ABSTRACT
Epithelial cells lining the male excurrent duct contribute to male fertility by employing a number of physiological mechanisms that generate a luminal microenvironment conducive to spermatozoa maturation and storage. Among these mechanisms, male duct epithelia establish intercellular tight junctions that constitute a barrier to paracellular diffusion of water, solutes, large molecules, and cells. Mechanisms regulating the male duct epithelial barrier remain unidentified. Transforming growth factor beta (TGFB) is a regulatory cytokine present in high concentrations in human semen. This study examined whether TGFB has any effects on epithelial function exhibited by primary cultures of porcine vas deferens epithelia. TGFB1 exposure caused a 70%–99% decrease in basal transepithelial electrical resistance (RTE, a sensitive indicator of barrier integrity), while a significant decrease in anion secretory response to forskolin was detected at the highest levels of TGFB1 exposure employed. SB431542, a selective TGFB receptor I (TGFBR1) inhibitor, prevented decreases in barrier function. Results also demonstrated that TGFB1 exposure modifies the distribution pattern of tight junction proteins occludin and claudin 7. TGFBR1 is localized at the apical border of the native porcine vas deferens epithelium. Pharmacological inhibition of mitogen-activated protein kinase (MAPK) 11 (also known as p38-MAPK) did not alter the effect of TGFB1 on RTE significantly. These data suggest that epithelia lining the vas deferens are subject to disruptions in the physical barrier if active TGFB becomes bioavailable in the luminal fluid, which might be expected to compromise fertility.
Keywords: cytokines, growth factors, male epithelial barrier, male reproductive tract, vas deferens
Epithelia lining the vas deferens respond to Transforming Growth Factor Beta 1 exposure with severe losses in transepithelial resistance and tight junction organization.
INTRODUCTION
Epithelia lining the male excurrent duct (i.e., efferent ducts, epididymis, and vas deferens) contribute to the generation of specific luminal fluid compositions that are required for sperm cell maturation and male fertility, as recently emphasized by studies demonstrating infertility or subfertility in human and animal models carrying certain genetic mutations [1–4]. In addition, transport mechanisms employed by these specialized epithelia to promote sperm cell maturation—regulation of luminal water content [5, 6], ion secretion and absorption [7], pH regulation [8, 9], and protein secretion [10]—would not be successful without an effective physical barrier that restricts permeability [11]. Epithelial cells lining the male excurrent duct establish tight junctions, which function as an anatomical physical barrier to the movement of water, solutes, and cells, such as defense cell types and spermatozoa [12, 13]. Moreover, the male excurrent duct epithelial barrier is thought to include an immune-protective component that prevents autoimmune responses to sperm cells as they transit, mature, or remain stored in the lumen of the excurrent system [13–15].
Transforming growth factor beta (TGFB) can induce pathological signaling in differentiated epithelial cells and lead to fundamental changes in cellular phenotype via epithelial-to-mesenchymal transformation (EMT). This process is at the causal core of life-threatening diseases, such as kidney and lung fibrosis [16–18]. Furthermore, TGFB receptor (TGFBR) 1 is colocalized with epithelial tight junctions, where it is structurally linked to occludin, a tight junction integral protein [19–21]. Importantly, activation of TGFBRs and receptor complex formation lead to rapid tight junction dissociations via a transcription-independent mechanism [19, 20].
The male reproductive tract synthesizes and secretes relatively high amounts of TGFB. While normal human plasma concentrations are reportedly 2–18 ng/ml [22, 23], TGFB concentrations in human semen have been reported at 85–238 ng/ml [24, 25]. TGFB exists as a latent or inactive form, and as an active form. Active TGFB1 can reach 2 ng/ml in human semen [24]. TGFB in semen is thought to exert an immune-regulatory effect on the female reproductive tract that contributes to spermatozoa survival and fertilization [26]. Additional participation of this signaling pathway in male fertility has been described with the TGFB1-null male mice kept alive via anti-inflammatory therapy [27]. These mice exhibit major hypothalamic-pituitary-gonadal dysfunction and are infertile [27].
The origin of TGFB in semen has not been determined with certainty. Importantly, high levels of TGFB immunoreactivity is present in rat epididymal epithelia [28], and coding sequences for TGFB isoforms and receptors are expressed in this tissue [29]. However, it remains unknown whether TGFB can regulate physiological parameters of male excurrent duct epithelia, and mechanisms and/or mediators of this signaling have not been identified in the male excurrent system. This study tested whether primary cultures of porcine vas deferens epithelial cells (1°PVDs) [30] respond to TGFB1 exposure with any significant changes in epithelial barrier function and/or ion transport. Results from initial experiments led us to conduct additional assays to test for the selectivity of the effect detected, and to identify a disruptive effect of TGFB signaling on vas deferens tight junctions.
MATERIALS AND METHODS
Tissue Acquisition and Epithelial Cell Isolation
Porcine vas deferens were surgically excised immediately postmortem from sexually mature boars at a local swine production facility, placed in ice-cold Hank buffered salt solution (137 mM NaCl, 5.4 mM KCl, 0.4 mM KH2PO4, 0.6 mM Na2HPO4, 5.5 mM glucose) and transported to the laboratory where isolation of epithelial cells was performed as described in detail previously [30]. Segments of porcine vas deferens and skeletal muscle were harvested onto cryogenic tubes and snap frozen in liquid nitrogen within seconds of excision and also transported to the laboratory, where they were kept at −80°C until further use.
