Abstract
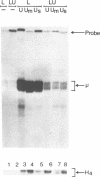
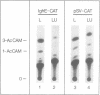
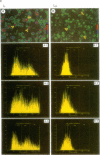
Stimulation of small, resting, splenic B cells with bacterial lipopolysaccharide (LPS) induces proliferation, differentiation to plasma cell formation, and the expression of immunoglobulin heavy chain (IgH). When this is combined with agents which crosslink surface Ig, differentiation and the induction of surface immunoglobulin are suppressed even though proliferation proceeds. We find that anti-mu antibodies suppresses Ig gene expression of transfected mu constructs, even if either the membrane or secretory segments have been deleted. We examined the effects of anti-mu treatment on the IgH enhancer (IgHE) attached to a heterologous test gene (CAT). Indeed the IgH enhancer alone was subject to anti-mu suppression, while the SV40 enhancer was insensitive. To determine what was responsible for suppression of enhancer function by anti-mu we examined nuclear extracts from stimulated splenic B cells for the presence of sequence-specific DNA binding activities to various sites within the enhancer. We found two specific differences--an induction in mu E5 binding activity, and a reduction in octamer transcription factor 2 (OTF2) binding activity, after anti-mu treatment. Analysis of these cells by in situ immunofluorescence with anti-OTF2 antibodies suggests that the nuclear localization of OTF2 in anti-mu treated cells may change, as well as its absolute level.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Bullock W. W., Melchers F. Inhibition of mitogenic stimulation of mouse lymphocytes by anti-mouse immunoglobulin antibodies. I. Mode of action. Eur J Immunol. 1974 Nov;4(11):715–722. doi: 10.1002/eji.1830041103. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Beckmann H., Su L. K., Kadesch T. TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev. 1990 Feb;4(2):167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- Berger C. N. In situ hybridization of immunoglobulin-specific RNA in single cells of the B lymphocyte lineage with radiolabelled DNA probes. EMBO J. 1986 Jan;5(1):85–93. doi: 10.1002/j.1460-2075.1986.tb04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Chen-Bettecken U., Wecker E., Schimpl A. IgM RNA switch from membrane to secretory form is prevented by adding antireceptor antibody to bacterial lipopolysaccharide-stimulated murine primary B-cell cultures. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7384–7388. doi: 10.1073/pnas.82.21.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Bettecken U., Wecker E., Schimpl A. Transcriptional control of mu- and kappa-gene expression in resting and bacterial lipopolysaccharide-activated normal B cells. Immunobiology. 1987 Mar;174(2):162–176. doi: 10.1016/s0171-2985(87)80036-0. [DOI] [PubMed] [Google Scholar]
- Chen U. Anti-IgM antibodies inhibit IgM expression in lipopolysaccharide-stimulated normal murine B-cells: study of RNA metabolism and translation. Gene. 1988 Dec 10;72(1-2):209–217. doi: 10.1016/0378-1119(88)90146-1. [DOI] [PubMed] [Google Scholar]
- Dennert G., Hyman R., Lesley J., Trowbridge I. S. Effects of cytotoxic monoclonal antibody specific for T200 glycoprotein on functional lymphoid cell populations. Cell Immunol. 1980 Aug 1;53(2):350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- Fiering S., Northrop J. P., Nolan G. P., Mattila P. S., Crabtree G. R., Herzenberg L. A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990 Oct;4(10):1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Matthias P., Thali M., Jiricny J., Schaffner W. Cell type-specificity elements of the immunoglobulin heavy chain gene enhancer. EMBO J. 1987 May;6(5):1323–1330. doi: 10.1002/j.1460-2075.1987.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Downward J., Waterfield M. D. Antibodies to the autophosphorylation sites of the epidermal growth factor receptor protein-tyrosine kinase as probes of structure and function. EMBO J. 1985 Nov;4(11):2869–2877. doi: 10.1002/j.1460-2075.1985.tb04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P., Kiledjian M., Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990 Jan 26;247(4941):467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Klein J., Bockman D. E., Cooper M. D., Lawton A. R. B cell differentiation induced by lipopolysaccharide. V. Suppression of plasma cell maturation by anti-mu: mode of action and characteristics of suppressed cells. J Immunol. 1978 Jan;120(1):158–166. [PubMed] [Google Scholar]
