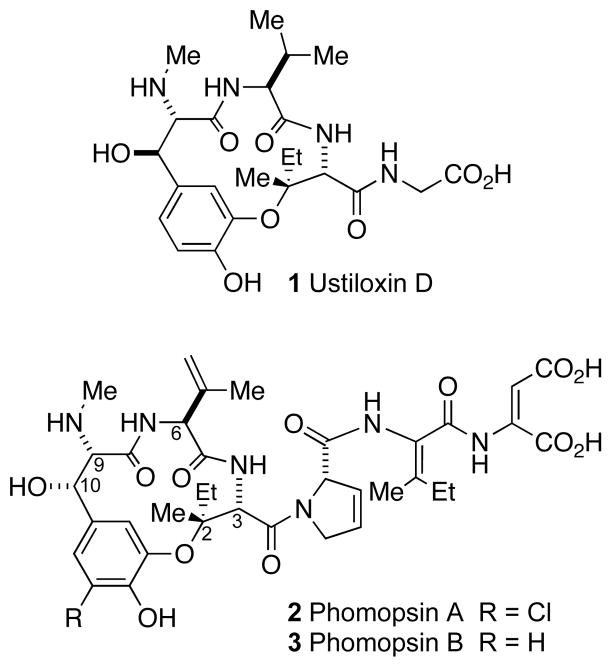
A variety of natural products and synthetic molecules cause mitotic arrest by interfering with microtubule function, and a few molecules of this mechanistic class are anticancer treatments.[1] The ustiloxin and phomopsin families of natural products (Scheme 1) are potent microtubule depolymerizers isolated from the fungus species Ustilaginoidea virens and Phomopsis leptostromiformis, respectively.[2] Members of both families possess a similar 13-membered macrolactam. Relative to the ustiloxins, the phomopsins contain an unsaturated sidechain, display dehydration of the valine residue within the macrocycle, and possess the opposite configuration of the secondary alcohol at the C10 position. Competitive tubulin binding assays demonstrated that one binding site for these compounds overlaps with the vinca alkaloid binding site. A secondary tubulin binding site for the phomopsins is as yet unidentified.[3] Because ustiloxin D (1) is only slightly less active than phomopsin A (2), it has been suggested that the macrocycle alone may be responsible for much of the biological activity.[4] Three total syntheses of ustiloxin D have been reported, one from this laboratory and two from the Joullié group.[5] We have used this knowledge, along with our previously reported progress, to synthesize the complex antimitotic phomopsin B (3).[6]
Scheme 1.
Structures of ustiloxin D and phomopsins.
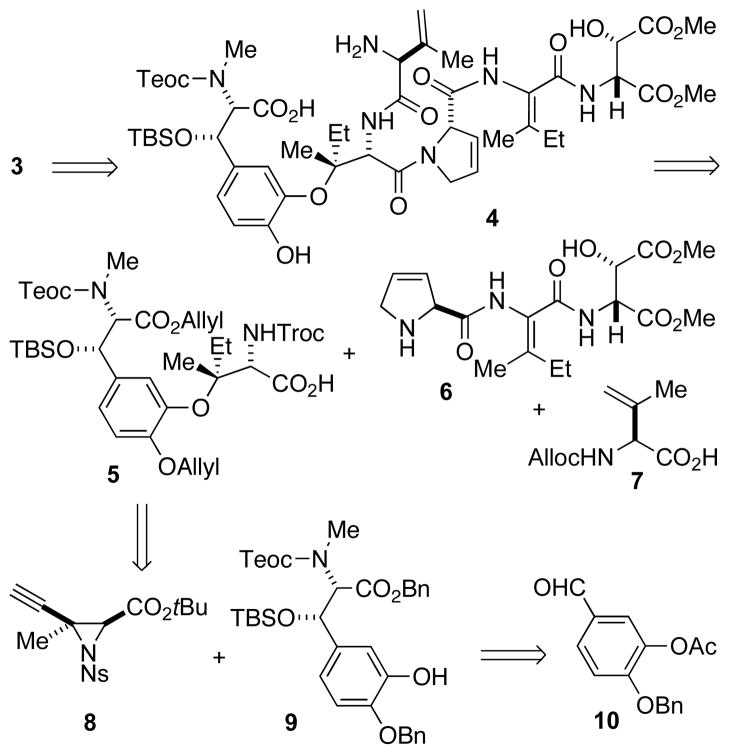
Phomopsin B is a hexapeptide although it contains none of the standard proteinogenic amino acids. Rather, there are four unsaturated residues, two oxidized residues, and an aryl-alkyl ether sidechain connection. A logical initial disconnection for phomopsin B would involve independent construction of both the macrocycle acid and sidechain amine, to be joined by peptide coupling. Unfortunately, such a strategy could not be realized[5c] and our present synthesis necessitated incorporation of the sidechain, prior to macrocycle closure (Scheme 2). In spite of this accomodation, our established syntheses of both the tripeptide sidechain precursor 6[6c] and ΔVal 7[6b] could be used when coupled in sequence to intermediate 5 (Scheme 2). We chose to incorporate a precursor to the last residue of the tripeptide sidechain because E-ΔAsp isomerizes to Z-ΔAsp in alkaline solution.[7]
Scheme 2.
