed from diagnosis to 6-months did not show any statistically significant change in PTG with the exception of Relating to Others in which a significant change over time was observed. These results are preliminary and with a larger sample followed prospectively for greater than 6-months may yield changes in the frequency or level of PTG. Also, although these changes over time were not statistically significant, the changes may have been clinically meaningful differences. Further understanding of the development and process of PTG is warranted, however the findings regarding the stability of PTG overtime wais consistent with the literature on PTG in breast cancer patients (Manne, Ostroff et al. 2004).
To demonstrate the observability of growth and to potentially differentiate the construct of PTG from positive reappraisal or reframing, caregiver ratings of patient PTG were examined. High levels of agreement were observed between the patient and caregiver on nearly all of the subscales of the PTGI, including Relating to Others, Spiritual Change, Personal Strength, and total PTGI score. No other known studies have analyzed this relationship among proxy ratings of patient PTG.
Alternative explanations may include that the (1) caregiver ratings of the patient are also correlated with caregiver ratings of their own growth, which suggests that caregivers may have simply viewed patients through their own experiences; or (2) patient growth was correlated with optimism, which again suggests that growth is more a product of reframing or reattribution, and (3) that the caregivers simply observe that the patient seems to feel as if he or she had grown. Further research is warranted to better understand the associations observed in the present study.
Prior trauma (within the past 3 years including the loss of a loved one or serious injury) was found to be associated with PTG at the time of diagnosis. Although the assessment of PTG in this study was in regard to the patients' cancer diagnosis, recent trauma may have primed the experience of PTG when diagnosed with cancer. Prior research has suggested that previous traumatic event may be instrumental in allowing individuals to cope with future stressors (Park, Mills-Baxter et al. 2005).
Optimism was also highly correlated with PTG, including significant associations with Appreciation of Life, Personal Strength and total PTGI subscales. The results of this study are consistent with previous research with head and neck cancer patients in which optimism was found to be a predictor of PTG (Harrington, McGurk et al. 2008). Similarly, PTG and optimism were found to be significantly associated in former Vietnam prisoners of war (Feder, Southwick et al. 2008) and patients diagnosed with breast cancer (Antoni, Lehman et al. 2001). With the exception of the expression of anxiety and association with the Appreciation subscale of the PTGI, no significant associations were found between expressed emotion and overall PTG in this study. Although a paucity of research exists regarding PTG and expressed emotion, our results were not consistent with other reports in the literature which may suggest an association between these two constructs (Park, Aldwin et al. 2008).
Posttraumatic growth was not found to be associated with quality of life and only patients scoring at least a 16 on the CES-D were found to be more likely to report higher scores on the Appreciation subscale scores. This lack of an association between PTG and depression is consistent with the findings in breast and colorectal cancer populations (Cordova, Cunningham et al. 2001; Salsman, Segerstrom et al. 2009), but the lack of an association with quality of life is inconsistent with the literature on breast cancer (Stanton, Danoff-Burg et al. 2002; Schwarzer, Luszczynska et al. 2006). In a sample of women with breast cancer post-surgery (at 1- and 4-7 years), higher initial benefit finding predicted better quality of life at follow-up (Carver and Antoni 2004). Due to the poor prognosis, high rates of depression, and poor quality of life at the time of diagnosis in this population, a lack of association between PTG and these clinical outcomes may have been observed. It may be that the process of PTG may require time and emotional resources that may not be available in patients who are confronted with such a life threatening diagnosis.
Alternatively, a recent paper by Frazier and colleagues (2009) reported in a study of colleague students who reported PTG before and after a traumatic experience, that perceived growth was associated with increased distress (Frazier, Tennen, Gavian, Park, Tomich, & Tashiro, 2009). In contrast, actual growth was related to decreased distress, suggesting that perceived and actual growth reflect different processes (Frazier et al, 2009). These authors also found that perceived (but not actual) growth was related to positive reinterpretation coping (Frazier et al, 2009). These findings may explain some of the inconsistencies found in the literature regarding the association between PTG and psychological and health outcomes.
