Abstract
The N-myc downstream-regulated gene 2 (NDRG2) is involved in tumor cell differentiation and apoptosis, but its function in tumor angiogenesis remains to be established. Here, we employed adenovirus overexpressing NDRG2 (Ad-NDRG2) to efficiently up-regulate target gene expression in the NDRG2-low-expressing, breast cancer cell line MCF-7. Moreover, VEGF secretion was decreased in MCF-7 cells infected by Ad-NDRG2, and medium conditioned by these infected cells could significantly inhibit the proliferation, tube formation and invasion of human umbilical vein endothelial cells (HUVECs). Further study indicated that the angiogenesis promoting factors VEGF and HIF-1α were down-regulated, whereas the angiogenesis suppressing factors p53 and VHL were up-regulated in MCF-7 cells infected by Ad-NDRG2. Finally, in a nude mouse model, intratumoral injections of Ad-NDRG2 every 3 days for 20 days significantly inhibited the growth and angiogenesis of xenografted MCF-7 tumors. In summary, these data indicate that NDRG2 may be involved in angiogenesis by impacting the expression of angiogenesis related factors. Thus, specific overexpression of NDRG2 by adenovirus represents a promising approach for the treatment of tumor angiogenesis.
Introduction
Breast cancer neovascularization (BCN), involving both angiogenesis and vasculogenesis, is a key event in tumorigenesis, invasion and metastasis. In recent decades, significant progress has been made toward understanding the pathophysiologic mechanisms of BCN. However, the development of BCN is a complicated and dynamic process, involving multiple stimuli, multiple cytokines and multiple cell-type participation, and the specific mechanisms underlying its progression are largely unknown.
Hypoxia, a common feature of malignant tumors, can be detected in central regions of solid tumors as well as during embryonic development [1]. Hypoxia regulates many transcription factors, including hypoxia-inducible factor (HIF)-1α, which controls hypoxia-induced angiogenic factors such as vascular endothelial growth factor (VEGF) [2]. VEGF functions as a survival factor in endothelial cells and tumor cells via VEGF receptors that are also up-regulated by hypoxia [3]. Thus, increased VEGF expression by hypoxia, and thereby promotion of angiogenesis, may be a prerequisite for progressive growth and metastasis of solid tumors, such as breast cancer. The tumor suppressor genes p53 and VHL inhibit angiogenesis [4], [5]. Mutation or loss of function of these suppressors results in high vascularization in malignant tumors. VHL and p53 could promote degradation of HIF-1α by ubiquitin-mediated proteolysis and suppress HIF-1α-stimulated transcription [6]. VEGF expression is negatively regulated by p53 and VHL and is frequently overexpressed along with HIF-1α in human cancers [4], [7]. To understand the interactions between angiogenesis promoting factors and angiogenesis suppressing factors, it is necessary to develop treatment strategies that specially target BCN.
NDRG2, a member of the N-myc downstream-regulated gene family, belongs to the alpha/beta hydrolase superfamily. It was first cloned in our laboratory from a normal human brain cDNA library by subtractive hybridization (GenBank accession no. AF159092) and is regarded as a tumor suppressor gene (TSG) that is transcriptionally repressed by c-Myc [8], [9], [10]. Accumulated evidence indicates that NDRG2 is down-regulated or undetectable in many human cancers [11], [12]. The role of NDRG2 in breast cancer has also been studied. Recently, it has been shown that breast cancer cells have low or undetectable expression of NDRG2 compared with the high expression of NDRG2 in normal tissues [13]. Further studies have found that NDRG2 is able to inhibit proliferation and enhance apoptosis in many malignant tumors [14]. In addition, NDRG2 could induce BMP-4 expression and inhibit the metastatic potential of breast cancer cells, specifically via suppression of MMP-9 activity [15]. In our previous study, we have verified also that, as a regulator, NDRG2 could modulate the process of breast cancer cell adhesion and invasion through regulating CD24 expression [16]. However, the specific effect of NDRG2 on breast cancer angiogenesis is still unknown.
In this study, we first demonstrate that NDRG2 is a regulator participating in the progression of breast cancer angiogenesis. Using adenovirus NDRG2 expression, we verified that Ad-NDRG2 could decrease VEGF secretion by MCF-7 cells, and this MCF-7 conditioned medium significantly inhibited HUVEC proliferation, tube formation and invasion. Furthermore, Ad-NDRG2 up-regulated the expressions of p53 and VHL while down-regulating the expressions of VEGF and HIF-1α. Ad-NDRG2 also inhibited tumor growth and angiogenesis in a nude mouse model. Our results present a novel role for NDRG2 in the regulation of breast cancer angiogenesis.