Cell Culture
Isolated vas deferens epithelial cells were seeded on 25-cm2 tissue culture flasks and grown in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA) and 1% penicillin and streptomycin (Invitrogen), where they became confluent in 2–5 days. Subsequently, cells were lifted with PBS containing trypsin and ethylene-diaminetetraacetic acid (Invitrogen), suspended in medium, and seeded on 1.13-cm2 Snapwell permeable supports (Corning-Costar; Cambridge, MA) where they formed confluent monolayers. Paired monolayers from each boar were fed every other day for at least 10 days postseeding, and then assigned to experimental groups in a given assay. Exposure to active TGFB1 (R&D Systems, Minneapolis, MN) was achieved by its inclusion in the basal compartment medium, while SB431542 (Sigma, St. Louis, MO) and protein kinase inhibitor SKF86002 (Sigma) were included in both the basal and apical culture media. Monolayers exposed to both TGFB1 and SKF86002 were first exposed to SKF86002 for 30–60 min prior to receiving media that contained TGFB1 in addition to the inhibitor. NMuMG and Cos-7 cells were also seeded on permeable supports and cultured with the same medium formulation and feeding protocol described above. NMuMG cells were cultured as epithelial cell monolayers for 10 days before lysis and protein isolation, while Cos-7 cells were cultured until confluence (2–3 days) and then fixed.
Electrophysiology
Epithelial cell monolayers were mounted in modified Ussing flux chambers (model DCV9 [Navicyte, San Diego, CA] and model P2300 [Physiologic Instruments, San Diego, CA]), bathed symmetrically in Ringer solution (120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.83 mM K2HPO4, 1.2 mM CaCl2, 1.2 mM MgCl2), maintained at 39°C, and bubbled with 5% CO2/95% O2 to provide mixing and pH stability. Monolayers were clamped to 0 mV and short-circuit current (ISC) was measured continuously with a voltage-clamp apparatus (model 558C; Dept. of Bioengineering, University of Iowa, Iowa City, IA). Data were acquired digitally at 0.1–1.0 Hz with an Intel-based computer using an MP100A-CE interface and AcqKnowledge software (ver. 3.7.3; BIOPAC Systems, Santa Barbara, CA). Once recordings began, this system applied a 5-sec bipolar voltage pulse every 100 sec, and the resulting change in ISC was used to calculate transepithelial electrical resistance (RTE) via Ohm law. After at least 10 min of recording in basal conditions, monolayers were exposed to forskolin (EMD Chemicals, Gibbstown, NJ). The assay was terminated once ISC tracings became stable.
Immunohistochemistry and Confocal Microscopy
Monolayers were fixed in 4% paraformaldehyde for 1 h, washed in PBS five times, incubated twice in 0.2% Triton X-100 (Sigma) for 5 min, and blocked in 5% bovine serum albumin (BSA; Thermo Scientific, Rockford, IL) and 0.2% Triton X-100 for 1 h. Native tissues were snap frozen in liquid nitrogen minutes after dissection, blocked in freezing medium, and sectioned (CM3050S Cryostat; Leica Microsystems Inc., Buffalo Grove, IL). Sections were collected onto slides, fixed in 4% paraformaldehyde for 15 min, washed in PBS, and subject to the same subsequent procedure described above for monolayers, except that blocking with BSA was conducted overnight. Primary antibody incubations were conducted overnight at 4°C with the following specifications: anti-occludin (3 μg/ml; catalog no. 331500; Invitrogen), anti-claudin 7 (3 μg/ml; catalog no. 349100; Invitrogen), anti-TGFBR1 (10 μg/ml; catalog no. sc-398G; Santa Cruz Biotechnology), and anti-phosphorylated mitogen-activated protein kinase (MAPK) 11 (0.3 μg/ml; catalog no. 9215; Cell Signaling Technology). Secondary immunofluorescent labeling was conducted with Alexa 488 and/or 594 (2 μg/ml; Invitrogen) for 1 h. Finally, monolayers or tissue sections received a fluorescent nucleic acid stain (4′,6-diamidino-2-phenylindole; Invitrogen) and final washes prior to mounting on slides. Monolayers were positioned so that the permeable plastic support faced the slide and the apical cellular membranes faced the cover slip. Scanning confocal microscopy was performed (LSM 510 META and LSM 700; Carl Zeiss Microimaging Inc., Thornwood, NY). Fluorescent signals were acquired independently by different lasers and incorporated in each image. From monolayers, at least 10 different unique images were acquired and at least one stack of images was derived along the Z axis (apical-to-basolateral). Analysis of confocal microscopy images and Z-Stack projections were conducted with Zeiss LSM Image Browser (version 4.2.0.121; Carl Zeiss), then exported and compiled with Corel Draw X3 (version 13.0.0.739; Corel Corp., Ottawa, ON, Canada). Individual fluorescent signal intensity was measured using ZEN 2010 (version 6.0; Carl Zeiss).
