ABSTRACT
The requirement for vitamin A in reproduction and development was first determined from studies of nutritional deficiencies. Subsequent research has shown that embryonic development and both male and female reproduction are modulated by retinoic acid (RA), the active form of vitamin A. Because RA is active in multiple developmental systems, its synthesis, transport, and degradation are tightly regulated in different tissues. A growing body of evidence implicates RA as a requirement for the initiation of meiosis in both male and female mammals, resulting in a mechanistic model involving the interplay of RA, RA synthesis enzymes, RA receptors, and degradative cytochrome P450 enzymes in this system. Recently, that model has been challenged, prompting a review of the established paradigm. While it remains possible that additional molecules may be involved in regulating entry into meiosis, the weight of evidence supporting a key role for RA is incontrovertible.
Keywords: germ cells, meiosis, ovary, retinoic acid, retinoids, testis
This review summarizes the evidence that supports a role for retinoic acid in meiotic initiation in both sexes.
INTRODUCTION
Nearly a century ago, E.V. McCollum established that a fat-soluble micronutrient (“factor A”) was necessary to sustain life and prevent blindness in cows and rats [1]. Since that time, vitamin A has been found to have essential roles not only in vision but also in skin, bone, immune system, and reproductive health, as well as in many aspects of embryonic development. Because of the essential nature of this vitamin, all steps in its metabolism—including the absorption of precursors, storage of retinol esters, oxidation of these esters to the primary active metabolite retinoic acid (RA), and degradation of RA to inactive metabolites—are subject to tight biological controls and are protected by genetic redundancy. Retinoic acid commonly acts in a paracrine manner, with one cell type controlling the storage and oxidation of retinol (ROL), while a second cell type serves as the target for the action of RA [2]. In the signaling cells, conversion of ROL to RA requires two sequential oxidative steps, catalyzed by retinol or alcohol dehydrogenases and by retinaldehyde dehydrogenases (RALDHs), respectively. In the responding cells, RA serves as a ligand for two families of nuclear receptors, the RA receptors (RARs) and the retinoid X receptors (RXRs). The RA:RAR/RXR complex binds to RA response elements (RAREs) in target genes, recruiting corepressors or coactivators and thereby bringing about transcriptional changes. The level of RA present in a given tissue is finely tuned by the balance between its synthesis by RALDHs and its oxidative degradation by the following cytochrome P450 enzymes: cytochrome P450, family 26, subfamily a, polypeptide 1 (CYP26A1); cytochrome P450, family 26, subfamily b, polypeptide 1 (CYP26B1); and cytochrome P450, family 26, subfamily c, polypeptide 1 (CYP26C1) [2].
In mammals, the timing of germ cell entry into meiosis differs between the sexes. The first histological evidence that germ cells in the fetal mouse ovary have embarked upon meiosis I is seen at about 13.5 days postcoitum (dpc) [3]. The formation of meiotic chromosomal figures is preceded by expression of the premeiotic marker gene stimulated by RA gene 8 (Stra8) at about 12.5 dpc [4–6]. On the other hand, testicular germ cells do not enter meiosis during fetal life; in mice, they first express Stra8 shortly after birth, and expression peaks at about 10 days postpartum (dpp), coincident with the onset of meiosis I [7]. The upregulation of Stra8 is critical for both oogenesis and spermatogenesis: in the absence of Stra8 function, the progression of germ cells through meiosis in both the fetal ovary and the postnatal testis is blocked [4, 8, 9].