Retrosynthesis of phomopsin B. Ac = acetate, Alloc = allyloxycarbonyl, Bn = benzyl, Ns = 2-nitrobenzenesulfonyl, TBS = t-butyldimethylsilyl, Teoc = 2-(trimethylsilyl)ethoxycarbonyl, Troc = 2,2,2-trichloroethoxycarbonyl.
Although innovative approaches to prepare the vicinal C2-C3 stereocenters of aryl-alkyl ether 5 had been reported, these strategies suffered from a lack of brevity and selectivity, respectively.[6a,5b] We envisioned that a recently described use of a phenolate to open a copper(I)-activated alkynyl aziridine[5d] could be used to construct this fragment. This convergent strategy would reduce the complexity to aziridine 8, a derivative of a published intermediate,[5d] and phenol 9 (Scheme 2). Phenol 9 could be formed using an aldol reaction between 3,4-dihydroxybenzaldehyde derivative 10 and a glycine equivalent that we used in our synthesis of ustiloxin D.[5b]
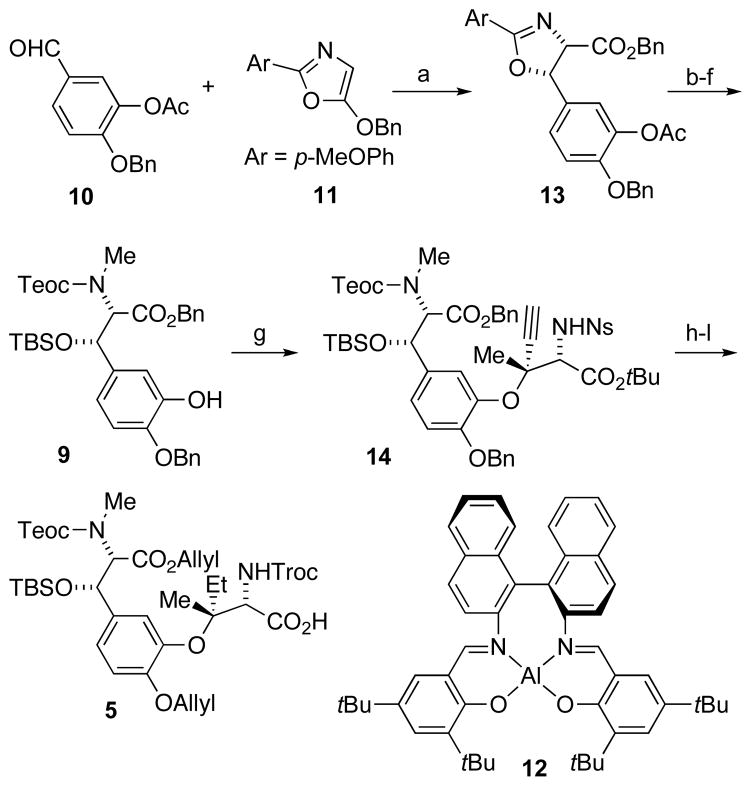
Our synthesis began by reacting benzaldehyde derivative 10 with oxazole 11[8] using Evans’ chiral salen-Al catalyst[9] to produce the cis-oxazoline 13 (Scheme 3). As evidence of the versatility of this strategy, we had previously used the opposite enantiomer of the Al catalyst to construct a diastereomeric trans-oxazoline for the synthesis of ustiloxin D.[5b] In contrast to ustiloxin’s robust trans-oxazoline, however, phomopsin’s cis-oxazoline undergoes rapid isomerization under alkaline conditions, mandating its early unmasking to the underlying N-methyl amino alcohol. Fluoride-labile groups were installed as part of a global end-game deprotection strategy, followed by deacetylation under mild conditions to furnish phenol 9 in excellent yield over five steps. Gentle deacetylation conditions were required to prevent epimerization of the C9 Teoc-protected secondary amine.
