ABSTRACT
Proper functioning of the ovary is critical to maintain fertility and overall health, and ovarian function depends on the maintenance and normal development of ovarian follicles. This review presents evidence about the potential impact of oxidative stress on the well-being of primordial, growing and preovulatory follicles, as well as oocytes and early embryos, examining cell types and molecular targets. Limited data from genetically modified mouse models suggest that several antioxidant enzymes that protect cells from reactive oxygen species (ROS) may play important roles in follicular development and/or survival. Exposures to agents known to cause oxidative stress, such as gamma irradiation, chemotherapeutic drugs, or polycyclic aromatic hydrocarbons, induce rapid primordial follicle loss; however, the mechanistic role of ROS has received limited attention. In contrast, ROS may play an important role in the initiation of apoptosis in antral follicles. Depletion of glutathione leads to atresia of antral follicles in vivo and apoptosis of granulosa cells in cultured antral follicles. Chemicals, such as cyclophosphamide, dimethylbenzanthracene, and methoxychlor, increase proapoptotic signals, preceded by increased ROS and signs of oxidative stress, and cotreatment with antioxidants is protective. In oocytes, glutathione levels change rapidly during progression of meiosis and early embryonic development, and high oocyte glutathione at the time of fertilization is required for male pronucleus formation and for embryonic development to the blastocyst stage. Because current evidence suggests that oxidative stress can have significant negative impacts on female fertility and gamete health, dietary or pharmacological intervention may prove to be effective strategies to protect female fertility.
Keywords: follicle, oocyte, ovary, oxidative stress, toxicology
Oxidative stress can have significant impacts on follicular atresia and oocyte quality.
REACTIVE OXYGEN SPECIES AND OXIDATIVE STRESS
Reactive oxygen and nitrogen species (ROS and RNS, respectively) include superoxide anion radicals, hydroxyl radicals, hydrogen peroxide (H2O2), peroxynitrite and other peroxides, nitric oxide, and others (Fig. 1). ROS are formed through leakage of electrons from the inner mitochondrial membrane during oxidative phosphorylation and ATP generation. In steroidogenic tissues such as the ovary, steroidogenic cytochrome P450 enzymes are also sources of ROS [1, 2]. Oxidative and nitrosative damage occur when ROS and RNS react with cellular lipids, proteins, and nucleic acids [3–5]. The redox (reduction-oxidation) state of a cell describes the relative concentrations of reduced versus oxidized states of proteins, enzymes, sulfhydryl-containing molecules, and other factors. The relationship between intracellular levels of ROS relative to endogenous antioxidants has a significant impact on this measure. Oxidative stress occurs when increased ROS levels disrupt cellular redox circuits, resulting in disturbances of redox-regulated cellular processes and/or oxidatively damage cellular macromolecules [6, 7].
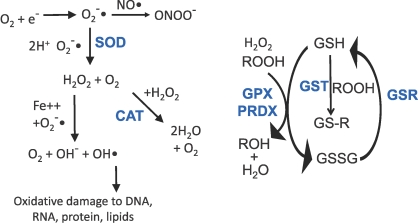
FIG. 1.
Reactive oxygen species (ROS) generation and detoxification. ROS are formed by the sequential addition of electrons to molecular oxygen, forming superoxide anion radical, H2O2, and hydroxyl radical. Peroxynitrite (ONOO−) is formed when superoxide anion radical reacts with nitric oxide (NO). Key antioxidant enzymes (in bold blue) and the reactions they catalyze are shown. CAT, catalase; GPX, glutathione peroxidase; GSR, glutathione reductase; GST, glutathione-S-transferase; PRDX, peroxiredoxin; SOD, superoxide dismutase. GPXs, PRDXs, and GSTs require glutathione (GSH) as a cofactor, and GSH can also scavenge free radicals through direct chemical reactions. GSR reduces the oxidized form of GSH (GSSG, glutathione disulfide).
ANTIOXIDANT DEFENSE MECHANISMS
Multiple enzyme systems and soluble factors maintain the redox state of cells. Some of these are shown in Figure 1. The antioxidant defense mechanisms in cells are complex and appear to be compartmentalized, such that nuclear, cytoplasmic, and mitochondrial levels of antioxidants behave independently [8, 9]. Ascorbate (vitamin C), present at millimolar levels in ovarian cells and at 50–200 micromolar in human follicular fluid [10, 11], reacts directly with ROS to detoxify them, as do tocopherols (vitamin E). Ascorbate is concentrated in granulosa cells, theca cells, luteal cells, and the oocyte, and its uptake is hormonally regulated [10–14]. Glutathione (GSH), a cysteine-containing tripeptide, is another key antioxidant present in millimolar concentrations in cells. It can scavenge free radicals through either direct chemical reactions or reduction of peroxides as a cofactor for GSH peroxidases, cycling between its reduced form (GSH) and an oxidized, dimerized form (GSSG) [15, 16]. The activity of GSH reductase, which requires NADPH as an energy source, ensures that most GSH is present in cells in the reduced form. Furthermore, glutathione transferases (GSTs), a large family of enzymes that covalently link reactive chemicals with GSH, aid in detoxification and excretion of toxic substances. The thioredoxin/thioredoxin reductase system serves a similar function, providing reducing potential for multiple biochemical reactions. Although homeostatic mechanisms ensure that the reduced forms of cysteine, GSH, and thioredoxins predominate, these various systems are not in thermodynamic equilibrium, as shown by the different Nernst potentials of oxidized and reduced forms of cysteine, GSH, and thioredoxins [8]. Together, these systems maintain proteins in the optimal reduced state and conformation, permit proper redox signaling, and even control some signal transduction pathways and gene expression [8, 9].
Other enzymes directly detoxify ROS (Fig. 1). Superoxide dismutases (SOD) react with superoxide anion radicals to form oxygen and H2O2. The three main SOD enzymes are copper-zinc SOD (CuZnSOD, SOD1, in cytoplasm), manganese SOD (MnSOD, SOD2, in mitochondria), and an extracellular form (ECSOD, SOD3) [17]. Catalase, various peroxidases and peroxiredoxins (PRDXs), including GSH peroxidases (GPXs) and some GSTs, can convert peroxides to water. Together, these interacting defense mechanisms permit cells to live in an oxidative environment, perform necessary biochemical processes, and even use these ROS/RNS as signaling molecules [6, 7]. For example, in the ovary there is a transient rise in ROS levels and decline in antioxidant expression after the preovulatory gonadotropin surge, and the rise in ROS is a necessary signal for ovulation [18–21].
EVIDENCE FOR A ROLE OF ANTIOXIDANT PROTECTIVE MECHANISMS IN OVARIAN FOLLICLE SURVIVAL
Numerous mouse models with deletions of antioxidant genes have been created in recent years and have been used to explore the regulation and function of redox control. With respect to reproductive function, normal fertility has been reported in mice that lack Gpx1 [22] or catalase [23] or that bear an inactivating mutation in glutathione reductase [24–26]. However, detailed studies of ovarian function have not been conducted in these mice. Deletion of the entire Gpx4 gene resulted in embryonic lethality [27], whereas deletion of the nuclear form [28] or of the mitochondrial form [29] reportedly had no effects on female fertility. This contrasts with the pronounced adverse effects of mitochondrial Gpx4 deletion [29] or of overexpression of Gpx4 [30] on male fertility and spermatogenesis.