Western Blot Analyses
Protein lysates were derived from 1°PVDs, NMuMG epithelial cell monolayers, native porcine vas deferens, and skeletal muscle tissues via direct homogenization and lysis in a Laemmli sample buffer containing 2% SDS, Tris-HCl (62.5 mM), and proteinase inhibitors. Segments of native porcine vas deferens, which had been stored at −80°C, were thawed and their lumens were flushed with PBS prior to homogenization. Following homogenization, samples were aspirated repeatedly through 25- or 30-gauge needles and centrifuged at 14 000 rpm for 15 min at 4°C. Protein concentration was determined by the micro BCA colorimetric assay (Pierce, Rockford, IL) and/or a spectrophotometer (ND 8000; NanoDrop Products, Wilmington, DE). Prior to electrophoresis, βmercaptoethanol, glycerin, and bromophenol-blue were added and samples incubated at 95°C for 5 min. Equal protein masses from each sample were resolved on 4%–18% Tris-Hepes-SDS gradient gels (Pierce). Blotting onto polyvinylidene fluoride membranes (Millipore, Billerica, MA) was conducted at 40 V for 2 h at 4°C. Membranes were blocked in 5% molecular-grade dry milk overnight at 4°C, and subsequently incubated overnight at 4°C with primary antibodies: anti-occludin (1 μg/ml; catalog no. 331500; Invitrogen), anti-claudin 7 (1 μg/ml; Invitrogen; catalog no. 349100), anti-TGFBR1 (10 μg/ml; catalog no. sc-398G; Santa Cruz Biotechnology), and anti-actin (1:1000 dilution; catalog no. A2066; Sigma). Incubations in peroxidase-conjugated secondary antibodies (Pierce) were conducted for 1 h at 20 ng/ml. Chemiluminescent signals were obtained with a suitable substrate (Pierce) and acquired digitally (Imagestation 4000R [Eastman Kodak Co., Rochester, NY] or FluorChem HD2 imager [Alpha Innotech, San Leandro, CA]). Measurements of band signal intensity were conducted with UN-SCAN IT Gel (version 6.1; Silk Scientific Inc., Orem, UT) and AlphaEase FC (version 6.0.0; Alpha Innotech).
Statistical Analysis
Paired t-tests and ANOVA were performed as appropriate. These tests and calculation of means and SEM were performed with Microsoft Excel (Office Suite 2007; Microsoft Corp., Redmond, WA). Graphs were made with SigmaPlot (version 10.0; Systat Software Inc., Point Richmond, CA).
RESULTS
TGFB1 Disrupts Transepithelial Resistance and Epithelial Transport Across Porcine Vas Deferens Epithelia
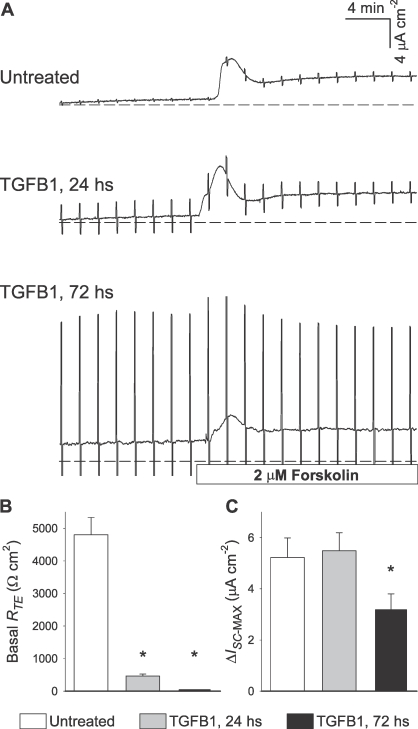
To test whether TGFB can induce changes in epithelial function in 1°PVDs, initial experiments employed paired monolayers that were either kept untreated or exposed to TGFB1 (100 ng/ml) for 24 and 72 h in conditions that have been reported previously [30]. Typical outcomes acquired with three monolayers of cells derived from a single vas deferens are depicted in Figure 1A. The magnitude of periodic bipolar pulses is inversely proportional to the monolayer's RTE. TGFB1-treated monolayers exhibited substantially reduced RTE; RTE was least in monolayers subject to the longest exposure. Overall, TGFB1 exposure for 24 h caused a 90% decrease in RTE, while 99% of basal RTE was ablated in 1°PVD monolayers exposed to TGFB1 for 72 h (Fig. 1B). Longer-term exposure significantly impaired forskolin-induced anion secretion across 1°PVDs (Fig. 1, A and C). Therefore, these initial outcomes suggested that TGFB1 induces a severe and time-dependent loss of barrier function in vas deferens epithelial cells, and that epithelial anion secretion can also be affected.
FIG. 1.
TGFB1 (100 ng/ml) causes time-dependent RTE decreases across primary cultures of porcine vas deferens epithelial cells (1°PVD). A) Vertical deflections in these Ussing chamber recordings correspond to preset electrical pulses (5 mV), and their magnitude is inversely proportional to RTE. Note that very small pulses are present throughout the untreated recording. Pulses shown defined that RTE at basal state was 4800, 400, and 50 Ohms · cm2 in untreated, 24 h, and 72 h, respectively. B) Basal RTE was significantly reduced after 24 and 72 h of exposure to TGFB1 (100 ng/ml). C) The ability to respond to intracellular cAMP generation (forskolin) with increases in ISC (indicating net anion secretion or cation absorption) was significantly reduced after 72-h exposure to TGFB1. Values are shown as mean ± SEM. *Statistically different from untreated (n = 6, P < 0.05).
