ABSTRACT
Men and women differ in their susceptibility to sexually transmittable infections (STIs) such as human immunodeficiency virus (HIV). However, a paucity of published information regarding the tissue structure of the human genital tract has limited our understanding of these gender differences. We collected cervical, vaginal, and penile tissues from human adult donors. Tissues were prepared with hematoxylin and eosin stains or immunofluorescence labeling of epithelial cell proteins and were analyzed for structural characteristics. Rhesus macaque genital tissues were evaluated to assess the use of this model for HIV/simian immunodeficiency virus transmission events. We found the stratified squamous epithelia of the male and female genital tract shared many similarities and important distinctions. Expression of E-cadherins, desmogleins 1/2, and involucrin was seen in all squamous epithelia, though expression patterns were heterogeneous. Filaggrin and a true cornified layer were markedly absent in female tissues but were clearly seen in all male epithelia. Desmogleins 1/2 were more consistent in the outermost strata of female squamous genital epithelia. Macaque tissues were similar to their respective human tissues. These initial observations highlight how male and female genital epithelia resemble and differ from one another. Further information regarding tissue structural characteristics will help to understand how STIs traverse these barriers to cause infection. This knowledge will be essential in future HIV pathogenesis, transmission, and prevention studies.
Keywords: barrier function, cervix, developmental origins of health and disease, filaggrin, intercellular junctions, keratin, male reproductive tract, vaginal epithelium
The expression of filaggrin, involucrin, desmogleins 1/2, and E-cadherin in male and female genital epithelia are characterized and compared using high-definition epifluorescence microscopy.
INTRODUCTION
There are many obvious differences between men and women. Some of these differences become apparent early in embryogenesis, when systemic levels of sex hormones guide the development of genital tract tissues [1]. In the female embryo's early weeks, the absence of androgen promotes Müllerian duct expansion, which forms the uterus and uterovaginal canal. Columnar, mucus-secreting epithelial cells predominantly line these surfaces. Around the 10th wk, squamous epithelial cells from the urogenital sinus migrate towards the uterovaginal canal, forming the vagina and portio vaginalis (ectocervix) [2]. In contrast, the male external genitalia arises entirely from the urogenital sinus and consists of only stratified squamous epithelia—this is attributed to the continued presence of testosterone and its derivative hormone during male embryogenesis [3].
Men and women thus share many similarities, i.e., the predominant stratified squamous epithelial lining of the genital tract. In the setting of unprotected intercourse with a partner harboring a sexually transmitted infection (STI) such as herpes simplex virus (HSV) or human immunodeficiency virus (HIV), these surfaces are most exposed to the pathogen. The squamous epithelium in these areas is technically very similar to the epidermis on most of the body's surface: it contains a single layer of basal cells (stratum basale) and overlying layers of spinous cells (stratum spinosum) and granular cells (stratum granulosum) [4]. Certain cell surface proteins can interact with similar proteins on adjacent cells to form intercellular junctions (ICJs). These ICJs allow epithelial cells to communicate with one another and prevent toxins and pathogens from entering the underlying dermis, vasculature, or immune system [5]. On most stratified squamous epithelial surfaces found in the human body, there is a final layer of cornified cells and keratin (stratum corneum) [6, 7]. The stratum corneum is thought to play a large, if not the largest, role in epithelial barrier function, keeping moisture and nutrients within the dermis and unwanted agents out.
In contrast, the uterine and endocervical epithelium are composed of single layers of columnar cells, drawing parallels to the rectal mucosal epithelium and suggesting a heightened vulnerability to pathogens such as Chlamydia trachomatis and Neisseria gonorrhea [3, 8]. However, the uterine and endocervical surfaces anatomically lie in shielded, relatively unexposed areas. Furthermore, certain antimicrobial factors appear to be secreted at higher levels by uterine as compared to vaginal epithelial cells [9]. Finally, thick mucus secretions with immune cells and immunoglobulins are typically found in the endocervical canal overlying the epithelium. The endocervical surface is also highly convoluted and contains numerous goblet cells that are continually producing more mucus. These features may all serve protective roles in the setting of an exposure to an infectious agent [10].
In this study, we sought to highlight major histological and structural characteristics between male and female genital epithelia. While many studies have explored the role of innate and adaptive immune cells within these tissues as a correlate to STI susceptibility, few have looked beyond these factors. We believe that a clearer knowledge of these structures will be fundamental to understanding the pathogenesis of many STIs and their primary sites of infection.
