Abstract
Myc plays an important role in tumor development, including acute myeloid leukemia (AML). However, MYC is also a powerful inducer of apoptosis, which is one of the major failsafe programs to prevent cancer development. To clarify the relative importance of the extrinsic (death receptor-mediated) versus the intrinsic (mitochondrial) pathway of apoptosis in MYC-driven AML, we coexpressed MYC together with anti-apoptotic proteins of relevance for AML; BCL-XL/BCL-2 (inhibiting the intrinsic pathway) or FLIPL (inhibiting the extrinsic pathway), in hematopoietic stems cells (HSCs). Transplantation of HSCs expressing MYC into syngeneic recipient mice resulted in development of AML and T-cell lymphomas within 7–9 weeks as expected. Importantly, coexpression of MYC together with BCL-XL/BCL-2 resulted in strongly accelerated kinetics and favored tumor development towards aggressive AML. In contrast, coexpression of MYC and FLIPL did neither accelerate tumorigenesis nor change the ratio of AML versus T-cell lymphoma. However, a change in distribution of immature CD4+CD8+ versus mature CD4+ T-cell lymphoma was observed in MYC/FLIPL mice, possibly as a result of increased survival of the CD4+ population, but this did not significantly affect the outcome of the disease. In conclusion, our findings provide direct evidence that BCL-XL and BCL-2 but not FLIPL acts in synergy with MYC to drive AML development.
Introduction
Acute myeloid leukemia (AML) represents a clonal expansion of hematopoietic stem and myeloid progenitor cells that have undergone malignant transformation [1], [2]. The leukemic cells are typically blocked in their differentiation and exhibit an abnormal extension of life span. Clinically it is a heterogeneous group of tumors. However, genetic studies during recent years have highlighted two common types of genetic changes, named class I and II, in AML [1], [2]. Class I comprise point mutations in genes involved in signal transduction, such as Ras, FLT3 and Kit, which control cell growth, survival and migration. Class II include a number of different translocations that all involves transcription factors such as MLL, AML1, CBF, EVI1 and RARα, which control the myeloid differentiation program, resulting in fusion proteins with aberrant activities [1], [2]. A common target gene for the class II translocation products and for deregulated signal transduction caused by some class I mutations is the oncogene/transcription factor MYC [3], [4]. MYC controls numerous genes involved in stem cell functions, differentiation, growth, immortalization, metabolism, survival and other fundamental cellular processes, and deregulated expression of MYC is very often implicated in human tumor development including hematological malignancies [5], [6]. For instance in Burkitt's lymphoma, MYC expression is deregulated through chromosomal translocations juxtaposing MYC and one of the immunoglobulin loci. Amplifications of MYC family genes (MYC, MYCN, MYCL) are common in a number of tumor types and are also found in AML although at a low frequency [5], [6]. Trisomy of chromosome 8 (where MYC resides) is common in AML, and the resulting increased MYC gene dosage has been suggested as a leukemogenesis mechanism [7]. In addition, both MYC and MYCN are frequently overexpressed in AML as a result of oncogenic events such as the class I and II mutations mentioned above [8], [9], [10]. As a consequence, the tumorigenic effect of the MLL-ENL translocation, one of the most devastating translocations in AML, was shown to be dependent on MYC expression [4]. Further evidence linking MYC to AML are the findings that enforced expression of MYC or MYCN in hematopoietic stem cells induce AML in mouse models [10], [11].
Overexpression of MYC in cells in culture or in animal models fuels cell growth and proliferation but is also a potent trigger of the apoptotic machinery, which acts as a failsafe mechanism to suppress tumorigenesis [5], [6]. Both the intrinsic (mitochondrial) pathway and the extrinsic (death receptor-mediated) pathways of apoptosis have been shown to be affected by deregulated MYC expression [12], [13]. Firstly, Myc is known to induce p19Arf, which activates p53, one of the main pathways controlling apoptosis [14]. p53 frequently becomes mutated during progression of MYC-induced tumors and targeted deletion of p53 accelerates MYC-induced B-lymphoma development in the Eμ-myc transgenic mouse model [15]. Secondly, MYC suppresses the expression of the anti-apoptotic proteins such as BCL-XL and BCL-2, which regulate the intrinsic pathway. This mechanism is often bypassed during lymphoma progression of Eμ-myc mice [16]. Further, overexpression of BCL-2 or BCL-XL in these mice [17], [18] or in transplanted bone marrow cells [11], [19] accelerates MYC-driven tumor development, suggesting that BCL-2 and BCL-XL act in synergy with MYC in tumorigenesis. Moreover, Myc induces expression of pro-apoptotic proteins such as Bim and Bax [20], [21]. Thirdly, MYC sensitizes cells to death receptor-mediated apoptosis mediated by Fas-ligand, TNF-α or TRAIL [12], [13], [22], [23]. One reason for this could be that MYC upregulates expression of the TRAIL receptor DR5 and the Fas-ligand [5], [23], [24]. Further, epigenetic silencing of the caspase 8, which is the downstream effector of the death receptors, has been implicated in escape from apoptosis in MYCN-amplified neuroblastoma [25]. In support of this view, cells lacking Fas or TRAIL-R show increased survival in the Eμ-myc lymphoma model [26], [27]. Part of the increased response to death receptor signaling could be accounted for through MYC-induced suppression of NFκB and activation of Bak, facilitating caspase 8-mediated activation of Bax [28]. FLIP (CFLAR), another important regulator of death receptor-mediated apoptosis [29], inhibits apoptosis induced by death receptors of the TNFR superfamily by binding to caspase 8 at the death-inducing signaling complex (DISC) thereby blocking its activation [30]. FLIP can act as a tumor progression factor by promoting tumor establishment and growth in vivo [31], [32], [33]. Overexpression of MYC in myeloid cell lines has been shown to cause premature down regulation of FLIPL, thereby triggering the death receptor pathway [34]. Overexpression of FLIPL in these cells could completely rescue the cells from apoptosis [34]. Further, MYC was shown to repress FLIPL expression in a number if cells types both in culture and in vivo by binding to the FLIPL gene promoter region, resulting in increased sensitivity to TRAIL-induced apoptosis [35]. Several of these pathways are important for AML development. The class I mutations described above synergize with class II translocations at least in part by suppressing apoptosis, for instance by activating the RAS, MAPK and PI3 kinase pathways [1]. Overexpression of BCL2, BCL-XL and/or MCL-1 and reduced expression of Bax is frequently observed in AML. AML cells are often resistant to TRAIL- and Fas-induced apoptosis, which has been attributed to death receptor or FADD downregulation, caspase 8 mutations or overexpression of FLIPL [36].
Although the contribution of the extrinsic pathway to MYC-induced apoptosis has been studied extensively in different cell types in culture, only a few studies of the importance of this pathway for MYC-driven tumorigenesis in vivo have been performed (26, 27, 50), all using B- or T-lymphoma models. The potential impact of this pathway on MYC-induced AML is therefore unknown. In particular, the relative importance of the intrinsic and the extrinsic anti-apoptotic pathways in tumorigenesis fueled by MYC in vivo, including AML, remains to be determined.
The aim of this work was to assess the in vivo-impact of these two apoptotic pathways during MYC-induced malignant transformation of hematopoietic stem cells. We therefore coexpressed MYC together with anti-apoptotic gene products of either the extrinsic death receptor-mediated pathway (FLIPL) or the intrinsic (mitochondrial) pathway of apoptosis (BCL-2 or BCL-XL) in hematopoietic stem cells (HSC) followed by transplantation of the cells into syngeneic recipient mice. Expression of MYC alone in HSC resulted in development of both myeloid and T-lymphoid tumors within two months after transplantation. Expression of MYC together with BCL-XL or BCL-2 resulted in almost immediate development of AML-like disease. Surprisingly and contrary to our expectations, expression of MYC together with FLIPL did not accelerate tumorigenesis. However, while the ratio of AML versus T-cell lymphoma was not altered, the incidence of mature CD4+ T-cell lymphoma increased at the expense of immature CD4+/CD8+ T-cell lymphoma in MYC/FLIPL mice. In conclusion, anti-apoptotic proteins of the intrinsic pathway accelerate the development of MYC-driven hematopoietic tumors to a larger extent than those of the extrinsic pathway of apoptosis.