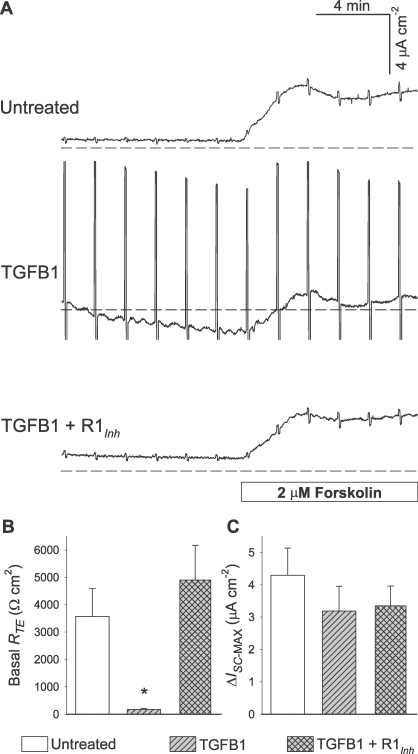
A subsequent set of assays was conducted to test for reproducibility of the TGFB1 effect on RTE when exposure is limited to 5 ng/ml for 24 h. Concurrently, we sought to determine whether this effect was linked to selective TGFBR activation. Consistent with the initial observations, TGFB1-treated 1°PVDs exhibited a large decrease (95%) in RTE (Fig. 2, A and B). SB43152, a selective TGFBR1 inhibitor, abrogated the TGFB1 effect on RTE. In fact, 1°PVDs exposed to both TGFB1 and TGFBR1 inhibitor had slightly greater RTE on average than untreated 1°PVDs, although no statistically significant difference was detected between these two groups. Mean forskolin-induced ISC changes were reduced by TGFB exposure, although statistically significant differences were not observed (Fig. 2, A and C). Collectively, results from these experiments provide compelling evidence that selective activation of TGFBRs in vas deferens epithelial cells leads to severe losses of barrier function, although the underlying mechanism is not fully defined.
FIG. 2.
A selective TGFBR1 inhibitor (R1Inh) abolishes the TGFB1 (5 ng/ml, 24 h) effect on RTE across 1°PVDs. A) ISC tracings depict the impact of 5-mV pulses on the three experimental groups, and reveal that RTE in 1°PVDs exposed to TGFB1 plus R1Inh SB431542 (10 μM) was similar to that in untreated 1°PVDs. B) Summary of observations reveal that monolayers exposed to TGFB1 only had a significant RTE decrease, and monolayers exposed to TGFB1 plus R1Inh exhibited mean basal RTE that was greater than untreated, although not significantly different. C) Forskolin-induced anion secretion is not significantly altered across experimental groups. Values are shown as mean ± SEM. *Statistically different from the other two groups (n = 5, P < 0.05).
TGFB1 Induces Disruptions in Tight Junction Protein Immunoreactivity in Porcine Vas Deferens Epithelial Cells
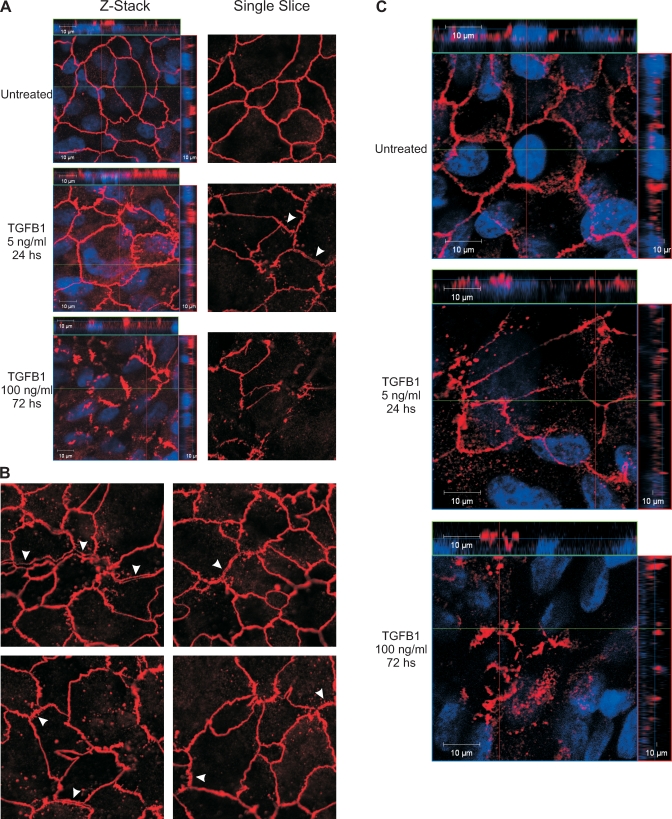
TGFBR1 localization has been shown to be restricted to tight junctions where it has direct protein-protein interactions with occludin, and binding of TGFB to TGFBRs reportedly leads to the formation of a phosphorylated receptor complex that induces tight junction dissociations [19–21]. Thus, we hypothesized that changes in occludin immunoreactivity might be associated with TGFB1-induced decreases of RTE in 1°PVDs, and tested this via scanning confocal microscopy. Severe disruptions of occludin immunoreactivity were detected in 1°PVDs exposed to 100 ng/ml for 72 h in that the typical reticular pattern was unrecognizable and largely absent (Fig. 3A). Only incomplete and irregular occludin strands were detected. At first examination, monolayers exposed to 5 ng/ml for 24 h appeared to preserve the reticular pattern, but abnormalities were clearly present under close examination. Punctate occludin immunoreactivity that was localized away from tight junction strands and largely distributed below the apical region (as seen in Z-stack projection, Fig. 3A) was consistently detected in this experimental group. In addition, a number of occludin strands were apparently dissociated in a manner that seemed to have generated hemistrands that pulled apart and remained with each of the neighboring cells (Fig. 3, A and B). Based on these results, it was hypothesized that other integral tight junction proteins are similarly affected by TGFB1 exposure. Among the claudins expressed in more distal portions of the male excurrent duct, claudin 7 is one of the most abundant [12]. Therefore, the immunoreactivity pattern of claudin 7 was also tested. Outcomes from these experiments suggest that severe disruptions in the reticular pattern of claudin 7 occur after exposure to TGFB1 exposure (Fig. 3C).
