Abstract
Expression of class III β-tubulin (βIII-tubulin) correlates with tumor progression and resistance to taxane-based therapies for several human malignancies but its use as a biomarker of tumor behaviour in prostate cancer (PCa) remains largely unexplored. Here, we describe βIII-tubulin immunohistochemical staining patterns of prostate tumors obtained from a broad spectrum of PCa patients, some of whom subsequently received docetaxel therapy for castration-resistant prostate cancer (CRPC). Elevated βIII-tubulin expression was significantly associated with tumor aggressiveness in PCa patients with presumed localized disease as it was found to be an independent marker of biochemical recurrence after treatment. Additionally, βIII-tubulin expression in tumor cells was an independent predictor of lower overall survival for patients receiving docetaxel-based chemotherapy for CRPC. Manipulation of βIII-tubulin expression in human prostate cancer cell lines using a human βIII-tubulin expression vector or βIII-tubulin siRNA altered cell survival in response to docetaxel treatment in a manner that supports a role for βIII-tubulin expression as a mediator of PCa cell resistance to docetaxel therapy. Our findings suggest a role for βIII-tubulin as candidate theranostic biomarker to predict the response to docetaxel-based chemotherapy as well as to target for treatment of docetaxel-resistant CRPC.
Keywords: Aged; Antineoplastic Agents; therapeutic use; Cell Line, Tumor; Cell Survival; drug effects; genetics; Drug Resistance, Neoplasm; genetics; Humans; Immunoblotting; Immunohistochemistry; Male; Middle Aged; Multivariate Analysis; Neoplasm Invasiveness; Orchiectomy; Prognosis; Prostate; drug effects; metabolism; surgery; Prostatectomy; Prostatic Neoplasms; drug therapy; metabolism; pathology; RNA Interference; Survival Analysis; Taxoids; therapeutic use; Tubulin; biosynthesis; genetics; Tumor Markers, Biological; biosynthesis; genetics
Keywords: class III β-tubulin, prostate cancer, predictive value, docetaxel, therapeutic resistance, prognosis
INTRODUCTION
Prostate cancer (PCa) is the most common solid malignancy and the second leading cause of death attributable to cancer in men (1). Despite the widespread use of PSA testing to screen for early stage PCa, men continue to be diagnosed with locally advanced or metastatic disease, and approximately 30% of newly diagnosed patients treated with curative intent will eventually relapse during follow-up (2). Treatments at this advanced stage usually include androgen deprivation therapy to deplete systemic androgens in patients, thus reducing the levels of a known PCa growth factor. This treatment is most often only transiently effective because prostate tumor cells can progress to a seeming androgen-independent growth phase now diagnosed as castration resistant prostate cancer (CRPC) (3). Once at this stage, docetaxel-based chemotherapy has been shown to improve though the survival advantage is relatively limited (4, 5). At this time, several molecular markers have been proposed to have dependent or independent utility for PCa patient prognostic assessments (6, 7) but these latter biomarkers remain unproven. Here, we discuss our efforts to evaluate the potential utility of class III β-tubulin for purposes of prognostication of PCa patient response to early, localized therapy or to late-stage, taxane-based chemotherapy.
Taxanes constitute an important group of chemotherapeutic agents that specifically target the β-tubulin subunit of microtubules. By targeting microtubule activity, taxanes can block cell mitosis and induce apoptosis, especially in tumor cells (8). A growing body of preclinical and clinical data now suggests that increased expression of one particular β-tubulin isoform, class III β-tubulin (βIII-tubulin), confers cancer cell resistance to taxanes (9–11) and that βIII-tubulin resistance to taxanes is clinically relevant for human lung, breast, and ovarian cancers (12). For these human malignancies, high tumor cell expression of βIII-tubulin was associated with significantly poorer survival rates in patients treated with taxane-based chemotherapy (13–17). For lung cancer, one study suggested that tumor cell βIII-tubulin expression was a prognostic factor for men who did not receive adjuvant chemotherapy (18)
For PCa, despite evidence from in vitro studies showing that β-tubulin isotype expression was altered in paclitaxel-resistant cells, there has been only little informative clinical data evaluating the prognostic or predictive value of βIII-tubulin expression in patient tumor cells (19, 20). Previously, we showed that expression of βIII-tubulin was increased in castration-resistant prostate cancers and that expression of this tubulin isoform might have a role in progression to CRPC (21). The aim of the present study was to determine whether βIII-tubulin expression might have prognostic value for hormone-naïve PCa patients treated by surgery or for CRPC patients treated with taxane-based therapy (docetaxel). Here we evaluated clinical prostate cancer specimens for expression levels of βIII-tubulin by immunohistochemistry and we manipulated βIII-tubulin expression in prostate cancer cell lines to determine the effects of this manipulation on in vitro responsiveness to docetaxel.
MATERIALS AND METHODS
Cell culture
Human prostate cancer cell lines LNCaP (clone FGC), 22Rv1, and DU145 were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were maintained in RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin. For this study low passage cells were used (<20 passages). The LNCaP-AI variant (passage 16 to 20) was derived from LNCaP cultures maintained more than 18 months growth in androgen depleted medium (phenol red-free RPMI supplemented with 10% charcoal-stripped FBS). LNCaP and 22Rv1 were authenticated through cell morphology monitoring, response to androgen treatment, and expression of Androgen Receptor which harbor mutations in these lines (22–24). DU145 was authenticated by lack of AR expression, expression of mutant TP53 (24), and assessment of the invasive behavior using Boyden chamber assays.
Western blot analysis
Protein lysates were prepared in the RIPA buffer (radioimmunoprecipitation assay lysis buffer) supplemented with protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) and phosphatase inhibitors (25mmol/L orthovanadate and 50 mmol/L NaF) (Sigma-Aldrich). The total protein concentration of the soluble extract was determined using the Bicinchoninic Acid Kit (Sigma-Aldrich). Each protein sample (30 μg) was resolved to SDS–PAGE, transferred onto a polyvinylidene difluoride membrane (Millipore, Molsheim, France) and incubated with a monoclonal antibody against βIII-tubulin (1 : 10 000; clone TUJ1; Covance, Emeryville, CA) or β-actin (1 : 16 000; AC-15; Sigma-Aldrich). Primary antibodies against the other β-tubulin isotypes were from Abcam (San Francisco, CA). Immune complexes were visualised by enhanced chemiluminescence detection (ECL plus kit, GE Healthcare, Little Chalfont, UK).
cDNA synthesis and real-time PCR
Quantitative PCR was carried out using SYBR Green dye on an Applied Biosystems 7000 Real Time PCR system (Applied Biosystems, Foster City, CA, USA). The conditions for RT–PCR have been described previously (25). The amount of βIII-tubulin mRNA levels relative to the housekeeping gene Ribosomal Protein, large, P0 (RPLP0) was determined on the basis of the comparative threshold cycle CT method (2−Δ ΔCT). The primer sequences for βIII-tubulin and RPLP0 have been described previously (25, 26).