Materials and Methods
Cell lines and mice
The human retroviral packaging cell line Phoenix-Eco (kindly provided by Dr. G. P. Nolan, Stanford University, CA, USA) was grown as described [37]. Female age-matched (6–8 weeks) inbred DBA/2 and BALB/c mice were purchased from Taconic (Lille Skensved, Denmark) or from the animal facility at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Swedish Board of Agriculture and the Department of Microbiology, Tumor and Cell Biology (MTC), Karolinska Institutet. The protocol was approved by the Ethical Committee of the Swedish board of Agriculture, North of Stockholm (Permit Number N99/11). All efforts were made in order to minimize animal suffering.
Expression vectors
The pMSCV-IRES-EYFP vector was produced by replacing the enhanced green fluorescent protein (EGFP) of the pMSCV-IRES-EGFP vector (kindly provided by Dr. A. Nienhuis, St. Jude Children's Research Hospital, Memphis, USA) with the enhanced yellow fluorescent protein (EYFP) of pEYFP (Clontech, Palo Alto, CA) using the NcoI and NotI sites. The pMSCV-BCL-XL-IRES-EGFP vector was obtained by subcloning the EcoRI fragment containing human BCL-XL from the pLXIN-BCL-XL expression vector [38] into pMSCV-IRES-EGFP. The pMSCV-FLIPL-IRES-EGFP vector was generated as described previously [39]. MYC was isolated by PCR from cDNA from muscle cells from the Human Multiple Tissue cDNA (MTC) Panel 1 (Clontech) using the primers 5′-acgtgaattccaccatgcccctcaacgttagcttc and 5′-tacgtctcgagcttacgcacaagagttccgtag and subsequently cloned into the EcoRI and XhoI sites of pMSCV-IRES-EYFP to obtain the pMSCV-MYC-IRES-EYFP expression vector. pMSCV-BCL-2-IRES-EGFP was obtained by subcloning human BCL-2 from the cDNA clone MGC:21366 into the EcoRI site of pMSCV-IRES-EGFP.
Production of retroviral particles
Ten micrograms of the plasmids pMSCV-IRES-EGFP (GFP), pMSCV-IRES-EYFP (YFP), pMSCV-BCL-XL-IRES-EGFP (BCL-XL-GFP), pMSCV-BCL-2-IRES-EGFP (BCL-2-GFP), pMSCV-FLIPL-IRES-EGFP (FLIPL-GFP) or pMSCV-MYC-IRES-EYFP (MYC-YFP) expression vectors were used to transiently transfect Phoenix-Eco packaging cell line using Lipofectamine 2000 Reagent (Invitrogen Ltd, Paisley UK). Supernatants containing recombinant viral particles were harvested 48 and 72 hours after transfection, passed through a 0.45 µ M filter, and kept in aliquots at −80°C until used for viral transduction.
Hematopoietic stem cell enrichment and retroviral transduction
Bone marrow was extracted from the femur and tibia of mice 48 h after i.p. injection of 150 mg/kg of 5-fluorouracil (Mayne Pharma Pic, Warwickshire, UK). Bone marrow cells were enriched for hematopoietic progenitors and stem cells by negative selection using the StemSep kit (StemCell Technologies Inc., Vancouver, BC, Canada) according to the manufacturer's specifications. Cells were cultured for 24 hours in OPTIMEM (Invitrogen) supplemented with 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 µg/ml streptomycin, IL-6, IL-3 and SCF containing supernatants [40]. The hematopoietic stem cells were then co-transduced by two rounds of spin infection with combinations of GFP, YFP, BCL-XL-GFP, BCL-2-GFP, FLIPL-GFP and MYC-YFP in the presence of 8 µg/ml polybrene (Sigma, St Louis, MO, USA). This procedure was repeated for three consecutive days and the cells were then cultured for another three days. The percentage of GFP and YFP positive cells were measured by flow cytometry prior to transplantation. Non-transduced cells were sometimes used to dilute GFP+/YFP+ cells to frequencies below 5%. Transplantation was performed by intravenous injection of 1×106 cells into lethally irradiated (800rad for BALB/c or 960 rad for DBA/2) syngeneic recipient mice. The mice were given 8 µg/ml of doxycycline (Doxyferm; Merckle GmbH, Blaubeuren, Germany) in the drinking water and kept in filter top cages.
Analysis of mice
Mice were monitored three times a week for signs of disease by palpation and observation and were judged as terminally ill when they displayed signs of paralysis in limbs or persistently hunched posture and slow movements. Mononuclear cell suspensions of spleen, thymus, liver, bone marrow and lymph nodes were obtained by passing tissues through nylon mesh cell strainers (BD Biosciences, Bedford, MA, USA). Tissues (lymph node, spleen, thymus, liver, kidney, heart, lung, bone) from the mice were fixed in buffered 4% formaldehyde.
Flow cytometry analysis
Single cell suspensions from spleen, thymus, femoral bone marrow and liver were incubated with mAbs including anti-CD4-APC, anti-Gr1-APC, anti-CD19-bio, and anti-CD71-bio (BD PharMingen San Diego CA, USA). Pacific blue-labeled anti-CD8 (clone 2.43), anti-CD11b (clone M1/70.15) anti-Ter119 and anti-IgM (clone M41) MAb were prepared in our laboratory according to standard procedures. Biotinylated samples were then incubated with streptavidin-APC (BD PharMingen) before being analyzed on a Cyan ADP flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). EGFP and EYFP were detected using 510/21-nm and 550/30-nm bandpass filters separated by a dichroic 525-nm mirror. Flow cytometric data were analyzed with FlowJo (Tree Star, Inc., Ashland, OR, USA).
Results
Transduction of hematopoietic stem cells with retroviruses expressing MYC alone or together with the anti-apoptotic genes BCL-XL or FLIPL
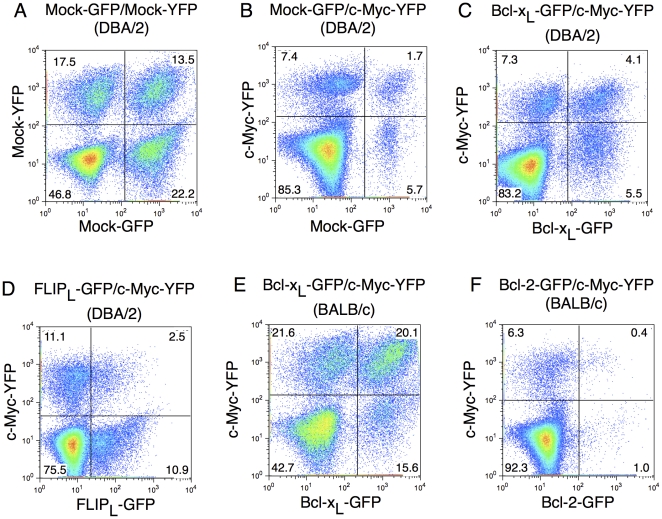
To study the effects of anti-apoptotic gene products regulating the intrinsic and extrinsic apoptosis pathway, respectively, on MYC-driven transformation of hematopoietic stem cells, murine lineage-negative (Lin−) hematopoietic stem cells (HSC) were enriched from bone marrow and transduced with replication incompetent retroviral expression vectors, followed by transplantation to lethally irradiated syngeneic recipient mice. Expression of the gene of interest is in this system driven by the retroviral LTR promoter that gives expression in all lineages of hematopoietic cells [37], [41]. The gene of interest is followed by an internal ribosomal entry site (IRES) and GFP or YFP reporter genes, resulting in the production of a bicistronic mRNA. HSC were transduced with combinations of two different retroviruses, one carrying MYC-YFP and the other either BCL-XL (regulating the intrinsic apoptotic pathway) or FLIPL (regulating the extrinsic pathway) linked to GFP. As controls the same GFP and YFP vectors lacking other genes were used. Previously, using vectors expressing GFP or YFP, we have shown that this results in a near to random frequency of single and double expressing hematopoietic cells after transduction and transplantation [37]. This experimental approach allows for a competitive repopulation strategy, whereby the effects of overexpression of two genes of interest, either alone or in combination can be studied in vivo. The expression of YFP and GFP as determined by flow cytometry after transduction of various combinations of MYC, BCL-XL, FLIPL or control vector into Lin− bone marrow cells prior to transplantation is shown in Figure 1. Western blot analysis confirmed that MYC, BCL-XL and FLIPL were all expressed in cells transduced with the respective retroviral constructs (Figure S1A).
Figure 1. Expression of the various combinations of genes in Lin− cells prior to transplantation.