Scheme 3.
Reagents and conditions: a) Na2SO4, 12, AgSbF6, 3 Å MS, PhCH3, 25 h, 96%, 98% ee; b) MeOTf, CH2Cl2, 2 h then NaBH4, NaHCO3, H2O, 0 °C, 15 min, 72%; c) 0.1 N (COOH)2, THF, 24 h, 99%; d) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C, 30 min, 87%; e) Teoc-Cl, NaHCO3, CH3CN, 16 h, 98%; f) NH2NH2·H2O, THF, 0 °C, 3 h, 98%; g) 8, CuOAc, DBU, PhCH3, 0 °C, 12 d, 87%; h) PhSH, Cs2CO3, DMF, 1 h, 92%; i) 2,2,2-trichloroethyl chloroformate, Na2CO3, THF, 1 h, 96%; j) H2, 20% Pd(OH)2/C, EtOAc, 4 d, 99%; k) allyl bromide, DBU, DMF, 5 h, 70%; l) SiO2, PhCH3, 90 °C, 20 h. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; DMF = N,N-dimethylformamide; MS = molecular sieves; Tf = trifluoromethanesulfonate.
Minor modifications to published procedures enabled facile construction of aziridine 8 (see Supporting Information), setting the stage for the aryl-alkyl etherification. Our initial attempts to form the Tyr-Ile ether followed the Joullié conditions of 2:1 phenol:aziridine stoichiometry with 1% copper(I) acetate.[5d] However, these conditions produced only 18% of the desired product. The majority of phenol 9 was recovered, but the aziridine was completely consumed. Optimized conditions required stoichiometric inversion using two equivalents of aziridine 8 and 1% copper(I) acetate to provide the desired aryl-alkyl ether 14 in 87% yield. The perfect regioselectivity of the aziridine opening sets the vicinal stereochemical centers at C2 and C3 in one step.
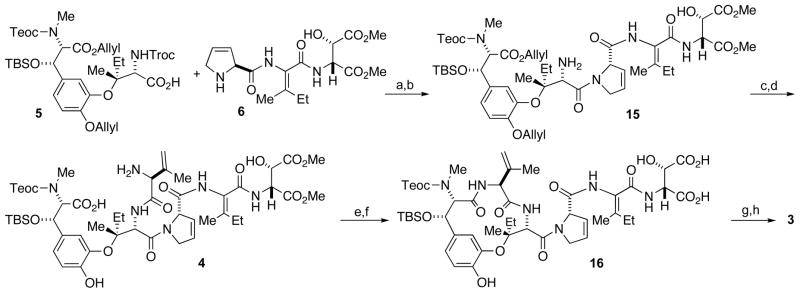
The next major synthetic hurdle involved appending the tripeptide sidechain and ΔVal. However, before these unsaturated fragments could be incorporated, a redox adjustment of the alkynyl group was required. We therefore exchanged the C3-amine protecting group and exposed the fully protected Tyr-Ile dipeptide to hydrogen and Pearlman’s catalyst to hydrogenate the alkyne and concomitantly hydrogenolyze the two benzyl groups in 99% yield. We next installed an orthogonal set of blocking agents to mask the newly liberated oxygens. We could now selectively reveal the C17 carboxylic acid by treatment with silica gel in 90 °C toluene[10] and produce intermediate 5, which is poised for further elaboration with the dehydrated amino acids.