MATERIALS AND METHODS
Human tissue specimens were collected from a total of 16 adult donors undergoing an elective hysterectomy (cervical and vaginal) or circumcision (foreskin) at Northwestern Memorial Hospital (Chicago, IL). The Northwestern Institutional Review Board approved all consent forms and protocols prior to tissue collection. All cervical specimens were obtained from premenopausal women, though no other clinical information was obtained. Three intact penile specimens were additionally obtained from tissue donation banks (LifeLegacy and Science Care) and the National Disease Research Institute. The penile tissues were shipped on ice within 24 h of death. All tissues were immediately dissected, snap-frozen in OCT compound (Sakura), and stored at −80°C until ready for use.
Five female and three male tissue specimens from rhesus macaques (Macaca mulatta) were obtained from collaborators at the International AIDS Vaccine Initiative (IAVI), Tulane National Primate Research Center, and Yerkes National Primate Research Center. The animals were fed and housed according to regulations set forth by the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. The animal experiments in this study were approved by the Institutional Animal Care and Use Committees at IAVI, Tulane, and Yerkes. Human cervical and foreskin tissue samples and macaque penile tissues were removed during surgery or necropsy and snap-frozen in OCT compound.
Frozen tissue cryosections (5–10 μm thick) were placed onto glass slides (VWR International, LLC) and fixed with either 3.7% formaldehyde/PIPES buffer or methanol/acetone mixtures. Sodium borohydride (0.05%; Sigma) was used to decrease tissue autofluorescence, and 0.2% cold fish skin gelatin (Sigma) combined with 10% normal goat serum and 0.1% Triton-X were used to enhance specific antigen-antibody binding. One set of tissues was stained with a primary mouse α-filaggrin antibody (1–5 μg/ml; Santa Cruz Biotechnology) and secondary goat anti-mouse IgG1 Rhodamine Red-X antibody (2.125 μg/ml; Jackson-ImmunoResearch). This was followed by staining with an α-involucrin (0.5–2 μg/ml; Santa Cruz Biotechnology) antibody fluorescently labeled with a Zenon Alexa-Fluor kit (Invitrogen). Another set was labeled with antibodies against E-cadherins (1–2 μg/ml; 488 nm-fluorescence labeled; BD Pharmingen) and desmogleins (1:10 dilution; labeled with Zenon Alexa-Fluor kit; courtesy of Kathy Green). Cell nuclei were stained with Hoescht. Each type of tissue had antibody concentrations titrated prior to final imaging to achieve optimal signal-to-background ratios. Tissue sections were mounted with mounting medium (DAKO), sealed with a glass coverslip, and kept at 4°C until ready for imaging. Every set included control slides stained with the appropriate isotype control (Ms IgG1 or Ms IgG2a at the same concentration as the primary antibody; BD Pharmingen). A separate set of tissues was fixed in formalin and embedded in paraffin to be used for hematoxylin and eosin (H&E) stains. The Northwestern Skin Diseases Research Core Facility performed these stains using their standard protocol.
Tissue was imaged using DeltaVision RT systems and softWoRx software (Applied Precision Instruments). The software includes the ability to stitch together separate frames to create a larger frame of view. All images were deconvolved using softWoRx, which enhances the fluorescence of the brightest pixels from surrounding pixels to reduce signal-to-noise ratios. Light microscopy was also performed with the above system, a Moticam 2300 system, and Images Plus software (Motic).
RESULTS
For this study, we collected adult human cervical, vaginal, and foreskin tissue from consenting donors undergoing a routine hysterectomy or circumcision. We also obtained penile specimens from cadaveric human donors. A total of eight cervical, two vaginal, eight foreskin, and three (uncircumcised) penile specimens were analyzed. To examine the basic histology of these protective layers, we performed standard H&E stains on human female and male genital tissues. The stratified squamous epithelia of the male and female lower reproductive tract were similar to one another, and representative images are shown in Figure 1. One notable difference was the lack of cornification in the female ectocervical and vaginal tissues as compared to male squamous epithelia. In contrast to the other surfaces, the convoluted endocervical epithelium is lined with a single layer of columnar cells.
FIG. 1.