Results
NDRG2 expression in breast cancer cell lines
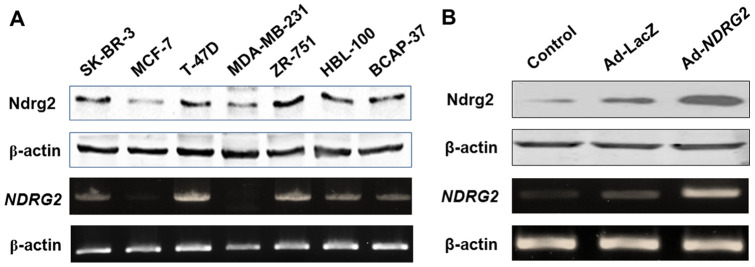
The tumor suppressor gene NDRG2 is down-regulated or undetectable in many human cancers. Here, NDRG2 expression in seven breast cancer cell lines was investigated by western blot and RT-PCR. The results showed that the expression of NDRG2 was higher in ZR-751 and T47D cell lines and lower in MDA-MB-231 and MCF-7 cell lines (Fig. 1A). To further determine the role of NDRG2, we chose to use MCF-7 cells (with wild-type p53) as our experimental model in the following studies.
Figure 1. Expression of NDRG2 in breast cancer cell lines and the effect of Ad-NDRG2.
(A) T-47D, MCF-7, BCAP-37, HBL-100, ZR-751, SK-BR-3 and MDA-MB-231 cells were collected for extraction of protein and RNA and analyzed for NDRG2 expression using western blot and RT-PCR. (B) NDRG2 low-expression MCF-7 breast cancer cells were infected by adenovirus carrying NDRG2 (Ad-NDRG2) or negative control LacZ (Ad-LacZ). Thereafter, protein and RNA from these cells were extracted and subjected to western blot and RT-PCR analysis. β-actin was used as a loading control. The data are representative of three independent experiments.
NDRG2 up-regulation by Ad-NDRG2 in MCF-7 cells
To evaluate the up-regulation of NDRG2 expression, western blot and RT-PCR analyses were performed. As shown in Figure 1B, after infection by Ad-NDRG2, the expression of NDRG2 was successfully increased at both the protein and mRNA levels in MCF-7 cells, which express a low level of endogenous NDRG2.
Suppression of HUVEC proliferation by up-regulating NDRG2 in MCF-7 cells
To investigate the effect of NDRG2 up-regulation in MCF-7 cells on HUVEC proliferation, an MTT assay was designed. First, HUVECs grown in serum-free medium conditioned by MCF-7 cells showed an increase in the proliferation rate, as revealed by the culture time (Fig. 2A). Next, medium conditioned by Ad-NDRG2 infected MCF-7 cells markedly inhibited HUVEC proliferation, which correlated with the conditioning time of the medium (Fig. 2B). Notably, compared to normoxic conditions, these effects were much more significant under hypoxic conditions.
Figure 2. Effect of MCF-7 preconditioned media on HUVEC proliferation.
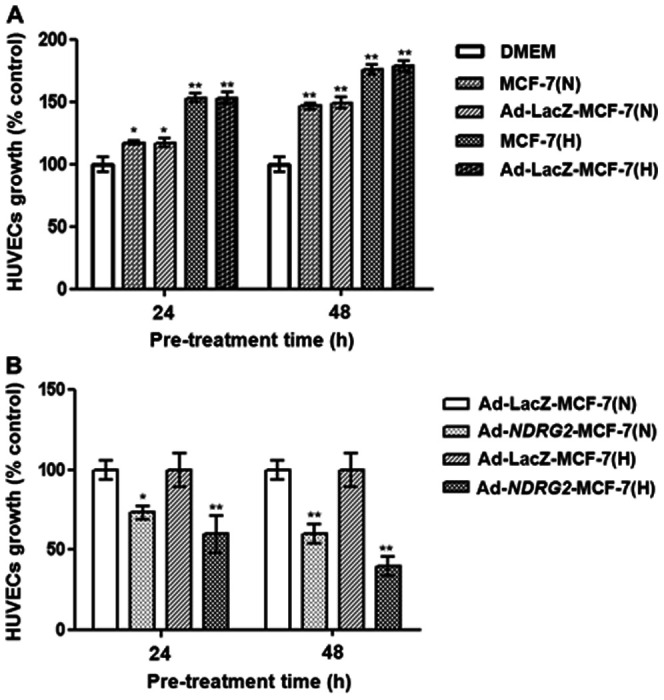
(A) HUVECs were grown to confluence and were then cultured in preconditioned media (derived from MCF-7 and Ad-LacZ-MCF-7 cells pretreated under normoxia and hypoxia for 24 or 48 h) for 24 h, and serum-free DMEM was used as a control. (B) HUVECs were grown to confluence and were then cultured in preconditioned media (derived from Ad-LacZ-MCF-7 or Ad-NDRG2-MCF-7 cells pretreated under normoxia and hypoxia for 24 or 48 h) for 24 h. Ad-LacZ -MCF-7(N) or Ad-LacZ-MCF-7 (H) was used as controls. N, normoxia; H, hypoxia. The data are the mean ± standard deviation (SD) for three independent experiments. Statistical significance was assessed using one-way ANOVA and Student's t-test. * or ** indicates P<0.05 or P<0.01, respectively, when compared with the control.
