Abstract
It has been found that the bacterial endotoxins (lipopolysaccharides, LPSs) contain some amino acids and glycine is the most abundant amino acid in the polysaccharide core preparations of LPSs of gram-negative bacteria. Until now nothing was known about the mechanism of amino acid incorporation into the lipopolysaccharide core. We found that one out of three glycyl-tRNAs(Gly) from Escherichia coli is the donor of amino acid and is the substrate for a putative aminoacyl-tRNA:LPS transferase. We have isolated, purified this tRNA and determined its nucleotide sequence to be major E.coli tRNA(3Gly). This tRNA(Gly) (anticodon GCC) conserved the tRNA structural features. The assay for determination of the specific incorporation of glycine into the lipopolysaccharide was also invented and described.
Full text
PDF
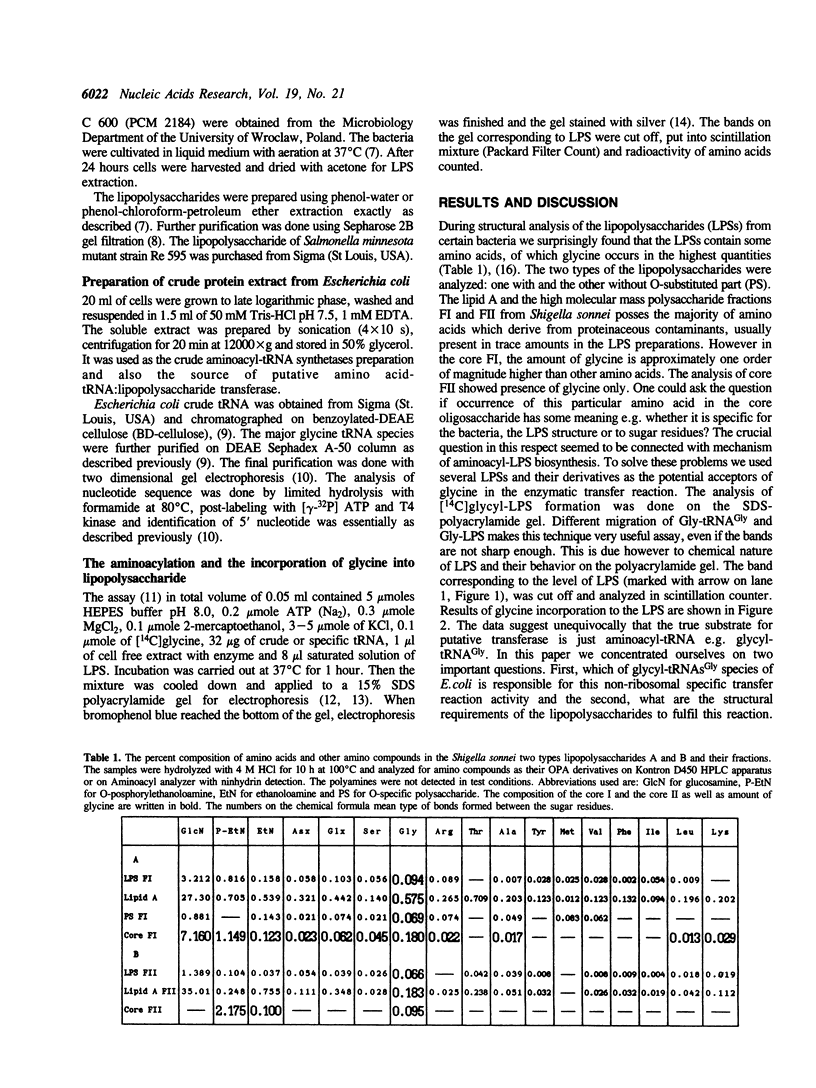
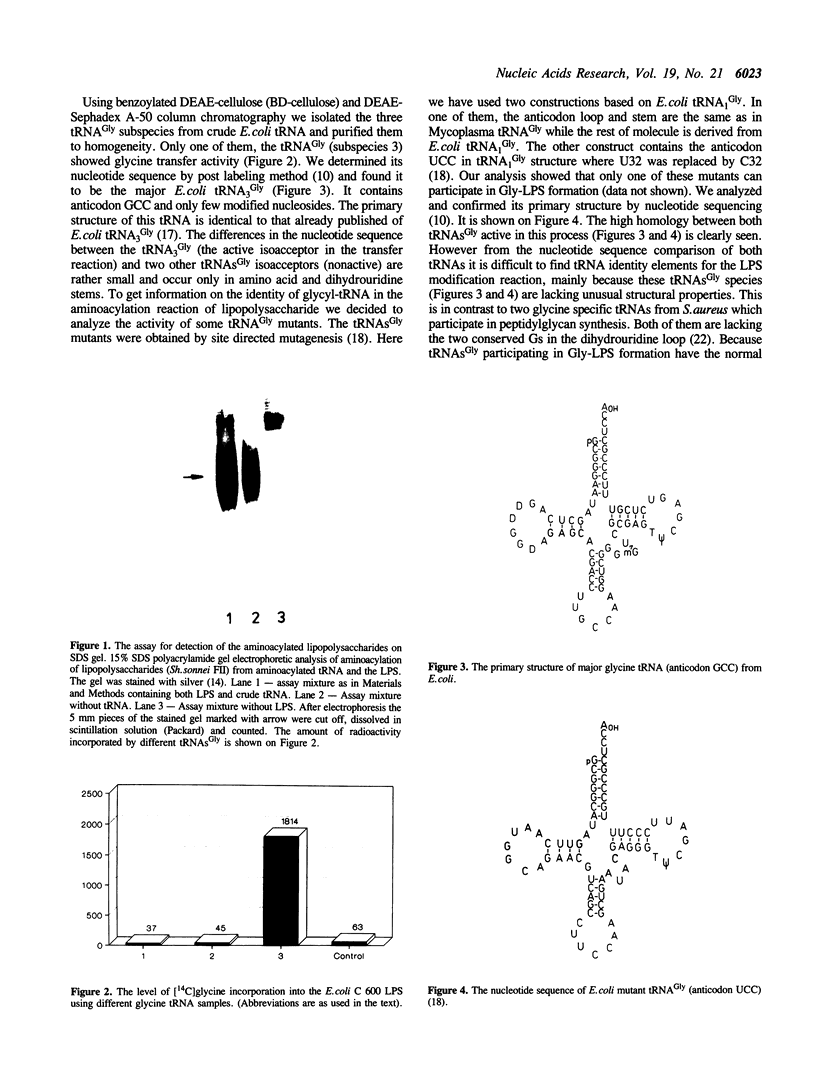
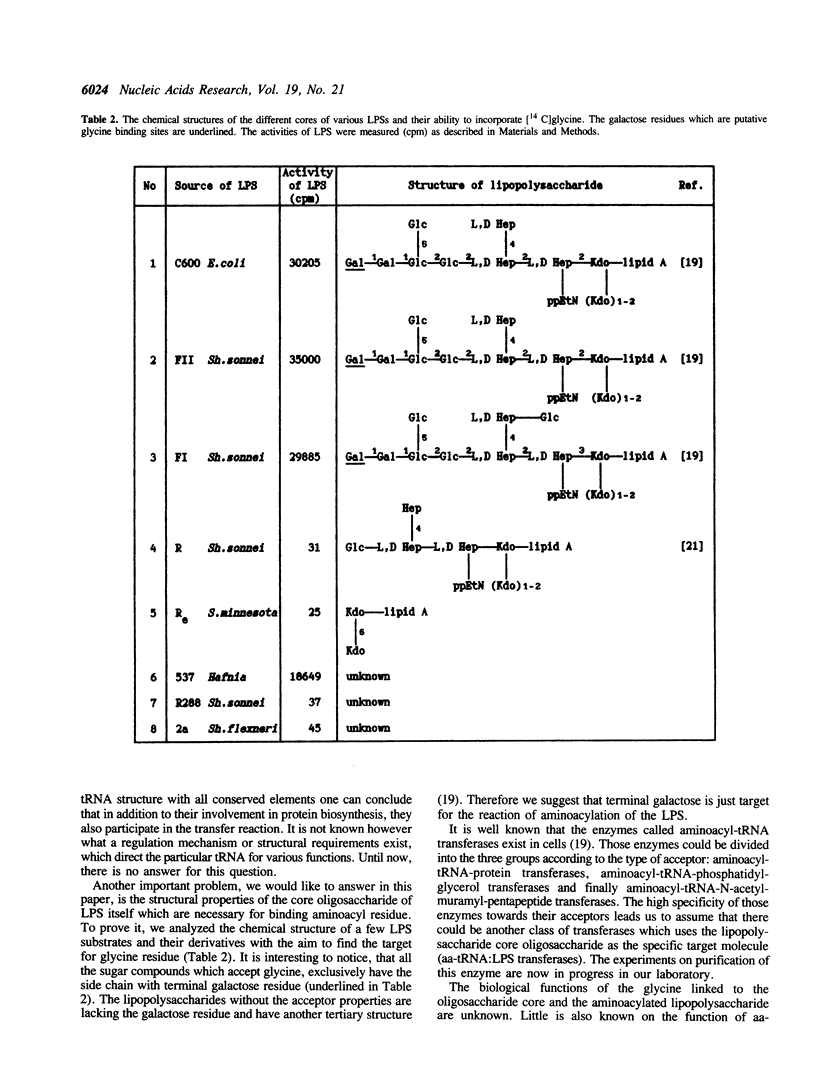

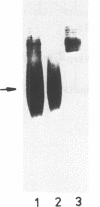
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barciszewska M., Dirheimer G., Keith G. The nucleotide sequence of methionine elongator tRNA from wheat germ. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1161–1168. doi: 10.1016/0006-291x(83)90684-8. [DOI] [PubMed] [Google Scholar]