Construction of bIII-tubulin expression vector and Generation of stable bIII-tubulin overexpressing cells
The ORF encoding bIII-tubulin was purchased from Invitrogen (Ultimate ORF clone, clone ID IOH3755, NM 00608.2). The bIII-tubulin ORF was provided in the Gateway entry vector pENTR 221. The bIII-tubulin expression was constructed by recombining the bIII-tubulin pENTR 221 into the destination vector pcDNA 3.2/V5-DEST (Invitrogen) via the LR reaction according to the manufacturer’s instructions (Gateway LR clonase II enzyme mix, Invitrogen). The resulting vector was designated as pcDNA-TUBB3. LNCaP and DU145 cells were seeded at the density of 1.5x105 cells in 100-mm culture dishes in RPMI 1640 supplemented with 10% FBS. The next day, cells were transfected with 4 μg of pcDNA-TUBB3 vector using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instructions. Cells were selected in geneticin (G418, 400μg/mL) for 3 weeks. Resistant colonies were isolated and allowed to grow as monoclonal population. Clones expressing the different levels of bIII-tubulin were selected, as determined by western blot analysis, for further studies.
siRNA transfection
Small interfering RNA (siRNA) against TUBB3, and control NonTargeting siRNA were obtained from Invitrogen, Inc. Three specific Stealth RNAi sequences were tested: TUBB3HSS115886 (5′-GACAUCUCUUCAGGCCUGACAAUUU-3′), TUBB3HSS115887 (5′-GCAUCAUGAACACCUUCAGCGUCGU-3′), and TUBB3HSS173592 (5′-CAGCUGGAGCGGAUCAGCGUCUACU-3′). The non silencing control siRNA, which has no sequence homology to any known human gene sequence, was used as a control for nonsequence-specific effects in all experiments.
Subconfluent human prostate cells were transfected with siRNA by using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. 72 hours after the transfection, the efficacy of the siRNA knock down was assessed by qRT-PCR and by immunoblotting. The optimal amount of siRNA used for transfection was determined as being 50 nmol/L and the best siRNA sequence allowing to reduce more than 70% of bIII-tubulin expression was identified as the sequence TUBB3HSS115887.
Docetaxel dose response curve
To assess the effect of βIII-tubulin overexpression on chemoresistance, 1x104 cells were seeded in 96-well microtiter plates. The next day, cells were treated with docetaxel at growing concentrations for 72 hours. Cell viability was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay.
To assess the effect of the combination treatment of βIII-tubulin silencing plus docetaxel, 22Rv1 cells were transfected with 50 nM of stealth siRNA against βIII-tubulin or control vector as described above and then treated with docetaxel at various concentrations for three days. Cell viability was then determined by MTT assay.
The cell survival curve was presented as the percentage of surviving cells versus the concentration of docetaxel. The IC50 values were defined as the concentration of drug required for 50% cell survival, and were calculated using a logarithmic regression. Results were expressed as means ± SEM. Each assay was done in triplicate and was repeated on three separate experiments.
Patients and Tissue Samples
Written informed consent was obtained. The study included :
(i) Two hundred and fifty eight patients who had undergone RP for localized PCa between November 1988 and May 2007. Demographics, clinical and biological data, pathologic parameters, and outcomes in terms of PSA recurrence and adjuvant treatment of 258 patients with hormone-naïve PCa (HNPC) was collected prospectively in a database and reviewed in a retrospective manner. Clinical, biological, and pathologic parameters and follow-up data are listed in Table 1. No patient had received neoadjuvant therapy.
Table 1.
Patient’s cohort characteristics of the HNPC cohort.
| Age, years : | |
| Mean | 64.5 |
| Median | 65.0 |
| Range | 47.1–75.0 |
| PSA, ng/ml: | |
| Mean | 14.2 |
| Median | 9.4 |
| Range | 1.4–113.0 |
| Prostate weight, gr: | |
| Mean | 55.6 |
| Median | 50.0 |
| Range | 10.0–210.0 |
| Clinical stage, No (%): | |
| T1 | 175 (67.8) |
| T2 or more | 83 (32.2) |
| Gleason score, No (%): | |
| ≤6 | 123 (47.7) |
| 7 | 73 (28.3) |
| ≥8 | 62 (24.0) |
| pT stage, No (%): | |
| pT2 | 150 (58.1) |
| pT3 | 86 (33.4) |
| pT4 | 22 (8.5) |
| Extraprostatic extension, No (%): | 96 (37.2) |
| Positive margins, No (%): | 56 (21.7) |
| Seminal vesicle invasion, No (%): | 56 (21.7) |
| Positive lymph nodes, No (%): | 23 (8.9) |
| Duration of follow-up, months: | |
| Mean | 61.5 |
| Median | 48.3 |
| Range | 0.6–170.7 |
| Recurrence, No (%): | 62 (24.0) |
Clinico-pathological characteristics of patients with clinically hormone naïve prostate cancer (n=258).
HNPC: hormone naive prostate cancer.
(7) Thirty seven patients with CRPC stage and analyzable initial hormone-naive tissue who received docetaxel-based chemotherapy as first-line treatment between January 2002 and July 2008. Demographics, clinical and biological data, pathologic parameters, and outcomes in terms of PSA were collected prospectively in a database and reviewed in a retrospective manner. The mean age was 68.1 years and the mean PSA level was 135.0 ng/ml at PCa diagnosis. The median Gleason score was 8 at diagnosis. Bone metastases were present in 21 patients at PCa diagnosis. Docetaxel-based chemotherapy was administered for clinical and/or biochemical progression in patients with CRPC after a mean duration of androgen-ablation therapy of 33.2 months. The patient’s hormone-refractory status was defined as a progressive increase in PSA level after androgen blockade. Hormonal castration had to be biologically confirmed. Chemotherapy was combined with low-dose orally administered prednisone.
Formalin-fixed, paraffin-embedded specimens were obtained from the Department of Pathology at the Henri Mondor Hospital, Créteil, France. Immunostaining was performed: (i) on the initial prostatic biopsies of 37 patients who had received first-line docetaxel-based chemotherapy; and (7) on tissue microarrays (TMA) for the HNPC patient’s cohort. When considering TMAs, for each PCa case, 4 replicate cores (diameter 0.6 mm) were obtained from cancer foci and four additional cores were also taken from nonneoplastic areas as previously described [25].
Immunohistochemistry (Supplementary Method)
Immunostaining was done on 5-μm tissue sections mounted on silane-coated slides. βIII-tubulin protein expression was evaluated using a monoclonal antibody specific for the βIII-tubulin isotype (1:500; clone TUJ1; Covance, Emeryville, CA).
A numerical score was assigned for the epithelial cells of each specimen. Samples with no stained tumour cells were scored as 0. A score of 1, 2, or 3 was assigned to samples with weak, moderate, or strong staining, respectively, independently of the proportion of stained tumour cells. The proportion of immunostained tumour cells was also assessed. For TMAs analysis, the mean of staining in the 4 neoplastic cores was considered. Only cytoplasmic staining was taken into account. All of the slides were independently evaluated by 3 observers (GP, ST, YA). Observers were blinded to the patients’ adjuvant treatment, final pathologic assessment, and outcome. Interrater reproducibility was 95%. Different scores were reassessed and consensus between observers was defined. Staining intensity seen in nerves and axons served as an internal positive control. The absence of immunostaining in red cells was used as negative control (27). Photomicrographs were taken using a Zeiss Axioplan2 microscope (Carl Zeiss, Le Pecq, France) from imaging platform (INSERM, U955, UPEC).