Alterations of HUVEC tube formation and invasion by regulating NDRG2 in MCF-7 cells
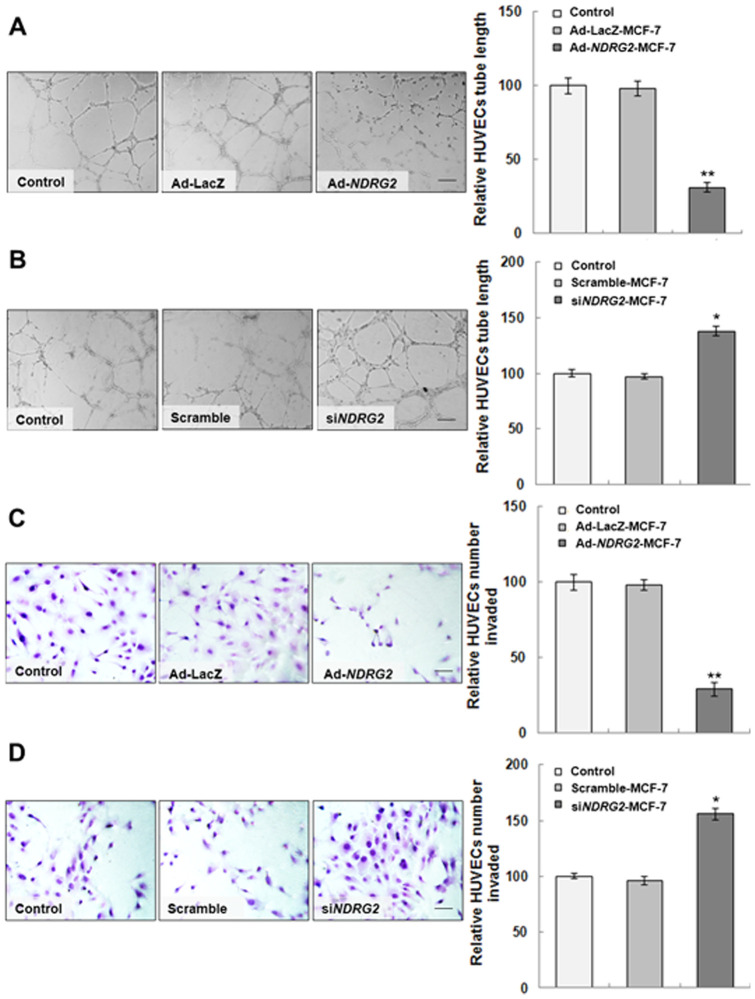
To test the effect of NDRG2 on angiogenesis, tube formation and invasion assays of HUVECs were performed on Matrigel under hypoxia. Ad-NDRG2 and NDRG2 siRNA (siNDRG2) were applied respectively to overexpress or knockdown NDRG2. As the control group, Ad-LacZ-MCF-7 or scramble-MCF-7 cells, HUVECs associated with one another and formed microtubes after 6 h. The medium from Ad-NDRG2-MCF-7 cells could repress the tube formation of HUVECs, but that from siNDRG2-MCF-7 cells promoted the tube formation of HUVECs (Fig. 3A and 3B). The invasion assay revealed that the medium from Ad-NDRG2-MCF-7 cells inhibited HUVECs' invasion ability; however, that from siNDRG2-MCF-7 cells increased a lot compared with the Ad-LacZ-MCF-7 or scramble-MCF-7 cells respectively (Fig. 3C and 3D). These data raised the possibility that NDRG2 overexpression or knockdown in MCF-7 cells might mediate the production of extracellular matrix components that in turn are involved in the tube formation and invasion of HUVECs.
Figure 3. Effect of MCF-7 preconditioned media on HUVEC tube formation and invasion.
(A and B) The tube formation assay was performed as described in Materials and Methodss. HUVECs were plated on Matrigel and incubated under hypoxia for 6 h. Tube lengths were measured with Image-Pro Plus software; the histograms represent the quantification of the tube length of HUVECs. (C and D) The invasion assay was performed as described in Materials and Methods. After 4 h under hypoxia, the amount of invaded cells was calculated in five random high-power fields (400×), and the number from the control group was used as a control. The histogram represents the quantification of cells that invaded. The data are the mean ± standard deviation (SD) for three independent experiments. Statistical significance was assessed using one-way ANOVA and Student's t-test. * or ** indicates P<0.05 or P<0.01, respectively, when compared with the control. The Ad-NDRG2 panel of Figure 3A is excluded from this article's CC-BY license. See the accompanying retraction notice for more information.