FIG. 3.
TGFB1 exposure induces disruptions in tight junction protein organization in vas deferens epithelia. A) Untreated 1°PVD monolayers exhibit a uniform reticular occludin immunoreactivity pattern (red) that is localized apically, above nuclei (blue), as seen in orthogonal projections displayed as banners located at the top and right of each of the left column images. Severe disruption of the reticular occludin immunoreactivity pattern is present in monolayers exposed to TGFB for 72 h (100 ng/ml), while exposure for 24 h (5 ng/ml) induced abnormalities, such as punctuate distribution and strand irregularity. Arrowheads indicate locations where occludin strands were apparently dissociated onto two hemistrands. Single images (second column in A) are derived from a plane where occludin immunoreactivity was most abundant. B) Additional single Z-slice images derived from 1°PVDs exposed to 5 ng/ml for 24 h show length-wise tight junction dissociations (arrowheads). C) Claudin 7 immunoreactivity (red) is also affected by TGFB1 exposure in a similar pattern as that of occludin. Results shown are representative of five paired observations (cells isolated from five boars).
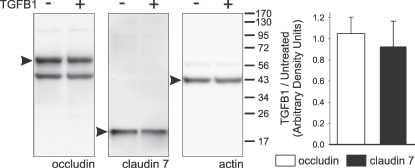
The possibility that TGFB1 exposure and signaling induce cellular changes that result in decreases in the abundance of tight junction proteins in 1°PVDs was tested. Results from Western blot analyses targeting occludin and claudin 7, and respective densitometry, indicate that no detectable changes in abundance of these proteins occur after 72-h exposure (Fig. 4).
FIG. 4.
TGFB1 exposure (100 ng/ml for 72 h) does not alter occludin or claudin 7 abundances in whole-cell lysates derived from 1°PVD monolayers. Western blot membranes probed with antibodies targeting occludin (65 kDa), claudin 7 (22 kDa), and actin (42 kDa) revealed bands of expected mobilities (arrowheads) that had no discernable intensity differences. Digitized images were subject to densitometry, and the summarized outcome (bar chart) supports that no changes in the abundances of these proteins occur after TGFB1 exposure. Results shown are representative of six paired observations (cells isolated from six boars). Values are shown as mean ± SEM.
Together with the results from the functional assays, these outcomes suggest that, if TGFB1 becomes bioavailable to receptors at vas deferens epithelial cells, it will increase permeability across this epithelium by inducing tight junction dissociations.
TGFBR1 Is Expressed in Apparent High Abundance in Native Vas Deferens Epithelium
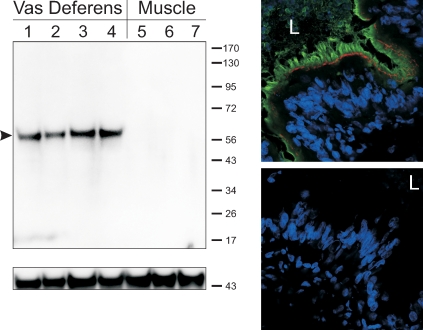
To test for the presence and localization of the TGFBR1 in native vas deferens, Western blot and immunohistochemistry coupled to confocal microscopy were conducted. Prominent single bands were evident in lanes loaded with whole vas deferens tissue lysates (Fig. 5). Lanes loaded with 1°PVD cell lysates or lysate derived from the NMuMG cell line also exhibited the equivalent bands, but were much fainter (data not shown). A distinct immunochemical signal was also detected on transverse sections of porcine vas deferens. TGFBR1 immunoreactivity was present in virtually all principal cells of the native epithelium, and was localized at the apical border of the epithelium (Fig. 5).
FIG. 5.
TGFBR1 is expressed apically in native vas deferens epithelium. Western blot analyses (top left) revealed a single band of expected mobility (predicted 56 kDa porcine protein, arrowhead) on lanes loaded with vas deferens tissue lysates (lanes 1–4). No bands were detected on lanes loaded with tissue lysates derived from skeletal muscle (lanes 5–7). Actin immunoreactivity was employed as a loading control (bottom left). Protein lysates were derived from different boars (n = 4 vasa deferentia and 3 muscle samples). Results from dual-labeling immunohistochemical analyses (top right) demonstrate that TGFBR1 immunoreactivity (green) is localized at the apical border of the native epithelium. By employing occludin immunoreactivity (red) as a reference, these results suggest that the TGFBR1 is localized largely at the epithelial brush border. No immunoreactive signals are detected if primary antibodies are subtracted from the preparation (bottom right). The 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei are seen in blue. L, lumen. n = 3 (tissues derived from three different boars).
These results suggest that TGFB signaling can occur in the native vas deferens epithelium and potentially lead to the effects observed in cultured vas deferens epithelial cells.