- Kemler I., Schaffner W. Octamer transcription factors and the cell type-specificity of immunoglobulin gene expression. FASEB J. 1990 Mar;4(5):1444–1449. doi: 10.1096/fasebj.4.5.2407588. [DOI] [PubMed] [Google Scholar]
- Koshland M. E. Presidential address: molecular aspects of B cell differentiation. American Association of Immunologists April 1983. J Immunol. 1983 Dec;131(6):i–ix. [PubMed] [Google Scholar]
- Köhrer K., Grummt I., Horak I. Functional RNA polymerase II promoters in solitary retroviral long terminal repeats (LTR-IS elements). Nucleic Acids Res. 1985 Apr 11;13(7):2631–2645. doi: 10.1093/nar/13.7.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanderson T., Hsu E. Anti-IgM treatment influences immunoglobulin heavy and light chain mRNA levels in mitogen-stimulated B lymphocytes. Eur J Immunol. 1985 Jun;15(6):641–643. doi: 10.1002/eji.1830150621. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Lenardo M., Baltimore D. Involvement of a second lymphoid-specific enhancer element in the regulation of immunoglobulin heavy-chain gene expression. Mol Cell Biol. 1990 Jun;10(6):3155–3162. doi: 10.1128/mcb.10.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Marcuzzi A., Van Ness B., Rouse T., Lafrenz D. Effects of anti-IgM suppression on polyclonally activated murine B cells: analysis of immunoglobulin mRNA, gene specific nuclear factors and cell cycle distribution. Nucleic Acids Res. 1989 Dec 25;17(24):10455–10472. doi: 10.1093/nar/17.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Mosthaf L., Pawlita M., Gruss P. A viral enhancer element specifically active in human haematopoietic cells. Nature. 1985 Jun 13;315(6020):597–600. doi: 10.1038/315597a0. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960 Dec 9;132(3441):1770–1771. doi: 10.1126/science.132.3441.1770. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Kadesch T., Sen R. Complex regulation of the immunoglobulin mu heavy-chain gene enhancer: microB, a new determinant of enhancer function. Mol Cell Biol. 1990 Jun;10(6):3145–3154. doi: 10.1128/mcb.10.6.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. L., Calame K. L. Complex protein binding within the mouse immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1987 Dec;7(12):4194–4203. doi: 10.1128/mcb.7.12.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. L., Calame K. Proteins binding to site C2 (muE3) in the immunoglobulin heavy-chain enhancer exist in multiple oligomeric forms. Mol Cell Biol. 1989 Feb;9(2):776–786. doi: 10.1128/mcb.9.2.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. L., Orth K., Calame K. L. Binding in vitro of multiple cellular proteins to immunoglobulin heavy-chain enhancer DNA. Mol Cell Biol. 1986 Dec;6(12):4168–4178. doi: 10.1128/mcb.6.12.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A., Heguy A., Fujii H., Roeder R. G. Activation of octamer-containing promoters by either octamer-binding transcription factor 1 (OTF-1) or OTF-2 and requirement of an additional B-cell-specific component for optimal transcription of immunoglobulin promoters. Mol Cell Biol. 1990 Dec;10(12):6204–6215. doi: 10.1128/mcb.10.12.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn J. M., van der Vliet P. C., Dathan N. A., Mattaj I. W. Anti-OTF-1 antibodies inhibit NFIII stimulation of in vitro adenovirus DNA replication. Nucleic Acids Res. 1989 Mar 11;17(5):1845–1863. doi: 10.1093/nar/17.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruezinsky D., Beckmann H., Kadesch T. Modulation of the IgH enhancer's cell type specificity through a genetic switch. Genes Dev. 1991 Jan;5(1):29–37. doi: 10.1101/gad.5.1.29. [DOI] [PubMed] [Google Scholar]
- Scheuermann R. H., Chen U. A developmental-specific factor binds to suppressor sites flanking the immunoglobulin heavy-chain enhancer. Genes Dev. 1989 Aug;3(8):1255–1266. doi: 10.1101/gad.3.8.1255. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Seyschab H., Friedl R., Schindler D., Hoehn H., Rabinovitch P. S., Chen U. The effects of bacterial lipopolysaccharide, anti-receptor antibodies and recombinant interferon on mouse B cell cycle progression using 5-bromo-2'-deoxyuridine/Hoechst 33258 dye flow cytometry. Eur J Immunol. 1989 Sep;19(9):1605–1612. doi: 10.1002/eji.1830190913. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Webb C. F., Gathings W. E., Cooper M. D. Effect of anti-gamma 3 antibodies on immunoglobulin isotype expression in lipopolysaccharide-stimulated cultures of mouse spleen cells. Eur J Immunol. 1983 Jul;13(7):556–559. doi: 10.1002/eji.1830130708. [DOI] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]