Two groups have developed Sod1 null mice, and both groups reported that the female mice were subfertile; however, the mechanistic basis for the reduced fertility of female Sod1 null mice remains unclear. Matzuk et al. [31] reported that ovaries of adult female Sod1 null mice had reduced numbers of preovulatory follicles and corpora lutea. They concluded that these mice were subfertile because of a defect in late follicular development or ovulation. In contrast, Ho et al. [17] reported that Sod1 null female mice had normal ovarian histology and ovulated similar numbers of ova during a natural estrous cycle but displayed increased postimplantation embryonic lethality. Perhaps the different genetic backgrounds of these two Sod1 knockout models accounts for these different findings. A study by Wong et al. [32] of copper chaperone for superoxide dismutase null mice, which have decreased ability to incorporate copper into SOD1, found a similar phenotype as Matzuk et al. [31], with abnormal development of antral follicles and no corpora lutea. Taken together, the evidence seems to support a role for SOD1 in antral follicle development. Sod2 knockout is lethal prior to puberty. However, transplantation of ovaries from Sod2 knockout juvenile mice to the ovarian bursa of wild-type mice, in which the ipsilateral ovaries had been removed and the contralateral oviducts had been cut, resulted in all stages of follicular development, ovulation, and fertility, suggesting that this enzyme is not critical for ovarian function [31].
Mice null for γ-glutamyl transpeptidase 1 (Ggt1) display a shortened life span, stunted growth, and a severe female reproductive phenotype, with complete infertility and lack of ovarian large antral follicles, corpora lutea, and ovulatory response to exogenous gonadotropins [33–35]. These mice have decreased ovarian cysteine concentrations but normal ovarian GSH concentrations compared to wild-type controls, and the female reproductive phenotype is completely rescued by cysteine replacement [33].
Glutamate cysteine ligase (GCL; formerly called gamma-glutamylcysteine synthetase), the rate-limiting enzyme in glutathione synthesis, is a heterodimer composed of a catalytic (GCLC) and a modifier (GCLM) subunit. Gclc knockout is embryonic lethal before Gestational Day 8.5 [36, 37]. Gclm null mice survive and reproduce [38, 39]; however, more recent detailed studies have revealed that female Gclm null mice have oocyte GSH concentrations less than 20% those of wild-type females and have markedly decreased fertility [40]. Gclm−/− females mated with wild-type males had smaller litter sizes and fewer uterine implantation sites compared to Gclm+/+ littermates [40]. This was due not to fewer ovulated oocytes but to decreased progression of zygotes to the two pronucleus stage at 0.5 days postcoitum and decreased progression to the blastocyst stage at 3.5 days postcoitum. In vitro fertilization and embryo culture studies showed that embryos derived from oocytes of Gclm−/− females fertilized with wild-type sperm had very low rates of progression to the blastocyst stage compared to those from Gclm+/+ females [40]. The latter results show that the decreased preimplantation development of embryos of Gclm−/− females is due mainly to low oocyte GSH concentrations as opposed to low GSH concentrations in other parts of the female reproductive tract.
SENSITIVITY OF OVARIAN FOLLICLES TO ROS
Antioxidant Depletion Increases and Antioxidant Supplementation Decreases Atresia of Antral Follicles
The tripeptide antioxidant GSH is present at moderate concentrations of 3–4 nmol/mg tissue or about 40–50 nmol/mg protein in the ovaries of adult rats and mice [41–44]. Treatment of adult cycling rats on estrus or proestrus with two doses of 5 mmol/kg buthionine sulfoximine (BSO; a specific inhibitor of GSH synthesis) administered 12 h apart suppressed ovarian GSH concentrations by greater than 50% compared to saline treated controls [41]. This acute depletion of ovarian GSH resulted in statistically significant increases in the percentage of atretic antral follicles and, though statistically nonsignificant, increases of a similar magnitude in the percentage of apoptotic antral follicles at 12 h after the second dose [41]. Further evidence that GSH may play a role in preventing antral follicle atresia comes from experiments using cultured rat antral follicles. Large (preovulatory) and small antral rat follicles spontaneously undergo apoptosis when cultured in the absence of gonadotropin hormone support, and treatment with follicle-stimulating hormone (FSH) prevents the initiation of apoptosis in these cultured follicles [45, 46]. A role for oxidative stress in the initiation of apoptosis by gonadotropin withdrawal was suggested by the observations that follicular ROS increased prior to any indicators of apoptosis and that an antiapoptotic FSH stimulus suppressed this ROS generation in cultured large antral follicles [47]. Moreover, in both small and large antral follicles, FSH treatment stimulated GSH synthesis [47, 48]. In large antral follicles, depletion of GSH with BSO in the presence of FSH partially but statistically significantly inhibited the antiapoptotic effect of FSH on granulosa cell apoptosis [47]. Moreover, each of the antioxidants ascorbic acid, N-acetylcysteine, SOD, and catalase protected against apoptosis of rat large antral follicles cultured without gonadotropin support [49].
H2O2
An ovarian culture system using Postnatal Day 4 (PND4) ovaries has been used to examine toxicity of chemicals on small ovarian follicles [50]. This system is quite useful because it contains a more concentrated population of small follicle stages, from primordial to small secondary follicles, than even young adult ovaries. PND4 mouse ovaries were exposed to multiple concentrations of H2O2 in culture for 8 days to determine their sensitivity to ROS. Following histological processing, the morphology of the ovaries was examined. Pyknotic cells were observed at concentrations of ≥3 mM (P. Devine, unpublished data). The lower concentrations affected cells around the periphery of the ovary. In spite of these signs of toxicity, primordial and small primary follicles maintained a normal appearance even in areas where other cells were pyknotic. At 6-mM concentrations of H2O2, morphological changes were observed throughout the ovary, except for a small amount of normal tissue in the center of the ovary. It was not until concentrations of 6 mM were used that small ovarian follicles demonstrated visible changes. Thus, primordial and small primary follicles seem to be more resistant to H2O2 than other ovarian cell types. Further work is needed to determine if comparable results would be produced by longer-lived or cell-permeable peroxides or oxidants.
4-Vinylcyclohexene Diepoxide
The chemical 4-vinylcyclohexene diepoxide (VCD) has been studied as a model compound that can accelerate ovarian follicle loss when given repeatedly to rodents [51, 52]. This metabolite of 4-vinylcyclohexene, a volatile by-product of chemical syntheses, had been identified by the National Toxicology Program to be a reproductive toxicant at high doses in rats and mice [53, 54]. VCD was found to specifically decrease numbers of primordial and smallest primary follicles [55, 56], with decreases in larger follicles occurring as a result of depletion of primordial follicles [57]. The mechanism of action by which VCD induces this effect has not been fully elucidated, although changes in apoptotic signaling [58–61], MAP kinase/AP-1 signaling [62], and KIT/KIT ligand signaling have been identified [63–65]. Further work is needed to better characterize VCD-induced changes in small ovarian follicles. The specificity of this ovotoxicant makes it a useful tool for studying small ovarian follicle susceptibility to chemical exposures.