MAPK11 Inhibition Has No Significant Effect on TGFB1-Induced Decreases in Transepithelial Resistance Across Vas Deferens Epithelial Cells
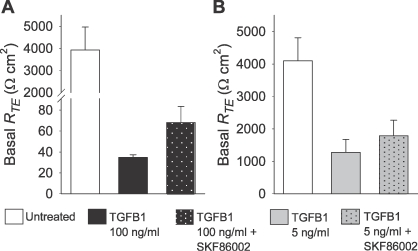
Studies addressing TGFB signal transduction in Sertoli cell primary cultures have demonstrated that MAPK11 (also known as p38-MAPK) has a prominent role in mediating this signaling [31]. Thus, we tested whether inhibition of this kinase could prevent TGFB1-induced decreases in RTE across 1°PVDs. Consistent again with initial experiments, TGFB1-exposed 1°PVDs (100 ng/ml for 72 h) exhibited a near total loss of RTE. Monolayers exposed to a combination of TGFB1 and the MAPK11 inhibitor SKF86002 (10 μM) also exhibited drastic decreases of RTE (Fig. 6A). Although SKF86002-treated monolayers had RTE means that were approximately twice that of 1°PVDs exposed to TGFB1 alone, the increment observed with the MAPK11 inhibitor only approached statistical significance (P < 0.08). A subsequent set of assays was conducted based on similar experimental design, but TGFB1 exposure was restricted to 5 ng/ml for 24 h. TGFB1-treated 1°PVDs had 70% less RTE when compared with untreated monolayers (Fig. 6B). TGFB1 plus SKF86002-treated monolayers again, on average, had RTE that remained greater than that of TGFB1 only, but their differences were not statistically significant (P < 0.06). To test if 10 μM SKF86002 induced significant MAPK11 inhibition, we conducted an assay in which Cos-7 cells were cultured in the presence or absence of anisomycin (25 μg/ml) and SKF86002 (10 μM). Localization and abundance of phosphorylated MAPK11 (pMAPK11) were then accessed via immunofluorescence detection and scanning confocal microscopy, which employed a primary antibody known to be selective for pMAPK11 [32]. Untreated Cos-7 cells exhibited no discernable pMAPK11 immunoreactivity, while anisomycin-treated cells presented marked nuclear pMAPK11 immunoreactivity, and anysomycin plus SKF86002-treated cells had significantly less pMAPK11 immunoreactivity than anisomycin-treated cells (data not shown).
FIG. 6.
Pharmacological inhibition of MAPK11 does not prevent TGFB1-induced decreases in RTE across 1°PVDs. A) RTE is severely reduced after 72 h of exposure to TGFB1 (100 ng/ml) and SKF86002 (10 μM). B) MAPK11 inhibition by SKF86002 (10 μM) also fails to prevent RTE decreases after 24 h of exposure in which TGFB1 concentration was 5 ng/ml. Values are shown as mean ± SEM (n = 6 in A, and 11 in B).
Collectively, these outcomes suggest that MAPK11 has a marginal role in the TGFB signaling events leading to decreases in RTE across vas deferens epithelial cells. MAPK11 seems to be expressed in 1°PVDs, and to be phosphorylated downstream from TGFBRs activation, but its effect in promoting tight junction disruption is suggestively secondary.
DISCUSSION
The goal of this study was to determine what effects, if any, TGFB exerts on epithelia lining the vas deferens. Outcomes reported here support a scenario in which activation of TGFBRs expressed in vas deferens epithelia leads to a severe loss in electrical barrier resistivity, while anion secretion can also be affected. Furthermore, losses in barrier function were associated with disruptions in epithelial tight junction protein organization and/or localization. These data are novel, and demonstrate that a better understanding of the relationship between TGFB signaling and the physiology of cells lining the male excurrent duct is needed. Moreover, these data and the known fibrogenic effects of TGFB [17, 33] suggest that this signaling pathway may participate in the etiology of human diseases of the male reproductive duct, such as epididymal and vas deferens obstructions and congenital bilateral absence of the vas deferens. It is also thought that breaches in the male barrier can promote the synthesis of anti-sperm antibodies, which are arguably responsible for poor spermatozoa motility and male infertility [34].
Our results show that high levels of barrier resistivity exhibited by cultured vas deferens epithelia are virtually eliminated via selective TGFBR activation. RTE was decreased over time and, by 72 h, it was nearly undetectable. Such severe loss of functional parameters is consistent with TGFB-induced EMT [18, 33]. Although these data reveal a high degree of vulnerability to the effects of this growth factor, it remains to be determined the magnitude of in vivo TGFB signaling in vas deferens epithelia and in epithelial cells lining other segments of the male excurrent duct in either physiological or pathological conditions. It is likely that, in a physiological context, the effective concentration of active TGFB is less than in the conditions employed in these assays. It is possible that, in vivo and under physiological conditions, the concentration of active TGFB is low enough to have minimal effects on barrier resistivity. Under physiological conditions, TGFB signaling is known to induce an inhibitory effect on tissue growth, therefore generating a local tumor-suppressive effect rather than a tumor- or fibrosis-promoting effect [35, 36]. However, epithelial tissues can develop resistance to TGFB's growth-inhibitory effect and switch to a state in which TGFB promotes pathological EMT and tumor formation and/or metastasis [37]. Tumors of primary growth in the vas deferens or epididymis are extremely rare [38, 39], and this is consistent with the possibility that signaling pathways may exist in these tissues to prevent abnormal tissue growth. Moreover, signaling by TGFB is known to induce an anti-inflammatory effect [40]. Thus, it is possible that this signaling reduces inflammatory responses to spermatozoa present in the excurrent duct luminal environment [26, 33]. Nevertheless, the results presented in this study suggest that the potential exists for induction of pathological processes by activation of the TGFB signaling pathway in vas deferens epithelia.