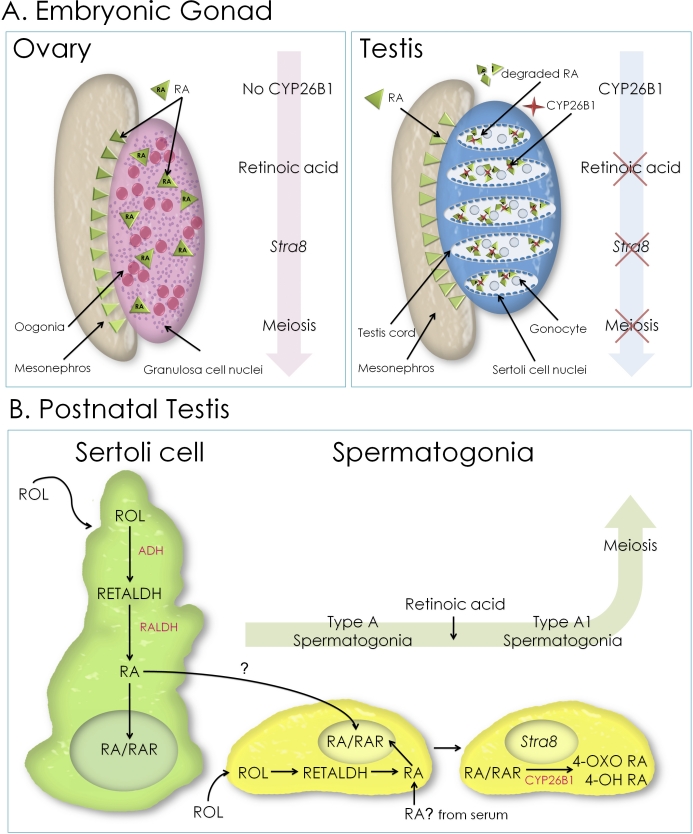
In recent years, independent investigations from numerous laboratories have yielded a large body of evidence demonstrating that RA triggers the onset of meiosis in both male and female mammals, including mice and rats (references detailed herein), as well as in other vertebrates, including chickens and amphibians [10, 11]. In humans, the role of RA in meiosis has been demonstrated only in the ovary [12, 13]. In the mouse, the organism most thoroughly studied thus far, the evidence suggests that RA, produced in the adjacent mesonephros, acts in the fetal ovary to trigger the onset of meiosis in germ cells. In the fetal testis, endogenous RA is evidently cleared by CYP26B1, ensuring that the onset of meiosis in male germ cells is delayed until after birth [5, 14, 15]. In the early postnatal and adult testis, RA is also required to upregulate Stra8 and sustain meiosis [14, 16]. As a result of these studies, the paradigm has become that the balance between RA synthesis and degradation in the developing reproductive system is required for appropriate control of the induction of Stra8 expression and hence the timing of meiotic initiation (Fig. 1).
FIG. 1.
The current paradigm for RA driving the onset of meiosis in males and females. A) In the embryonic ovary, RA (green triangles) generated in the mesonephros drives the expression of Stra8 and the onset of meiosis in the oogonia (pink). In the male embryo, RA is still produced in the mesonephros; however, the action of CYP26B1 (red stars), an RA-metabolizing enzyme that is made by Sertoli cells and testicular interstitial cells, acts to degrade this RA, thereby preventing Stra8 expression and the onset of meiosis. B) In the postnatal testis, there are several possible ways in which RA might be generated and delivered to spermatogonia. Retinol (ROL) may be internalized by either Sertoli cells or spermatogonia and converted first to retinaldehyde (RETALDH) and then to RA via a two-step process catalyzed by the alcohol dehydrogenases (ADH) and the aldehyde/retinaldehyde dehydrogenases (RALDH). Retinoic acid may also be delivered directly from the serum. Once inside the cell, RA can interact with RARs and stimulate transcription of a number of genes, including Stra8. The expression of Stra8 in spermatogonia, stimulated by RA, is coincident with the transition from undifferentiated type A spermatogonia to differentiated type A1 spermatogonia. Excess RA can be metabolized by the enzyme CYP26B1 into 4-oxo (OXO) and 4-hydroxy (OH) forms. These retinoid forms are then secreted from the cell.
Data from a recent study [17] have challenged this view. In that study, meiosis was examined in fetal ovaries null for either aldehyde dehydrogenase family 1, subfamily A2 (Aldh1a2) (also known as RALDH 2 [Raldh2]) alone or in combination with aldehyde dehydrogenase family 1, subfamily A3 (Aldh1a3) (also known as RALDH 3 [Raldh3]). Because Stra8 was still expressed and meiosis markers synaptonemal complex protein 3 (Scp3) transcript and H2A histone family, member X (γH2AFX) protein were still detectable, the authors concluded that meiosis had occurred normally, despite the absence of two major RA-synthesizing enzymes. These authors were unable to detect RA in the developing ovary using a transgenic RARE-LacZ reporter mouse line, nor were they able to demonstrate strong binding of RARs to Stra8 regulatory sequences. Curiously, they did find that the inhibition of CYP26B1 action in fetal testes led to the ectopic upregulation of Stra8 and that this occurred only when the mesonephros was present. The authors concluded 1) that RA is not present in the developing mouse ovary and is therefore not required for meiosis to begin in XX fetal germ cells and 2) that the role of CYP26B1 in the developing fetal testis is to degrade a nonretinoid meiosis-inducing factor that diffuses in from the mesonephros.