Statistical analysis
Null expression (0% of stained cells) and weak to strong expression (score 1 to 3, and ≥5% of stained cells) were grouped separately as dichotomic variables for statistical analysis.
The Student-t test was used for continuous data. The Mann-Whitney test and the Kruskal-Wallis test were used when data were not normally distributed. Qualitative data were tested using a Chi-square test or Fisher’s test as appropriate. The Gleason score was dichotomized according to the definition of high-risk prostate cancer as follows: Gleason score<8 versus ≥8 (2). Survival curves were generated by the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazards model was used to evaluate the independent value of βIII-tubulin expression among commonly used prognostic factors. Hazard ratios (HR) were presented with 95% CI.
(i) In the CRPC cohort, the starting point of the analysis was the first cycle of chemotherapy. The PSA Working Group criteria were used to evaluate PSA responses: we chose a decrease of >75% from the baseline PSA level as the criterion for PSA response (28). The primary endpoint was overall survival (OS) defined as the time from the start of chemotherapy until death from any cause or last follow-up for censored patients. Time-to-progression was defined as the time between the first cycle of chemotherapy and an elevated PSA finding.
(7) In the HNPC cohort, Biochemical RFS was analyzed. The day of surgery was reported as the starting point of analysis. Recurrence was defined as the first detectable elevation of PSA above 0.20 ng/ml (at least two consecutive measurements).
A value of p<0.05 was considered statistically significant, and all p values were two-sided. SPSS 13.0 (Chicago, Illinois) software was used for statistical analyses.
RESULTS
Prognostic value of βIII-tubulin expression for prostate cancer recurrence in hormone-naïve PCa patients treated by radical prostatectomy
We had previously reported that βIII-tubulin was expressed significantly lower in hormone naïve PCa (HNPC) as compared to castration resistant PCa (CRPC). In our previous study, however, the small patient sample size in the HNPC group (n=74) prevented us from deriving any statistically reliable association with other patient prognostic factors (21). Here, we extended our assessment of βIII-tubulin expression to PCa-containing specimens obtained from 258 PCa patients that were treated by radical prostatectomy (Table 1). We identified βIII-tubulin expression in 43 of the 258 specimens from patients with HNPC (16.7%, Table 2). Strong βIII-tubulin immunostaining (score of 3.0) was detected in 6.2% of tumors. When βIII-tubulin expression was observed, the percentage of positively-stained tumor cells showed a wide range of variability (range: 5–100%; mean 25.8%; median 10%). Representative examples of immunostaining are shown in Fig. 1A–C. βIII-tubulin expression was not detected in non-malignant prostate basal or luminal epithelial cells adjacent to the tumor. Correlations of βIII-tubulin immunostaining with histoprognostic parameters are described in Table 2. We found that positive immunostaining was significantly associated with a Gleason score ≥8 (p=0.001; OR 3.17), a primary Gleason grade of 4 or 5 (p=0.013; OR 2.28), a pT stage ≥ 3 (p=0.042; OR 1.97), an extraprostatic extension (p=0.028; OR 2.10) and positive lymph nodes (p=0.034; OR 3.05). It is noteworthy that βIII-tubulin expression was also significantly associated with a high risk for biochemical recurrence (p=0.029: OR 2.15). The intensity of immunostaining was also correlated with the histoprognostic parameters. A strong immunostaining, defined by a staining score of 3 was also markedly associated with a Gleason score ≥8 (p<0.001), extraprostatic extension (p<0.001), positive surgical margins (p=0.025), pT stage (p<0.001), and positive lymph nodes (p=0.043), when compared with a null-to-moderate staining (score 0, 1, or 2). The 3-year and 5-year recurrence free survival (RFS) was 84.5% and 75.4%, respectively, in patients with no βIII-tubulin expression in the prostate tumor. By contrast, the 3-year and 5-year RFS was 72.4% and 59.4%, respectively, in patients expressing βIII-tubulin in prostate tumor cells. The log-rank test was significant with a p value of 0.002 (Fig. 1D). In patients with favorable pathological features (Gleason <8, pT2 cancer, and negative surgical margin), the 5-year RFS was 91.7% in βIII-tubulin-negative patients versus 79.6% in βIII-tubulin-positive patients (p=0.006). Furthermore, in multivariate analysis using a Cox model taking into account Gleason score, pT stage, and surgical margin status, βIII-tubulin expression was an independent predictor of biochemical recurrence (p=0.029; HR 1.95 [1.07–3.55]).
Table 2.
Association between βIII-tubulin expression and clinico-pathologic parameters.
| Patients with no TUBB3 expression n=215 | Patients with TUBB3 expression n=43 | Univariate analysis | ||
|---|---|---|---|---|
| p | OR [95%CI] | |||
| Age, No (%): | ||||
| <65 years | 105 (48.8) | 19 (44.2) | 0.577 | |
| >65 years | 110 (51.2) | 24 (55.8) | ||
| Age, years (mean) | 64.6 | 63.8 | 0.399 | |
| Clinical stage, No (%): | ||||
| T1 | 151 (70.2) | 25 (58.2) | 0.068 | |
| T2 or more | 64 (29.8) | 18 (41.9) | ||
| PSA, No (%): | ||||
| <10 ng/ml | 119 (55.3) | 19 (44.2) | 0.180 | |
| >10 ng/ml | 96 (44.7) | 24 (55.8) | ||
| PSA, ng/ml (mean) | 13.1 | 19.5 | 0.066 | |
| Gleason score, No (%): | ||||
| ≤7 | 172 (80.0) | 24 (55.8) | 0.001 | 3.17 [1.59–6.30] |
| ≥8 | 43 (20.0) | 19 (44.2) | ||
| Primary Gleason grade 4 or 5, No (%): | 72 (33.5) | 23 (53.5) | 0.013 | 2.28 [1.18–4.43] |
| Prostate weight, No (%): | ||||
| <50 gr | 88 (40.9) | 22 (51.2) | 0.264 | |
| >50 gr | 127 (59.1) | 21 (48.9) | ||
| Prostate weight, gr (mean) | 55.9 | 54.2 | 0.724 | |
| pT stage, No (%): | ||||
| pT2 | 131 (60.9) | 19 (44.2) | 0.042 | 1.97 [1.02–3.82] |
| pT3-4 | 84 (39.1) | 24 (55.8) | ||
| Extracapsular extension, No (%): | 74 (34.4) | 22 (51.2) | 0.028 | 2.10 [1.08–4.09] |
| Positive surgical margins, No (%): | 43 (20.0) | 13 (30.2) | 0.116 | |
| Seminal vesicle invasion, No (%): | 42 (19.5) | 14 (32.6) | 0.059 | |
| Positive lymph nodes, No (%): | 15 (7.0) | 8 (18.6) | 0.034 | 3.05 [1.20–7.73] |
| Recurrence, No (%): | 46 (21.4) | 16 (37.2) | 0.029 | 2.15 [1.07–4.33] |
| Duration of follow-up, months (mean) | 62.1 | 58.4 | 0.603 | |
Clinical data, pathologic features, PSA failure: correlations with βIII-tubulin expression in HNPC samples (univariate analysis). HNPC: hormone naive prostate cancer; PSA: prostate specific antigen.