Regulation of the expression of hypoxia-induced, angiogenesis related factors by up-regulation of NDRG2 in MCF-7 cells
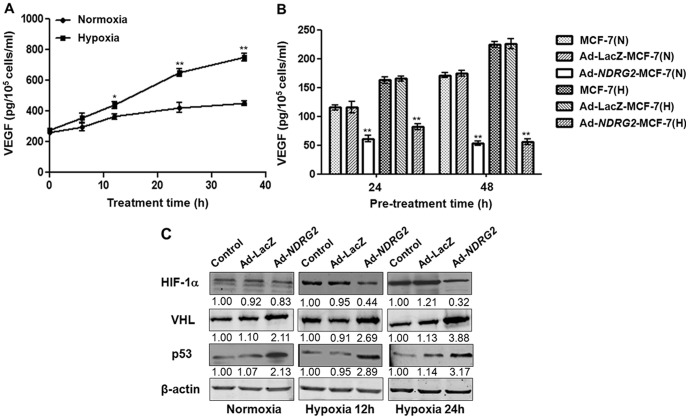
To further explore whether products of resulting from NDRG2 up-regulation in MCF-7 cells may be responsible for inhibition of HUVEC growth, we measured the VEGF level in cell culture medium under normoxic or hypoxic conditions. ELISA assay results showed that, compared to normoxic conditions, hypoxia could significantly increase VEGF secretion into the cell culture medium in a time-dependent manner (Fig. 4A). Furthermore, VEGF secretion was decreased in MCF-7 cells infected by Ad-NDRG2 in a time-dependent manner, especially under hypoxic conditions (Fig. 4B). To determine whether these hypoxia-induced, angiogenesis related factors are involved in NDRG2-mediated cell activities in breast cancer, western blot was performed to examine the expression levels of these factors. The results showed that HIF-1α expression was decreased in Ad-NDRG2-MCF-7 cells, and this was in accordance with the decreased VEGF secretion. In contrast to the effect on VEGF and HIF-1α, the expressions of p53 and VHL were up-regulated by Ad-NDRG2 under hypoxia (Fig. 4C). Thus, it is possible that NDRG2 influences HIF-1α and VEGF expression by indirectly regulating p53 and VHL expression or by directly controlling the transcription of HIF-1α and VEGF.
Figure 4. Effect of Ad-NDRG2 on the expression of VEGF, HIF-1α, p53, and VHL under normoxia or hypoxia.
(A) MCF-7 cells were incubated for the indicated time (0, 6, 12, 24, 36 h) in normoxic (21% O2) or hypoxic (1% O2) conditions. After incubation, the media were analyzed for VEGF levels by ELISA assay, and the cell number was counted. (B) MCF-7, Ad-LacZ-MCF-7 and Ad-NDRG2-MCF-7 cells were incubated for the indicated time (24, 48 h) in normoxic or hypoxic conditions. After incubation, the media were analyzed for VEGF levels by ELISA assay, and the cell number was counted. The data are the mean ± standard deviation (SD) for three independent experiments. Statistical significance was assessed with one-way ANOVA and Student's t-test. * or ** indicates P<0.05 or P<0.01, respectively, when compared with the normoxia or Ad-LacZ-MCF-7 group. N, normoxia; H, hypoxia. (C) MCF-7, Ad-LacZ-MCF-7 and Ad-NDRG2-MCF-7 cells were incubated under hypoxia for 0, 12, or 24 h. After incubation, the cells were analyzed by western blot assay. β-actin protein levels were used as a loading control.
Suppression of tumor growth and angiogenesis in a nude mouse model by intratumoral injection of Ad-NDRG2
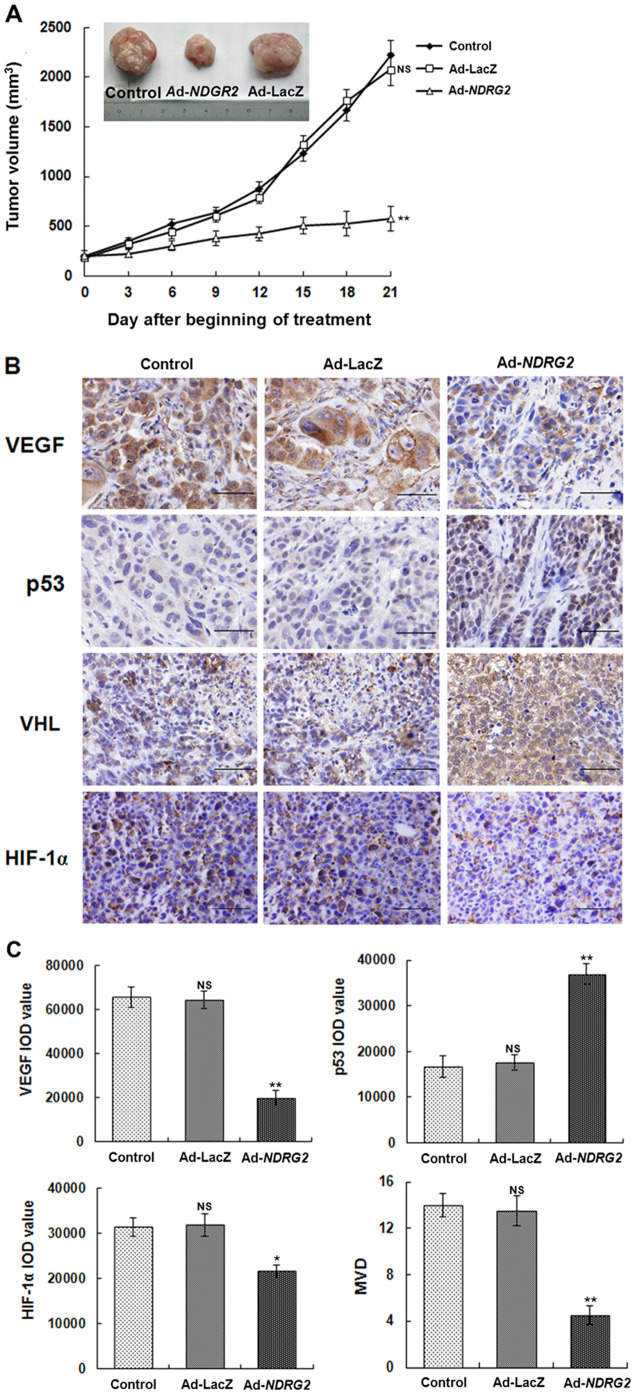
To investigate the effects of Ad-NDRG2 on tumor growth and angiogenesis in vivo, we injected 1×109 PFU Ad-NDRG2, Ad-LacZ or PBS every 3 days into pre-established, human MCF-7 breast tumors (approximately 200 mm3) grown in nude mice. As shown in Figure 5A, the Ad-NDRG2 group achieved a sustained and significant arrest of tumor growth (71% decrease in mean tumor volume on day 21 compared with the control group), whereas the growth of tumors injected with Ad-LacZ was not significantly inhibited (3.4% decrease compared with the control group). The mice were sacrificed at 21 days after beginning intratumoral injections, and the tumors were removed for analysis of the vascularization. Immunolabeling for VEGF and HIF-1α were lower in tumors excised from mice in the Ad-NDRG2 group. Moreover, the p53 and VHL expression levels in the Ad-NDRG2 group were dramatically increased dramatically compared to the control group. The microvessel density (MVD) of the tumors was examined after immunolabeling for CD31, a typical vascular endothelial cell marker. In the Ad-NDRG2 group, the MVD of the tumors was inhibited compared with the control and Ad-LacZ groups. There was no significant difference between the control and Ad-LacZ groups (Fig. 5B and 5C).