Clearly, these data do not fit comfortably with the established model for RA-driven control of germ cell entry into meiosis (Fig. 1) and force a reconsideration of available evidence. In this review, we will evaluate the data supporting the established role for RA as a molecular regulator of germ cell entry into meiosis and the new data underpinning the challenge proposed by Kumar et al. [17] in the hope of reconciling the apparently conflicting observations.
THE CASE FOR RA
Evidence for the involvement of RA in germ cell meiosis derives from observations and experiments in a number of species spanning the last decade. These investigations have led to seven major conclusions supporting the model shown in Figure 1, as discussed in the following sections.
Initiation of Meiosis I in Both Spermatogenesis and Oogenesis Is Blocked by Dietary Retinoid Deficiency
The effects of vitamin A deficiency or retinoid supplementation on spermatogenesis in rats and mice are well documented. Long-term vitamin A deprivation results in spermatogenic arrest at the spermatogonial A to A1 transition (undifferentiated to differentiated spermatogonia) or at the preleptotene spermatocyte stage in rats, and primarily at the A to A1 transition in mice [18–23]. When ROL is provided to vitamin A-deficient (VAD) rodents, meiosis is reinitiated promptly and synchronously [18, 20, 22]. Large doses of RA can also induce resumption of meiosis in this system, suggesting that RA, and not its precursor ROL, is the active factor [23]. Recent findings have extended our understanding of this phenomenon: ROL injection into VAD mice dramatically induced Stra8 expression over a 24-h period, indicating that ROL rescue in VAD rodents involves induction of expression of this critical premeiotic gene [14]. Retinoic acid is also required for initiation of meiosis during the first wave of mouse spermatogenesis. In postnatal male mice null for lecithin:retinol acyltransferase (Lrat), which are particularly susceptible to becoming VAD, dietary depletion of vitamin A resulted in loss of Stra8 expression, the accumulation of undifferentiated spermatogonia, and meiotic failure [24].
Li and Clagett-Dame [25] used VAD rats to study germ cells in the developing ovary. They observed that the majority of germ cells in ovaries from severely VAD embryos failed to induce Stra8, failed to enter meiosis, and remained undifferentiated. In addition, in a group of animals that was moderately deficient in RA, only about 30% of the oogonia entered meiosis compared with 75% in the controls. These in vivo experiments demonstrated a dose-dependent requirement for RA to initiate meiosis in the fetal gonad at the exact developmental point that the established paradigm predicts. Moreover, they demonstrated that RA is necessary, not just sufficient, to initiate meiosis.
RA Induces Expression of the Meiotic Gatekeeper Gene Stra8
Gene knockout studies have firmly established that expression of the premeiotic gene Stra8 is indispensable for entry into meiosis in the fetal ovary [4] and for the switch from mitosis to meiosis in the adult testis [8, 9]. When Stra8 was knocked out in mice on a pure C57BL/6 background, preleptotene germ cells did not enter meiosis [8]; however, if the knockout was done on a mixed background, some of the cells completed meiotic replication and entered meiosis but failed to complete prophase [9]. Stra8 was identified as an RA-responsive gene in P19 embryonal carcinoma cells: RA treatment leads to upregulation of Stra8 within 2 h [26, 27]. Indeed, the gene designation Stra8 is an acronym for “stimulated by RA gene 8.” Treatment of mouse fetal gonadal tissue or adult testis tissue with RA ex vivo also leads to upregulation of Stra8 [5, 7, 14, 28–30].
Meiosis Is Triggered by Exogenous Retinoids
An early indication that retinoids might be involved in meiotic initiation came from the observation that treatment of 14.5-dpc rat ovary explants with RA or an RARα-specific agonist accelerated entry into meiosis [31]. More recently, it has been demonstrated that exogenous RA can stimulate germ cells in the embryonic testis to enter meiosis, as determined by expression of bona fide meiotic markers Scp3, DMC1 dosage suppressor of mck1 homolog, meiosis-specific homologous recombination (yeast) (Dmc1), and γH2afx and by morphological analysis [5, 14, 15, 29]. Exogenous RA cannot rescue the loss of meiosis in Stra8-null fetal ovaries [14], implying that the role of RA is to induce Stra8 that then triggers chromatin condensation characteristic of meiotic prophase.