Figure 1. Prognostic value of βIII-tubulin expression in predicting the biochemical recurrence after radical prostatectomy in HNPC prostate cancer patients.
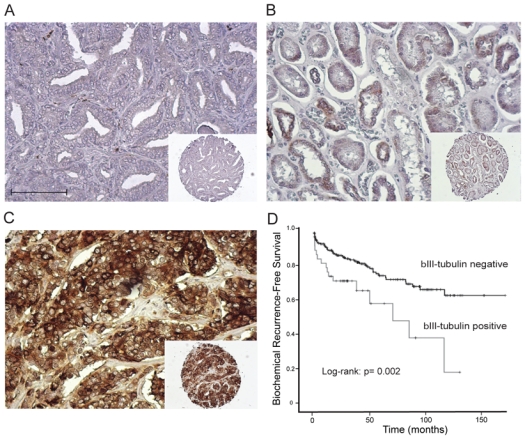
A, B, C. Representative staining for βIII-tubulin in HNPC TMAs: absence of staining (A), moderate staining (B), strong staining (C). Photomicrographs are taken at x20 objective magnification (A–C) and x10 objective magnification; insets (A–C). Scale Bar, 100μm.
D. Recurrence-free survival (RFS) curves stratified by the expression of βIII-tubulin (negativity versus positivity of immunostaining, log-rank test; p=0.002). HNPC: hormone naive prostate cancer; TMA: tissue micro-array.
Increased expression of βIII-tubulin in prostate cancer cell lines in response to in vitro docetaxel treatment
We previously reported that βIII-tubulin expression was increased in LNCaP cells, (21) that were grown in androgen depleted medium or in tumor xenografts from these cells after castration of the host mouse. In the present work, we tested whether docetaxel treatment could affect βIII-tubulin expression in two androgen-independent (AI) PCa cell lines; LNCaP-AI that expresses low levels of βIII-tubulin or 22Rv1 that endogenously expresses higher levels of βIII-tubulin. LNCaP-AI variant cells were treated with docetaxel at 3 nmol/L for up to 30 days. As shown in Figure 2A, using Western blots to assess the levels of expressed βIII-tubulin protein, expression was continuously elevated by exposure to docetaxel over the time course of the experiment. It is noteworthy that the class II β-tubulin, an isotype known to be predominantly expressed in the brain also increased along with total β-tubulin under docetaxel exposure whereas no significant increase was seen for βI and βIV isotypes (Fig. 2A and Supplementary Fig. S1). To corroborate these findings, we set out to further characterize the expression of β-tubulin isotypes in 22Rv1 under exposure to docetaxel at 15 nM for 72 hrs. The 22Rv1 PCa cell line is derived from a primary tumor xenograft, CWR22, that relapsed during androgen ablation (29). These cells express at least three androgen receptor isoforms that appear to be constitutively active, likely promoting proliferation as well as the expression of multiple AR-dependent genes in a ligand-independent manner (23). Consistent with earlier observations, upregulation of βIII-tubulin protein (Fig. 2B and Supplementary Fig. S2A) as well as mRNA levels (Supplementary Fig. S2B) was evident in docetaxel-treated 22Rv1 cells. The βII- and total protein levels were similarly increased in docetaxel-treated cells in contrast to that found for βI and βIV isotypes. Together, these data demonstrate that both acute and chronic exposure of AI prostate cancer cells to docetaxel upregulates βIII-tubulin along with a seeming general increase of total β-tubulin.
Figure 2. Links between βIII-tubulin expression and the acquisition of docetaxel resistance in androgen-sensitive and androgen-insensitive prostate cancer cells.
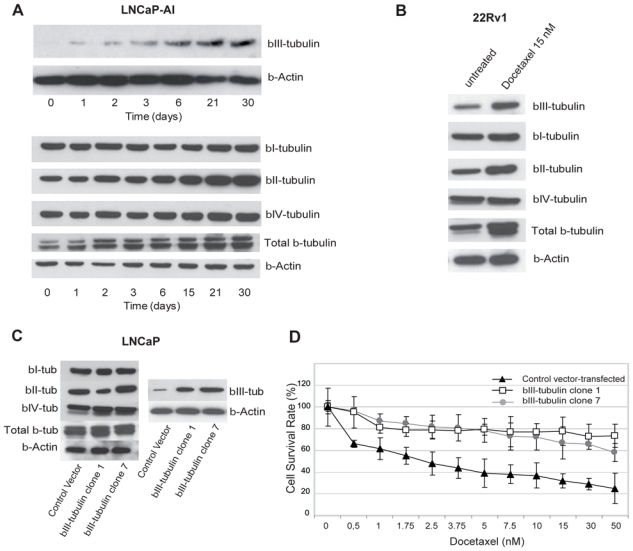
A. Time course expression of βIII-tubulin, total β-tubulin and β-tubulin isotypes in LNCaP-AI cells cultivated at 3 nmol/L docetaxel. B. Increased protein expression was confirmed by Western blotting: untreated 22Rv1 compared with 22Rv1 cultivated in the presence of 15 nmol/L docetaxel. C. Differential expression of βIII-tubulin between control vector-transfected cells and βIII-tubulin transfected clones was assessed by immunoblotting. A significant increase in levels of βIII-tubulin protein was observed in the βIII-tubulin transfected LNCaP cells (βIII-tubulin clone 1 and βIII-tubulin clone 7) compared with the control vector-transfected LNCaP cells. D. Dose response curve assessing the effect of βIII-tubulin overexpression in LNCaP cells. Cell viability assays showed that βIII-tubulin transfected LNCaP cells (clone 1 and 7) were significantly more resistant to docetaxel treatment than control vector-transfected LNCaP cells. Points, mean; bars, SEM.