Figure 5. Effects of intratumoral injections of Ad-NDRG2 on the growth and vascularization of MCF-7 cell xenografts in mice.
These experiments were described in “Materials and Methods”. (A) Tumor growth curve. The tumor growth was assessed every 3 days until Day 21 of treatment by measuring two perpendicular diameters and calculating the volume in mm3. Statistical analysis was performed using Day 21 values only, with one-way ANOVA and Student's t-test. ** indicates P<0.01 when compared with the control. The typical photographs of tumors are shown. (B) Intratumoral vascularization was assessed by VEGF, p53, VHL, and HIF-1α immunolabeling (400× magnification) on paraffin-embedded MCF-7 cell tumor sections. Representative images are shown. (C) Integrated optical density (IOD) values of VEGF, p53, VHL, HIF-1α protein expression and MVD of the tumors were evaluated. ImagePro Plus software was utilized to analyze the IOD values of the positive areas of immunohistochemical staining, and the resulting histograms are shown. After areas containing dense vascularity, either within the tumor or during different pathological stages, were selected using a microscopic field with 100× power, all brown-stained vessels were counted under three fields with 200× power. The mean value of the vessels in the three fields was taken as the MVD count of a tumor. The histograms are shown. Statistical analysis was carried out using one-way ANOVA. The results are shown as the mean ± SD from a total of six mice. *P<0.05 or **P<0.01.
Discussion
Although surgical resection, adjuvant and endocrine therapy are commonly used to treat breast cancer patients, the overall survival rate for breast cancer requires improvement. Therefore, the development of biological therapies for breast cancer is urgently required. Recent reports have shown, in breast cancer patients, that NDRG2 expression is significantly reduced compared to breast normal tissue [13]. Further studies have found that NDRG2 inhibits the proliferation, adhesion and invasion of breast cancer cells [14], [16]. In addition, our previous study found that NDRG2 could inhibit the metastatic potential of breast cancer cells, specifically via suppression of CD24 or MMP-9 expression [16]. Interestingly, silencing NDRG2 attenuates p53-mediated apoptosis, whereas overexpression of NDRG2 suppresses tumor cell growth regardless of the presence or absence of p53 [14]. Therefore, NDRG2 is required for p53-mediated apoptosis but not for proliferation. These data indicate that an association exists between NDRG2 expression and reduced breast cancer malignant behavior and that NDRG2 may be a novel target for future biological therapies for breast cancer. However, to date, the roles of NDRG2 in breast cancer angiogenesis have not been explored. In this paper, our data revealed that NDRG2 overexpression in breast cancer cells could suppress HUVEC proliferation, tube formation and invasion by reducing VEGF and HIF-1α expression and increasing p53 and VHL expression. Importantly, tumor growth and angiogenesis in a nude mouse model were inhibited by intratumoral injection of Ad-NDRG2.