Both the caregivers' rating of the patients' PTG as well as the caregiver's own PTG was highly correlated with patient PTG. Posttraumatic growth is often a measure of much controversy, yet this association supports the construct of PTG as observable in individuals diagnosed with hepatobiliary carcinoma. The duration as well as the proximity of the relationship between patients and caregivers included in this study likely contributed to the high level of agreement. The duration of the majority of relationships was over 30 years and many of the caregivers were intensively involved in the patients' end of life care. Alternatively, the high level of agreement may suggest that (1) an individual who has greater levels of PTG may be more likely to report a loved one's PTG, (2) people who have PTG may be more likely to partner with people who are more likely to experience PTG after a traumatic event, or (3) the patient or the caregiver may influence the others' level of PTG after a traumatic event.
The present study has several limitations. The results should be considered preliminary as the sample size at follow-up is small and data collection is on-going. Additionally, although females were included in this study, different conclusions might have been reached if the sample constituted a greater number of women. This study found no association between PTG and clinical outcomes, although additional patient follow-up data and more detailed medical, psychological, and family history data could reveal an association similar to other reports.
In summary, PTG is not commonly studied in cancer populations that present at end of life stages. The present study not only contributes to the literature on the association of proxy ratings of PTG, but also reinforces the role of caregivers in the growth process as indicated by agreement between self-reported patient PTG and the caregiver's proxy rating of patient PTG. Ongoing data collection will examine if caregiver PTG is preventative in regard to complicated bereavement after caregiving has ended.
References
- Allan MM, Giles M. Psychometric properties of Scheier and Carver's Life Orientation Test in a sample of Australian prisoners. Psychol Rep. 2008;103(1):305–22. doi: 10.2466/pr0.103.1.305-322. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lehman JM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology. 2001;20(1):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa KT, Kim J, et al. Measuring quality of life among cervical cancer survivors: preliminary assessment of instrumentation validity in a cross-cultural study. Qual Life Res. 2008;17(1):147–57. doi: 10.1007/s11136-007-9276-3. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Alderfer MA, et al. Posttraumatic growth in adolescent survivors of cancer and their mothers and fathers. J Pediatr Psychol. 2006;31(4):413–9. doi: 10.1093/jpepsy/jsj058. [DOI] [PubMed] [Google Scholar]
- Cadell S. The Sun Always Comes Out after It Rains: Understanding Posttraumatic Growth in HIV Caregivers. Health & Social Work. 2007;32(3):169–176. doi: 10.1093/hsw/32.3.169. [DOI] [PubMed] [Google Scholar]
- Carver CS, Antoni MH. Finding benefit in breast cancer during the year after diagnosis predicts better adjustment 5 to 8 years after diagnosis. Health Psychology. 2004;23(6):595–8. doi: 10.1037/0278-6133.23.6.595. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cordova MJ, Cunningham LL, et al. Posttraumatic growth following breast cancer: a controlled comparison study. Health Psychology. 2001;20(3):176–85. [PubMed] [Google Scholar]
- Duncan E, Gidron Y, et al. The effects of guided written disclosure on psychological symptoms among parents of children with cancer. J Fam Nurs. 2007;13(3):370–84. doi: 10.1177/1074840707303843. [DOI] [PubMed] [Google Scholar]
- Elliot DM. Traumatic Events Survey. University of California, Los Angeles, School of Medicine; 1992. [Google Scholar]
- Feder A, Southwick SM, et al. Posttraumatic Growth in Former Vietnam Prisoners of War. Psychiatry. 2008;71(4):359–370. doi: 10.1521/psyc.2008.71.4.359. [DOI] [PubMed] [Google Scholar]