Several other studies had either found no effect of RA on meiosis or overlooked any possible effect. When Best and colleagues [32] cultured 11.5-dpc urogenital ridges with exogenous RA, they found that Stra8 was expressed but that only 1% of XY germ cells had entered meiosis. It remains unclear why the observations in that study differ from those by other groups. Other investigators looking at the effects of RA on rat testis development did not find that RA triggered meiosis, but they did not specifically look for such an effect [33–35]. Those studies found that RA treatment for 3 days induced dose-dependent apoptosis of the germ cells: such an observation is not at odds with the possibility that XY germ cells in the testis entered meiosis and were subsequently removed by apoptosis, a well-known phenomenon [15, 36–38].
Meiosis Is Triggered by Endogenous Retinoids
The effects of increasing endogenous RA levels have been tested in a number of studies in which the degradation of RA in mouse fetal testes has been inhibited using strategies aimed at blocking CYP26B1 activity. When CYP26B1 is inhibited pharmacologically using the potent but nonspecific cytochrome P450 inhibitor ketoconazole, resident germ cells of the fetal testis upregulate Stra8, Scp3, and Dmc1 and take on morphological features of meiotic germ cells [5, 14]. Although ketoconazole is capable of inhibiting P450 enzymes other than CYP26B1, it is clear that the action of ketoconazole in the induction of meiosis in this system relies on signaling through RARs: when ketoconazole and the RAR panantagonist BMS-204493 were used in tandem in ex vivo organ culture, meiosis was not induced [14]. The same conclusion was drawn when a more specific inhibitor of CYP26 enzymatic activity, R115866, was tested in parallel with the RAR panantagonist [14]. In the prepubertal testis, inhibition of CYP26 with R115866 has also been used to demonstrate an increase in differentiated spermatogonia (Stra8 and kit oncogene (Kit) positive) and a decrease in undifferentiated spermatogonia (POU domain, class 5, transcription factor 1 [Pou5f1] positive) [16]. Taken together, these studies strongly imply that induction of Stra8 and entry into meiosis require RAR signaling and that a CYP26 enzyme opposes this action.
More specific evidence for the importance of CYP26B1 comes from studies of Cyp26b1-knockout mice. These mice die immediately after birth of respiratory distress [15, 39]. In Cyp26b1-null fetal testes, germ cells strongly upregulated Stra8 and Scp3, and by 16.5 dpc some germ cells had progressed as far as the pachytene stage of meiosis I [5, 15]. Importantly, a threefold increased level of RA was detected, confirming that removal of Cyp26b1 had, as expected, allowed endogenous RA to build up in the tissue [15]. To demonstrate that the meiotic induction observed in the Cyp26b1-knockout mouse was the result of the presence of ectopic RA accumulation (rather than some other effect of the loss of Cyp26b1), wild-type fetal testes were treated with an RAR agonist (AM580) that is resistant to the actions of CYP26B1 [15]. As expected, XY germ cells in such testes promptly expressed Stra8 and entered meiosis. Even later in development, when XY germ cells in an embryonic testis have already entered mitotic arrest, they can be triggered to upregulate Stra8 and enter meiosis by ectopic exposure to endogenous RA. When Cyp26b1 was deleted specifically in Sertoli cells from about 15.5 dpc, Stra8 was induced at about 16.5 dpc, and XY germ cells entered meiosis [25].
Meiosis Is Inhibited by RAR Antagonists and Stimulated by RAR Agonists
If RA directly induces Stra8 expression and thereby triggers entry into meiosis, it is necessary that RARs are expressed by germ cells. This has been demonstrated for both fetal ovarian germ cells [5, 40, 41] and prepubertal/adult testis germ cells [40, 42, 43]. Furthermore, studies have shown that both Stra8 induction and the entry into meiosis can be inhibited in the embryonic ovary by the action of RAR antagonists. In two separate studies [5, 14], the RAR panantagonists BMS-204493 and AGN193109 were shown to inhibit Stra8, Scp3, and Dmc1 expression in 11.5-dpc mouse ovaries in culture. Conversely, Stra8 was induced in embryonic testes cultured with agonists specific for each of the three RARs, demonstrating that any one of the three RAR isotypes is capable of activating Stra8 expression in the developing gonad [14].