Recently, several reports have shown that NDRG2 is a target gene of HIF-1α, and hypoxia treatment dramatically up-regulated NDRG2 expression in many human cancer cell lines [17], [18]. Knockdown of HIF-1α expression did not change NDRG2 expression but could abolish hypoxia-induced up-regulation of NDRG2. In our study, infection by Ad-NDRG2 reduced HIF-1α expression in a time-dependent manner in MCF-7 cells. Based on these results, we believe that NDRG2 is a hypoxia-regulated gene, NDRG2 and HIF-1α could regulate each other, and there might be feedback loops between them. Furthermore, our data showed that VEGF secretion was decreased in MCF-7 cells infected by Ad-NDRG2 in a time-dependent manner, especially under hypoxic conditions. This suggests that NDRG2 may be involved in hypoxia-induced angiogenesis.
Loss of wild type p53 and VHL could promote angiogenesis by relieving inhibition of VEGF production. Several findings demonstrated that p53 and VHL could inhibit VEGF expression by regulating the transcriptional activity of Sp1 and by down-regulating Src kinase activity under hypoxia [19], [20]. Moreover, both p53 and VHL can promote the degradation of HIF-1α to indirectly suppress VEGF expression [21], [22], [23]. There is evidence indicating that the expression of VEGF is negatively regulated by p53 and VHL and is frequently overexpressed along with HIF-1α in human cancers.
The present study has shown that NDRG2 overexpression in breast cancer cells can inhibit the proliferation, tube formation and invasion of HUVECs, preventing the participation of these cells in hypoxia-induced, tumor angiogenesis. This effect may be related to the inhibition of VEGF secretion. Both in vitro and in vivo up-regulation of NDRG2 by Ad-NDRG2 increased p53 and VHL expression and decreased VEGF expression. It is possible that the down-regulation of VEGF by NDRG2 overexpression might be the consequence of the up-regulation of the tumor suppressor genes p53 and VHL. In summary, our results provide the first evidence that NDRG2 contributes to breast cancer angiogenesis by controlling the expression of angiogenesis related factors. Further investigation into the molecular mechanism of NDRG2 action may offer a novel approach for treating breast cancer, specifically by targeting breast cancer angiogenesis.
Materials and Methods
Cell lines and reagents
Human breast cancer cell lines T-47D, MCF-7, BCAP-37, HBL-100, ZR-751, SK-BR-3 and MDA-MB-231, were obtained from the American Type Culture Collection (ATCC, USA) and cultured according to their directions and guidelines. Human umbilical vein endothelial cells (HUVECs) were obtained and cultured as described previously [21], [22]. HUVECs were suspended in HuMedia-EG2 medium (Kurabo Industries) and then plated on plastic culture dishes. HuMedia-EG2 medium consisted of the base medium (HuMedia-EB2) supplemented with 2% fetal bovine serum, 10 ng/ml hEGF, 5 ng/ml hFGF-B, 1 µg/ml hydrocortisone, 50 µg/ml gentamicin, 50 ng/ml amphatericin B, and 10 µg/mL heparin. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Before experiments, cells were cultured under serum-free conditions in M199 medium for 18 to 24 h. For hypoxia treatment, cells were cultured under hypoxic conditions (1% O2, 5% CO2 and 94% N2) and harvested at different times. Anti-NDRG2 (1∶500 dilution) antibodies were purchased from BD Corporation (BD, NY, USA). Anti-HIF-1α (1∶200 dilution) and anti-p53 (1∶1000 dilution) antibodies, and ECL reagents were from Santa Cruz Technology (Santa Cruz, CA, USA). Anti-VHL antibody (1∶200 dilution) was from Neomarkers Corporation (Taipei, China). Anti-β-actin antibody (1∶5000 dilution) was from Sigma (Sigma, USA).
Gene infection
A multiplicity of infection (MOI) of 40 was determined experimentally for MCF-7 cells. Cells were seeded in 6-well plates at a density of 5×105 cells/well and incubated to reach approximately 80% confluence. After removing the medium, adenovirus expressing NDRG2 (Ad-NDRG2) or the negative control gene LacZ (Ad-LacZ) was added in serum-free DMEM, incubated for 2 h, replaced with fresh DMEM supplemented with 10% FBS and incubated for 48 h.
Gene transfection
MCF-7 cells were seeded in 6-well plates at a density of 5×105 cells/well and incubated to reach approximately 80% confluence. The gene transfection assay was performed with the following constructs using Lipofectamine 2000 (Invitrogen, Carlsbad, Calif) according to the manufacturer's instructions: a small interfering RNA (siRNA) that targeted NDRG2 (siNDRG2: forward, GCUCUCUGGAAAUUCUGAGUUGAUA; reverse, UUAAGAGCAUAUCUCGCCAGGAUGU) and its scramble siRNA (scramble: forward, UUCUCCGAACGUGUCACGUTT; reverse, ACGUGACACGUUCGGAGAATT; Biomics Inc., AgouraHills, Calif). Cells were exposed to siRNA in DMEM for 6 h, after which the medium was replaced with DMEM containing 10% FBS and the cells were incubated for 48 h.