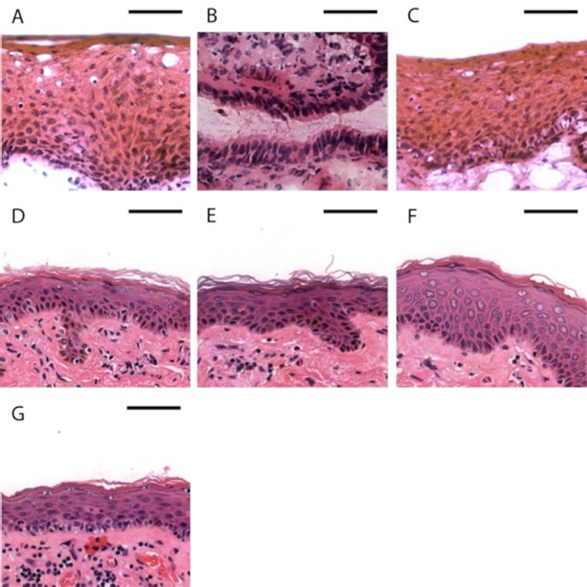
Basic structure of female and male genital tract epithelia. H&E stained genital tract epithelia from a female and uncircumcised male donor. Vaginal (A) and ectocervical (C) tissues are lined by stratified squamous epithelia. Endocervical epithelia (B) consist of single layers of columnar cells. Inner foreskin (D) and outer foreskin (E), shaft (F), and glans (G) surfaces are all composed of keratinized stratified squamous epithelia. Bar = 50 μm.
To further explore differences between the tissues, we examined the superficial strata in greater detail. We evaluated two proteins, filaggrin and involucrin (discussed further below), using immunofluorescent labeling of these proteins and epifluorescent microscopy (Fig. 2). Four cervical, two vaginal, four foreskin, and three penile donor specimens were stained for these two markers. Each donor specimen had three to five sections imaged and analyzed for epithelial protein expression, with an average area of 0.128 mm2 surveyed per sample. Each panel (field of view) was deconvolved and stitched together to create larger surveillance areas. In all female epithelia, we found only scant (≤1 layer per field of view) expression of filaggrin (Fig. 2, A–C). Involucrin expression was robust and could be seen throughout the stratum granulosum. This was consistent for all cervical and vaginal tissues examined. In contrast, male genital epithelia showed higher levels of filaggrin expression in the stratum corneum, with intra- and interindividual variation (as we have observed previously) [11]. Involucrin expression was consistent throughout both male and female genital epithelia.
FIG. 2.
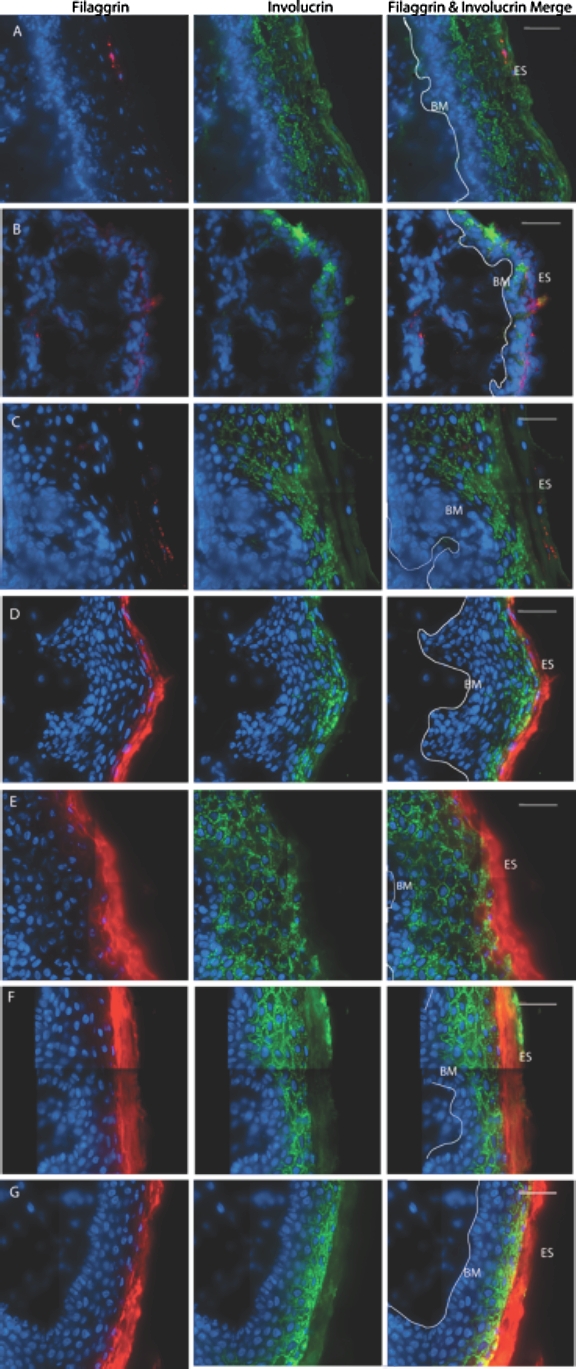
Superficial strata in genital tract epithelia. Deconvolved, stitched fluorescent images of human adult donor tissues. Filaggrin (red) and involucrin (green) proteins fluorescently labeled in ectocervical (A), endocervical (B), vaginal (C), outer foreskin (D), inner foreskin (E), shaft (F), and glans (G) tissues (from an uncircumcised donor). ES, epithelial surface; BM, basement membrane; white line, basal edge of stratum basale. Bar = 40 μm.