- Frazier P, Tennen H, Gavian M, Park C, Tomich P, Tashiro Ty. Does Self Reported Posttraumatic Growth Reflect Genuine Positive Change? Psychological Science. 2009;20(7):912–919. doi: 10.1111/j.1467-9280.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- Hann D, Winter K, et al. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46(5):437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Harrington S, McGurk M, et al. Positive consequences of head and neck cancer: key correlates of finding benefit. J Psychosoc Oncol. 2008;26(3):43–62. doi: 10.1080/07347330802115848. [DOI] [PubMed] [Google Scholar]
- Heffernan N, Cella D, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20(9):2229–39. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- Ho RT, Chan CL, et al. Emotional control in Chinese female cancer survivors. Psychooncology. 2004;13(11):808–17. doi: 10.1002/pon.799. [DOI] [PubMed] [Google Scholar]
- Ho SM, Chan CL, et al. Posttraumatic growth in Chinese cancer survivors. Psycho-Oncology. 2004;13(6):377–89. doi: 10.1002/pon.758. [DOI] [PubMed] [Google Scholar]
- Jorngarden A, Mattsson E, et al. Health-related quality of life, anxiety and depression among adolescents and young adults with cancer: a prospective longitudinal study. Eur J Cancer. 2007;43(13):1952–8. doi: 10.1016/j.ejca.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Kim Y, Schulz R, et al. Benefit-finding in the cancer caregiving experience. Psychosom Med. 2007;69(3):283–91. doi: 10.1097/PSY.0b013e3180417cf4. [DOI] [PubMed] [Google Scholar]
- Lechner SC, Carver CS, et al. Curvilinear associations between benefit finding and psychosocial adjustment to breast cancer. J Consult Clin Psychol. 2006;74(5):828–40. doi: 10.1037/0022-006X.74.5.828. [DOI] [PubMed] [Google Scholar]
- Lee EH, Chun M, et al. Validation of the Functional Assessment of Cancer Therapy-General (FACT-G) scale for measuring the health-related quality of life in Korean women with breast cancer. Jpn J Clin Oncol. 2004;34(7):393–9. doi: 10.1093/jjco/hyh070. [DOI] [PubMed] [Google Scholar]
- Levine EG, Aviv C, et al. The benefits of prayer on mood and well-being of breast cancer survivors. Support Care Cancer. 2008 doi: 10.1007/s00520-008-0482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MA, Golant M, et al. Electronic support groups for breast carcinoma: a clinical trial of effectiveness. Cancer. 2003;97(4):920–5. doi: 10.1002/cncr.11145. [DOI] [PubMed] [Google Scholar]
- Manne S, Ostroff J, et al. Posttraumatic growth after breast cancer: patient, partner, and couple perspectives. Psychosomatic Medicine. 2004;66(3):442–54. doi: 10.1097/01.psy.0000127689.38525.7d. [DOI] [PubMed] [Google Scholar]
- Martin LL, Kleiber DA. Letting Go of the Negative: Psychological Growth From a Close Brush with Death. Traumatology. 2005;11(4):221–232. [Google Scholar]
- McMillen C, Zuravin S, et al. Perceived benefit from child sexual abuse. Journal of Consulting & Clinical Psychology. 1995;63(6):1037–43. doi: 10.1037//0022-006x.63.6.1037. [DOI] [PubMed] [Google Scholar]
- Morrill EF, Brewer NT, et al. The interaction of post-traumatic growth and post-traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology. 2008;17(9):948–53. doi: 10.1002/pon.1313. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Danoff-Burg S, et al. Post-traumatic growth and psychosocial adjustment of daughters of breast cancer survivors. Oncol Nurs Forum. 2006;33(3):543–51. doi: 10.1188/06.ONF.543-551. [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Parpa E, et al. Traumatic distress and positive changes in advanced cancer patients. Am J Hosp Palliat Care. 2007;24(4):270–6. doi: 10.1177/1049909107299917. [DOI] [PubMed] [Google Scholar]
- Mystakidou K, Tsilika E, et al. Post-traumatic growth in advanced cancer patients receiving palliative care. Br J Health Psychol. 2008;13(Pt 4):633–46. doi: 10.1348/135910707X246177. [DOI] [PubMed] [Google Scholar]
- Okun A, Stein RE, et al. Content validity of the Psychiatric Symptom Index, CES-depression Scale, and State-Trait Anxiety Inventory from the perspective of DSM-IV. Psychol Rep. 1996;79(3 Pt 1):1059–69. doi: 10.2466/pr0.1996.79.3.1059. [DOI] [PubMed] [Google Scholar]
- Park CL. Overview of Theoretical Perspectives. In: Park CL, Lechner SC, Stanton AL, Antoni MH, editors. Medical Illness and Positive Life Change: Can Crisis Lead to Personal Transformation. American Psychological Association; 2008. [Google Scholar]
- Park CL, Aldwin CM, et al. Pathways to posttraumatic growth versus posttraumatic stress: coping and emotional reactions following the September 11, 2001, terrorist attacks. Am J Orthopsychiatry. 2008;78(3):300–12. doi: 10.1037/a0014054. [DOI] [PubMed] [Google Scholar]