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Brade H., Rietschel E. T. Alpha-2----4-interlinked 3-deoxy-D-manno-octulosonic acid disaccharide. A common constituent of enterobacterial lipopolysaccharides. Eur J Biochem. 1984 Dec 3;145(2):231–236. doi: 10.1111/j.1432-1033.1984.tb08543.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gamian A., Romanowska E. The core structure of Shigella sonnei lipopolysaccharide and the linkage between O-specific polysaccharide and the core region. Eur J Biochem. 1982 Dec;129(1):105–109. doi: 10.1111/j.1432-1033.1982.tb07027.x. [DOI] [PubMed] [Google Scholar]
- Kato M., Nozawa Y. Complete purification of arginyl-tRNA:protein arginyltransferase from hog kidney and production of its antibody. Anal Biochem. 1984 Dec;143(2):361–367. doi: 10.1016/0003-2697(84)90675-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebbar S., Cavaillon J. M., Caroff M., Ledur A., Brade H., Sarfati R., Haeffner-Cavaillon N. Molecular requirement for interleukin 1 induction by lipopolysaccharide-stimulated human monocytes: involvement of the heptosyl-2-keto-3-deoxyoctulosonate region. Eur J Immunol. 1986 Jan;16(1):87–91. doi: 10.1002/eji.1830160117. [DOI] [PubMed] [Google Scholar]