The preparation of tripeptide 6 has been outlined previously.[6c] Using our methodology, the E-ΔIle residue was synthesized by the anti-elimination of cyclic sulfamidite diastereomers. Carboxylic acid 5 and tripeptide 6 were coupled in satisfactory yield to produce adduct 15 (Scheme 4). Following Troc deprotection the final amino acid, Alloc-ΔVal-OH 7, was coupled to provide the complete carbon skeleton. We next employed palladium to deprotect the C8 allyl ester, the N7-Alloc-protected amine, and phenolic allyl ether to reveal the desired macrocycle precursor in amino acid 4.[11] Macrocyclization was favored over intermolecular coupling by using high dilution conditions (3 mM) to provide the desired macrocycle in moderate yield.
Scheme 4.
Reagents and conditions: a) PyBOP, iPr2NEt, CH3CN, 14 h, 28% (2 steps); b) Zn, HOAc, 12 h, 77%; c) 7, PyBOP, iPr2NEt, CH3CN, 1 h, 79%; d) Pd(PPh3)4, nBu3SnH, AcOH, CH2Cl2, 1 h, 74%; e) PyAOP, iPr2NEt, DMF, 12 h, 48%; f) LiOH, THF, 0 °C to rt, 75 min; g) Ac2O, pyr, 0 °C, 1.5 h then LiOH, THF, 15 min; h) TAS-F, DMF, 15 h, 17% (3 steps); PyAOP = (7-azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate; PyBOP = (benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate; TAS-F = tris(dimethylamino)sulfonium difluorotrimethylsilicate.
Saponification of the terminal methyl esters provided the β-hydroxy-Asp intermediate 16. Model studies had shown that use of acetic anhydride and pyridine successfully dehydrated the β-hydroxyaspartic acid residue to produce a mixture of the desired E-ΔAsp and the doubly-dehydrated cyclic anhydride. This mixture could be converged into the desired E-ΔAsp species following brief exposure to lithium hydroxide. Care was taken in the hydrolysis step because prolonged alkaline exposure results in isomerization to the undesired, thermodynamic Z-ΔAsp product. Finally, the TBS and Teoc groups were removed in one step using anhydrous fluoride conditions of TAS-F in DMF to yield phomopsin B (3) following reverse phase HPLC purification.[12]
The identity of the synthetic phomopsin B was confirmed by MS, HPLC, and NMR. Interestingly, the 1H, 1H-13C HSQC, and 1H-13C HMBC spectra of the HPLC-purified synthetic and natural compounds were similar but not identical.[13] Upon mixing equimolar amounts of the two samples together the spectra display one set of resonances, demonstrating that both samples are phomopsin B (see Supporting Information). HPLC analysis supports this conclusion (see Supporting Information). The HPLC analysis further revealed that phomopsin B exists as an equilibrium mixture of two species of significantly distinct polarities. We isolated each of the peaks by HPLC and observed that the two species interconvert, suggesting that one is not a decomposition product of the other (see Supporting Information). Additionally, LC-MS analysis shows that both peaks report identical molecular ions (M+H=755 and M–H=753 for C36H46N6O12). It is possible that the acidic HPLC conditions are responsible for the establishment of an equilibrium mixture, as there is no evidence of two distinct species by NMR analysis. Alternatively, it is possible that any spectroscopic differences between the two species are so slight as to prevent detection by NMR, especially given the limited quantities of sample and the existence of atropisomers (i.e., amide rotamers) within phomopsin B that complicate spectroscopic analyses.
In summary, we accomplished the first total synthesis of phomopsin B in a longest linear sequence of 26 steps. Our convergent synthetic strategy will allow us to probe structure-activity relationships between members of this family of molecules and their microtubule targets. Of the key steps in our synthesis, the aldol reaction, the regioselective aziridine ring opening, and the modular coupling of a valine derivative can be exploited to introduce alternate stereochemistries and altogether new connectivities within the macrocycle.
Supplementary Material
Footnotes
Financial support was provided by the NIH (GM073046), the California Tobacco-Related Disease Research Program (15DT-0015) to J.S.G. and by NSERC Canada for a PGSB Fellowship to A.M.S.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the
Contributor Information
Joshua S. Grimley, Department of Chemistry, Stanford University, Clark Center W350A, 318 Campus Drive, Stanford, CA 94305.
Dr. Andrew M. Sawayama, Department of Chemistry, Stanford University, Clark Center W350A, 318 Campus Drive, Stanford, CA 94305.