While the stratum corneum contributes greatly to the epithelial barrier function, intercellular proteins found in other strata also play key roles [5]. We chose to focus on two classes of ICJ proteins known to contribute to epithelial adhesion and stability: adherens junctions and desmosomes (discussed further below). We specifically examined desmogleins 1/2 and E-cadherin expression using immunofluorescence microscopy (Fig. 3). Seven cervical, two vaginal, seven foreskin, and three penile donor specimens were used in this analysis. We imaged three to five sections per donor sample, with an average survey area of 0.191 mm2 per specimen. There was intra- and interindividual heterogeneity, but both proteins of interest were expressed in all tissues examined. In both male and female genital tissues, E-cadherins were found throughout the stratum basale, spinosum, and granulosum. In the latter two strata, its expression overlapped that of desmogleins 1/2. In female squamous epithelia, dense layers of superficial epithelial cells were seen expressing only desmogleins at the surface, which were generally not observed in male tissues. We also found desmogleins 1/2 and E-cadherin expression in the endocervical simple columnar epithelium (Fig. 3B), though less so than in squamous epithelia.
FIG. 3.
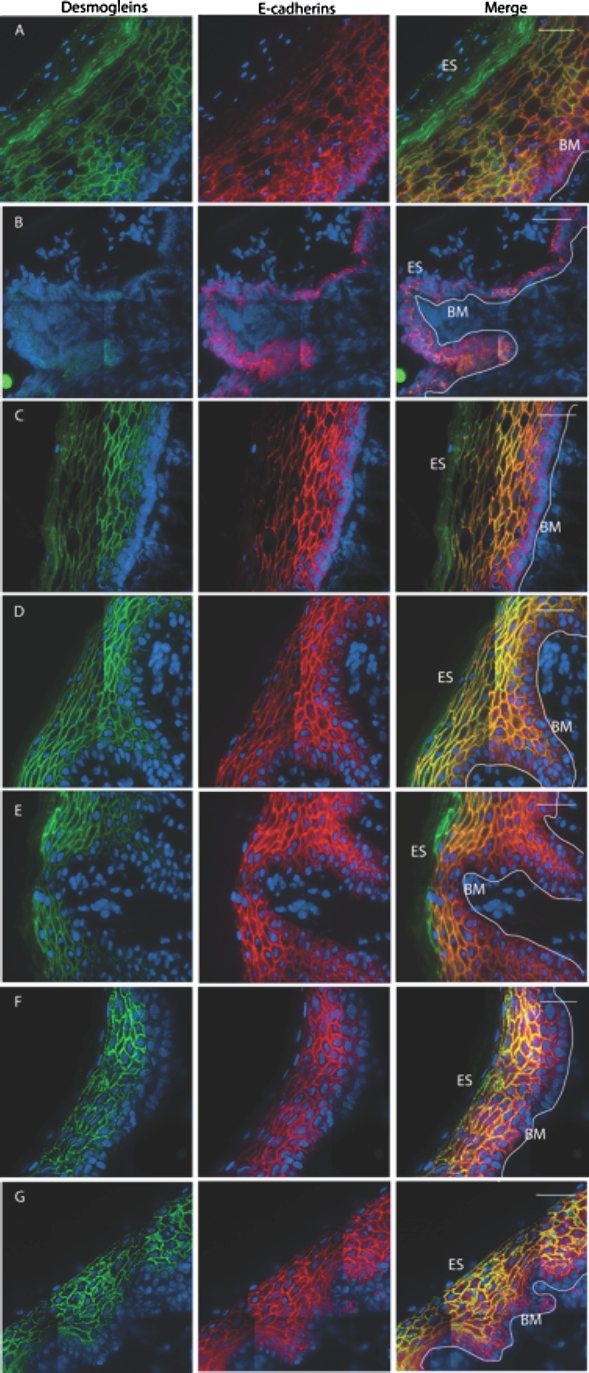
Intercellular junctions in genital tract epithelia. Deconvolved, stitched fluorescent images of human adult genital tissues. Desmogleins 1/2 (4B2; green) and E-cadherins (red) in the squamous epithelia of male and female genital tract tissues: ectocervical (A), endocervical (B), vaginal (C), outer foreskin (D), inner foreskin (E), shaft (F), and glans (G) tissues. ES, epithelial surface; BM, basement membrane; white line, basal edge of stratum basale. Bar = 40 μm.