- Park CL, Mills-Baxter MA, et al. Post-Traumatic Growth from Life's Most Traumatic Event: Influence on Elders' Current Coping and Adjustment. Traumatology. 2005;11(4):297–306. [Google Scholar]
- Polatinsky S, Esprey Y. An assessment of gender differences in the perception of benefit resulting from the loss of a child. J Trauma Stress. 2000;13(4):709–18. doi: 10.1023/A:1007870419116. [DOI] [PubMed] [Google Scholar]
- Powell T, Ekin-Wood A, et al. Post-traumatic growth after head injury: a long-term follow-up. Brain Inj. 2007;21(1):31–8. doi: 10.1080/02699050601106245. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The Center for Epidemiological Studies Depression Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rosenbach C, Renneberg B. Positive change after severe burn injuries. J Burn Care Res. 2008;29(4):638–43. doi: 10.1097/BCR.0b013e31817de275. [DOI] [PubMed] [Google Scholar]
- Salsman JM, Segerstrom SC, et al. Posttraumatic growth and PTSD symptomatology among colorectal cancer survivors: a 3-month longitudinal examination of cognitive processing. Psychooncology. 2009;18(1):30–41. doi: 10.1002/pon.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter E, Stallard P. Posttraumatic growth in child survivors of a road traffic accident. Journal of Traumatic Stress. 2004;17(4):335–40. doi: 10.1023/B:JOTS.0000038482.53911.01. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4(3):219–47. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, et al. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schulz U, Mohamed NE. Turning the tide: benefit finding after cancer surgery. Soc Sci Med. 2004;59(3):653–62. doi: 10.1016/j.socscimed.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Luszczynska A, et al. Changes in finding benefit after cancer surgery and the prediction of well-being one year later. Social Science & Medicine. 2006;63(6):1614–24. doi: 10.1016/j.socscimed.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sears SR, Stanton AL, et al. The yellow brick road and the emerald city: benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. Health Psychology. 2003;22(5):487–97. doi: 10.1037/0278-6133.22.5.487. [DOI] [PubMed] [Google Scholar]
- Shakespeare-Finch J, Enders T. Corroborating evidence of posttraumatic growth. J Trauma Stress. 2008;21(4):421–4. doi: 10.1002/jts.20347. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, et al. Randomized, controlled trial of written emotional expression and benefit finding in breast cancer patients. Journal of Clinical Oncology. 2002;20(20):4160–8. doi: 10.1200/JCO.2002.08.521. [DOI] [PubMed] [Google Scholar]
- Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. Journal of Traumatic Stress. 1996;9(3):455–71. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Thornton AA, Perez MA. Posttraumatic growth in prostate cancer survivors and their partners. Psychooncology. 2006;15(4):285–96. doi: 10.1002/pon.953. [DOI] [PubMed] [Google Scholar]
- Tomich PL, Helgeson VS. Is finding something good in the bad always good? Benefit finding among women with breast cancer. Health Psychology. 2004;23(1):16–23. doi: 10.1037/0278-6133.23.1.16. [DOI] [PubMed] [Google Scholar]
- Watson M, Greer S. Development of a questionnaire measure of emotional control. J Psychosom Res. 1983;27(4):299–305. doi: 10.1016/0022-3999(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Weinrib AZ, Rothrock NE, et al. The assessment and validity of stress-related growth in a community-based sample. J Consult Clin Psychol. 2006;74(5):851–8. doi: 10.1037/0022-006X.74.5.851. [DOI] [PubMed] [Google Scholar]
- Weiss T. Posttraumatic growth in women with breast cancer and their husbands: an intersubjective validation study. Journal of Psychosocial Oncology. 2002;20(2):65–80. [Google Scholar]
- Weiss T. Correlates of posttraumatic growth in husbands of breast cancer survivors. Psychooncology. 2004;13(4):260–8. doi: 10.1002/pon.735. [DOI] [PubMed] [Google Scholar]
- Widows MR, Jacobsen PB, et al. Predictors of posttraumatic growth following bone marrow transplantation for cancer. Health Psychol. 2005;24(3):266–73. doi: 10.1037/0278-6133.24.3.266. [DOI] [PubMed] [Google Scholar]
- Zhu ZC, Lang QB, et al. Evaluation of Chinese version of the Functional Assessment of Cancer Therapy-Hepatobiliary questionnaire. Zhong Xi Yi Jie He Xue Bao. 2008;6(4):341–5. doi: 10.3736/jcim20080403. [DOI] [PubMed] [Google Scholar]