RT-PCR
RNA extracted from cells with Trizol reagent (Invitrogen, Life Technologies, US) was converted to cDNA using the RevertAid™ First Strand cDNA Synthesis Kit (Fermantas, CA). Total RNA (5 µg) was then reverse transcribed into cDNA using an AMV reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer's instructions. The cDNA was subjected to PCR amplification using the following primers: NDRG2, 5′-ATGGCGGAGCTGCAGGAGGTGC-3′ (sense) and 5′-TGAGGAACGAGGTCTGGGTGGG-3′ (antisense); β-actin, 5′-GATCATTGCTCCTCCTGAGC-3′ (sense) and 5′- TGTGGACTTGGGAGAGGACT- 3′ (antisense). The PCR mixture (25 µl) containing a Taq polymerase from Promega was denatured at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, with a final extension at 72°C for 10 min. The PCR products were separated in 3% agarose gels by electrophoresis and visualized with ethidium bromide under a UV light.
Western blot
MCF-7 cells were homogenized in RIPA lysis buffer and insoluble material was removed by centrifugation at 4°C. From each sample, 80–100 µg of total protein extract was resolved by 12% SDS-PAGE and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). The membranes were blocked in 5% milk and probed with the antibodies overnight at 4°C. Membranes were washed and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotech) for 1 h at 37°C. Chemiluminescent HRP substrate solution (Millipore, USA) was used to develop the images.
MTT assay
HUVECs were plated at a density of 4×104 cells/cm2 in 24-well plates in complete medium and allowed to adhere overnight. The conditioned culture medium from MCF-7 cells was placed in the wells, and the whole plate was incubated in a hypoxic chamber. HUVEC proliferation was measured using a modified MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. After the inserts were removed, 100 µl MTT was added per well, and HUVECs were incubated for 4 h. The formazan crystals formed were dissolved in 750 µl dimethyl sulfoxide after the medium was aspirated. The solution was transferred to a 96-well plate and the optical density value was recorded at 570 nm on a Microplate Reader (Model 680, Bio-Rad, USA). The results were expressed relative to the OD value of HUVEC monocultures on day 1 of the assay.
ELISA assay
To assess VEGF secretion in the supernatants of infected cells, the cells were incubated in serum-free medium under normoxia or hypoxia. After 0–36 h, the media were collected, centrifuged to remove cellular debris, and stored at −70°C until assayed for VEGF. VEGF secreted in the culture medium was measured by ELISA according to the manufacturer's instructions. Data were expressed in pg VEGF/105 cells/ml.
Tube formation assay
Sterile 24-well plates were coated with 200 µl Matrigel and incubated at 37°C for 1 h to form gels. After polymerization of the gels, 1.0×105 HUVECs were seeded into each well and incubated with 1.0 ml DMEM containing 1% FBS. Next, media from MCF-7 cells were placed in the wells under hypoxia for 6 h. Five different fields were chosen randomly in each well, and photographs were taken. The length of the tubes was measured using Image-Pro Plus software (Media Cybernetics, L.P., Silver Spring, MD, USA) and was expressed as total length (mm) per microscopic field for each well.
Invasion assay
The HUVECs migration assay was performed using Matrigel-coated, Costar Transwell inserts with an 8 µm pore-size. Briefly, 5.0×104 HUVECs were seeded onto inserts and incubated with M199 medium containing 1% FBS. After a 1 h attachment, the inserts were transferred to 24-well plates containing media from Ad-LacZ-MCF-7 or Ad-NDRG2-MCF-7 cells. After incubation for 4 h under hypoxic condition, the inserts were fixed with 4% paraformaldehyde and stained with hematoxylin and eosin. The number of invaded cells was counted using phase contrast microscopy (400×). Five randomly chosen fields were counted per insert.
Xenograft study in nude mice
For inoculation into nude mice, MCF-7 cells were washed with PBS, digested with trypsin, and resuspended in serum-free DMEM medium. After centrifugation (800 rpm), cell pellets were resuspended in DMEM. The cell suspension (5×106 cells in a volume of 100 µl PBS) was injected subcutaneously into the hind legs of 4-week-old female BALB/C athymic (nu/nu) mice (SLAC Laboratory Animal Company, Shanghai, China) [24]. When tumors reached a volume of 200 mm3, the mice were arbitrarily assigned to different groups (n = 6 each) to receive intratumoral injections of 109 PFU Ad-NDRG2, Ad-LacZ, or PBS. Intratumoral injections were repeated every 3 days for a total of 20 days. Tumors were measured (perpendicular diameters) every day and their volumes calculated. On day 20, the mice were sacrificed and their tumors removed for analysis. Tumor volumes were calculated based on caliper measurements of the length and width of the lesions using the following formula: 0.5×length×width2. The growth curve was then derived from these data.
All the experimental procedures were conducted in accordance with the Detailed Rules for the Administration of Animal Experiments for Medical Research Purposes issued by the Ministry of Health of China and received ethical approval by the Animal Experiment Administration Committee of the Fourth Military Medical University (Xi'an, P. R. China). All efforts were made to minimize the animals' suffering and to reduce the number of animals used.