Finally, we sought to examine the characteristics of the rhesus macaque female and male genital tracts. Five female and three male macaque genital tissue specimens were collected during necropsy. For each specimen, three to five separate sections were examined, with an average surface area of 0.437 mm2 for the stratum corneum analysis and 0.818 mm2 for the intercellular junction analysis. These tissues were processed and analyzed in the same way as we had done with the human tissues. We found that male and female macaque genital epithelia were very similar to that of their human counterparts in terms of structural protein expression (Fig. 4). There was only scant expression of filaggrin and robust expression of involucrin in the macaque female ectocervix, whereas the macaque male foreskin had high levels of both involucrin and filaggrin expression. We also found similar patterns of E-cadherin and desmoglein 1/2 expression in macaque and human genital tissues. One notable difference was between the outermost layers of human and native macaque female squamous epithelia, which were denser and more proliferative in the latter. In medroxyprogesterone (DMPA)-treated female macaques, these outer layers were substantially reduced (data not shown).
FIG. 4.
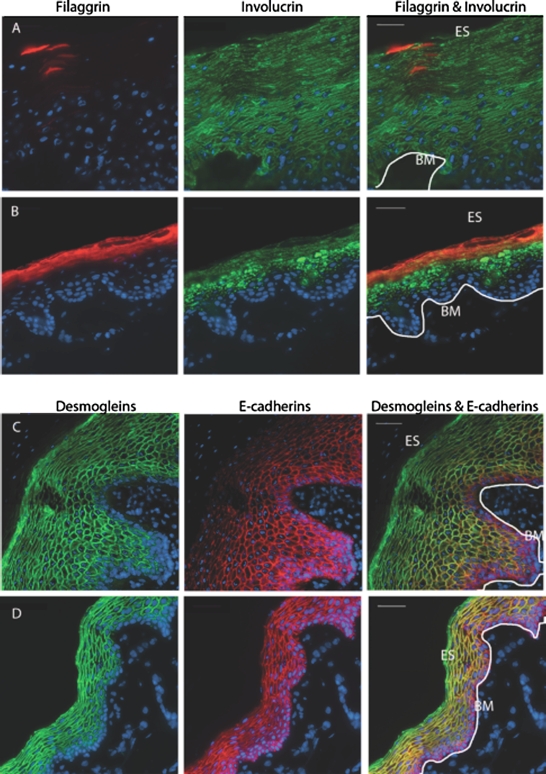
Keratinization and ICJs in macaque genital tissues. Deconvolved, stitched fluorescent images of macaque genital tissues. Ectocervical (A, C) and foreskin (B, D) tissue expression of filaggrin (red in A, B), involucrin (green in A, B), E-cadherins (red in C, D), and desmogleins 1/2 (green in C, D). ES, epithelial surface; BM, basement membrane; white line, basal edge of stratum basale. Bars = 40 μm.
DISCUSSION
Some differences between men and women are obvious, others are more subtle. While much of the basic male and female genital tract histology has been discussed in textbooks, their details, including similarities and differences, are often less well described. An incomplete understanding of these specifics can lead to incorrect assumptions about where transmission events take place. For example, a recent review in Nature Reviews Immunology equated inner foreskin epithelia with vaginal and ectocervical epithelia and stated that these areas were similarly susceptible to infectious pathogens [12]. While these areas arise from a common precursor during embryonic development and share many similarities, they also become distinct from one another in the absence or presence of certain androgens during development [1]. These distinctions may help explain why N. gonorrhea and C. trachomatis preferentially affect the uterus and cervix in the female and the prostate and urethra in the male. In contrast, shared characteristics may be why HSV-2 and Treponema pallidum (syphilis) appear commonly in all types of stratified squamous epithelia. By analyzing the expression of four unique epithelial proteins in this study, we characterized major similarities and potentially important differences between male and female genital epithelia.