FACS analysis of expression of YFP and GFP in HSC prior to transplantation in HSC derived from (A) DBA/2 mice transduced with Mock-GFP and Mock-YFP, (B) DBA/2 mice transduced with Mock-GFP and MYC-YFP, (C) DBA/2 mice transduced with BCL-XL-GFP and MYC-YFP, (D) DBA/2 mice transduced with FLIPL-GFP and MYC-YFP, (E) BALB/c mice transduced with BCL-XL-GFP or MYC-YFP and (F) BALB/c mice transduced with BCL-2-GFP and MYC-YFP. The percentage of cells expressing the genes in each quadrant, gated on PI negative cells, is indicated.
Inhibition of the intrinsic but not the extrinsic pathway of apoptosis accelerates MYC-driven tumorigenesis and shortens survival
Transplantation of Lin- bon marrow cells transduced with Mock-GFP/MYC-YFP resulted in reconstitution of myeloid, erythroid and lymphoid cells (Figure S2) but 7–9 weeks after transplantation, mice suddenly started to display signs of disease. Early signs of disease were ruffled fur, wasting and slow movements that rapidly developed into hind limb paralysis within two days. In some cases, also front leg paralysis was detected, and these animals showed mediastinal tumors invading the spine. Necropsy of moribund animals showed splenomegaly, grossly enlarged livers, non-coagulating blood, spotted lungs and in many animals also enlarged lymph nodes and thymus. This was in sharp contrast to mice receiving HSC transduced with Mock-GFP/Mock-YFP that remained healthy during the whole study period of 6 months (data not shown), despite higher frequencies of retroviral transduction (Figure 1A). The median survival after transplantation of Mock-GFP/MYC-YFP recipient mice was 56 days after transplantation (Figure 2A). This is in good agreement with previous reports on transplantation of MYC-transduced HSC into irradiated mice [11], [19], [42], [43].
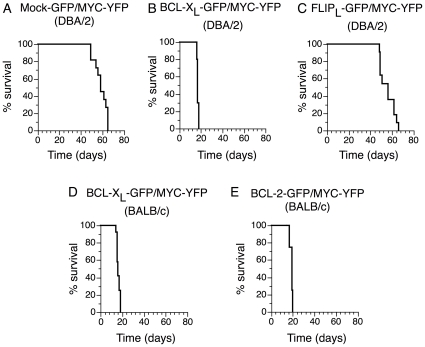
Figure 2. BCL-XL and BCL-2 but not FLIPL accelerate Myc-induced hematopoietic tumorigenesis.
Kaplan-Meier survival analysis of mice transplanted with HSC over-expressing (A) Mock-GFP/MYC-YFP in DBA/2, (B) BCL-XL -GFP/MYC-YFP in DBA/2, (C) FLIPL-GFP/MYC-YFP in DBA/2, (D) BCL-XL-GFP/MYC-YFP in BALB/c and (E) BCL-2-GFP/MYC-YFP in BALB/c. Mice were monitored daily for tumors or signs of paralysis and killed if showing signs of sickness. The percentage of mice surviving at daily intervals is shown.
In order to examine the influence of the intrinsic pathway of apoptosis during MYC-induced malignant transformation HSC were transduced with a combination of MYC and BCL-XL (Figure 1C). BCL-XL/MYC recipient mice rapidly became moribund and had a median survival of only 17 days (Figure 2A, B and Table 1). BCL-XL therefore significantly accelerated MYC-induced tumorigenesis. BCL-XL/MYC mice displayed similar signs of disease as Mock/MYC mice, namely ruffled fur and slow movements that developed into hind limb paralysis. Necropsy of moribund BCL-XL/MYC mice showed that tumors were disseminated throughout the hematopoietic tissues with extensive involvement of spleen and bone marrow and tumor infiltration of the liver. We further tested whether over-expression of FLIPL, a cellular inhibitor of death receptor-mediated apoptosis, would influence MYC-driven malignant transformation of hematopoietic cells. HSC were co-transduced with retroviral expression vectors containing MYC-YFP and FLIPL-GFP (Figure 1D). Surprisingly, FLIPL/MYC mice displayed similar signs of disease and with a similar kinetics as Mock/MYC mice (Figure 2A and 2C). All FLIPL/MYC mice died or were euthanized due to severe signs of sickness within 9 weeks with a 58 days median survival time for FLIPL/MYC to be compared with 56 days for Mock/MYC mice. The difference in survival time between Mock/MYC mice and FLIPL/MYC was not statistically significant whereas the difference in survival times between Mock/MYC and BCL-XL/MYC mice was highly significant (Table 1). Necropsy of moribund FLIPL/MYC animals showed similar features as Mock/MYC mice, including splenomegaly, hepatomegaly, non-coagulating blood, spotted lungs and in many animals also enlarged lymph nodes and thymus.
Table 1. Statistical analysis of survival data after transplantation of hematopoietic stem cells expressing MYC, BCL-XL, BCL-2 and/or FLIPL.
| Mock-GFP/MYC-YFP (DBA/2) | FLIPL-GFP/MYC-YFP (DBA/2) | BCL-XL-GFP/MYC-YFP (DBA/2) | BCL-XL-GFP/MYC-YFP (BALB/c) | |
| FLIPL-GFP/MYC-YFP (DBA/2) | NS* p = 0.5077 | - | ||
| BCL-XL-GFP/MYC-YFP (DBA/2) | S p<0.0001 | S p<0.0001 | - | |
| BCL-XL-GFP/MYC-YFP (BALB/c) | S p<0.0001 | S p<0.0001 | NS p = 0.2156 | - |
| BCL-2-GFP/MYC-YFP (BALB/c) | S p<0.0001 | S p<0.0001 | S p = 0.0004 | S P<0.0001 |
Statistical analysis of the survival data presented in Figure 2.
Log-rank (Mantel-Cox) test. NS = non significant. S = significant. P-value indicated below.
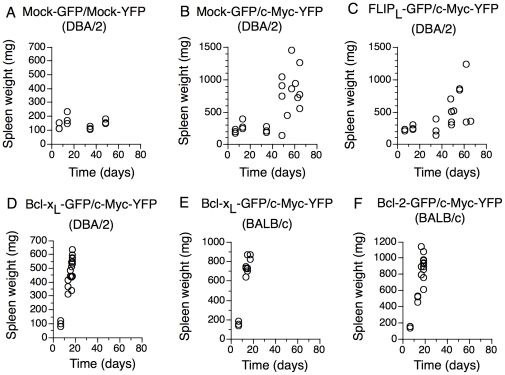
Spleen weights of recipient mice (showing no signs of disease) were recorded at 7, 14, 35 and 49 days after transplantation. Figure 3 shows spleen weights at these time points as well as spleen weights of all moribund mice. Mock-GFP/Mock-YFP recipient mice did not show signs of splenomegaly at any time although they had somewhat larger spleens 14 days after transplantation likely due to intensive reconstitution after irradiation and transplantation (Figure 3A). Mock-GFP/MYC-YFP mice only had slightly increased spleen sizes at early time points up to 5 weeks after transplantation (mean weight: 236 mg compared to 138 mg for Mock-GFP/Mock-YFP recipients). However, at later time points (+7 weeks), Mock-GFP/MYC-YFP recipient mice developed severe splenomegaly (mean weight: 816 mg) (Figure 3B). Co-expression of BCL-XL together with MYC resulted in accelerated development of severe splenomegaly in all animals 2 weeks after transplantation with mean values for moribund animals at 504 mg compared to 180 mg in control Mock/Mock animals and 260 mg in control Mock/MYC mice at 2 weeks after transplantation (Figure 3A, B and D).
Figure 3. Over-expression of BCL-XL and BCL-2 accelerate Myc-induced splenomegaly whereas co-expression of FLIPL does not influence MYC-induced splenomegaly.
Spleen weight of animals transplanted with HSC expressing (A) Mock-GFP/Mock-YFP into DBA/2 mice, (B) Mock-GFP/MYC-YFP into DBA/2 mice, (C) FLIPL-GFP/MYC-YFP into DBA/2 mice, (D) BCL-XL-GFP/MYC-YFP into DBA2 mice, (E) BCL-XL-GFP/MYC-YFP into BALB/c mice and (F) BCL-2-GFP/MYC-YFP into BALB/c. Spleens of mice at control time points (7 days, 14 days, 35 days and 49 days after transplantation) and spleens of all moribund mice were weighed. Each dot represents one mouse.
As in Mock/Myc mice, FLIPL/MYC mice appeared to have a latency period before development of splenomegaly (average spleen weight at 5 weeks after transplantation, 237 mg for FLIPL/MYC compared to 236 mg for Mock/MYC and 138 mg for Mock/Mock recipient mice) (Figure 3). Moribund FLIPL/MYC mice at later time points (+7 weeks) had grossly enlarged spleens (average 579 mg), to a similar extent as Mock/MYC mice (average 816 mg).