Stable over-expression of βIII-tubulin in prostate cancer cell lines confers resistance to docetaxel
To assess whether over-expression of βIII-tubulin was sufficient to confer resistance to docetaxel in prostate cancer cells, we established PCa cell clones, from LNCaP or DU145 cells, stably expressing the human TUBB3 gene (βIII-tubulin transfected clones) under the control of a CMV promoter. The AR negative DU145 line is derived from a brain metastasis. These cells might represent a very aggressive stage of prostate cancer as reflected by their growth rate and their invasive behaviour in vitro and in vivo (30). LNCaP cells are androgen-sensitive prostate cancer cells originated from a lymph node metastasis. Lymph nodes are the most common and earliest sites for prostate cancer metastasis thus rendering this model particularly attractive for the research community (22). Differential expression of βIII-tubulin protein between control vector-transfected LNCaP cells and TUBB3-transfected clones was established by Western blot analysis (Fig. 2C). Despite some decrease of βII isotype was seen in βIII-tubulin transfected LNCaP cells (βIII-tubulin clone 1), forced expression of TUBB3 did not seem to affect consistently the expression of the β-tubulin isotypes. Interestingly, the βIII-tubulin overexpressing LNCaP cells had a neuroendocrine-like appearance (Supplementary Fig. S3) suggesting that forced expression of βIII-tubulin is associated with a trans-differentiation process that has been frequently described for these cells (31, 32). To examine the effects of βIII-tubulin over-expression, we measured the half-time inhibitory concentration (IC50) of docetaxel assessed by measuring cell viability at 72 hrs after exposure. The cell viability assays showed that βIII-tubulin transfected LNCaP cells (βIII-tubulin clone 1 and βIII-tubulin clone 7) were significantly more resistant to docetaxel treatment than control vector-transfected LNCaP cells with a 6.6-fold increase in the IC50 (33.1 versus 5.0 nmol/L, p<0.001, Fig. 2D). A significant difference between clones and control cell survival was already achieved at 2 nmol/L docetaxel and persited up to 50 nmol/L docetaxel treatment (p<0.001). βIII-tubulin transfected LNCaP cells were again poorly sensitive to docetaxel when higher doses were tested (Supplementary Fig. S4) Having established that βIII-tubulin has severe implications for docetaxel resistance in LNCaP cells, we sought to determine if this could be due in part to an effect of βIII-tubulin on cell proliferation. To this end, cell proliferation was assayed in the absence of drug. Cell doubling times for vector-transfected LNCaP and βIII-tubulin transfectants were estimated at 31h and 44h, respectively (supplementary Table 1). Two βIII-tubulin transfected DU145 clones were also selected for subsequent treatment including 1 clone expressing high levels of βIII-tubulin (βIII-tubulin clone 7), and 1 clone expressing moderate βIII-tubulin (βIII-tubulin clone 5) at a level similar to that found in parental or control vector-transfected DU 145 cells (Fig. 3A). The cell viability assays showed that bIII-tubulin-transfected clone 7 was significantly more resistant to docetaxel treatment compared to clone 5 or control vector-transfected DU 145 cells with a 2-fold increase in IC50 (11.1 versus 5.1 nmol/L, p<0.001, Fig. 3B). A significant difference in cell viability between clone 7 and control cells was already achieved at 5 nmol/L (p<0.001). There were no noticeable morphological differences between the parental vector-transfected DU145 and either of the two sublines. Nor were there any evident differences in growth rates noted during standard passage and cell proliferation assays of the sublines compared to the vector-transfected or parental cells (supplementary Table1).
Figure 3. Functional overexpression and knockdown of βIII-tubulin modulates the androgen-independent prostate cancer cells sensitivity to docetaxel.
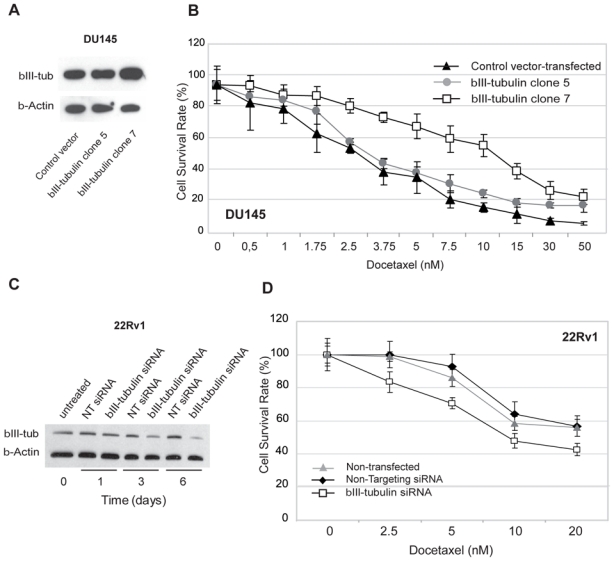
A. Differential levels of βIII-tubulin protein between control vector-transfected and βIII-tubulin transfected (clone 5 and 7) DU145 cells: Western blot analysis showed that. The βIII-tubulin clone 5 expressed a similar level of βIII-tubulin compared with control vector-transfected cells, whereas the βIII-tubulin clone 7 showed a higher level of βIII-tubulin expression.
B. Dose response curve assessing the effect of βIII-tubulin overexpression in androgen-independent DU145 cells. Cell viability assays showed that clone 7 was significantly more resistant to docetaxel treatment relative to βIII-tubulin clone 5 and control vector-transfected DU 145 cells. Points, mean; bars, SEM. NT: non targeting.
C. Western blot confirmation of βIII-tubulin knockdown in 22Rv1 cells after siRNA transfection. The βIII-tubulin protein level was decreased 72 hours after transfection and lasted at least 6 days after a single transfection.
D. Dose response curve assessing the effect of βIII-tubulin silencing in androgen-independent 22Rv1 cells. Cell viability assays showed that βIII-tubulin siRNA-transfected 22Rv1 cells were significantly more sensitive to docetaxel treatment relative to non targeting siRNA-transfected and non-transfected 22Rv1 cells. Points, mean; bars, SEM.
βIII-tubulin silencing increases sensitivity to docetaxel
To further ascertain a role for βIII-tubulin in chemoresistance to docetaxel, siRNAs directed against βIII-tubulin was used to knockdown endogenous βIII-tubulin expression in 22Rv1 cells. The targeting siRNA with the highest reduction of βIII-tubulin was selected for further study based on RT-PCR and Western-blot analysis for effects on βIII-tubulin mRNA and protein. βIII-tubulin mRNA levels were decreased by 70% using this siRNA and βIII-tubulin knockdown was confirmed by Western blot analysis after 3 and 6 days (Fig. 3C). siRNA mediated loss of βIII-tubulin did not appear to affect the other βIII-tubulin isotypes (supplementary Figure S5). An assessment of the doubling times revealed that concomitantly with the loss of βIII-tubulin upon βIII-tubulin siRNA treatment, there was a trend towards an increase in cell growth (supplementary table 1). Docetaxel was then added to the growth medium of control cells or targeting siRNA-treated cells 72 hours after the transfection. Seventy hrs later, targeting siRNA-transfected 22Rv1 cells were found to be more sensitive to docetaxel treatment at all doses tested compared to the control 22Rv1 cells. Overall, βIII-tubulin siRNA-transfected 22Rv1 cells were found to be 2-fold more sensitive to docetaxel (IC50 of 4.4 versus 8.5 nmol/L, p<0.001, Fig. 3D) than controls. These results indicate that βIII-tubulin silencing sensitizes AI PCa cells to docetaxel.