The Stratum Corneum Differs Between the Male and Female Genital Epithelia
The stratum corneum plays a crucial role in the skin's barrier function, protecting underlying tissues and retaining vital nutrients [7, 13]. It consists of proteins, lipids, and enucleated, terminally differentiated epithelial cells. Among the most notable proteins found in this layer are loricrin, small proline-rich proteins, and filaggrin [14]. Filaggrin, or filament aggregation protein, is cleaved from its precursor protein in the stratum granulosum [15]. It is responsible for aggregating keratin filaments and flattening epithelial cells as they differentiate outward, creating the cornified layer of certain types of stratum corneum. Studies in knockout mice have shown that filaggrin is indispensible for the protective barrier function of the skin. In fact, the human skin diseases ichthyosis vulgaris and atopic dermatitis are both linked to genetic mutations leading to abnormal filaggrin expression. Involucrin is another structural protein expressed in the stratum granulosum, acting as a scaffold to which proteins and lipids bind as they form the outermost layers [7, 16]. In contrast to filaggrin, involucrin is found in almost all types of stratified squamous epithelia, including corneal and esophageal epithelium [16]. Transgenic mice expressing human involucrin and triple knockout mice (including involucrin) have also shown abnormal epidermal differentiation, desquamation, and barrier function [17, 18].
Our standard H&E stains of the genital epithelia showed subtle and known differences between the male and female stratum corneum. A detailed analysis with fluorescently labeled antibodies revealed that the female stratum corneum generally contains little to no filaggrin. In contrast, there was strong filaggrin expression in all aspects of the external male genital epithelia. Thus, the presence of filaggrin and a distinct cornified layer in the male tissues appear to be the important distinctions between the two sexes. Epidemiologic reports and studies have reported higher rates of C. trachomatis and human papillomavirus infections in women than men, suggesting that male-to-female transmission is more effective [19, 20]. Our observed differences may be one factor contributing to this greater susceptibility in women.
Other studies have also explored the role of keratinization in vaginal transmission: Marx et al. [21] demonstrated that progesterone treatment in rhesus macaques yielded thinner vaginal epithelia and greater rates of simian immunodeficiency virus (SIV) infection. Conversely, estrogen treatment enhanced vaginal keratinization in ovariectomized macaques, and this correlated with protection against SIV vaginal challenges [22, 23]. Mouse models have shown similar results when treated with hormones and challenged with HSV-2 [24]. However, studies with human subjects have failed to demonstrate significant changes in response to progesterone therapy [25–27]. Thus, the effects of hormonal therapy, keratin layers, and their relationship to one another are largely unknown in the context of STIs.
Beyond dermatologic diseases, filaggrin loss-of-function mutations have also been linked to peanut allergies [28]. The favored hypothesis is that a filaggrin-deficient upper gastrointestinal tract is more vulnerable to allergen penetration, leading to systemic sensitization and heightened immune responses upon reexposure to the allergen [29]. One could surmise that a similar response occurs in the filaggrin-deficient female genital tract. Supporting this hypothesis is the finding that triple involucrin knockout mice exhibited higher numbers of CD4+ T-cells in the epidermis than wild-type mice [18]. This interplay between epithelial structures and the immune system may be important in understanding the transmission of pathogens such as HIV, whose primary target is CD4+ T-cells [30].
Many Similarities Exist Between the Male and Female Genital Epithelia
Adherens junctions are integral ICJs and the first form to guide the subsequent formation of tight junctions and desmosomes [5]. Inhibition of E-cadherins (an adherens protein) in differentiating cells leads to hair loss, water loss, and even early death in mouse models [31, 32]. Desmosomes, another class of ICJs, are also important for cell-cell adhesions and are composed of cadherins, armadillo proteins, and plakins forming intercellular desmosomal plaques [31]. While adherens junction complexes interact with cellular actin, desmosomes interact with cellular intermediate filaments. Exfoliative toxin A, produced by Staphylococcus aureus, is known to specifically target desmoglein 1 (DSG1), and toxin-mediated degradation of DSG1 results in the blister formation seen in staph scalded-skin syndrome [6, 33].