To ensure that the FLIPL construct was functionally active, Fas-sensitive A20 cells were transduced with the FLIPL-GFP construct and thereafter incubated with or without agonistic anti-Fas mAb for 20 hours and thereafter analyzed by flow cytometry. Figure S1B shows that FLIPL efficiently inhibited Fas-induced apoptosis in this system. This suggested that inhibition of the intrinsic but not the extrinsic pathway of apoptosis synergized with MYC to promote tumorigenesis of HSC.
Inhibition of the intrinsic but not the extrinsic pathway of apoptosis drives MYC-driven tumorigenesis towards acute myeloid leukemia
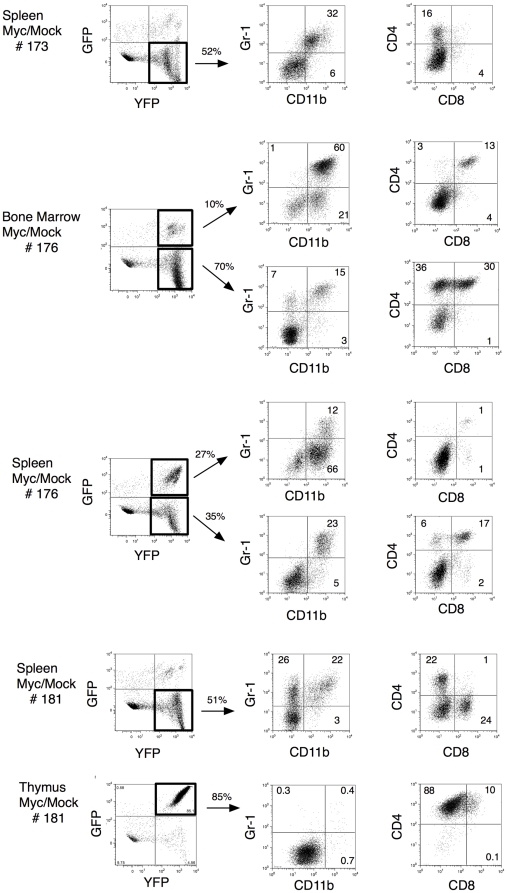
We next investigated the spectrum of tumors generated by expression of MYC alone and by coexpression of MYC together with BCL-XL or FLIPL, respectively, using flow cytometry. Single cell suspensions from femoral bone marrow, spleen, thymus, liver and (in a few individual mice) enlarged lymph nodes of mesenteric or cervical origin were stained with the myeloid markers anti-CD11b and anti-Gr1, the T-cell markers anti-CD4 and anti-CD8, the B-cell markers anti-CD19 and anti-IgM, and the erythroid markers anti-CD71 and Ter119. Mock/Mock animals all showed similar staining patterns with normal frequencies of myeloid as well as lymphoid cells (Table S1a). Each Mock/MYC animal displayed an individual setup of tumors of both myeloid lineages as well as tumors of T-cell lineage origins (Figure 4, Table S1b). Moreover, each Mock/MYC animal typically displayed diverse tumors in different cellular compartments. Bone marrow often contained tumors of myeloid origin whereas thymus and lymph nodes (if enlarged) contained T-cell tumors. Spleen and infiltrating cells of the liver frequently showed a mixed population of both myeloid and lymphoid origin with increased numbers in separate animals of both T-cells (in most cases CD4+, but also individuals with increased numbers of CD8+ and CD4+CD8+ cells) and often myeloid cells (Gr1+CD11b+, CD11b+ and/or Gr1+). Analyses of spleen cells from a few representative Mock/MYC mice are shown in Figure 4. Individual animals could have increased numbers of both MYC (GFP−YFP+) and Mock/MYC (GFP+YFP+) cells as exemplified by MYC/Mock #176 spleen (Figure 4) where the GFP+YFP+ subpopulation was mainly CD11b+ and the GFP−YFP+ subpopulation could be further divided into a Gr1+CD11b+ and a CD4+CD8+ population, indicating that these tumors were of oligoclonal nature.
Figure 4. Overexpression of MYC in HSC induces both myeloid and lymphoid leukemia.
Flow cytometry analysis of bone marrow and spleen cells from two individual moribund Mock/MYC mice (indicated with # in the left). In the middle, percentage of GFP+YFP+ (Mock/MYC expressing) cells or GFP−YFP+ (MYC expressing) cells are indicated. These cells were further characterized with anti-CD11b, anti-Gr1, anti-CD4, anti-CD8, anti-CD19, anti-IgM anti-CD71 and anti-Ter119. The percentage of cells in each quadrant is indicated.
In contrast, the vastly dominating cell type among BCL-XL-GFP/MYC-YFP expressing cells were myeloid CD11b+Gr1+ cells, which accounted for around 50% of the cells in bone marrow and spleen (Table S1d and Figure 5). Also the livers of moribund mice were heavily infiltrated with BCL-XL-GFP/MYC-YFP expressing myeloid CD11b+Gr1+ cells (Figure 5). Mice transplanted with MYC/BCL-XL had a pronounced leukocytosis compared to Mock/Mock transplanted mice (Figure S3). A differential count of the blood confirmed that approximately 50% of cells in the MYC/BCL-XL mice were blasts (Figure S3 and Table S2). These results indicate that BCL-XL drives MYC-driven tumorigenesis towards acute myeloid leukemia. These results differ from a report by Luo et al [11] that suggested that expression of MYC alone in bone marrow cells transplanted into recipient hosts give rise to AML, while coexpression of MYC and BCL-2 gave rise to a mixture of AML and pre-B acute lymphoid leukemia in BALB/c mice (see Discussion). In order to generalize our finding to other anti-apoptotic members of the BCL-2 family and also to other strains of mice, MYC was over expressed together with BCL-XL or BCL-2 in HSC of BALB/c mice followed by transplantation (Figure 1E-F). Mice transplanted with this combination rapidly developed a similar AML-like disease as did DBA/2 mice transplanted with HSC over expressing BCL-XL and MYC. Mean survival time was 16 days for BCL-XL/MYC BALB/c mice (Figure 2D) and 19 days for BCL-2/MYC BALB/c mice (Figure 2E). This minor difference in survival time between BCL-XL/MYC and BCL-2/MYC mice was statistically significant but is most likely a reflection of the lower proportion of BCL-2/MYC double expressing cells among the transplanted cells (Table 1, Figure 1). Co-expression of BCL-XL or BCL-2 together with MYC resulted in splenomegaly in all animals with mean values for moribund animals at 757 mg for BCL-XL/MYC BALB/c (Figure 3E) and 899 mg for BCL-2/MYC BALB/c mice (Figure 3F). All tumors predominantly expressed the granulocyte markers Gr1 and CD11b and were GFP+/YFP+ (Figure 5, Table S1e-f), thus confirming the results from the DBA/2 mice. For MYC alone, similar results concerning the kinetics of tumor development and the frequencies of myeloid versus lymphoid tumor cells were obtained using BALB/c mice (data not shown).
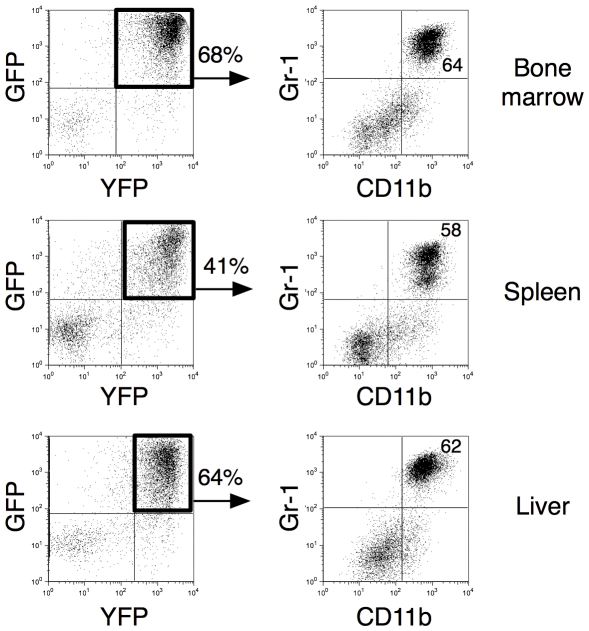
Figure 5. Overexpression of BCL-XL-GFP/MYC-YFP induces AML.