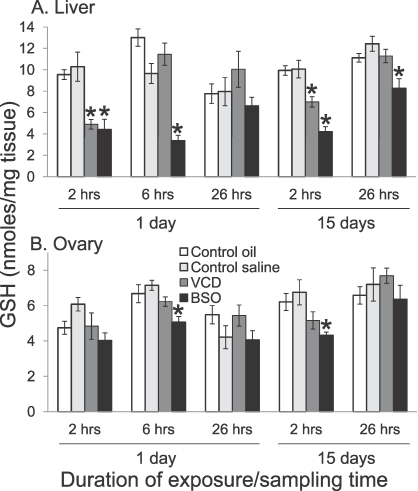
Oxidative stress was examined as another possible mechanism underlying VCD-induced ovarian follicle loss. Polar conjugates in urine of VCD-exposed rats and mice suggested that GSH might be involved in detoxifying VCD (Salyers and Sipes, unpublished data). GSH levels were measured in liver and ovary following single or repeated (15 days) exposures of Fischer 344 rats to 80 mg/kg VCD, a dose and duration known to specifically reduce primordial and primary follicle numbers [66]. Hepatic GSH was significantly decreased 2 h after a single i.p. exposure to VCD, with recovery occurring within 6 h (Fig. 2A). In contrast, a specific inhibitor of the rate-limiting step in GSH synthesis, BSO (450 mg/kg; 2 mmol/kg), caused a more persistent decrease in hepatic GSH levels (2–6 h) that returned to normal by 26 h. VCD alone had no effect on ovarian GSH levels, and BSO reduced ovarian GSH by only approximately 25% and only at 6 h after exposures (Fig. 2B). No increases in lipid peroxidation products, measured as thiobarbituric acid-reactive substances, were observed in either ovarian or hepatic tissues 2–24 h following single VCD exposures. Furthermore, 15 days of i.p. injections with BSO did not affect small ovarian follicle numbers, even though it did significantly reduce both hepatic and ovarian GSH levels [66]. Overall, these results suggest that VCD does not cause overall changes in ovarian GSH levels. It is possible that VCD causes only localized changes in redox status in target follicles, but other methods would need to be used to better characterize such changes.
FIG. 2.
VCD-induced reductions in hepatic (A) but not ovarian (B) GSH levels. GSH levels were measured by HPLC in tissue homogenates of female Fischer 344 rats 2, 6, or 26 h after a single i.p. dose or 2 or 26 h following 15 days of dosing of 80 mg/kg VCD in sesame oil, 450 mg/kg (2 mmol/kg) BSO in saline, or vehicle control (sesame oil or saline). Values represent means ± SEM; n = 8–12 animals per group. *Significantly different from control, P < 0.05. Modified from Devine et al. [66] with permission of the Society of Toxicology and Oxford University Press.
Subsequent experiments tested whether antioxidants could protect against VCD-induced follicle loss in vitro [67]. Cultured PND4 rat ovaries exposed to 30 μM VCD for 15 days exhibited 90% fewer primordial follicles. No significant protection was provided by including the antioxidants vitamin E, vitamin C, or GSH (1 mM) in the culture medium with or without VCD. However, because GSH does not easily penetrate cells, a more cell-permeable form should be tested.
17β-Estradiol (E2) has been reported to have antioxidant properties at pharmacologic levels [68]. In contrast to the antioxidants used above, exogenous E2 was found to have protective effects against VCD in vivo. Thompson et al. [69] demonstrated that daily dosing of 0.1 mg/kg E2 or genistein alone to adult rats did not affect small follicle populations but did protect primary follicles against the effects of 15 days of dosing with 80 mg/kg VCD. There was no protective effect on primordial follicles. Binding to the receptor seemed to be involved because cotreatment with E2, VCD, and the estrogen receptor antagonist 4-hydroxytamoxifen blocked the protective effect of E2 on VCD-induced primary follicle loss [69]. Whether this effect of E2 or genistein on the ovarian toxicity of VCD involves protection against ROS is not known. Overall, results with VCD do not suggest a strong mechanistic link between VCD-induced follicle loss and oxidative stress or ROS.
Cyclophosphamide
Alkylating chemotherapeutic drugs and radiation are used in treating cancers, and their activity is thought to be through induction of DNA damage to tumor cells. These treatments, although necessary for survival of the patient, often have negative side effects, including detrimental effects on the reproductive system [70–72]. Depletion of the ovarian follicle pool and subsequent permanent infertility have been reported [71, 73], suggesting that human primordial follicles are sensitive to at least some types of treatments. Also, the rapid but temporary loss of cyclicity in some patients suggests toxicity to antral follicles from certain drugs [71, 73, 74]. The use of mixed exposures (multiple drugs and radiation) complicates the characterization of potential reproductive toxicity of each drug.
Cyclophosphamide (CP), an alkylating chemotherapeutic drug used often against cancers and autoimmune disorders, has been known for decades to cause temporary or permanent amenorrhea, reduced fertility, or infertility [73, 75–78]. Destruction of follicles at all stages of development has been reported in rodents and humans [79–83]. Dose-dependent decreases in primordial follicles were induced by CP in vivo in mice [83, 84]. Oxidative stress has been implicated in CP-induced toxicity to granulosa cells of antral follicles (see below), but the mechanism underlying small ovarian follicle loss remains unknown. CP requires metabolic activation via oxidation by cytochrome P450 enzymes to 4-hydroxycyclophosphamide, which then undergoes ring opening to aldophosphamide, which spontaneously decomposes to the reactive metabolite phosphoramide mustard (PM) [85–87]. PM is thought to be the active metabolite responsible for CP's anticancer activity as well as its ovarian toxicity [84, 87].
To evaluate CP's mode of action more specifically, the role of DNA damage in CP-induced ovarian follicle loss was examined [88]. Primordial ovarian follicle loss has been reported in multiple strains of mice. Rapid primordial and primary follicle loss were characterized in CD-1 mice at ≥150 mg CP/kg. Furthermore, primordial follicle loss occurred in both cultured PND4 mouse and rat ovaries at concentrations of ≥3 or 30 μM, respectively, of PM [88, 89]). Also, in mice, small primary follicles were sensitive to concentrations as low as 0.1 μM PM [88]. Loss of primordial and primary follicles was independent of caspase activation, as culture with the pan-caspase inhibitor z-VAD-fmk did not prevent small follicle degeneration [89]. Under the same conditions, a marker for DNA double-strand breaks was detected in oocytes of cultured ovaries. The histone H2AFX is incorporated into nucleosomes throughout the chromatin of all organisms, and this protein becomes phosphorylated at sites of DNA double-strand breaks. This occurs above background levels before morphological changes are observed (24–48 h after exposures) at approximately the same PM concentrations that cause significant follicle loss [88]. Although DNA double-strand breaks have been observed in response to oxidative stress, further work is needed to provide evidence for or against the involvement of ROS in PM-induced DNA damage [88].
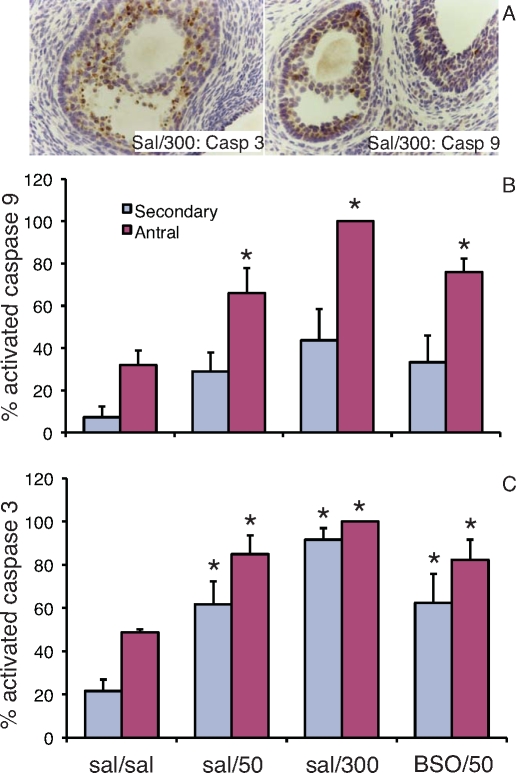
Although as discussed above, CP targets primordial and primary follicles in mice, in adult rats a single injection of 200 mg/kg CP destroyed secondary and antral follicles but not primary and primordial follicles [79]. This destruction of secondary and antral follicles was shown to be caused by the dose-dependent induction of apoptosis in granulosa cells of these follicle types at 24 h after a single injection of 50 or 300 mg/kg CP [41]. Subsequent studies revealed that induction of apoptosis by CP in granulosa cells of ovarian follicles was associated with activation of caspase 9 and caspase 3 (Fig. 3). This contrasts with the absence of caspase activation during primordial follicle death induced by CP [89]. Treatment with BSO, a specific inhibitor of GSH synthesis, 2 h before administration of CP did not enhance the induction of apoptosis in growing follicles by CP. However, the low solubility of BSO limited the dose of BSO that could be administered, and therefore the maximal depletion of ovarian GSH concentrations achievable in vivo with BSO was limited to about 50% of control levels at 12 h after injection with levels recovering thereafter [41]; it is possible that more complete depletion of GSH would have potentiated the effects of CP.