Meiosis Is Blocked by ALDH Inhibitors
Bis-(dichloroacetyl)-diamines (BDADs) are compounds that inhibit spermatogenesis. Recent investigations have demonstrated that the BDAD WIN 18,446 acts by inhibiting the biosynthesis of RA from ROL and, consequently, blocks the expression of Stra8 in embryonic gonads, neonatal and adult testes, and isolated 2-dpp germ cells [44]. Expression of Stra8 can be rescued by exogenous RA but not by ROL. In a complementary study [45], WIN 18,446 was shown to inhibit RALDH2, the major enzyme of RA biosynthesis, in vitro and in vivo, in rabbit testes. After treatment, intratesticular RA levels were decreased, spermatogenesis was impaired, and rabbits became infertile. The RALDH inhibitor citral also blocks meiosis in mouse and human fetal tissues [5, 13, 46].
RA Is Present at the Right Place and Time to Drive Meiosis
Given that germ cells enter meiosis in the fetal ovary but not in the fetal testis, one would expect to be able to detect the presence of RA in the ovary but not in the testis. Using the RARE-hsplacZ indicator mouse [47], RA was indeed detected in the developing mouse ovary [5]. Although β-galactosidase reporter staining in the developing ovaries was weak, no staining at all was observed in stage-matched fetal testes, in which Cyp26b1 is strongly expressed. Presumably, low levels of endogenous RA are sufficient to drive meiosis in the context of the fetal gonad.
The presence of RA detected by RARE-lacZ reporter mice does not reveal where the RA is actually made but rather its sites of action. To resolve this issue, gonads and mesonephroi were cultured on a lawn of F9 RARE reporter cells that respond to tissues that actively synthesize RA but not those that merely harbor RA. This assay was used to show that the mesonephros, but neither the ovary nor the testis, synthesizes RA [5]. This result was in agreement with the finding of strong Aldh1a2 expression in the mesonephros [5]. Thus, it was hypothesized that RA is produced in the mesonephric duct and tubules and that it diffuses into the adjacent gonad, triggering Stra8 expression and meiosis in resident germ cells. A recent study that seemingly challenges this hypothesis was published by Livera and colleagues [31]. They found that, when the mesonephros was removed from 11.5-dpc ovaries and those ovaries were then cultured, germ cells still enter meiosis. As the authors point out, however, this does not prove that RA is not involved in meiotic initiation, because it cannot be ruled out that some RA is produced within the fetal ovary itself [30]. In other words, this study challenges the source of RA but not the model that RA is the driver of entry into meiosis.
In recent investigations, Snyder et al. [16] used the same RARE-lacZ reporter mice to examine the role of RA in the initiation of meiosis during the first round of spermatogenesis in neonatal mice. They found that all germ cells expressing β-galactosidase, indicating response to endogenous RA, also expressed Stra8. When the neonates received injections of exogenous RA, the number of germ cells positive for RA activity and for Stra8 increased. Positive cells were predominantly differentiated premeiotic germ cells.
In summary, the combined weight of published data presents a strong case for the action of RA in triggering germ cell entry into meiosis. In particular, the block in meiotic progression in males and females as a result of dietary vitamin A insufficiency provides unequivocal evidence for the requirement for RA to complete this crucial biological process. Nonetheless, the recent findings by Kumar et al. [17] potentially cloud the issue.
MEIOSIS IN THE ABSENCE OF RA SIGNALING?
Somewhat surprisingly, given the case already outlined, Kumar et al. [17] found that germ cells in the fetal ovary could enter meiosis in Aldh1a2-knockout or Aldh1a2/Aldh1a3-double-knockout mice. They were unable to detect RA in the developing ovary using a transgenic RARE-LacZ reporter mouse line, nor were they able to demonstrate strong binding of RARs to Stra8 regulatory sequences. However, consistent with other studies, inhibition of CYP26B1 in fetal testes led to the ectopic upregulation of Stra8, albeit only when the mesonephros was present. Based on their observations, Kumar et al. do not dispute the central role of Stra8 in the onset of meiosis, nor do they disagree that CYP26B1 is key to the avoidance of meiosis in the fetal testis. They postulate that a substance, not a retinoid, diffuses into the gonad from the mesonephros and is degraded by CYP26B1. The suggestion that entry into meiosis is completely independent of RA is controversial and has attracted a great deal of interest in the field. Just how sound is the evidence that RA is not involved?