Predictive value of early βIII-tubulin expression in a docetaxel-treated patient cohort
By examining the βIII-tubulin status in the initial prostatic biopsies of patients subsequently treated for CRPC disease with docetaxel chemotherapy, we investigated the usefulness of βIII-tubulin expression as a potential predictive tumor biomarker for response to docetaxel. Of the 37 cases, 17 were positive for βIII-tubulin (9 with moderate and 8 with strong staining) whereas 20 were negative. Representative examples of staining are shown in Fig. 4A and 4B. In this cohort, βIII-tubulin expression was significantly correlated with a Gleason score >7 at diagnosis; 29.4% of βIII-tubulin-negative cancers were graded 8 or more as compared to 63.2% of βIII-tubulin-positive cancers (p=0.043). PSA responses were observed in 52% of βIII-tubulin-negative patients compared with 35% of βIII-tubulin-positive patients (p=0.337). Patients with βIII-tubulin positive tumours experienced shorter time-to-progression. Median time-to-progression was 4.7 months in βIII-tubulin-positive patients compared with 9.8 months in βIII-tubulin-negative patients (Breslow p=0.149; log-rank p=0.522). Overall, the median survival time for the entire cohort was 25.3 months (95% CI: 16.5–34.2) and the observed cumulative probabilities at years 1, 2, and 3 were 79.5%, 57.8%, and 43.7%, respectively. Median OS was significantly shorter for patients with βIII-tubulin positive tumours than those with βIII-tubulin-negative hormone-naïve tumours (13.5 versus 41.6 months, p=0.019; Fig. 4C). Finally, baseline PSA levels measured prior to the first cycle of chemotherapy in these patients were also significantly related to OS. Multivariate analysis taking βIII-tubulin expression, baseline PSA, age, and duration of androgen deprivation therapy into account showed that βIII-tubulin expression (HR 2.93; p=0.037) and baseline PSA level (HR 4.09; p=0.012) were independent predictors of OS.
Figure 4. Predictive value of βIII-tubulin expression for response to docetaxel in castration-resistant prostate cancer patients.
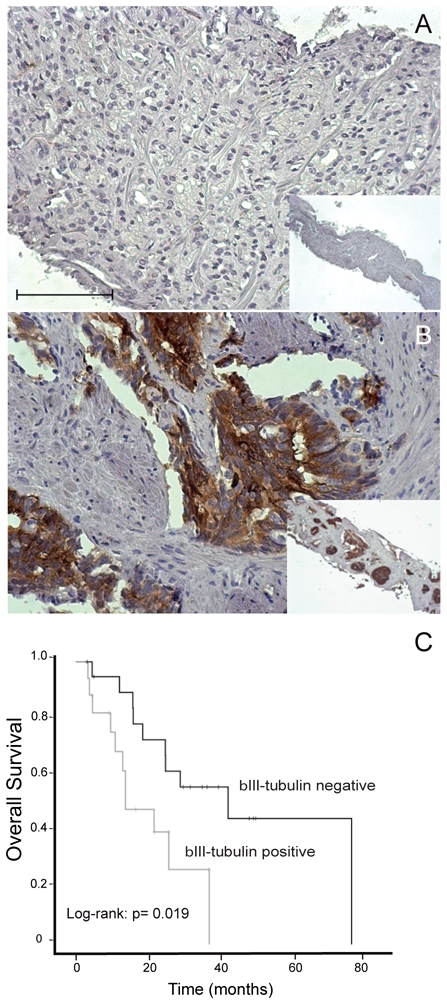
A, B. Representative staining for βIII-tubulin in prostate biopsy cores: absence of staining (A), staining positivity (B). Photomicrographs are taken at x20 objective magnification (A–B), insets x5 objective magnification (AC). Scale Bar, 100μm.
C. Overall survival in docetaxel-treated patients: stratification by βIII-tubulin expression in prostatic tissue (negativity versus positivity of immunostaining, log-rank test; p=0.019): expression of βIII-tubulin in prostate cancer tissue is significantly predictive for poorer survival in docetaxel-treated patients.
DISCUSSION
In prostate cancer, clinical data and histoprognostic parameters, separated or integrated into nomograms still fail at the individual level to accurately determine the risk of biochemical or clinical relapse after local treatment (33, 34). At CRPC stage, docetaxel-based chemotherapy has proven to have some effectiveness in terms of overall response rates and survival in CRPC patients (4, 5). However, any selection of patients likely to benefit most from this form of chemotherapy is difficult and is often based simply upon patient age or the presence of co-morbidities in individuals.
Numerous preclinical studies have reported that the selective overexpression of βIII-tubulin constitutes an important mechanism for resistance to tubulin-binding agents in various cancer cell lines (19, 35–38). Several clinical studies have shown that high levels of βIII-tubulin expression in tumor cells are associated with low response rates and poorer survival in patients treated with taxane-based chemotherapies (13–17, 39). Our findings in prostate cancer were consistent with the previous studies and highlight that the expression of βIII-tubulin is associated with non organ-confined disease, metastatic lymph nodes and PSA failure. Thus, tumor cell βIII-tubulin expression might characterize a general subclass of PCa patients with aggressive behavior and poor prognosis. This could give reason to consider adjuvant treatments for patients with βIII-tubulin positive tumors.
Characteristics of our HNPC patient’s cohort differed slightly from characteristics of patients who actually undergo a radical prostatectomy. The advent of PSA testing has lead to a considerable stage migration with an increase of low-risk prostate cancers. In our HNPC cohort study, we included patients who underwent radical prostatectomy since 1988. This constitutes a selection bias due a more important proportion of high-risk prostate cancer before the PSA era. These discrepancies may limit the study of βIII-tubulin expression as the number of high-risk prostate cancers decreases over time. However, we showed that the prognostic impact of βIII-tubulin remained significant in organ-confined PCa and therefore, we posit that the βIII-tubulin is of additional value to well-established histoprognostic parameters even in the assessment of presumed low-risk PCa.
We also observed here that the βIII-tubulin expression in AI PCa cell lines was increased in response to acute or chronic exposure to docetaxel. In line with our findings, Ranganathan et al. previously showed an increase in βIII-tubulin expression in response to paclitaxel treatment of DU145 cells (19). The exact mechanism by which increased expression of βIII-tubulin mediates drug resistance remains open to debate. In some instances, we found that alterations of βIII-tubulin expression were associated with changes in cell morphology and/or cell proliferation rate. Thus, there is reason to believe that these events might be key determinants of drug resistance. Additionally, evidence is accumulating that microtubules containing βIII-tubulin exhibit an aberrant dynamicity. These microtubules are less stable than microtubules composed of other β-tubulin isotypes (9, 10, 40, 41) Since the primary effect of taxane is to bind microtubules thereby enhancing the microtubule polymerization and decreasing microtubule dynamicity, it has been suggested that βIII-tubulin-containing microtubules are more prone to overcome the suppressive effects of taxanes on microtubule dynamics. Although it should be noted that all the above studies (12,13, 43) have focused on the assembly of purified microtubules in cell free systems and therefore remain controversial given contradictory reports obtained in intact cells, and hence more biologically relevant, that βIII-tubulin did not intrinsically affect microtubule dynamic, at least in some instances (38, 42). Interestingly, in these last studies, the effects of paclitaxel on microtubule dynamics were altered, suggesting a role for βIII-tubulin in drug-microtubule interactions. Consistent with this, Mozetti et al. have reported that βIII-tubulin overexpression was the prominent mechanism of paclitaxel resistance in ovarian cancer patients, as compared with other mechanisms of drug resistance such as overexpression of MDR-1 and point mutations in tubulin at the binding site of paclitaxel (43). Although many studies have focused on paclitaxel, such characteristics might also be pertinent for docetaxel. Interestingly, recent reports further showed that βIII-tubulin confers resistance to microtubule-destabilizing agents such as vinorelbine in breast cancer cells (44). In the setting of non-small cell lung cancer, βIII-tubulin was shown to impact significantly on the response to both tubulin targeting-agents and DNA-damaging agents (45). The investigators proposed that βIII-tubulin may serve as a survival factor to rescue tumor cells from death signals triggered by chemotherapeutic agents. Therefore, it is conceivable that elevated expression of βIII-tubulin can exert similar effects in other malignancies, including prostate cancer, and future work should explore this question.