FIG. 3.
Cyclophosphamide (CP)-induced apoptosis in granulosa cells of ovarian follicles involves activation of caspases 9 and 3. Proestrous female rats received sequential i.p. injections 2 h apart: normal saline followed by normal saline with 10% DMSO (sal/sal), saline followed by 50 mg/kg CP in DMSO (sal/50), saline followed by 300 mg/kg CP (sal/300), or 5 mmol/kg buthionine sulfoximine followed by 50 mg/kg CP (BSO/50) [41]. The animals were euthanized 24 h later and ovaries (n = 3 per group) were processed for immunostaining with antibodies to activated (cleaved) caspase 9 or activated caspase 3 (both from Cell Signaling Technologies, Beverly, MA) using the Vectastain ABC Kit. Secondary and antral follicles with more than three positively stained granulosa cells per largest cross section were counted in 16 sections per ovary blind to treatment group. A) Representative images of antral follicles with positively stained (brown) granulosa cells in ovaries from rats treated with 300 mg/kg CP. The graphs show the mean ± SEM percentages of secondary and antral follicles that stained positively for activated caspase 9 (B) and activated caspase 3 (C). *Significantly different from respective sal/sal control, P < 0.05. (U. Luderer, previously unpublished data obtained on same ovaries as TUNEL data published in Lopez and Luderer [41]).
In order to further study the mechanism by which CP induces apoptosis in granulosa cells and how this is modulated by GSH, a human granulosa cell tumor line, COV434 cells, was used [90]. This cell line was chosen because the cells possess many of the characteristics of normal granulosa cells, including expressing FSH receptors and synthesizing E2 in response to FSH and androstenedione [90]. A preactivated form of CP, 4-hydroperoxycyclophosphamide (4HC), which spontaneously breaks down in solution to 4-hydroxycyclophosphamide [87, 91], was used to study the effects of CP in COV434 cells. Treatment of these cells with 4HC at concentrations of 1–50 μM rapidly and dose dependently depleted intracellular GSH with a concomitant rapid rise in ROS, followed by initiation of apoptosis as measured by activation of caspase 3, TUNEL, and Hoechst 33342 staining [92]. Depletion of GSH with BSO enhanced and supplementation of GSH with GSH ethyl ester or supplementation with other antioxidants prevented the initiation of apoptosis by 4HC [92]. Taken together, these findings demonstrate that the rise in ROS mediates 4HC-induced apoptosis in granulosa cells [92].
Ionizing Radiation
Women treated with ionizing radiation to the pelvis often suffer from amenorrhea or premature ovarian failure [73]. Treatment of nonovarian cells with ionizing radiation has been shown to result in the generation of ROS [5, 93]. In vivo studies show that ionizing radiation destroys small follicles as well as antral follicles in rats and mice [71, 94, 95] and rhesus monkeys [96]. High doses of gamma irradiation (8.3 Gy) to Postnatal Day (PND) 21 mice caused significant and rapid increases in degenerating primordial and primary follicles, as determined by morphological changes at 2, 8, and 14 h after irradiation [95, 97]. Pretreatment with 100 μg melatonin, which is a good antioxidant, partially and significantly protected against radiation-induced primordial follicle loss at all time points; 10 μg melatonin was less protective [95]. Melatonin pretreatment provided less consistent protective effects for primary and secondary/antral follicles [95].
In women, temporary amenorrhea, with eventual resumption of menstrual cycling, may be caused by destruction of growing follicles by ionizing radiation without complete destruction of the primordial follicle pool. Studies in COV434 granulosa tumor cells showed that gamma irradiation caused a rapid (within 30 min) and sustained increase in ROS that was followed by apoptotic death at 6 h [98]. COV434 cells that were stably transfected with constructs directing overexpression of one or both subunits of the rate-limiting enzyme in GSH synthesis, glutamate cysteine ligase, had increased levels of GSH and were protected against the generation of ROS and the initiation of apoptosis by gamma irradiation [98].
Chromium
The role of oxidative stress in the ovarian toxicity of the heavy metal chromium (Cr) has been examined. This metal can be present naturally, leaching into drinking water supplies as chromium (CrVI) [99]. Occupational exposures are also possible since Cr is used in multiple industrial applications, such as leather tanning, electroplating, and wood preservatives, and in the production of steel alloys used in orthopedic implants and other applications [100]. Cr induces follicular atresia in adult mice when given in drinking water [101]. The mechanism of action is thought to involve ROS as the metal cycles through various oxidation states within cells, and treatment with ascorbate has been shown to protect against reproductive toxicity in male monkeys [102]. Banu et al. [103] examined the effects of in vivo lactational exposures to chromium on female reproduction and the ovary. From birth until weaning on PND 21, mothers of pups were given 200 mg/L potassium chromate with or without 500 mg/L ascorbate in their drinking water. Pups thus exposed to Cr via lactation had delayed puberty (PND 55 versus PND 33 in controls) and longer estrous cycles, and ascorbate protected against these effects [103]. In these animals, numbers of each follicle type were significantly reduced in Cr-treated rats at PND 21 and PND 45, but secondary and antral follicles recovered by PND 65. Serum E2, testosterone, progesterone, growth hormone, and prolactin were reduced at all time points examined, and FSH was increased (PND 21 and PND 45) with Cr treatments. The mechanism of follicular destruction was examined in cultured rat granulosa cells; Cr increased expression of proapoptotic BCL2 family proteins, decreased expression of antiapoptotic BCL2 family proteins and AKT, increased phosphorylation of TRP53 and MAPK3/1, caused cleavage of caspase 3 and PARP, and induced apoptotic cell death in a time-dependent manner [104]. Ascorbate prevented these effects both in vivo and in vitro [103, 104]. Lower doses must be examined, as well as the possible mechanisms by which Cr can have such effects, but oxidative stress is likely involved. Primordial and small primary follicles do appear targeted by these exposures, although not necessarily specifically. The most sensitive follicle type may be identified when reduced exposures are tested.
9,10-Dimethyl-1,2-Benzanthracene
The polycyclic aromatic hydrocarbons 9,10-dimethyl-1,2-benzanthracene (DMBA), benzo[a]pyrene, and 3-methylcholanthrene are well known to destroy primordial and primary follicles following in vivo treatment of mice and rats [105–107]. Early studies also noted that DMBA treatment destroyed antral follicles [108]. A more recent study investigated the mechanism by which DMBA destroys antral follicles. Culture of rat large antral follicles with DMBA at concentrations greater than or equal to 1 μM in the presence of an antiapoptotic concentration of FSH resulted in increased expression of the proapoptotic protein BAX in granulosa cells by 24 h, followed by increased caspase 3 activation and DNA fragmentation detected by TUNEL staining by 48 h [109]. ROS were already significantly increased relative to follicles treated with FSH alone by 12 h of DMBA treatment and remained elevated through 48 h, but GSH concentrations were not decreased. Depletion of GSH with BSO enhanced the initiation of apoptosis by DMBA [109]. Supplementation with GSH ethyl ester but not with the antioxidants butylated hydroxytoluene and dithiothreitol prevented the initiation of apoptosis by DMBA [109]. These results are consistent with a role for ROS in the initiation of apoptosis by DMBA in granulosa cells of antral follicles and demonstrate an antiapoptic effect of GSH.