We suggest that alternative interpretations are possible for several of the observations by Kumar et al. [17]. First, Kumar et al. did not factor aldehyde dehydrogenase family 1, subfamily A1 (ALDH1A1) (also known as retinal dehydrogenase 1 [RALDH1]) into their system. Aldh1a1 is highly expressed in the fetal testis, and although expression is much lower in the fetal murine ovary, it is detectable via both quantitative RT-PCR and microarray analysis [48]. In the human embryonic ovary, all three RALDHs have been shown to be expressed, and it is postulated that ALDH1A1 may be the one that actually drives meiosis in that system [12, 13].
But if ALDH1A1 is present at any significant level in the embryonic ovary, why was no RA signaling activity detected in the study by Kumar et al. [17]? The RARE-hsplacZ reporter mouse line is notoriously prone to losing responder activity [49], and while it is clear that RA levels in the Aldh1a2-knockout and Aldh1a2/3-knockout mice are reduced relative to wild type, no quantitative evidence has been presented that RA is completely absent from these gonads. A negative result showing failure to find something is not the same as proving that something is not there. We suggest that, given that Aldh1a1 mRNA can be detected in the embryonic ovary, the action of this enzyme may generate enough RA to explain the results seen in the mutant mice in the study by Kumar et al.
Second, the mouse model used by Kumar et al. [17] relies on the supply of exogenous RA to pregnant mothers for a period around gastrulation to overcome lethality associated with RA deprivation during development. The necessity of deliberately adding a molecule when one is attempting to assay the consequences of the absence of that molecule complicates the experimental system, and the presence of residual RA at low levels, or the delayed effects on germ cells of early exposure to RA, cannot be completely discounted.
Third, Kumar et al. [17] performed chromatin immunoprecipitation (ChIP) on 13.5-dpc tissue from mesonephros and ovary to assay the ability of RARs to bind to the Stra8 promoter. They showed that the binding of all three RARs to the RARE present upstream of Stra8 was significantly less than the binding of these receptors to the RARE present in the RAR beta (Rarb) gene. However, the weakness of the ChIP signal could be due to the low representation of germ cells in the samples assayed, which comprised all mesonephric cells and somatic cells of the ovary in addition to the target germ cell population. We speculate that RARs might be shown to bind more convincingly if the starting material had consisted of purified germ cells; other studies [14, 28] clearly indicate that only germ cells, at a particular time during their development, are able to respond to RA by upregulating Stra8. We do not concur that the data by Kumar et al. convincingly establish that Stra8 is not a normal target of RA, but it is clear that further studies will be required to address this point.
Fourth, Kumar et al. [17] postulate that a meiosis-inducing substance diffuses into the gonad from the mesonephros and is degraded by CYP26B1. Given that CYP26B1 is not known to degrade nonretinoid compounds, and that these properties match exactly those established for RA, we suggest that the simplest explanation for the findings by Kumar et al. is that their observations are attributable to RA.
RECONCILING ESTABLISHED AND NEW FINDINGS
Skepticism regarding an established model is not a bad thing. Although it is possible to question some of the experimental findings that make up the body of evidence in support of the role of RA in driving entry into meiosis, we are unable to identify any issues that discount a role for RA completely. For example, it has been suggested that the induction of Stra8 and the initiation of meiosis by exogenous RA reported in various publications result from the “supra-physiological” concentrations used [17]. Most of the ex vivo organ culture studies have used concentrations of RA ranging from 0.7 to 1 μM RA. However, given that whole tissues were treated in these experiments, it is likely that target cells are exposed to lower concentrations of RA than those initially present in the media. Furthermore, at least three studies [7, 50, 51] have demonstrated that RA acts directly on isolated germ cells (i.e., not via Sertoli or any other gonadal somatic cell type) to trigger Stra8 expression. When individual target cells (i.e., purified germ cells) are studied in culture, far lower RA concentrations are required to trigger meiosis [5]. In these experiments, as little as 1 nM RA is sufficient to induce Stra8 expression; the effect is dose dependent, and the levels of RA required are in keeping with concentrations estimated to be present in embryonic tissues (10–40 nM in most target tissues and 11 nM in the adult murine testis) [47, 52].