Another interesting open question regarding antimicrotubule drug resistance associated with changes in βIII-tubulin expression in cancer cells is: what is the contribution of other isotypes in response to taxane-based regimens? Ranganathan et al. have previously noted that class IVb β-tubulin was increased collaterally in βIII-tubulin-transfected DU145 cells in response to paclitaxel treatment (20). In a separate study, wherein paclitaxel-resistant DU145 variants were selected after chronic exposure to the drug, examination of the β-tubulin isotype composition revealed increased expression of βIII-tubulin but no changes were observed for IVb β-tubulin. Instead, some increase in IVa β-tubulin expression was noted (19). Although this previous work in DU145 has suggested that βIII-tubulin expression is upregulated in response to paclitaxel treatment, our survey using docetaxel treatment of two androgen-independent AR-positive PCa cells, 22Rv1 and LNCaP-AI provides additional evidence that this situation might be relevant in clinical settings. These studies further indicate that expression of the class βII tubulin can be altered in PCa cells and highlight a potential role for this isotype in the emergence of docetaxel resistance. The ectopic overexpression or silencing of βIII-tubulin resulted in a seeming specific up- or downregulation of the βII-tubulin expression, respectively. Surprisingly, under these conditions, we failed to find any significant changes in the expression of other β-tubulin isotypes. These instances strengthened the role of βIII-tubulin in contributing to chemosensitvity and illustrate the importance of monitoring the β-tubulin isotype profile to confirm the contribution of the target isotype in drug response studies. Although previous work has suggested that manipulation of βII- and βIVb-tubulin expression did not cause changes in paclitaxel sensitivity of CHO and lung cancer cells (46, 47), these cell lines differ from prostate cancer cells, and future work is warranted in order to determine the full functional implications of each β-tubulin isotype in the emergence of drug-resistance in prostate cancer cells.
Our findings in experimental prostate cancer cell lines were consistent with our clinical observations that βIII-tubulin expression in prostate tumors correlated significantly with outcomes of docetaxel chemotherapy for CRPC patients. Patients expressing βIII-tubulin at diagnosis had reduced survival. Thus it is tempting to speculate that the assessment of βIII-tubulin expression could determine which CRPC patients might benefit from taxane-based chemotherapy. Studies exploring potential molecular markers in response to chemotherapy are currently limited by the difficulty in obtaining tissues from patients with CRPC. Many changes occurred during the evolution to CRPC, and cancer tissue at diagnosis does not always reflect accurately the cancer tissue in its more advanced stage. In fact, obtaining tumor tissue at CRPC stage, or before and during therapy, though rarely feasible in clinical practice, would certainly be the best way to investigate the impact of biomarker on response to therapy. Nevertheless, by examining βIII-tubulin expression in the tumors of HNPC patients, which are easily accessible to tumor sampling, it seems possible to evaluate their response to docetaxel-based therapy once the disease has progressed to CRPC. If these findings are confirmed, the use of novel tubulin-targeted agents, such as epothilones, could be useful for βIII-tubulin-positive PCa patients (11, 48, 49). This might also offer the opportunity for therapeutic intervention by an anti-βIII-tubulin treatment in such patients.
Collectively, our results suggest that the functional and clinical biomarker aspects of βIII-tubulin expression are linked. Consistent with our previous findings and other studies reporting an increase in the βIII-tubulin isotype as a result of anti-microtubule drug treatments (19, 20, 50), it seems likely that adjuvant treatments other than the current taxane-based chemotherapy regimen may be required for this group of patients. More importantly, our results now support the idea that βIII-tubulin expression may be linked to multiple forms of PCa; progression to recurrence in HNPC patients, progression to CRPC in hormone-treated patients and, finally, progression to docetaxel resistance in docetaxel-treated CRPC patients. In the area of cancer treatment, clinicians have to deal with two limitations: the difficulty to predict accurately the relapse after local treatment; and the ability to anticipate ineffective adjuvant or systemic therapy. To date, in prostate cancer, new molecular markers are needed to better define the subset of patients who are most likely to benefit from an adjuvant strategy after radical treatment, and from chemotherapy at CRPC stage. Our findings were consistent with the role of βIII-tubulin expression as prognostic marker of biochemical recurrence at HNPC stage. Assessment of the βIII-tubulin expression in cancer tissue therefore could be useful to identify and monitor prostate cancer at high risk for recurrence after radical prostatectomy. If longer follow-up and rates of specific mortality confirm these results, the βIII-tubulin expression might be interesting for predicting recurrence rates and for proposing adjuvant therapy.
Functional overexpression or knockdown of βIII-tubulin modulates the prostate cancer cell lines sensitivity to docetaxel. The tissue βIII-tubulin expression status also has predictive value in terms of OS in patients receiving docetaxel-based chemotherapy for hormone-independent disease. These findings underline the importance of βIII-tubulin expression, which could be used in addition to clinicopathologic characteristics to select patients for docetaxel-based chemotherapy. Prospective studies incorporating βIII-tubulin immunohistochemistry are warranted to determine the clinical relevance of routine use of this assay.
Acknowledgments
This work was supported by INSERM, the ‘Conseil Général du Val de Marne’, the ‘Université Paris-Est Créteil Val de Marne’, grants from the ARTP (to G.P, S.T, F.V), the ‘Association pour la Recherche sur le Cancer’ (to G.P, F.V) and the FERCM (to G.P).