Methoxychlor
In vivo treatment of mice with the organochlorine insecticide methoxychlor at ≥32 mg/kg for 20 days was reported to induce atresia of antral follicles but not of primordial, primary, or secondary follicles [110]. At these doses, ovaries of mice treated with methoxychlor for 20 days had elevated levels of H2O2 and of oxidative protein and DNA damage assessed via nitrotyrosine and 8-hydroxy-2′-deoxyguanosine immunostaining, respectively [111]. Ovarian enzymatic activity and mRNA levels of SOD1, GPX1, and catalase were also significantly decreased in ovaries after 20 days of treatment with 32 or 64 mg/kg methoxychlor [111]. Similarly, antral follicles cultured with 10 or 100 μg/ml but not 1 μg/ml methoxychlor underwent atresia by 72 h of treatment, which was preceded by a rise in mRNA levels of Bax after 48 h and which was prevented by supplementation with the GSH precursor N-acetylcysteine [112, 113]. Follicular expression of the antioxidant genes Sod1, Gpx2, and catalase were unaltered after 24 h of methoxychlor treatment in vitro and were significantly decreased in all concentration groups when atresia was already well advanced at 96 h [112]. At 48 h, Sod1 mRNA levels were significantly decreased at the 1- and 10-μg/ml concentrations of methoxychlor, and glutathione peroxidase 1 and catalase were significantly increased at the 100-μg/ml methoxychlor concentration [112]. These observations provide support for a role of oxidative stress in the initiation of antral follicle atresia by methoxychlor.
Conclusions on Sensitivity of Small Follicles to Oxidative Stress
Overall, evidence regarding the roles of oxidative stress and ovarian antioxidant status in toxicant-induced destruction of small ovarian follicles is mixed. H2O2 did not induce atresia of small follicles in cultured neonatal ovaries, and antioxidants were not protective against the primordial follicle toxicity of VCD [67]. In contrast, oxidative stress may play a role in the destruction of primordial follicles by exposure to the heavy metal Cr or exposure to ionizing radiation since treatment with antioxidants was protective [95, 103, 104]. Future studies could involve altering expression of protective antioxidant defense mechanisms specifically in small follicles or oocytes using available transgenic mice expressing Cre recombinase only at certain follicle stages. Further research measuring the effects of toxicant exposure on ROS generation and the modulation of toxicant effects by antioxidant supplementation or depletion in isolated cultured follicles and ovaries would also help in characterizing small follicle sensitivity.
Conclusions on Sensitivity of Antral Follicles to Oxidative Stress
Antral follicles appear to be highly sensitive to oxidative stress-induced apoptosis of granulosa cells. The observations that GSH depletion enhances and antioxidant supplementation inhibits the initiation of apoptosis in antral follicles and granulosa cells by a variety of toxicants and by ionizing radiation support a role for ROS in this process. Direct measurements of rising ROS levels prior to any increases in markers of apoptosis further support the contention that ROS are involved in the initiation of apoptosis by various toxicants and ionizing radiation in antral follicles and granulosa cells. Because glutathione-S-transferase-catalyzed conjugation with GSH is an important phase II detoxification pathway for active metabolites of DMBA and CP [91, 114, 115], it is also possible that GSH depletion enhances toxicity by preventing the detoxification of these metabolites and that GSH supplementation enhances detoxification of these metabolites. Arguing against the latter explanation for CP is the observation that supplementation with other antioxidants besides GSH ethyl ester was also protective [92]. For DMBA, GSH supplementation was protective, but supplementation with butylated hydroxytoluene or dithiothreitol was not [109]. Therefore, additional studies are needed to clarify the role of ROS in the initiation of apoptosis in antral follicles by DMBA.
EFFECTS OF OXIDATIVE STRESS ON OOCYTES AND PREIMPLANTATION EMBRYONIC DEVELOPMENT
Oocyte GSH concentrations increase rapidly after the preovulatory gonadotropin surge, with maximal levels in ovulated MII oocytes, and oocyte GSH is important during fertilization and early embryonic development [116–124]. Biochemical depletion of GSH with BSO during oocyte maturation in vitro [116, 123] or in vivo [124] prevented sperm chromatin decondensation and formation of the male pronucleus after in vitro fertilization. The observation that the majority of zygotes of Gclm−/− female mice fail to form the second, male pronucleus at 0.5 days postcoitum in vivo [40] provides further evidence of the importance of oocyte GSH in formation of the male pronucleus. These effects of GSH depletion and GSH deficiency are believed to be due to the requirement for reduction of protamine disulfide bonds in the sperm nucleus for sperm nuclear reactivation to occur [116, 125]. Brief (and reversible) depletion of GSH following exposure to an oxidant (diamide) in ovulated hamster oocytes prior to in vitro fertilization disrupted hamster oocyte meiotic spindles, as well as sperm chromatin decondensation, leading to the formation of abnormal female pronuclei and suggesting that altered redox status can impact zygote formation in several ways [117]. In contrast, no spindle abnormalities were reported when bovine oocytes were depleted of GSH using BSO [123]. These results suggest that there may be a species difference in the importance of GSH for meiotic spindle function, that the presence of small amounts of reduced GSH in BSO-treated oocytes may be sufficient to support normal meiotic spindles under normal conditions when GSH turnover from the oxidized form occurs quickly, or that meiotic spindle function may be sensitive to oxidative stress but not to GSH depletion per se.
In hamsters, embryonic GSH concentrations decline from highest levels in the metaphase II oocyte to low levels in blastocysts [118], and similar declines are observed in mouse embryos [120, 126]. Two-cell mouse embryos do not normally express Gclc or synthesize GSH de novo, whereas blastocysts express Gclc and synthesize GSH [119–121]. Moreover, although two-cell embryos upregulated Gclc expression in response to an oxidant stimulus that depleted GSH, tert-butyl hydroperoxide, they were unable to do so in response to depletion of GSH by an electrophilic toxicant, diethylmaleate, and GSH depletion with BSO for 45 h beginning at the two-cell stage inhibited development to the blastocyst stage [119–121]. In vivo treatment with BSO beginning prior to initiation of a superovulation protocol significantly diminished concentrations of GSH in oocytes and increased the percentage of degenerating embryos retrieved at 1.5 days postcoitum [126]. In contrast, BSO injections beginning at 0.5 days postcoitum significantly decreased GSH concentrations in oviductal and uterine secretions but did not decrease zygote GSH concentrations or affect embryonic development at 1.5 or 2.5 days postcoitum [126]. Superovulated oocytes from Gclm−/− females have GSH concentrations less than 20% of wild-type oocytes [40]. Embryos derived from oocytes of Gclm−/− females and fertilized in vitro with sperm from wild-type males developed to the blastocyst stage in culture at very low rates compared to embryos derived from oocytes of Gclm+/+ females [40]. Together, these findings support the conclusion that GSH present in the oocyte is critical for normal zygote formation and preimplantation development, whereas GSH in reproductive tract secretions appears to play a lesser, supportive role.