Flow cytometry analysis of bone marrow (top), spleen (middle) and liver (bottom) cells from one representative moribund BCL-XL-GFP/MYC-YFP mouse. Single cell suspensions from femoral bone marrow, spleen and liver were stained with anti-CD11b and anti-Gr1. The percentage of dominating cells is indicated.
As in Mock/MYC mice, FLIPL/MYC mice primarily developed tumors of myeloid (Gr1+CD11b+, CD11b+ and/or Gr1+) or T-cell lineage origin (CD4+). FLIPL/MYC spleen and infiltrating cells of the liver frequently showed a mixed population of both myeloid and lymphoid origin as in Mock/MYC mice (Table S1b-c). One notable difference to cells expressing MYC alone was that the double positive CD4+CD8+ lymphoid tumor cells population was diminished among FLIPL/MYC-expressing cells in favor of the CD4+ population (Table S1c). This might suggest that FLIPL plays a role in the selection of MYC-induced lymphoid but not of myeloid tumor cells in this system.
Immunohistochemical analyses confirmed and extended the cytometrical results. Sections of spleen and liver were stained with Abs against the T-cell markers CD45R and CD3 and the myeloid marker myeloperoxidase (MPO) (See Materials and Methods S1). Extensive staining with CD3 and MPO could be observed in spleen and liver of Mock/MYC recipient mice. In liver, CD3+ cells were predominantly distributed as perivascular “cuffs” in contrast to a more diffuse distribution of myeloid cells. Splenic architecture was heavily distorted with an expanded red pulp, immunohistochemically dominated by CD3+ and MPO+ cells (Figure S4). No infiltration could be detected in livers of Mock/Mock recipient mice and spleens showed a normal architecture (Figure S5). In MYC/BCL-XL or MYC/BCL-2 mice tumor cells in spleen and liver expressed the myeloid marker MPO but not the T-cell markers (Figure S6 and S7). Immunohistochemical stainings of tumor cells in FLIPL/MYC mice showed similar staining patters as Mock/MYC (Figure S8).
Tumor cell lines could be established from all moribund Mock/MYC, MYC/BCL-XL, MYC/BCL-2 and MYC/FLIPL recipient mice. These cells were further characterized by flow cytometry and all MYC-YFP+ appeared with similar phenotypes as the primary tumors (data not shown).
These results are summarized in Figure 6 and suggest that Mock/MYC mice developed myeloid leukemia and lymphoblastic T-cell lymphoma. Coexpression of MYC and BCL-XL or BCL-2 strongly favored tumor development towards myeloid leukemia, whereas coexpression of MYC and FLIPL did not affect the distribution of myeloid versus lymphoid tumor cells, but changed the ratio between single CD4+ and double positive CD4+CD8+ T-cell lymphomas.
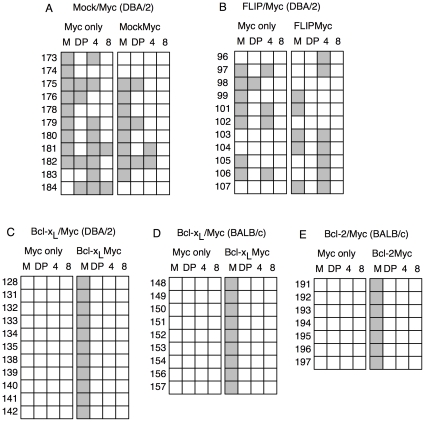
Figure 6. Tumor phenotype in Mock/MYC, FLIPL/MYC, BCL-XL/MYC and BCL-2/MYC recipient mice.
Summary of flow cytometry analysis of spleen cells from moribund mice transplanted with HSC expressing (A) Mock-GFP/MYC-YFP into DBA/2 mice, (B) FLIPL-GFP/MYC-YFP into DBA/2 mice, (C) BCL-XL-GFP/MYC-YFP into DBA2 mice, (D) BCL-XL-GFP/MYC-YFP into BALB/c mice and (E) BCL-2-GFP/MYC-YFP into BALB/c mice. Left panel specify tumors expressing MYC-YFP only and right panel specify cells expressing MYC-YFP together with either Mock-GFP (A), FLIPL-GFP (B), BCL-XL-GFP (C and D) or BCL-2-GFP (E). Filled box indicate tumor phenotype. M indicates tumors of myeloid lineage, DP indicate CD4+CD8+ lymphoid tumors, 4 indicate CD4+ lymphoid tumors and 8 indicate CD8+ lymphoid tumors. Numbers correspond to identity of individual animal.
Early expansion of myeloid leukemic cells in MYC and MYC/BCL-XL expressing mice at the expense of other hematopoietic cell populations
In order to follow leukemia/lymphoma development kinetically and to gain insight into how different blood cell populations were affected by MYC and MYC/BCL-XL expression, hematopoietic development was studied in detail at 7, 14 and 35 days after transplantation of Mock/MYC- or MYC/BCL-XL-transduced HSC into recipient mice. Figure S2 (middle columns, top row) shows that MYC-YFP-expressing cells dominated among cells in bone marrow and spleen already at day 7. In bone marrow and spleen, MYC-YFP-expressing cells constituted about 50% of all cells. This figure was rather constant between 7 and 35 days after transplantation. At the time of transplantation, only about 6% of the cells expressed MYC-YFP, indicating that these cells underwent a rapid expansion to reach high steady-state levels in vivo. Mock-GFP/Mock-YFP-expressing cells, on the other hand, did not expand and were kept at constant relative numbers reflecting the proportions of transduced cells before transplantation (Figure S2, left columns, top row). BCL-XL-GFP/MYC-YFP expressing cells in bone marrow expanded from about 20% of the cells at day 7 to 75% at day 14 after transplantation. In spleen, BCL-XL-GFP/MYC-YFP expressing cells expanded from 20% at day 7 to 40% at day 14 (Figure S2, right columns, top row).
Next, the phenotypes of cells expressing Mock-GFP, MYC-YFP and BCL-XL-GFP/MYC-YFP were analyzed. In figure S2 (middle panels) it can be seen that the dominating cell type among expanded MYC expressing cells was the myeloid CD11b+Gr1+ cell population, which accounted for around 60% of the cells in the bone marrow and 20–30% in spleen already at day 7 and thereafter. Corresponding figure for Mock-expressing cells was considerably lower. MYC expression was readily detectable in pre-B, B, CD4+ and CD8+ T cells and in erythroid Ter119+CD71hi and Ter119+CD71lo cells but did not result in their expansion. A tendency towards expansion of CD19+IgM− and CD8+ T cells could be recorded among MYC-expressing cells in the spleen at day 35. BCL-XL-GFP/MYC-YFP expressing cells could be found in pre-B, B, CD4+, CD8+ T cells and in erythroid Ter119+CD71hi and Ter119+CD71lo cells, in particular at day 14 (Figure S2, right panels). However, the vastly dominating cell type among expanded BCL-XL-GFP/MYC-YFP expressing cells already at day 7 were myeloid CD11b+Gr1+ cells, which accounted for around 60% of the cells in bone marrow and spleen at day 14.
Overall, these results indicate that the myeloid CD11b+Gr1+ population dominated at early time points both in mice transplanted with MYC-YFP- and BCL-XL-GFP/MYC-YFP-expressing HSCs. The expansion of other cell populations lagged behind and was only evident in MYC-expressing mice at day 35, a time point where the MYC/BCL-XL mice had already died from AML.