Immunohistochemical results suggest that TGFBR activation leads to disruptions in epithelial barrier organization and the localization of proteins that form tight junctions, which could account for the reduction in RTE. While greater concentration and longer duration of exposure revealed profound loss of occludin and claudin 7 from tight junctions, immunoreactivity of these proteins were likewise altered in 1°PVDs exposed to 5 ng/ml for 24 h, which was also accompanied by substantial losses in barrier function (70%–95%) measured electrically. Abnormalities in occludin immunoreactivity included hemistrands detected along the apical aspect of neighboring cells. This outcome seems to suggest that a mechanism for dissociation of tight junctions in vas deferens epithelia begins with a length-wise dissociation of the tight junction strand, which ‘unzips' the junctional complexes. Corroborating this possibility are reports indicating that TGFBR1 is structurally linked to occludin at tight junctions, and that TGFBR binding and phosphorylation lead to tight junction dissociations [19–21]. Moreover, the degree of disruption in occludin and claudin 7 organization was proportional to the level of TGFB exposure, suggesting that increases in local availability of active TGFB in vivo would cause increasing levels of disruption to the vas deferens epithelial barrier. Immunohistochemical results obtained with the claudin 7 antibody revealed more background immunoreactivity, and the signal from tight junction strands was less sharp than that obtained with the anti-occludin. This may have contributed to the lack of visualization of the length-wise tight junction strand dissociation that was typically detected with the anti-occludin antibodies.
Outcomes reported here also reveal that TGFBR1 immunoreactivity is markedly present in the apical border of the native porcine vas deferens epithelium. This supports the concept that these epithelia are subject to endogenous signaling if TGFB becomes bioavailable at the luminal fluid. No definite evidence is yet available on whether vas deferens epithelial cells synthesize and secrete TGFB, but immunohistochemical data are available to support that epididymal epithelia do [28]. Moreover, local activation of TGFB is a key regulatory mechanism for signaling [41, 42] and, interestingly, epididymal epithelia express proteases that activate TGFB [43, 44]. The luminal environment of the male excurrent duct also presents segmental pH variability, which, in segments such as the cauda epididymis, reaches fairly acidic levels. In the healthy duct, one would expect duct luminal pH levels that are more alkaline than those required to activate latent TGFB [45]. However, in pathologies such as cystic fibrosis, there may be increased TGFB activation as bicarbonate secretion is thought to be impaired.
MAPK11 mediates changes in barrier function induced by TGFB3 in Sertoli cells [31] and was shown to mediate TGFB signaling independently in other cell systems [46]. In addition, fundamental barrier function differences exist between Sertoli cell primary cultures and 1°PVDs: RTE levels are in the order of 60 [31] and >4000 Ohms/cm2, respectively. Nevertheless, in Sertoli cell primary cultures, MAPK11 inhibition prevented TGFB3-induced RTE decreases by 50% [31]. In 1°PVDs, MAPK11 inhibition reduced the effect of TGFB1 on RTE by 18%, and RTE values were not significantly rescued from a statistical perspective. These outcomes suggest that TGFB1-induced RTE decrease in vas deferens epithelial cells is largely independent of MAPK11. These results, considered together with the apparent tight junction dissociation effect, seem to suggest that a transcription-independent mechanism, as was shown to occur in the NMuMG cell line [19], may account for the extensive barrier function decreases detected in 1°PVD exposed to TGFB1. As demonstrated in NMuMG cells, receptor complex formation at tight junctions and subsequent Par-6 phosphorylation induces binding of the E3 ubiquitin ligase SMURF1. This leads to ubiquination and degradation of RHOA, and tight junction dissociations [19]. It remains an open question whether this mechanism is present in epithelia lining the male excurrent duct.
In summary, this study demonstrates that TGFBR activation in vas deferens epithelia can impair epithelial integrity and function. Specifically, the epithelial barrier to small solute movement becomes virtually absent. This is expected to compromise generation and maintenance of a luminal microenvironment conducive to spermatozoa maturation and male fertility.
ACKNOWLEDGMENT
The authors extend sincere thanks to Henrys Ltd. for making tissues available for this study, to the K-State COBRE Confocal Core Facility (RR017686), to Mr. Jimmie Stewart III (Kansas State University) for technical assistance, to Dr. Anna Zolkiewska (Kansas State University) for the NMuMG cells, and to Dr. Eric J. Sorscher (University of Alabama at Birmingham) for contributions to this study.
Footnotes
Supported by National Institutes of Health grants HD058398 and RR017686; this manuscript represents contribution #11-280-J from the Kansas Agricultural Experiment Station.