CONCLUSIONS
In recent years, there has been growing interest in the roles of ROS and oxidative stress in female reproduction. Endogenous ROS have been shown to play important roles as signaling molecules, for example, during ovulation. Accumulating evidence demonstrates that ROS are key signals in the initiation of apoptosis in antral follicles and granulosa cells of antral follicles by diverse stimuli, such as gonadotropin withdrawal, exposure to exogenous toxicants, and exposure to ionizing radiation, and that antioxidants protect against these stimuli. Studies have also demonstrated that the high concentrations of the antioxidant GSH in oocytes are necessary for normal fertilization and subsequent preimplantation embryonic development. These studies suggest that antral follicles and fertilization and early embryonic development may be particularly sensitive to exposures to environmental stressors and chemical toxicants that induce oxidative stress. In contrast, the current evidence is less consistent regarding the sensitivity of primordial and primary follicles to oxidative stress or changes in antioxidant status. Future studies should aim to clarify the roles of antioxidant defense mechanisms and ROS in the development and survival of small follicles and further explore the involvement of ROS and antioxidants in toxicant-induced destruction of small follicles. Future studies should also test the possible protective effects of in vivo antioxidant supplementation on female fertility.
Footnotes
Supported by National Institutes of Health (NIH) grants AG032087 and ES10963 to U.L. and National Sciences and Engineering Council of Canada (NSERC) and Canadian Institutes of Health Research (CIHR) grants to P.J.D.
REFERENCES
- Hall PF. Testicular steroid synthesis: organization and regulation. : Knobil E, Neill J. (eds.), The Physiology of Reproduction, vol. 1, 2nd ed. New York: Raven Press; 1994: 1335 1362 [Google Scholar]
- Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev 2006; 38: 171 196 [DOI] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EMG, Doorn JA, Slikker W. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci 2009; 112: 4 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen 2010; 51: 380 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996; 313: 17 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal 2006; 8: 1865 1879 [DOI] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008; 295: C849 C868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med 2008; 44: 921 937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 2008; 1780: 1273 1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck MR, Jeyaseelan I, Scholes RA. Minireview: ascorbic acid and fertility. Biol Reprod 1995; 52: 262 266 [DOI] [PubMed] [Google Scholar]
- Zreik TG, Kodaman PH, Jones EE, Olive DL, Behrman HR. Identification and characterization of an ascorbic acid transporter in human granulosa-lutein cells. Mol Hum Reprod 1999; 5: 299 302 [DOI] [PubMed] [Google Scholar]
- Musicki B, Kodaman PH, Aten RF, Behrman HR. Endocrine regulation of ascorbic acid transport and secretion in luteal cells. Biol Reprod 1996; 54: 399 406 [DOI] [PubMed] [Google Scholar]
- Aten RF, Duarte KM, Behrman HR. Regulation of ovarian antioxidant vitamins, reduced glutathione, and lipid peroxidation by luteinizing hormone and prostaglandin F2alpha. Biol Reprod 1992; 46: 401 407 [DOI] [PubMed] [Google Scholar]
- Behrman HR, Preston SL, Aten RF, Rinaudo P, Zreik TG. Hormone induction of ascorbic acid transport in immature granulosa cells. Endocrinology 1996; 137: 4316 4321 [DOI] [PubMed] [Google Scholar]
- Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Semin Liver Dis 1998; 18: 415 424 [DOI] [PubMed] [Google Scholar]
- Shan XQ, Aw TY, Jones DP. Glutathione-dependent protection against oxidative injury. Pharmacol Ther 1990; 47: 61 71 [DOI] [PubMed] [Google Scholar]
- Ho Y-S, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem 1998; 273: 7765 7769 [DOI] [PubMed] [Google Scholar]
- Laloraya M, Kumar PG, Laloraya MM. Changes in the levels of superoxide anion radical and superoxide dismutase during the estrous cycle of rattus norvegicus and induction of superoxide dismutase in rat ovary by lutropin. Biochem Biophys Res Commun 1988; 157: 146 153 [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE. Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in vitro perfused rabbit ovary. J Reprod Fertil 1991; 91: 207 212 [DOI] [PubMed] [Google Scholar]
- Sato EF, Kobuchi H, Edashige K, Takahashi M, Yoshioka T, Utsumi K, Inoue M. Dynamic aspects of ovarian superoxide dismutase isozymes during the ovulatory process in the rat. FEBS Lett 1992; 303: 121 125 [DOI] [PubMed] [Google Scholar]
- Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A 2011; 108: 1462 1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y-S, Magnenat J-L, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 1997; 272: 16644 16651 [DOI] [PubMed] [Google Scholar]
- Ho Y-S, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 2004; 279: 32804 32812 [DOI] [PubMed] [Google Scholar]
- Pretsch W. Glutathione reductase activity deficiency in homozygous Gr1a1Neu mice does not cause haemolytic anaemia. Genet Res 1999; 73: 1 5 [DOI] [PubMed] [Google Scholar]
- Rogers LK, Bates CM, Welty SE, Smith CV. Diquat induces renal proximal tubule injury in glutathione reductase-deficient mice. Toxicol Appl Pharmacol 2006; 217: 289 298 [DOI] [PubMed] [Google Scholar]
- Rogers LK, Tamura T, Rogers BJ, Welty SE, Hansen TN, Smith CV. Analyses of glutathione reductase hypomorphic mice indicate a genetic knockout. Toxicol Sci 2004; 82: 367 373 [DOI] [PubMed] [Google Scholar]
- Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein gpx4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med 2003; 34: 496 502 [DOI] [PubMed] [Google Scholar]
- Conrad M, Moreno SG, Sinowatz F, Ursini F, Kölle S, Roveri A, Brielmeier M, Wurst W, Maiorino M, Bornkamm GW. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol 2005; 25: 7637 7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Förster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, Neumüller C, Deutsch MJ, Walch A, Hrabé de Angelis M, Wurst W, Ursini F, et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J 2009; 23: 3233 3242 [DOI] [PubMed] [Google Scholar]
- Puglisi R, Bevilacqua A, Carlomagno G, Lenzi A, Gandini L, Stefanini M, Mangia F, Boitani C. Mice Overexpressing the mitochondrial phospholipid hydroperoxide glutathione peroxidase in male germ cells show abnormal spermatogenesis and reduced fertility. Endocrinology 2007; 148: 4302 4309 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 1998; 139: 4008 4011 [DOI] [PubMed] [Google Scholar]
- Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, Price DL, Rothstein J, Gitlin JD. Copper chaperone for superoxide dismutase is essential to activate mammalian cu/zn superoxide dismutase. Proc Natl Acad Sci U S A 2000; 97: 2886 2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wiseman AL, Kala G, Kala SV, Matzuk MM, Lieberman MW. Reproductive defects in γ-glutamyl transpeptidase deficient mice. Endocrinology 2000; 141: 4270 4277 [DOI] [PubMed] [Google Scholar]
- Lieberman MW, Wiseman AL, Shi Z-Z, Carter BZ, Barrios R, Ou C-N, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, et al. Growth retardation and cysteine deficiency in γ-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci 1996; 93: 7923 7926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will Y, Fishcher KA, Horton RA, Kaetzel RS, Brown MK, Hedstrom O, Lieberman MW, Reed DJ. γ-Glutamyltranspeptidase-deficient knockout mice as a model to study the relationship between glutathione status, mitochondrial function, and cellular function. Hepatology 2000; 32: 740 749 [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun 2000; 279: 324 329 [DOI] [PubMed] [Google Scholar]