Dr. Hiroko Tanaka, Department of Chemistry, Stanford University, Clark Center W350A, 318 Campus Drive, Stanford, CA 94305
Dr. Michelle M. Stohlmeyer, Department of Chemistry, Stanford University, Clark Center W350A, 318 Campus Drive, Stanford, CA 94305
Dr. F. Woiwode Thomas, Department of Chemistry, Stanford University, Clark Center W350A, 318 Campus Drive, Stanford, CA 94305
Prof. Thomas J. Wandless, Email: wandless@stanford.edu, Department of Chemical and Systems Biology, Stanford University, Clark Center W350A, 318 Campus Drive, Stanford, CA 94305, Fax: (+1) 650-725-4665
References
- 1.Jordan MA, Wilson L. Nat Rev Cancer. 2004;4:253. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Ustiloxins: Koiso Y, Natori S, Iwasaki S, Sato S, Sonoda R, Fujita Y, Yaegashi H, Sato Z. Tetrahedron Lett. 1992;33:4157.Koiso Y, Li Y, Iwasaki S. J Antibiot. 1994;47:765. doi: 10.7164/antibiotics.47.765.Phomopsins: Culvenor CCJ, Beck AB, Clarke M, Cockrum PA, Edgar JA, Frahn JL, Jago MW, Lanigan GW, Pane AL, Peterson JE, Smith LW, White RR. Aust J Biol Sci. 1977;30:269. doi: 10.1071/bi9770269.MacKay MF, Van Donkelaar A, Culvenor CCJ. J Chem Soc, Chem Commun. 1986:1219.Culvenor CCJ, Edgar JA, MacKay MF. Tetrahedron. 1989;45:2351.
- 3.Iwasaki S. Med Res Rev. 1993;13:183. doi: 10.1002/med.2610130205. [DOI] [PubMed] [Google Scholar]
- 4.Morisaki N, Mitsui Y, Yamashita Y, Koiso Y, Shirai R, Hashimoto Y, Iwasaki S. J Antibiot. 1998;51:423. doi: 10.7164/antibiotics.51.423. [DOI] [PubMed] [Google Scholar]
- 5.a) Cao B, Park H, Joullié MM. J Am Chem Soc. 2002;124:520. doi: 10.1021/ja017277z. [DOI] [PubMed] [Google Scholar]; b) Tanaka H, Sawayama AM, Wandless TJ. J Am Chem Soc. 2003;125:6864. doi: 10.1021/ja035429f. [DOI] [PubMed] [Google Scholar]; c) Sawayama AM, Tanaka H, Wandless TJ. J Org Chem. 2004;69:8810. doi: 10.1021/jo048854f. [DOI] [PubMed] [Google Scholar]; d) Li P, Evans D, Joullié MM. Org Lett. 2005;7:5325. doi: 10.1021/ol052287g. [DOI] [PubMed] [Google Scholar]
- 6.a) Woiwode TF, Rose C, Wandless TJ. J Org Chem. 1998;63:9594. [Google Scholar]; b) Woiwode TF, Wandless TJ. J Org Chem. 1999;64:7670. [Google Scholar]; c) Stohlmeyer MM, Tanaka H, Wandless TJ. J Am Chem Soc. 1999;121:6100. [Google Scholar]
- 7.Shin C, Obara T, Morita S, Yonezawa Y. Bull Chem Soc Jpn. 1988;61:3265. [Google Scholar]
- 8.Wipf P, Miller CP. J Org Chem. 1993;58:3604. [Google Scholar]
- 9.Evans DA, Janey JM, Magomedov N, Tedrow JS. Angew Chem. 2001;113:1936. [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:1884. [PubMed] [Google Scholar]
- 10.Jackson RW. Tetrahedron Lett. 2001;42:5163. [Google Scholar]
- 11.Dangles O, Guibé F, Balavione G, Lavielle S, Marquet A. J Org Chem. 1987;52:4984. [Google Scholar]
- 12.Noyori R, Nishida I, Sakata J, Nishizawa M. J Am Chem Soc. 1980;102:1223. [Google Scholar]
- 13.We thank Prof. John Edgar at Australia’s Commonwealth Scientific and Industrial Research Organization for providing us with samples of authentic phomopsins A and B. We also thank Prof. Justin Du Bois for his advice throughout this project.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.