MYC- and MYC/BCL-XL-expressing CD11b+Gr1+, pre-B and B cells become blast transformed early after transplantation
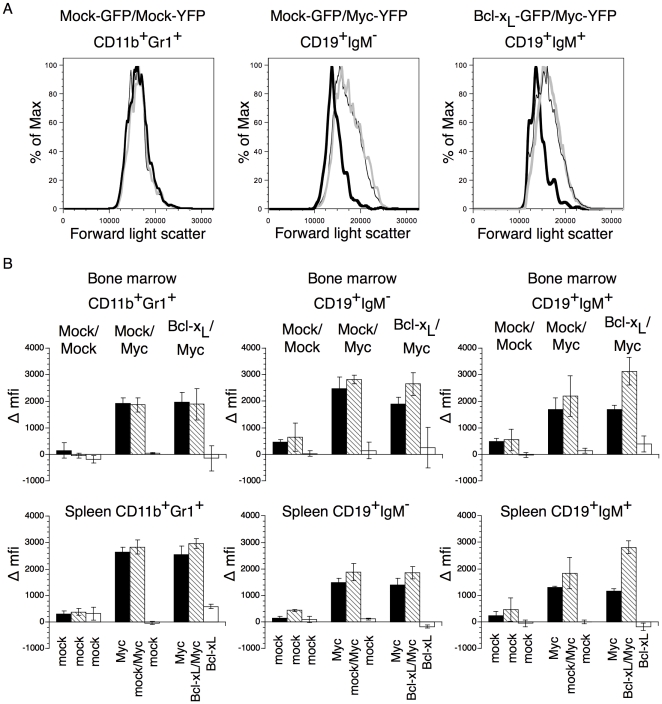
To study activation and blast transformation of myeloid and B lymphoid cells, differences in mean fluorescence intensity (Δmfi) in forward scatter were analyzed in CD11b+Gr1+, CD19+IgM− and CD19+IgM+ cells from bone marrow and spleen 14 days after transplantation. BCL-XL/MYC and MYC expressing cells of all these phenotypes were clearly blast transformed compared to non-transduced cells, cells expressing Mock-GFP or cells expressing only BCL-XL-GFP within the same animal (Figure 7, A–B middle and right panels). Similar results were obtained at 7 days and 35 days after transplantation (data not shown). For CD19+IgM+B cells, increased cell size was noted for MYC/BCL-XL cells compared to cells expressing MYC only, perhaps indicating increased survival of such cells in the presence of BCL-XL. It was, however, mainly MYC- and MYC/BCL-XL-expressing myeloid CD11b+Gr-1+ cells that expanded and rapidly reached elevated steady-state levels (Figure S2). In summary, mice transplanted with MYC- or MYC/BCL-XL-expressing cells, contained large number of pre-malignant MYC-expressing cells of various lineages. These populations remained at steady-state levels until about 6–7 weeks after transplantation in MYC-expressing mice, when exponential growth of some of these cell populations suddenly ensued. At this time point MYC/BCL-XL-expressing mice had died from AML.
Figure 7. Blast formation of Mock-GFP/Mock-YFP, Mock-GFP/MYC-YFP or BCL-XL-GFP/MYC-YFP bone marrow and spleen cells.
(A) Forward light scatter of CD11b+Gr1+ bone marrow cells from Mock-GFP/Mock-YFP, Mock-GFP/MYC-YFP and BCL-XL-GFP/MYC-YFP recipient mice 2 weeks after transplantation. Grey thick lines indicate GFP−YFP+ cells, thick black lines indicate GFP+YFP− cells and thin lines indicate GFP+YFP+ cells. (B) Difference in mean fluorescence intensity (Δmfi) in forward scatter of CD11b+Gr1+ (left), CD19+IgM− (middle) and CD19+IgM+ (right) cells in bone marrow (top) and spleen (bottom) between GFP−YFP− (non-transduced cells) and GFP−YFP+ (black bars), GFP+YFP+ (striped bars) or GFP+YFP− (white bars) cells are shown. Values indicate means of three individual mice and error bars indicate 1 SD.
Discussion
Several lines of evidence from the human disease and mouse models have implicated the MYC oncogene in development of AML [3], [4], [8], [10], [11], [19], [42], [43], [44], [45], [46]. However, deregulated MYC expression has also been shown to induce the two major pathways of apoptosis, the intrinsic and the extrinsic (death receptor) pathways [15], [20], [23], [34], [35], [47]. This anti-tumorigenic property of MYC needs to be overcome for tumor development to occur, and mutations or deregulated expression of components of both the intrinsic and extrinsic pathways have been documented in AML [36], although the relative contribution of these pathways for the disease has not been investigated systematically. In order to explore the role of these two pathways of apoptosis during MYC-induced AML, we have overexpressed MYC in HSC, the tumor-initiating cell of AML, either alone or together with cellular inhibitors of the intrinsic or the extrinsic pathways of apoptosis of particular relevance for AML; BCL-XL, BCL-2 and FLIPL. The transduced HSC were transplanted to lethally irradiated syngeneic recipient mice after which tumor development was monitored.
Our results show that mice reconstituted with HSC overexpressing MYC developed myeloid leukemia and T cell lymphoma within 9 weeks, in agreement with previous reports [11], [19], [42], [43]. Importantly, coexpression with BCL-XL or BLC-2 strongly accelerated MYC-induced tumorigenesis and all mice died or were moribund within 2 weeks after transplantation. Co-expression of MYC with BCL-XL or BCL-2 also influenced the tumor phenotype; Rather than a mixed phenotype of myeloid and T lymphoid tumors, development of an aggressive AML-like disease was favored (Figure 6). The synergistic effect of MYC and BCL-XL or BCL-2 overexpression on tumor development observed here is in agreement with previous observations in transgenic Eμ-MYC/BCL-2-induced B lymphoma [17], [18]. However, although using an AML mouse model based on transplantation of transduced bone marrow cells into irradiated recipient hosts similar to this study, Luo et al. [11] reported that BCL-2 did not accelerate MYC-induced AML, but rather stimulated development of pre-B acute lymphoid leukemia (ALL). We could not reproduce the results of Luo et al in our model using the same genes and mouse strain (see further discussion below). Our detailed studies of the kinetics of tumor development showed that overexpression of MYC, either alone or together with BCL-XL or BCL-2, rapidly resulted in blast transformation of various hematopoietic cell types, including myeloid and lymphoid cells. However, only myeloid cells expanded, and in the case of MYC expression they rapidly reached a steady-state level that was kept until about 7–9 weeks after transplantation, when suddenly a fatal expansion ensued. In the case of MYC and BCL-XL or BCL-2 co-expression, myeloid cells expanded exponentially already within a few days after transplantation. Our results indicate that different hematopoietic cell lineages are differentially permissive for oncogenic transformation. It is likely that an early expansion of the MYC-transformed myeloid leukemia cells is counteracted by MYC-induced apoptosis that is overcome by BCL-XL or BCL-2 expression, resulting in an almost immediate tumor transformation of myeloid CD11b/Gr1-positive cells, subsequently causing AML. Our results are in line with a recently published study by Beverly and Varmus [19] in which MYC was overexpressed together with each of the 6 known anti-apoptotic members of the BCL-2 family in bone marrow cells transplanted into irradiated hosts like here, all resulting in acceleration of MYC-induced AML-like disease. The reason for the discrepancy in relation to Luo et al. [11] is unclear, but may be due to differences in the purification of the retroviral target bone marrow cell population (see further discussion below).
In contrast to the effects of BCL-XL and BCL-2 overexpression, FLIPL although functional, did not enhance MYC-driven tumorigenesis, neither with respect to the kinetics nor the relative proportion of myeloid leukemia versus T cell lymphoma compared with MYC alone. This result was unexpected considering previous reports that Myc sensitizes cells, including hematopoietic cells, to death receptor-induced apoptosis [12], [13], [22], [23], [24], [25], [27], [35], [48], involving at least in part MYC-mediated repression of FLIPL expression [34], [35]. There could be several different explanations to this result. First, it should be noted that all previous studies on the relation between MYC and FLIPL has been performed in cell cultures in vitro, and may be less relevant for tumor development in vivo. Nevertheless, TRAIL-R deficiency has been shown to enhance MYC-induced lymphomagenesis in the Eμ-myc model [27]. However, recent studies suggest that MYC sensitizes death receptor signaling mainly by regulating BCL-2-family proteins of the intrinsic pathway and by suppressing NFκB [28], [49], which all are involved in crosstalk with the extrinsic pathway. Through this mechanism several pathways of MYC-induced apoptosis would be blocked by BCL-XL and BCL-2, which may explain their particular importance in Myc-driven AML as shown here. Still, even if MYC mainly sensitizes the death receptor pathway via regulating BCL-2-family proteins and NFκB, we would expect overexpression of FLIPL to have some impact if the death receptor pathway is important for suppressing AML development. Another possible explanation is that the death receptor pathway and its regulation by FLIPL is important for certain MYC-induced tumors, such as Eμ-myc driven B cell lymphoma, but less important in the case of AML. One difference between MYC and MYC/FLIPL mice was that the ratio between immature CD4+/CD8+ and mature CD4+ T-cell lymphoma was skewed towards CD4+ lymphoma. This might indicate that overexpression of FLIPL favors differentiation of T-cell lymphoma cells or increases the survival of mature CD4+ T-cell lymphoma cells, but does not change the overall tumor burden. Previous studies of Myc-induced CD4+/CD8+ T–cell lymphoma in the CD2-MYC model in a lpr genetic background showed that deletion of Fas did not accelerate T-cell lymphoma development, although an increased population of CD4+ T-cells was found [50]. Smith et al. [45] reported that MYC-expressing CD4+ T cells in the VavP-Myc model was more prone to apoptosis than the corresponding CD4+/CD8+ T-cells, suggesting that FLIPL might contribute to increased survival of this population in our model system, although this did not contribute to increased T-cell lymphoma over AML ratio. Any potential impact of FLIPL on MYC-induced B lymphomagenesis seems to be masked in this system by the rapid development of AML and T-cell lymphoma. This conclusion is not incompatible with FLIPL being important for Myc-driven development of other types of tumors, such as B lymphoma (26, 27), under other conditions.