- Shi Z-Z, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci 2000; 97: 5101 5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol 2006; 70: 2116 2126 [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate cysteine ligase modifier subunit Gclm (−/−) knockout mouse: novel model system for severely compromised oxidative stress response. J Biol Chem 2002; 277: 49446 49452 [DOI] [PubMed] [Google Scholar]
- Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, Luderer U. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology 2011; 152: 2806 2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez SG, Luderer U. Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic Biol Med 2004; 36: 1366 1377 [DOI] [PubMed] [Google Scholar]
- Luderer U, Kavanagh TJ, White CC, Faustman EM. Gonadotropin regulation of glutathione synthesis in the rat ovary. Reprod Toxicol 2001; 15: 495 504 [DOI] [PubMed] [Google Scholar]
- Mattison DR, Shiromizu K, Pendergrass JA, Thorgeirsson SS. Ontogeny of ovarian glutathione and sensitivity to primordial oocyte destruction by cyclophosphamide. Pediatr Pharmacol 1983; 3: 49 55 [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U. Gonadotropin regulation of glutamate cysteine ligase catalytic and modifier subunit expression in the rat ovary is subunit and follicle stage-specific. Am J Physiol Endocrinol Metab 2005; 289: E391 E402 [DOI] [PubMed] [Google Scholar]
- Chun S-Y, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsueh AJW. Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology 1994; 135: 1845 1853 [DOI] [PubMed] [Google Scholar]
- Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology 1996; 137: 1447 1456 [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U. Opposing effects of glutathione depletion and FSH on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 2006; 147: 1224 1236 [DOI] [PubMed] [Google Scholar]
- Hoang YD, Nakamura BN, Luderer U. Follicle-stimulating hormone and estradiol interact to stimulate glutathione synthesis in rat ovarian follicles and granulosa cells. Biol Reprod 2009; 81: 636 646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 1995; 136: 242 252 [DOI] [PubMed] [Google Scholar]
- Devine PJ, Petrillo SK, Cortvrindt R. In vitro ovarian model systems to study toxicology. : Richburg J, Hoyer P. (eds.), Reproductive and Endocrine Toxicology, vol. 11, : McQueen C (ed.) Comprehensive Toxicology. Oxford, UK: Elsevier; 2010. [Google Scholar]
- Hoyer PB, Devine PJ, Hu X, Thompson KE, Sipes IG. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol 2001; 29: 91 99 [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Sipes IG. Development of an animal model for ovotoxicity using 4-vinylcyclohexene: a case study. Birth Defects Res B Dev Reprod Toxicol 2007; 80: 113 125 [DOI] [PubMed] [Google Scholar]
- National Toxicology Program NTP Technical Report on the Toxicology and Carcinogenesis Studies of 4-Vinylcyclohexene (CAS No. 100-40-3) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Research Triangle Park, NC: National Toxicology Program, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1986. [PubMed] [Google Scholar]
- National Toxicology Program NTP Technical Report on the Toxicology and Carcinogenesis Studies of 4-Vinyl-1-Cyclohexene Diepoxide (CAS No. 106-87-6) in F344/N Rats and B6C3F1 Mice (Dermal Studies). Research Triangle Park, NC: National Toxicology Program, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1989. [PubMed] [Google Scholar]
- Flaws JA, Salyers KL, Sipes IG, Hoyer PB. Reduced ability of rat preantral ovarian follicles to metabolize 4-vinyl-1-cyclohexene diepoxide in vitro. Toxicol Appl Pharmacol 1994; 126: 286 294 [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattision DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol 1990; 105: 372 381 [DOI] [PubMed] [Google Scholar]
- Kao S-W, Sipes IG, Hoyer PB. Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol 1999; 13: 67 75 [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 1996; 139: 394 401 [DOI] [PubMed] [Google Scholar]
- Hu XM, Christian PJ, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax and Bcl-xl protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod 2001; 65: 1489 1495 [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod 2001; 65: 87 93 [DOI] [PubMed] [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol 1996; 139: 402 410 [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod 2002; 67: 718 724 [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Keating AF, Christian PJ, Sen N, Hoying JB, Brooks HL, Hoyer PB. Involvement of the KIT/KITL signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol Reprod 2008; 79: 318 327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Mark CJ, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7,12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol Appl Pharmacol 2009; 241: 127 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark-Kappeler CJ, Sen N, Keating AF, Sipes IG, Hoyer PB. Distribution and Responsiveness of rat anti-müllerian hormone during ovarian development and VCD-induced ovotoxicity. Toxicol Appl Pharmacol 2010; 249: 1 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Hoyer PB. Effect of 4-vinylcyclohexene diepoxide dosing in rats on GSH levels in liver and ovaries. Toxicol Sci 2001; 62: 315 320 [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Hoyer PB. Initiation of delayed ovotoxicity by in vitro and in vivo exposures of rat ovaries to 4-vinylcyclohexene diepoxide. Reprod Toxicol 2004; 19: 71 77 [DOI] [PubMed] [Google Scholar]
- Nathan L, Chaudhuri G. Antioxidant and prooxidant actions of estrogens: potential physiological and clinical implications. Semin Reprod Endocrinol 1998; 16: 309 314 [DOI] [PubMed] [Google Scholar]
- Thompson KE, Sipes IG, Greenstein BD, Hoyer PB. 17beta-estradiol affords protection against 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in Fischer-344 rats. Endocrinology 2002; 143: 1058 1065 [DOI] [PubMed] [Google Scholar]
- Byrne J. Long-term genetic and reproductive effects of ionizing radiation and chemotherapeutic agents on cancer patients and their offspring. Teratology 1999; 59: 210 215 [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 2001; 7: 535 543 [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab 2006; 91: 1723 1728 [DOI] [PubMed] [Google Scholar]
- Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am 1998; 27: 927 943 [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Matus-Riley M, Meadows AT. Gonadal effects of cancer therapy in girls. Cancer 1985; 55: 2364 2372 [DOI] [PubMed] [Google Scholar]
- Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, Donaldson SS, Byrne J, Robison LL. Fertility of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27: 2677 2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo RA. Potential options for preservation of fertility in women. N Engl J Med 2005; 353: 64 73 [DOI] [PubMed] [Google Scholar]
- Kumar R, Biggart JD, McEvoy J, McGeown MG. Cyclophosphamide and reproductive function. Lancet 1972; June 3: 1212 1214 [DOI] [PubMed] [Google Scholar]
- Warne GL, Fairley KF, Hobbs JB, Martin FIR. Cyclophosphamide-induced ovarian failure. N Engl J Med 1973; 289: 1159 1162 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Heindel JJ. Ovarian toxicants: multiple mechanisms of action. : Korach KS. (ed.), Reproductive and Developmental Toxicology. New York: Marcel Dekker; 1998: 373 395 [Google Scholar]
- Jarrell J. Young Lai EV, Barr R, McMahon A, Belbeck L, O'Connell G. Ovarian toxicity of cyclophosphamide alone and in combination with ovarian irradiation in the rat. Cancer Res 1987; 47: 2340 2343 [PubMed] [Google Scholar]
- Plowchalk DR, Mattison DR. Reproductive toxicity of cyclophosphamide in the C57BL/6N mouse: 1. Effects on ovarian structure and function. Reprod Toxicol 1992; 6: 411 421 [DOI] [PubMed] [Google Scholar]
- Shiromizu K, Thorgeirsson SS, Mattison DR. Effect of cyclophosphamide on oocyte and follicle number in Sprague-Dawley rats, C57BL/6N and DBA/2N mice. Pediatric Pharmacology 1984; 4: 213 221 [PubMed] [Google Scholar]
- Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod 1999; 14: 1903 1907 [DOI] [PubMed] [Google Scholar]