- Parent J. B. Membrane receptors on rat hepatocytes for the inner core region of bacterial lipopolysaccharides. J Biol Chem. 1990 Feb 25;265(6):3455–3461. [PubMed] [Google Scholar]
- Pollack M., Chia J. K., Koles N. L., Miller M., Guelde G. Specificity and cross-reactivity of monoclonal antibodies reactive with the core and lipid A regions of bacterial lipopolysaccharide. J Infect Dis. 1989 Feb;159(2):168–188. doi: 10.1093/infdis/159.2.168. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Carbon J. Nucleotide sequence studies of normal and genetically altered glycine transfer ribonucleic acids from Escherichia coli. J Biol Chem. 1975 Jul 25;250(14):5530–5541. [PubMed] [Google Scholar]
- Roberts R. J. Structures of two glycyl-tRNAs from Staphylococcus epidermidis. Nat New Biol. 1972 May 10;237(71):44–45. doi: 10.1038/newbio237044a0. [DOI] [PubMed] [Google Scholar]
- Romanowska E. Sepharose gel filtration method of purification of lipopolysaccharides. Anal Biochem. 1970 Feb;33(2):383–389. doi: 10.1016/0003-2697(70)90309-x. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Aminoacyl-tRNA transferases. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):91–139. doi: 10.1002/9780470122853.ch4. [DOI] [PubMed] [Google Scholar]