In our analysis of E-cadherin and desmoglein 1/2 expression, we observed greater levels of desmogleins expression at the superficial-most layers of the female genital stratified squamous epithelia as compared to male genital epithelia. In a study by Elias et al. [34], desmoglein isoform ratios were altered in the skin to resemble levels seen in oral mucosal membranes. This resulted in mice with defective skin barrier function, dehydration, and ultimately death. We postulate that higher desmogleins expression in the female outermost strata is due to the lack of a cornified layer, which can only occur in protected areas such as the vaginal vault. These epithelia contain fewer proteases and undergo less desquamation than the more exposed male genital tissues. Given the fate of the mice observed by Elias et al. [34], it would appear that this represents another disadvantage to the female genital mucosa in defense against certain STIs.
Macaque Genital Tissues Are Similar to Human Genital Tissues
Numerous scientific studies have used nonhuman primates to conduct studies that would be difficult if not impossible to carry out in human subjects. In particular, rhesus macaques are often used to study HIV/SIV transmission and infection. Vaginal and penile inoculations with SIV in macaque models are used to mimic exposures seen in heterosexual transmission events in humans [35, 36]. By examining the above-mentioned protein markers in the macaque male penile and female vaginal and cervical tissues, we determined that the two species are histologically very similar in regard to genital epithelium. While both species exhibited intra- and interindividual variation, the stratum corneum of the female macaque sqaumous epithelium was generally thicker than that of the human female. These layers were diminished to levels equal to or less than human tissues in animals treated with DMPA (data not shown) [21]. However, the use of DMPA-treated animals in SIV transmission studies remains controversial (due to these histologic as well as potential immunologic changes) [37]. The male macaque model is not as well established, though it has shown limited success with penile SIV inoculations [36, 38].
One drawback to our study is that we do not yet know the physiologic implications of our observations. Parameters such as transepithelial water loss, pH, capacitance, and permeability would be important in determining functional differences in the epithelial barrier. Our group has previously shown that HIV-1 target cells in the inner foreskin are more sensitive to certain chemokines than the outer foreskin, likely due to differences in permeability [39]. Prospective studies coordinating the measurement of these parameters in vivo and analyzing structural characteristics ex vivo would be potentially insightful. In addition, we have not yet explored hormonal influences on these structural proteins, which is particularly relevant to female tissues. Since all tissues are de-identified prior to transfer to the lab repository, clinical information such as hormonal status is not readily available, though this may change with future studies.
We did not quantify expression levels of these markers and simply present here a qualitative assessment of the tissues' structural characteristics. There was also significant intra- and interindividual variation, so we were careful to only highlight observations that held true for all samples. Further studies detailing the level of expression, along with the inclusion of a wider range of protein markers such as loricrin and tight junctional proteins, will help delineate other important characteristics. Finally, we support further studies using larger sample sizes, though this is often limited by the lack of viable tissues available for analysis.
In this paper, we have illustrated and outlined histologic differences and similarities between the male and female genital tract. We hope that the information provided here will provide some of the fundamental knowledge needed to design and understand future clinical and basic science studies in this field.
ACKNOWLEDGMENT
The authors would like to thank the donors who have contributed tremendously to this and many other studies, as well as the Northwestern Departments of Obstetrics and Gynecology (John Lurain), Urology, and Surgical Pathology for their continued collaboration. We would also like to thank the veterinary staff at the Tulane National Primate Research Center for their invaluable support, as well as the contributions of IAVI, Ruth Ruprecht, and the Yerkes Primate Research Center. Finally, we acknowledge the tremendous assistance of Hanz Blatt, Spiro Getsios, and the Northwestern Skin Disease Research Center on this project and manuscript.
Footnotes
Supported by National Institutes of Health grants 5R33AI076968, 5K08HD060451-02, and U19 AI076981 and the Northwestern Institute for Women's Health Research Pioneer Award.