It has also been suggested that the actual physiological role of RAR antagonists may be different from their reported functions because these compounds may exert nonspecific effects on other receptors [17], based on a known case involving the RAR antagonist Ro 41–5253 [53]. This is a fair point, although the two pan-RAR antagonists that have been shown to inhibit meiosis are BMS-204493 and AGN193109, not Ro 41–5253 [5, 14]. The same authors argue that commonly used RALDH inhibitors, including disulfiram compounds such as WIN 18,446 and citral, can also inhibit aldehyde dehydrogenases that have no relevance to retinoid metabolism. While this is possible, the reversal of the WIN 18,446 inhibition of Stra8 induction by RA but not ROL strongly indicates that the significant inhibitory action of WIN 18,446 on meiosis is on the RALDHs [44].
The identification of intrinsic factors and modifiers affecting meiosis entry is currently limited. Deleted in azoospermia-like (DAZL), an RNA-binding protein expressed in postmigratory germ cells, is one intrinsic factor that must be expressed if germ cells are to respond to RA by initiating meiosis [28]. Similarly, doublesex and mab-3 related transcription factor 1 (DMRT1) expressed in spermatogonia appears to restrict RA responsiveness and repress the transcription of Stra8, thereby opposing meiotic entry [54], while it seems to have the opposite role in the fetal ovary [55]. Clearly, the field has only just begun to understand the details of how the intrinsic and extrinsic factors that regulate meiosis are produced in or delivered to the embryonic ovary and postnatal testis and how signaling pathways are activated and gene expression and protein changes occur in germ cells in response to these factors.
The control of the onset of meiosis is obviously crucial for successful reproduction. The current paradigm shown in Figure 1 has been challenged by the findings by Kumar et al. [17], but there remains strong evidence for the action of RA in the induction of Stra8 and the onset of meiosis in both sexes. While there are ample experimental data supporting this paradigm, the entire story is likely incomplete. Retinoic acid is clearly necessary given the weight of evidence that deficiency of vitamin A or inhibition of RA action precludes normal meiotic behavior in both male and female germ cells. However, it may not be the only secreted inducer of meiosis. Future studies will determine if other inducers exist and how they may interact with RA action.
Footnotes
This review was supported by a Contraceptive Center Grant U54 42454 and by HD 10808 from the NIH to M.D.G.
REFERENCES
- Wolf G. Discovery of vitamin A. : eLS. Hoboken, NJ: John Wiley & Sons Ltd; 2001. [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008; 134: 921 931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol 2003; 262: 1 15 [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430 1434 [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596 600 [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 2003; 262: 303 312 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537 545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105: 14976 14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Feret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 2008; 121: 3233 3242 [DOI] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Bowles J, Koopman P, Sinclair AH. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev Biol 2008; 8: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallacides A, Chesnel A, Chardard D, Flament S, Dumond H. Evidence for a conserved role of retinoic acid in urodele amphibian meiosis onset. Dev Dyn 2009; 238: 1389 1398 [DOI] [PubMed] [Google Scholar]
- Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT. Retinoic acid signalling and the control of meiotic entry in the human fetal gonad. PLoS One 2011; 6: e20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouffant R, Guerquin MJ, Duquenne C, Frydman N, Coffigny H, Rouiller-Fabre V, Frydman R, Habert R, Livera G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum Reprod 2010; 25: 2579 2590 [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474 2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007; 148: 4560 4567 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod 2010; 83: 783 790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao X, Duester G. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun 2011; 2: 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci 1989; 564: 154 172 [DOI] [PubMed] [Google Scholar]
- Huang HF, Hembree WC. Spermatogenic response to vitamin A in vitamin A deficient rats. Biol Reprod 1979; 21: 891 904 [DOI] [PubMed] [Google Scholar]
- Morales C, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology 1987; 121: 432 434 [DOI] [PubMed] [Google Scholar]
- Thomson JN, Howell JM, Pitt GAJ, Vitamin A. and reproduction in rats. Proc R Soc Lond (Biol) 1964; 159: 510 535 [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod 1990; 43: 363 367 [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology 1991; 128: 697 704 [DOI] [PubMed] [Google Scholar]