REFERENCES
- Hoglund P, Hihnala S, Kujala M, Tiitinen A, Dunkel L, Holmberg C. Disruption of the SLC26A3-mediated anion transport is associated with male subfertility. Fertil Steril 2006; 85: 232 235 [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. Embo J 2006; 25: 4131 4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven K, Messer L, van der Ven H, Jeyendran RS, Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod 1996; 11: 513 517 [DOI] [PubMed] [Google Scholar]
- Traystman MD, Schulte NA, MacDonald M, Anderson JR, Sanger WG. Mutation analysis for cystic fibrosis to determine carrier status in 167 sperm donors from the Nebraska Genetic Semen Bank. Hum Mutat 1994; 4: 271 275 [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature 1997; 390: 509 512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Pietrement C, Brown D, Breton S. Segmental and cellular expression of aquaporins in the male excurrent duct. Biochim Biophys Acta 2006; 1758: 1025 1033 [DOI] [PubMed] [Google Scholar]
- Carlin RW, Lee JH, Marcus DC, Schultz BD. Adenosine stimulates anion secretion across cultured and native adult human vas deferens epithelia. Biol Reprod 2003; 68: 1027 1034 [DOI] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 2008; 135: 1108 1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Alves F, Duncan CL, Lillich JD, Schultz BD. Porcine vas deferens luminal pH is acutely increased by systemic xylazine administration. Biol Reprod 2010; 82: 132 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntin P, Dacheux F, Druart X, Gatti JL, Okamura N, Dacheux JL. Characterization and identification of proteins secreted in the various regions of the adult boar epididymis. Biol Reprod 1996; 55: 956 974 [DOI] [PubMed] [Google Scholar]
- Hinton BT, Howards SS. Permeability characteristics of the epithelium in the rat caput epididymidis. J Reprod Fertil 1981; 63: 95 99 [DOI] [PubMed] [Google Scholar]
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Androl 2007; 9: 463 475 [DOI] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 2011; 84: 851 858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee RA, Hickey DK, Beagley KW, Jones RC. Transport of IgG across the blood-luminal barrier of the male reproductive tract of the rat and the effect of estradiol administration on reabsorption of fluid and IgG by the epididymal ducts. Biol Reprod 2005; 73: 688 694 [DOI] [PubMed] [Google Scholar]
- Itoh M, Chen XH, Takeuchi Y, Miki T. Morphological demonstration of the immune privilege in the testis using adjuvants: tissue responses of male reproductive organs in mice injected with Bordetella pertussigens. Arch Histol Cytol 1995; 58: 575 579 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang X, Li Y, Liu Y. Downregulation of Smad transcriptional corepressors SnoN and Ski in the fibrotic kidney: an amplification mechanism for TGF-beta1 signaling. J Am Soc Nephrol 2003; 14: 3167 3177 [DOI] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004; 15: 1 12 [DOI] [PubMed] [Google Scholar]
- Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci 2008; 121: 1036 1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 2005; 307: 1603 1609 [DOI] [PubMed] [Google Scholar]
- Bose R, Wrana JL. Regulation of Par6 by extracellular signals. Curr Opin Cell Biol 2006; 18: 206 212 [DOI] [PubMed] [Google Scholar]
- Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, Luga V, Przulj N, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science 2005; 307: 1621 1625 [DOI] [PubMed] [Google Scholar]
- Stadnicki A, Machnik G, Klimacka-Nawrot E, Wolanska-Karut A, Labuzek K. Transforming growth factor-beta1 and its receptors in patients with ulcerative colitis. Int Immunopharmacol 2009; 9: 761 766 [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Heathcote K, Chiano M, Snieder H, Kemp PR, Metcalfe JC, Carter ND, Spector TD. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet 1999; 8: 93 97 [DOI] [PubMed] [Google Scholar]
- Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod 2007; 22: 2928 2935 [DOI] [PubMed] [Google Scholar]
- Nocera M, Chu TM. Transforming growth factor beta as an immunosuppressive protein in human seminal plasma. Am J Reprod Immunol 1993; 30: 1 8 [DOI] [PubMed] [Google Scholar]
- Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta—a mediator of immune deviation in seminal plasma. J Reprod Immunol 2002; 57: 109 128 [DOI] [PubMed] [Google Scholar]
- Ingman WV, Robertson SA. Transforming growth factor-beta1 null mutation causes infertility in male mice associated with testosterone deficiency and sexual dysfunction. Endocrinology 2007; 148: 4032 4043 [DOI] [PubMed] [Google Scholar]
- Desai KV, Flanders KC, Kondaiah P. Expression of transforming growth factor-beta isoforms in the rat male accessory sex organs and epididymis. Cell Tissue Res 1998; 294: 271 277 [DOI] [PubMed] [Google Scholar]
- Henderson NA, Cooke GM, Robaire B. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5alpha-reductase inhibition. J Endocrinol 2006; 190: 779 791 [DOI] [PubMed] [Google Scholar]
- Sedlacek RL, Carlin RW, Singh AK, Schultz BD. Neurotransmitter-stimulated ion transport by cultured porcine vas deferens epithelium. Am J Physiol Renal Physiol 2001; 281: F557 F570 [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 2003; 68: 1597 1612 [DOI] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol 2006; 177: 7579 7587 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871 890 [DOI] [PubMed] [Google Scholar]
- Samplaski MK, Agarwal A, Sharma R, Sabanegh E. New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol 2010; 17: 839 847 [DOI] [PubMed] [Google Scholar]
- Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M, Hill CS, Beug H, Downward J. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev 2000; 14: 2610 2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003; 3: 807 821 [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442 454 [DOI] [PubMed] [Google Scholar]
- Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP, King BF., Jr Imaging of the seminal vesicle and vas deferens. Radiographics 2009; 29: 1105 1121 [DOI] [PubMed] [Google Scholar]
- Bisceglia M, Dor DB, Carosi I, Vairo M, Pasquinelli G. Paratesticular mesothelioma: report of a case with comprehensive review of literature. Adv Anat Pathol 2010; 17: 53 70 [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992; 359: 693 699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol 2008; 40: 1068 1078 [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem 2005; 280: 7409 7412 [DOI] [PubMed] [Google Scholar]
- Monsees TK, Blocher S, Loddo C, Steger K, Schill WB. Tissue kallikrein and bradykinin B2 receptors in the reproductive tract of the male rat. Andrologia 2003; 35: 24 31 [DOI] [PubMed] [Google Scholar]
- Emami N, Diamandis EP. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF beta 1 in semen. Biol Chem 2010; 391: 85 95 [DOI] [PubMed] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol 1988; 106: 1659 1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J 2002; 21: 3749 3759 [DOI] [PMC free article] [PubMed] [Google Scholar]