REFERENCES
- Gilbert SF. Developmental Biology. Sunderland, MA: Sinauer Associates; 2000: 201 250 [Google Scholar]
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Saunders Elsevier; 2009: 2201 2210 [Google Scholar]
- Robboy SJ, Bentley RC, Russell P. Blaustein's Pathology of the Female Genital Tract. New York, NY; Springer; 2001. [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009; 9: 679 691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 2007; 127: 2525 2532 [DOI] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol 2007; 127: 2499 2515 [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005; 6: 328 340 [DOI] [PubMed] [Google Scholar]
- Fu YS, Reagan JW. Pathology of the Uterine Cervix, Vagina, and Vulva. Philadelphia, PA: Saunders; 1989. [Google Scholar]
- Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol 2011; 65: 196 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice PA, Schachter J. Pathogenesis of pelvic inflammatory disease. What are the questions? JAMA 1991; 266: 2587 2593 [PubMed] [Google Scholar]
- Dinh MH, McRaven MD, Kelley Z, Penugonda S, Hope TJ. Keratinization of the adult male foreskin and implications for male circumcision. AIDS 2010; 24: 899 906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 2010; 10: 699 711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison KC. Barrier function of the skin: “la raison d'être” of the epidermis. J Invest Dermatol 2003; 121: 231 241 [DOI] [PubMed] [Google Scholar]
- Steven AC, Steinert PM. Protein composition of cornified cell envelopes of epidermal keratinocytes. J Cell Sci 1994; 107: 693 700 [PubMed] [Google Scholar]
- McGrath JA, Uitto J. The filaggrin story: novel insights into skin-barrier function and disease. Trends Mol Med 2008; 14: 20 27 [DOI] [PubMed] [Google Scholar]
- Banks-Schlegel S, Green H. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol 1981; 90: 732 737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djian P, Easley K, Green H. Targeted ablation of the murine involucrin gene. J Cell Biol 2000; 151: 381 388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla LM, Nachat R, Groot KR, Klement JF, Uitto J, Djian P, Määttä A, Watt FM. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J Cell Biol 2007; 179: 1599 1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2009. Atlanta, GA: US Department of Health and Human Services; 2010: 1 123 [Google Scholar]
- Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis 2009; 200: 1059 1067 [DOI] [PubMed] [Google Scholar]
- Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 1996; 2: 1084 1089 [DOI] [PubMed] [Google Scholar]
- Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis 2000; 182: 708 715 [DOI] [PubMed] [Google Scholar]
- Smith SM, Mefford M, Klase Z, Sodora D, Alexander N, Hess D, Marx PA. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS 2004; 18: 1637 1643 [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott M, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 1994; 70: 369 380 [PubMed] [Google Scholar]
- Mauck C. Overview of why hormones may be an issue. J Acquir Immune Defic Syndr 2005; 38: S11 S12 [DOI] [PubMed] [Google Scholar]
- Bahamondes L, Trevisan M, Andrade L, Marchi NM, Castro S, Díaz J, Faúndes A. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception 2000; 62: 23 27 [DOI] [PubMed] [Google Scholar]
- Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol 2000; 96: 431 439 [DOI] [PubMed] [Google Scholar]
- Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, Northstone K, Henderson J, Alizadehfar R, Ben-Shoshan M, Morgan K, Roberts G, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol 2011; 127: 661 667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Qualia CM, Baroody FM, Beck LA. Filaggrin expression in oral, nasal, and esophageal mucosa. J Invest Dermatol 2008; 128: 1594 1597 [DOI] [PubMed] [Google Scholar]
- Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect 2003; 5: 59 67 [DOI] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol 2004; 5: 271 281 [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 1988; 107: 1575 1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med 2000; 6: 1275 1277 [DOI] [PubMed] [Google Scholar]
- Elias PM, Matsuyoshi N, Wu H, Lin C, Wang ZH, Brown BE, Stanley JR. Desmoglein isoform distribution affects stratum corneum structure and function. J Cell Biol 2001; 153: 243 249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Gardner M, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol 2000; 74: 6087 6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WW, Rao SS, Lim SY, Zhang J, Hraber PT, Brassard LM, Luedemann C, Todd JP, Dodson A, Shen L, Buzby AP, Whitney JB, et al. TRIM5 modulates penile mucosal acquisition of simian immunodeficiency virus in rhesus monkeys. J Virol 2011; 85: 10389 10398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol 2002; 128: 10 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZM, Keele B, Qureshi H, Stone M, Desilva V, Fritts L, Lifson JD, Miller CJ. SIVmac251 is inefficiently transmitted to rhesus macaques by penile inoculation with a single SIVenv variant found in ramp-up phase plasma. AIDS Res Hum Retroviruses 2011; 27: 1259 1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach KM, Barry SM, Anderson M, Hope TJ. Enhanced cellular responses and environmental sampling within inner foreskin explants: implications for the foreskin's role in HIV transmission. Mucosal Immunol 2010; 3: 410 418 [DOI] [PMC free article] [PubMed] [Google Scholar]