- Li H, Palczewski K, Baehr W, Clagett-Dame M. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol Reprod 2011; 84: 336 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Clagett-Dame M. Vitamin A deficiency blocks the initiation of meiosis of germ cells in the developing rat ovary in vivo. Biol Reprod 2009; 81: 996 1001 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier JM, Schuhbaur B, Dolle P, Chambon P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev Biol 1995; 170: 420 433 [DOI] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol 1996; 135: 469 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 2008; 322: 1685 1687 [DOI] [PubMed] [Google Scholar]
- Trautmann E, Guerquin MJ, Duquenne C, Lahaye JB, Habert R, Livera G. Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle 2008; 7: 656 664 [DOI] [PubMed] [Google Scholar]
- Guerquin MJ, Duquenne C, Lahaye JB, Tourpin S, Habert R, Livera G. New testicular mechanisms involved in the prevention of fetal meiotic initiation in mice. Dev Biol 2010; 346: 320 330 [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Valla J, Habert R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Mol Cell Endocrinol 2000; 165: 225 231 [DOI] [PubMed] [Google Scholar]
- Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development 2008; 135: 1415 1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kim KH. Retinoic acid inhibits rat XY gonad development by blocking mesonephric cell migration and decreasing the number of gonocytes. Biol Reprod 2004; 70: 687 693 [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Durand P, Habert R. Multiple effects of retinoids on the development of Sertoli, germ, and Leydig cells of fetal and neonatal rat testis in culture. Biol Reprod 2000; 62: 1303 1314 [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Habert R. Retinoid receptors involved in the effects of retinoic acid on rat testis development. Biol Reprod 2001; 64: 1307 1314 [DOI] [PubMed] [Google Scholar]
- Bowles J, Koopman P. Sex determination in mammalian germ cells: extrinsic versus intrinsic factors. Reproduction 2010; 139: 943 958 [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol 1984; 38: 7 23 [PubMed] [Google Scholar]
- Yao HH, DiNapoli L, Capel B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 2003; 130: 5895 5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell 2004; 6: 411 422 [DOI] [PubMed] [Google Scholar]
- Boulogne B, Levacher C, Durand P, Habert R. Retinoic acid receptors and retinoid X receptors in the rat testis during fetal and postnatal development: immunolocalization and implication in the control of the number of gonocytes. Biol Reprod 1999; 61: 1548 1557 [DOI] [PubMed] [Google Scholar]
- Morita Y, Tilly JL. Segregation of retinoic acid effects on fetal ovarian germ cell mitosis versus apoptosis by requirement for new macromolecular synthesis. Endocrinology 1999; 140: 2696 2703 [DOI] [PubMed] [Google Scholar]
- Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: identification of potential heterodimeric receptors. Biol Reprod 1999; 61: 1300 1308 [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006; 147: 96 110 [DOI] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory J, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod 2011; 84: 957 965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amory JK, Muller CH, Shimshoni JA, Isoherranen N, Paik J, Moreb JS, Amory DW, Sr, Evanoff R, Goldstein AS, Griswold MD. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl 2011; 32: 111 119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietruszko R, Abriola DP, Izaguirre G, Kikonyogo A, Dryjanski M, Ambroziak W. Aldehyde inhibitors of aldehyde dehydrogenases. Adv Exp Med Biol 1999; 463: 79 87 [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev 1991; 5: 1333 1344 [DOI] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Knight D, Smith CA, Roeszler KN, Bagheri-Fam S, Harley VR, Sinclair AH, Koopman P. Male-specific expression of Aldh1a1 in mouse and chicken fetal testes: implications for retinoid balance in gonad development. Dev Dyn 2009; 238: 2073 2080 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Drager UC. Detection of retinoic acid catabolism with reporter systems and by in situ hybridization for CYP26 enzymes. Methods Mol Biol 2010; 652: 277 294 [DOI] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 2010; 19: 440 449 [DOI] [PubMed] [Google Scholar]
- Ohta K, Lin Y, Hogg N, Yamamoto M, Yamazaki Y. Direct effects of retinoic acid on entry of fetal male germ cells into meiosis in mice. Biol Reprod 2010; 83: 1056 1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton C, Maden M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev Dyn 1995; 202: 312 323 [DOI] [PubMed] [Google Scholar]
- Schupp M, Curtin JC, Kim RJ, Billin AN, Lazar MA. A widely used retinoic acid receptor antagonist induces peroxisome proliferator-activated receptor-gamma activity. Mol Pharmacol 2007; 71: 1251 1257 [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell 2010; 19: 612 624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol 2011; 356: 63 70 [DOI] [PMC free article] [PubMed] [Google Scholar]