Immunohistochemistry
Immunohistochemical staining was performed to assess the protein expression of VEGF, HIF-1α, VHL, p53 and CD31, as described previously [25]. For immunohistochemistry, formalin-fixed tumor tissues were embedded in paraffin, and serial 4 µm sections were obtained using a Leica microtome. For staining, tumor sections were dewaxed in toluene, rehydrated in an alcohol gradient, permeabilized in citrate buffer (pH 6.0), quenched with 3% H2O2 for 5 min to eliminate endogenous peroxidase activity, washed in PBS, incubated overnight with different antibodies and then with biotinylated goat anti-rat or anti-rabbit IgG antibody for 15 min. After washing, sections were incubated with streptavidin–peroxidase, lightly counterstained with hematoxylin, and observed under a photomicroscope.
Statistical analysis
SPSS 13.0 software was used to perform statistical analyses. Data are presented as the mean ± SD, and statistical comparisons between groups were made using one-way ANOVA followed by Student's t-test. P<0.05 was considered statistically significant.
Funding Statement
This work was supported by grants from the National Nature Science Foundation of China (nos. 30830054, 81172292, 81071894, 81072050 and 30572100) and Nation Program on Key Basic Research Project (2009CB521704). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–2392. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 2.Yang XM, Wang YS, Zhang J, Li Y, Xu JF, et al. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:1873–1879. doi: 10.1167/iovs.08-2591. [DOI] [PubMed] [Google Scholar]
- 3.Dewhirst MW. Relationships between cycling hypoxia, HIF-1, angiogenesis and oxidative stress. Radiat Res. 2009;172:653–665. doi: 10.1667/RR1926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YQ, Luk JM, Ikeda K, Man K, Chu AC, et al. Regulatory role of vHL/HIF-1alpha in hypoxia-induced VEGF production in hepatic stellate cells. Biochem Biophys Res Commun. 2004;317:358–362. doi: 10.1016/j.bbrc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Sikora S, Godzik A. Combination of multiple alignment analysis and surface mapping paves a way for a detailed pathway reconstruction–the case of VHL (von Hippel-Lindau) protein and angiogenesis regulatory pathway. Protein Sci. 2004;13:786–796. doi: 10.1110/ps.03454904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem. 2003;278:13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 7.Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952–6957. [PubMed] [Google Scholar]
- 8.Deng Y, Yao L, Chau L, Ng SS, Peng Y, et al. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003;106:342–347. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- 9.Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, et al. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 10.Yao L, Zhang J, Liu X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin (Shanghai) 2008;40:625–635. doi: 10.1111/j.1745-7270.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Li F, Liu X, Shen L, Liu J, et al. The repression of human differentiation-related gene NDRG2 expression by Myc via Miz-1-dependent interaction with the NDRG2 core promoter. J Biol Chem. 2006;281:39159–39168. doi: 10.1074/jbc.M605820200. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Wu GJ, Liu XW, Zhang R, Yu L, et al. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;310:94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Lorentzen A, Lewinsky RH, Bornholdt J, Vogel LK, Mitchelmore C. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer. BMC Cancer. 2011;11:14. doi: 10.1186/1471-2407-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu N, Wang L, Liu X, Yang Q, Zhang J, et al. Promoter methylation, mutation, and genomic deletion are involved in the decreased NDRG2 expression levels in several cancer cell lines. Biochem Biophys Res Commun. 2007;358:164–169. doi: 10.1016/j.bbrc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 15.Shon SK, Kim A, Kim JY, Kim KI, Yang Y, et al. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009;385:198–203. doi: 10.1016/j.bbrc.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Zheng J, Liu Q, Li Y, Yang J, Ma J, et al. NDRG2 expression regulates CD24 and metastatic potential of breast cancer cells. Asian Pac J Cancer Prev. 2010;11:1817–1821. [PubMed] [Google Scholar]
- 17.Choi SC, Yoon SR, Park YP, Song EY, Kim JW, et al. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas-mediated cell death. Exp Mol Med. 2007;39:705–714. doi: 10.1038/emm.2007.77. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Liu N, Yao L, Li F, Zhang J, et al. NDRG2 is a new HIF-1 target gene necessary for hypoxia-induced apoptosis in A549 cells. Cell Physiol Biochem. 2008;21:239–250. doi: 10.1159/000113765. [DOI] [PubMed] [Google Scholar]
- 19.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 20.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg M, Haas R, Brauch H, Acker T, Flamme I, et al. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 22.Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, et al. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19:4621–4631. doi: 10.1038/sj.onc.1203814. [DOI] [PubMed] [Google Scholar]
- 23.Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, et al. p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem. 1998;273:11995–11998. doi: 10.1074/jbc.273.20.11995. [DOI] [PubMed] [Google Scholar]
- 24.Pille JY, Denoyelle C, Varet J, Bertrand JR, Soria J, et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267–274. doi: 10.1016/j.ymthe.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Li Y, Yang J, Liu Q, Shi M, et al. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression. BMC Cancer. 2011;11: 251:251–259. doi: 10.1186/1471-2407-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]