Although MYC was over expressed in cells of all hematopoietic lineages and resulted in blast transformation, morbidity was primarily due to development of myeloid and/or T-cell tumors.
T-cell lymphomas were similar both in phenotype (CD4+ or CD4+/CD8+) and latency (7–10 weeks) to those of VavP-MYC17 mice [51]. VavP-Myc17 transgenic animals with high expression of MYC in nucleated cells of hematopoietic origin have been reported to mainly develop T-cell lymphomas [51] whereas transgenic animals with low expression of c-Myc instead mainly developed myeloid monocytic tumors and only 7% T-cell lymphomas [45], suggesting that different expression levels of MYC may provoke different tumor phenotypes. In our experimental system, we were unable to find a direct correlation between MYC expression levels and myeloid or lymphoid phenotypes, but we cannot completely rule out that MYC expression levels may influence the disease phenotype. Most reports where MYC is expressed broadly in hematopoietic cell types in mice, it seems to generate either myeloid leukemia, T lymphoma or a mix of these two tumor types like here, which may reflect different levels of MYC depending on expression systems used [11], [19], [42], [43], [45], [46], [51]. Retroviral expression of MYC in bone marrow cells like here seems more often to result in predominance of myeloid leukemia [11], [42], [43], possibly due to lower levels of MYC than in the Vav promoter system. Another possibility is that the tumor initiating MYC target cell(s) differ between the systems. This may also explain differences in MYC-induced tumor types between different retroviral transduction systems. For instance, Luo et al. [11], who observed AML in the absence of T-cell lymphoma, used unfractionated mononuclear bone marrow cells, whereas we transduced Lin− bone marrow cells. In another transplantation model, overexpression of MYC resulted in development of aggressive pre-B cell lymphomas, with low penetrance and after long latency (>100 days) [21]. Here, fetal liver (E14.5) cells were used for retroviral transduction. Also other methodological aspects such as choice of retroviral backbone, the use of single or dual expression vectors or time of culture in vitro prior to transplantation may influence experimental outcome.
In conclusion, our results suggest that MYC-induced transformation of HSC accelerates and polarizes hematopoietic tumor development towards aggressive AML by coexpression of suppressors of the intrinsic but not the extrinsic pathway of apoptosis. Since all these players have been implicated in the development of human AML, these results may potentially be of relevance for further preclinical and clinical studies of this disease.
Supporting Information
Expression and functionality of retroviral constructs. A. Analysis of proteins from MYC/BCL-XL tumor cells and Fas-sensitive A20 cells transduced with MSCV-hFLIPL-IRES-GFP retroviral particles. B. Fas-sensitive A20 cells were transduced with MSCV-hFLIPL-IRES-GFP retroviral particles. Transduced or wt A20 cells were thereafter incubated with or without agonistic anti-Fas mAb (0.25 µg/ml of Jo2) for 20 hours and thereafter analyzed by flow cytometry using propidium iodide (PI) to discriminate between live and dead cells.
(TIF)
Early hematopoietic development of DBA/2 mice reconstituted with Mock-GFP/Mock-YFP, Mock-GFP/MYC-YFP or BCL-XL-GFP/Myc-YFP expressing HSCs. Phenotypic analysis of bone marrow (left) and spleen (right) at 7, 14 and 35 days after transplantation of Mock-GFP/Mock-YFP, Mock-GFP/MYC-YFP or BCL-XL-GFP/MYC-YFP expressing HSCs into DBA/2 mice was performed with FACS. The horizontal axis indicates days after transplantation and the vertical axis represents percentage of cells expressing the marker indicated to the left. Black triangles: GFP+YFP− cells, black squares; GFP−YFP+ cells and white circles; GFP+YFP+ cells.
(TIF)
Appearance of leukemic blasts in the blood of MYC/BCL-XL recipient mice. Blood smears stained with May-Grunewald-Giemsa. Blood smear from a Mock/Mock mouse shows a normal picture with few leukocytes (A) as compared to the leukocytosis seen in MYC/BCL-XL mice (B) (primary magnification 20×). Higher magnification shows a dominance of blast-like cells with only few maturing granulocytes in MYC/BCL-XL mice (C and D) (primary magnification 60×).
(TIF)
Immunohistochemical staining of sections of spleen and liver of Mock/MYC mice. The section slides were stained with antibodies directed against the T-cell markers CD45R and CD3 and the myeloid marker myeloperoxidase (MPO) and analyzed by immunohistochemistry as described in Materials and Methods S1.
(TIF)
Immunohistochemical staining of sections of spleen of Mock/Mock mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemical staining of sections of spleen and liver of MYC/BCL-XL mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemical staining of sections of spleen and liver of MYC/BCL-2 mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemical staining of sections of spleen, liver and thymus of MYC/FLIPL mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemistry.
(DOCX)
Flow cytometric analysis of bone marrow, thymus, spleen and liver in MYC, BCL-XL,, BCL-2, FLIPL or control virus recipient mice.
(DOCX)
Differential count of nucleated cells in peripheral blood smears in MYC/BCL-XL and control mice.
(DOCX)
Acknowledgments
We thank Birger Christensson for help with microscopy and Sören Lehmann and Vedrana Tabor for useful comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the Swedish Cancer Society (AG, LGL, BS), the Swedish Childhood Cancer Society (LGL, BS), the Swedish Research Council (AG, LGL, BS), the Stockholm Cancer Society (LGL, BS), KI Foundations (AG, LGL, BS), KI support to the Advanced Cancer Therapies (ACT!) consortium (LGL, BS), The County Council of Stockholm (BS), Olle Engkvist's Foundation (LGL), and Åke Olsson's Foundation for Hematology Research (AG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gilliland DG, Tallman MS. Focus on acute leukemias. Cancer Cell. 2002;1:417–420. doi: 10.1016/s1535-6108(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 2.Frankfurt O, Licht JD, Tallman MS. Molecular characterization of acute myeloid leukemia and its impact on treatment. Curr Opin Oncol. 2007;19:635–649. doi: 10.1097/CCO.0b013e3282f10e55. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Tidow C, Steffen B, Cauvet T, Tickenbrock L, Ji P, et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol Cell Biol. 2004;24:2890–2904. doi: 10.1128/MCB.24.7.2890-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiner S, Birke M, Garcia-Cuellar MP, Zilles O, Greil J, et al. MLL-ENL causes a reversible and myc-dependent block of myelomonocytic cell differentiation. Cancer Res. 2001;61:6480–6486. [PubMed] [Google Scholar]
- 5.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 6.Larsson LG, Henriksson MA. The Yin and Yang functions of the Myc oncoprotein in cancer development and as targets for therapy. Exp Cell Res. 2010;316:1429–1437. doi: 10.1016/j.yexcr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Jones L, Wei G, Sevcikova S, Phan V, Jain S, et al. Gain of MYC underlies recurrent trisomy of the MYC chromosome in acute promyelocytic leukemia. J Exp Med. 2010;207:2581–2594. doi: 10.1084/jem.20091071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larramendy ML, Niini T, Elonen E, Nagy B, Ollila J, et al. Overexpression of translocation-associated fusion genes of FGFRI, MYC, NPMI, and DEK, but absence of the translocations in acute myeloid leukemia. A microarray analysis. Haematologica. 2002;87:569–577. [PubMed] [Google Scholar]
- 9.Preisler HD, Guan WD, Khan S, Kinniburgh A. Differing patterns of proto-oncogene expression in immature and mature myeloid cells. Leuk Res. 1987;11:923–934. doi: 10.1016/0145-2126(87)90138-x. [DOI] [PubMed] [Google Scholar]