Footnotes
No financial disclosure.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 3.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–5. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Bamias A, Bozas G, Antoniou N, et al. Prognostic and predictive factors in patients with androgen-independent prostate cancer treated with docetaxel and estramustine: a single institution experience. Eur Urol. 2008;53:323–31. doi: 10.1016/j.eururo.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 7.Yoshino T, Shiina H, Urakami S, et al. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12:6116–24. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- 8.Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17:1061–70. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- 9.Lu Q, Luduena RF. Removal of beta III isotype enhances taxol induced microtubule assembly. Cell Struct Funct. 1993;18:173–82. doi: 10.1247/csf.18.173. [DOI] [PubMed] [Google Scholar]
- 10.Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci U S A. 1994;91:11358–62. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbatini P, Spriggs DR. Epothilones: better or more of the same? J Clin Oncol. 2009;27:3079–81. doi: 10.1200/JCO.2008.21.6481. [DOI] [PubMed] [Google Scholar]
- 12.Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–75. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 13.Ferrandina G, Zannoni GF, Martinelli E, et al. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res. 2006;12:2774–9. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 14.Galmarini CM, Treilleux I, Cardoso F, et al. Class III beta-tubulin isotype predicts response in advanced breast cancer patients randomly treated either with single-agent doxorubicin or docetaxel. Clin Cancer Res. 2008;14:4511–6. doi: 10.1158/1078-0432.CCR-07-4741. [DOI] [PubMed] [Google Scholar]
- 15.Seve P, Reiman T, Isaac S, et al. Protein abundance of class III beta-tubulin but not Delta2-alpha-tubulin or tau is related to paclitaxel response in carcinomas of unknown primary site. Anticancer Res. 2008;28:1161–7. [PubMed] [Google Scholar]
- 16.Seve P, Mackey J, Isaac S, et al. Class III beta-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther. 2005;4:2001–7. doi: 10.1158/1535-7163.MCT-05-0244. [DOI] [PubMed] [Google Scholar]
- 17.Urano N, Fujiwara Y, Doki Y, et al. Clinical significance of class III beta-tubulin expression and its predictive value for resistance to docetaxel-based chemotherapy in gastric cancer. Int J Oncol. 2006;28:375–81. [PubMed] [Google Scholar]
- 18.Seve P, Lai R, Ding K, et al. Class III beta-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR. 10. Clin Cancer Res. 2007;13:994–9. doi: 10.1158/1078-0432.CCR-06-1503. [DOI] [PubMed] [Google Scholar]
- 19.Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998;77:562–6. doi: 10.1038/bjc.1998.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan S, McCauley RA, Dexter DW, Hudes GR. Modulation of endogenous beta-tubulin isotype expression as a result of human beta(III)cDNA transfection into prostate carcinoma cells. Br J Cancer. 2001;85:735–40. doi: 10.1054/bjoc.2001.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry S, Ploussard G, Allory Y, et al. Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br J Cancer. 2009;101:951–6. doi: 10.1038/sj.bjc.6605245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horoszewicz JS, Leong SS, Kawinski E, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–18. [PubMed] [Google Scholar]
- 23.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–25. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 25.Azoulay S, Terry S, Chimingqi M, et al. Comparative expression of Hedgehog ligands at different stages of prostate carcinoma progression. J Pathol. 2008;216:460–70. doi: 10.1002/path.2427. [DOI] [PubMed] [Google Scholar]
- 26.Frigo DE, McDonnell DP. Differential effects of prostate cancer therapeutics on neuroendocrine transdifferentiation. Mol Cancer Ther. 2008;7:659–69. doi: 10.1158/1535-7163.MCT-07-0480. [DOI] [PubMed] [Google Scholar]
- 27.Ranganathan S, Salazar H, Benetatos CA, Hudes GR. Immunohistochemical analysis of beta-tubulin isotypes in human prostate carcinoma and benign prostatic hypertrophy. Prostate. 1997;30:263–8. doi: 10.1002/(sici)1097-0045(19970301)30:4<263::aid-pros6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 29.Sramkoski RM, Pretlow TG, II, Giaconia JM, et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–9. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 30.Mickey DD, Stone KR, Wunderli H, Mickey GH, Paulson DF. Characterization of a human prostate adenocarcinoma cell line (DU 145) as a monolayer culture and as a solid tumor in athymic mice. Prog Clin Biol Res. 1980;37:67–84. [PubMed] [Google Scholar]
- 31.Cindolo L, Cantile M, Vacherot F, Terry S, de la Taille A. Neuroendocrine differentiation in prostate cancer: from lab to bedside. Urol Int. 2007;79:287–96. doi: 10.1159/000109711. [DOI] [PubMed] [Google Scholar]
- 32.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–47. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 33.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 34.Walz J, Chun FK, Klein EA, et al. Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. J Urol. 2009;181:601–7. doi: 10.1016/j.juro.2008.10.033. discussion 607–8. [DOI] [PubMed] [Google Scholar]
- 35.Burkhart CA, Kavallaris M, Band Horwitz S. The role of beta-tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta. 2001;1471:O1–9. doi: 10.1016/s0304-419x(00)00022-6. [DOI] [PubMed] [Google Scholar]
- 36.Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer. 1999;80:1020–5. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akasaka K, Maesawa C, Shibazaki M, et al. Loss of class III beta-tubulin induced by histone deacetylation is associated with chemosensitivity to paclitaxel in malignant melanoma cells. J Invest Dermatol. 2009;129:1516–26. doi: 10.1038/jid.2008.406. [DOI] [PubMed] [Google Scholar]
- 38.Kamath K, Wilson L, Cabral F, Jordan MA. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280:12902–7. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 39.Seve P, Isaac S, Tredan O, et al. Expression of class III {beta}-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin Cancer Res. 2005;11:5481–6. doi: 10.1158/1078-0432.CCR-05-0285. [DOI] [PubMed] [Google Scholar]
- 40.Derry WB, Wilson L, Khan IA, Luduena RF, Jordan MA. Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified beta-tubulin isotypes. Biochemistry. 1997;36:3554–62. doi: 10.1021/bi962724m. [DOI] [PubMed] [Google Scholar]
- 41.Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III beta-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton. 2003;56:45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- 42.Gan PP, McCarroll JA, Po’uha ST, Kamath K, Jordan MA, Kavallaris M. Microtubule dynamics, mitotic arrest, and apoptosis: drug-induced differential effects of betaIII-tubulin. Mol Cancer Ther. 9:1339–48. doi: 10.1158/1535-7163.MCT-09-0679. [DOI] [PubMed] [Google Scholar]
- 43.Mozzetti S, Ferlini C, Concolino P, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 44.Stengel C, Newman SP, Leese MP, Potter BV, Reed MJ, Purohit A. Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br J Cancer. 102:316–24. doi: 10.1038/sj.bjc.6605489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67:9356–63. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 46.Blade K, Menick DR, Cabral F. Overexpression of class I, II or IVb beta-tubulin isotypes in CHO cells is insufficient to confer resistance to paclitaxel. J Cell Sci. 1999;112 ( Pt 13):2213–21. doi: 10.1242/jcs.112.13.2213. [DOI] [PubMed] [Google Scholar]
- 47.Gan PP, Kavallaris M. Tubulin-targeted drug action: functional significance of class ii and class IVb beta-tubulin in vinca alkaloid sensitivity. Cancer Res. 2008;68:9817–24. doi: 10.1158/0008-5472.CAN-08-1501. [DOI] [PubMed] [Google Scholar]
- 48.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 49.Hussain A, DiPaola RS, Baron AD, Higano CS, Tchekmedyian NS, Johri AR. Phase II trial of weekly patupilone in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20:492–7. doi: 10.1093/annonc/mdn665. [DOI] [PubMed] [Google Scholar]
- 50.Ranganathan S, Dexter DW, Benetatos CA, Chapman AE, Tew KD, Hudes GR. Increase of beta(III)- and beta(IVa)-tubulin isotopes in human prostate carcinoma cells as a result of estramustine resistance. Cancer Res. 1996;56:2584–9. [PubMed] [Google Scholar]