- Plowchalk DR, Mattison DR. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol Appl Pharmacol 1991; 107: 472 481 [DOI] [PubMed] [Google Scholar]
- Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 1993; 53: 5629 5637 [PubMed] [Google Scholar]
- Dirven HAAM, van Ommen B, van Bladeren PJ. Involvement of human glutathione S-transferase isoenzymes in the conjugation of cyclophosphamide metabolites with glutathione. Cancer Res 1994; 54: 6215 6220 [PubMed] [Google Scholar]
- Gamcsik MP, Dolan ME, Andersson BS, Murray D. Mechanisms of resistance to the toxicity of cyclophosphamide. Curr Pharm Des 1999; 5: 587 605 [PubMed] [Google Scholar]
- Petrillo SK, Desmeules P, Truong T-Q, Devine PJ. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl Pharmacol 2011; 253: 94 102 [DOI] [PubMed] [Google Scholar]
- Desmeules P, Devine PJ. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol Sci 2006; 90: 500 509 [DOI] [PubMed] [Google Scholar]
- Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. Characterization of an immortalized human granulosa cell line (COV434). Mol Hum Reprod 2000; 6: 146 153 [DOI] [PubMed] [Google Scholar]
- Flowers J, Ludeman SM, Gamcsik MP, Colvin OM, Shao K-L, Boal JH, Springer JB, Adams DJ. Evidence for a role of chloroethylaziridine in the cytotoxicity of cyclophosphamide. Cancer Chemother Pharmacol 2000; 45: 335 344 [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luong BT, Tan Y, Luderer U. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol Sci 2007; 98: 216 230 [DOI] [PubMed] [Google Scholar]
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular response to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev 2004; 23: 311 322 [DOI] [PubMed] [Google Scholar]
- Hanoux V, Pairault C, Bakalska M, Habert R, Livera G. Caspase-2 involvement during ionizing radiation-induced oocyte death in the mouse ovary. Cell Death Differ 2007; 14: 671 681 [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee CJ. Effect of exogenous melatonin on the ovarian follicles in γ-irradiated mouse. Mutat Res 2000; 449: 33 39 [DOI] [PubMed] [Google Scholar]
- Ataya KM, Valeriote FA, Ramahi-Ataya AJ. Effect of cyclophosphamide on the immature rat ovary. Cancer Res 1989; 49: 1660 1664 [PubMed] [Google Scholar]
- Lee CJ, Park HH, Do BR, Yoon YD, Kim JK. Natural and radiation-induced degeneration of the primordial and primary follicles in the mouse ovary. Anim Reprod Sci 2000; 59: 109 117 [DOI] [PubMed] [Google Scholar]
- Cortés-Wanstreet MM, Giedzinski E, Limoli CL, Luderer U. Overexpression of glutamate cysteine ligase protects human COV434 granulosa tumor cells against oxidative and γ-radiation-induced cell death. Mutagenesis 2009; 24: 211 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 2008; 21: 28 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan GM, Learmonth ID, Case CP. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit Rev Toxicol 2008; 38: 645 674 [DOI] [PubMed] [Google Scholar]
- Murthy RC, Junaid M, Saxena DK. Ovarian dysfunction in mice following chromium (VI) exposure. Toxicol Lett 1996; 89: 147 154 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Rajendiran G, Sekhar P, Gowri C, Govindarajulu P, Aruldhas MM. Reproductive toxicity of chromium in adult bonnet monkeys (Macaca radiata Geoffrey): reversible oxidative stress in the semen. Toxicol Appl Pharmacol 2006; 215: 237 249 [DOI] [PubMed] [Google Scholar]
- Banu SK, Samuel JB, Arosh JA, Burghardt RC, Aruldhas MM. Lactational exposure to hexavalent chromium delays puberty by impairing ovarian development, steroidogenesis, and pituitary hormone synthesis in developing Wistar rats. Toxicol Appl Pharmacol 2008; 232: 180 189 [DOI] [PubMed] [Google Scholar]
- Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, Burghardt RC. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl Pharmacol 2011; 251: 253 266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison DR. Difference in sensitivity of rat and mouse primordial oocytes to destruction by polycyclic aromatic hydrocarbons. Chem Biol Interact 1979; 28: 133 137 [DOI] [PubMed] [Google Scholar]
- Mattison DR. Nightingale. Oocyte destruction by polycyclic aromatic hydrocarbons is not linked to the inducibility of ovarian aryl hydrocarbon (benzo(a)pyrene) hydroxylase activity in (DBA/2N × C57BL/6N) F1 × DBA/2N backcross mice. Pediatr Pharmacol 1982; 2: 11 21 [PubMed] [Google Scholar]
- Mattison DR, Thorgeirsson SS. Ovarian aryl hydrocarbon hydroxylase activity and primordial oocyte toxicity of polycyclic aromatic hydrocarbons in mice. Cancer Res 1979; 39: 3471 3475 [PubMed] [Google Scholar]
- Mattison DR. Morphology of oocyte and follicle destruction by polycyclic aromatic hydrocarbons in mice. Toxicol Appl Pharmacol 1980; 53: 249 259 [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Nakamura BN, Luderer U. Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene (DMBA) in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol Reprod 2007; 77: 442 451 [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds DA, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian surface epithelium in the mouse. Toxicol Sci 2002; 68: 473 478 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol 2006; 216: 436 445 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci 2006; 93: 382 389 [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfield CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci 2005; 88: 213 221 [DOI] [PubMed] [Google Scholar]
- Jernström B, Funk M, Frank H, Mannervik B, Seidel A. Glutathione-S-transferase A1-1-catalysed conjugation of bay and fjord region diol epoxides of polycyclic aromatic hydrocarbons with glutathione. Carcinogenesis 1996; 17: 1491 1498 [DOI] [PubMed] [Google Scholar]
- Seidel A, Friedberg T, Löllman B, Schwierzok A, Funk M, Frank H, Holler R, Oesch F, Glatt H. Detoxification of optically active bay- and fjord-region polycyclic aromatic hydrocarbon dihydrodiol epoxides by human glutathione transferase P1-1 expressed in Chinese hamster V79 cells. Carcinogenesis 1998; 19: 1975 1981 [DOI] [PubMed] [Google Scholar]
- Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol 1988; 125: 181 186 [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod 1997; 57: 1413 1419 [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev 2003; 64: 106 112 [DOI] [PubMed] [Google Scholar]
- Gardiner CS, Reed DJ. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys 1995; 318: 30 36 [DOI] [PubMed] [Google Scholar]
- Gardiner CS, Reed DJ. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos. Arch Biochem Biophys 1995; 321: 6 12 [DOI] [PubMed] [Google Scholar]
- Stover SK, Gushansky GA, Salmen JJ, Gardiner CS. Regulation of γ-glutamate cysteine ligase expression by oxidative stress in the mouse preimplantation embryo. Toxicol Appl Pharmacol 2000; 168: 153 159 [DOI] [PubMed] [Google Scholar]
- Perreault SD, Goldman JM, Luderer U, Hunt PA. Targeting female reproductive function during follicular maturation, ovulation, and fertilization: critical windows for pharmaceutical or toxicant action. : Richburg J, Hoyer P. (eds.), Reproductive and Endocrine Toxicology, vol. 11, : McQueen C (ed.) Comprehensive Toxicology. Oxford, UK: Elsevier; 2010. [Google Scholar]
- Sutovsky P, Schatten G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol Reprod 1997; 56: 1503 1512 [DOI] [PubMed] [Google Scholar]
- Calvin HI, Grosshans K, Blake EJ. Estimation and manipulation of glutathione levels in prepuberal mouse ovaries and ova: relevance to sperm nucleus transformation in the fertilized egg. Gamete Res 1986; 14: 265 275 [Google Scholar]
- Perreault SD, Wolff RA, Zirkin BR. The role of disulfide bond reduction during mammalian sperm nuclear decondensation in vivo. Dev Biol 1984; 101: 160 167 [DOI] [PubMed] [Google Scholar]
- Salmen JJ, Skufca F, Matt A, Gushansky G, Mason A, Gardiner CS. Role of glutathione in reproductive tract secretions on mouse preimplantation embryo development. Biol Reprod 2005; 73: 308 314 [DOI] [PubMed] [Google Scholar]