- 10.Kawagoe H, Kandilci A, Kranenburg TA, Grosveld GC. Overexpression of N-Myc rapidly causes acute myeloid leukemia in mice. Cancer Res. 2007;67:10677–10685. doi: 10.1158/0008-5472.CAN-07-1118. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Li Q, O'Neal J, Kreisel F, Le Beau MM, et al. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106:2452–2461. doi: 10.1182/blood-2005-02-0734. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 13.Meyer N, Kim SS, Penn LZ. The Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol. 2006;16:275–287. doi: 10.1016/j.semcancer.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 18.Swanson PJ, Kuslak SL, Fang W, Tze L, Gaffney P, et al. Fatal acute lymphoblastic leukemia in mice transgenic for B cell-restricted bcl-xL and c-myc. J Immunol. 2004;172:6684–6691. doi: 10.4049/jimmunol.172.11.6684. [DOI] [PubMed] [Google Scholar]
- 19.Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009;28:1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hueber AO, Zornig M, Lyon D, Suda T, Nagata S, et al. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene. 2008;27:6462–6472. doi: 10.1038/onc.2008.312. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Engels IH, Knee DA, Nasoff M, Deveraux QL, et al. Synthetic lethal targeting of MYC by activation of the DR5 death receptor pathway. Cancer Cell. 2004;5:501–512. doi: 10.1016/s1535-6108(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 25.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 26.Zornig M, Grzeschiczek A, Kowalski MB, Hartmann KU, Moroy T. Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in E mu L-MYC transgenic mice but not in animals infected with MoMuLV. Oncogene. 1995;10:2397–2401. [PubMed] [Google Scholar]
- 27.Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–123. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- 30.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, et al. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medema JP, de Jong J, van Hall T, Melief CJ, Offringa R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J Exp Med. 1999;190:1033–1038. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Screpanti V, Wallin RP, Ljunggren HG, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol. 2001;167:2068–2073. doi: 10.4049/jimmunol.167.4.2068. [DOI] [PubMed] [Google Scholar]
- 34.Amanullah A, Liebermann DA, Hoffman B. Deregulated c-Myc prematurely recruits both Type I and II CD95/Fas apoptotic pathways associated with terminal myeloid differentiation. Oncogene. 2002;21:1600–1610. doi: 10.1038/sj.onc.1205231. [DOI] [PubMed] [Google Scholar]
- 35.Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Testa U, Riccioni R. Deregulation of apoptosis in acute myeloid leukemia. Haematologica. 2007;92:81–94. doi: 10.3324/haematol.10279. [DOI] [PubMed] [Google Scholar]
- 37.Nyakeriga AM, Djerbi M, Malinowski MM, Grandien A. Simultaneous expression and detection of multiple retroviral constructs in haematopoietic cells after bone marrow transplantation. Scand J Immunol. 2005;61:545–550. doi: 10.1111/j.1365-3083.2005.01607.x. [DOI] [PubMed] [Google Scholar]
- 38.Djerbi M, Darreh-Shori T, Zhivotovsky B, Grandien A. Characterization of the human FLICE-inhibitory protein locus and comparison of the anti-apoptotic activity of four different flip isoforms. Scand J Immunol. 2001;54:180–189. doi: 10.1046/j.1365-3083.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 39.Djerbi M, Malinowski MM, Yagita H, Zhivotovsky B, Grandien A. Participation of FLIP, RIP and Bcl-x(L) in Fas-mediated T-cell death. Scand J Immunol. 2007;66:410–421. doi: 10.1111/j.1365-3083.2007.01957.x. [DOI] [PubMed] [Google Scholar]
- 40.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 41.Persons DA, Allay JA, Allay ER, Smeyne RJ, Ashmun RA, et al. Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood. 1997;90:1777–1786. [PubMed] [Google Scholar]
- 42.Bonham L, MacKenzie K, Wood S, Rowe PB, Symonds G. Both myeloproliferative disease and leukemia are induced by transplantation of bone marrow cells expressing v-myc. Oncogene. 1992;7:2219–2229. [PubMed] [Google Scholar]
- 43.Dolnikov A, Shounan Y, Millington M, Mackenzie K, Symonds G. V-myc in a simple, single gene retroviral vector causes rapid induction of leukemia and concomitant apoptosis following bone marrow transplantation. Leukemia. 1998;12:542–553. doi: 10.1038/sj.leu.2400968. [DOI] [PubMed] [Google Scholar]
- 44.Preisler HD. The treatment of acute non-lymphocytic leukemia. Blood Rev. 1987;1:97–105. doi: 10.1016/0268-960x(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 45.Smith DP, Bath ML, Metcalf D, Harris AW, Cory S. MYC levels govern hematopoietic tumor type and latency in transgenic mice. Blood. 2006;108:653–661. doi: 10.1182/blood-2006-01-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 47.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 48.Zornig M, Busch G, Beneke R, Gulbins E, Lang F, et al. Survival and death of prelymphomatous B-cells from N-myc/bcl-2 double transgenic mice correlates with the regulation of intracellular Ca2+ fluxes. Oncogene. 1995;11:2165–2174. [PubMed] [Google Scholar]
- 49.Nieminen AI, Partanen JI, Hau A, Klefstrom J. c-Myc primed mitochondria determine cellular sensitivity to TRAIL-induced apoptosis. EMBO J. 2007;26:1055–1067. doi: 10.1038/sj.emboj.7601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cameron ER, Morton J, Johnston CJ, Irvine J, Bell M, et al. Fas-independent apoptosis in T-cell tumours induced by the CD2-myc transgene. Cell Death Differ. 2000;7:80–88. doi: 10.1038/sj.cdd.4400630. [DOI] [PubMed] [Google Scholar]
- 51.Smith DP, Bath ML, Harris AW, Cory S. T-cell lymphomas mask slower developing B-lymphoid and myeloid tumours in transgenic mice with broad haemopoietic expression of MYC. Oncogene. 2005;24:3544–3553. doi: 10.1038/sj.onc.1208399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression and functionality of retroviral constructs. A. Analysis of proteins from MYC/BCL-XL tumor cells and Fas-sensitive A20 cells transduced with MSCV-hFLIPL-IRES-GFP retroviral particles. B. Fas-sensitive A20 cells were transduced with MSCV-hFLIPL-IRES-GFP retroviral particles. Transduced or wt A20 cells were thereafter incubated with or without agonistic anti-Fas mAb (0.25 µg/ml of Jo2) for 20 hours and thereafter analyzed by flow cytometry using propidium iodide (PI) to discriminate between live and dead cells.
(TIF)
Early hematopoietic development of DBA/2 mice reconstituted with Mock-GFP/Mock-YFP, Mock-GFP/MYC-YFP or BCL-XL-GFP/Myc-YFP expressing HSCs. Phenotypic analysis of bone marrow (left) and spleen (right) at 7, 14 and 35 days after transplantation of Mock-GFP/Mock-YFP, Mock-GFP/MYC-YFP or BCL-XL-GFP/MYC-YFP expressing HSCs into DBA/2 mice was performed with FACS. The horizontal axis indicates days after transplantation and the vertical axis represents percentage of cells expressing the marker indicated to the left. Black triangles: GFP+YFP− cells, black squares; GFP−YFP+ cells and white circles; GFP+YFP+ cells.
(TIF)
Appearance of leukemic blasts in the blood of MYC/BCL-XL recipient mice. Blood smears stained with May-Grunewald-Giemsa. Blood smear from a Mock/Mock mouse shows a normal picture with few leukocytes (A) as compared to the leukocytosis seen in MYC/BCL-XL mice (B) (primary magnification 20×). Higher magnification shows a dominance of blast-like cells with only few maturing granulocytes in MYC/BCL-XL mice (C and D) (primary magnification 60×).
(TIF)
Immunohistochemical staining of sections of spleen and liver of Mock/MYC mice. The section slides were stained with antibodies directed against the T-cell markers CD45R and CD3 and the myeloid marker myeloperoxidase (MPO) and analyzed by immunohistochemistry as described in Materials and Methods S1.
(TIF)
Immunohistochemical staining of sections of spleen of Mock/Mock mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemical staining of sections of spleen and liver of MYC/BCL-XL mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemical staining of sections of spleen and liver of MYC/BCL-2 mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemical staining of sections of spleen, liver and thymus of MYC/FLIPL mice. The analysis was performed as described in the legend to Figure S4.
(TIF)
Immunohistochemistry.
(DOCX)
Flow cytometric analysis of bone marrow, thymus, spleen and liver in MYC, BCL-XL,, BCL-2, FLIPL or control virus recipient mice.
(DOCX)
Differential count of nucleated cells in peripheral blood smears in MYC/BCL-XL and control mice.
(DOCX)