ABSTRACT
The embryonic origins of ovarian granulosa cells have been a subject of debate for decades. By tamoxifen-induced lineage tracing of Foxl2-expressing cells, we show that descendants of the bipotential supporting cell precursors in the early gonad contribute granulosa cells to a specific population of follicles in the medulla of the ovary that begin to grow immediately after birth. These precursor cells arise from the proliferative ovarian surface epithelium and enter mitotic arrest prior to upregulating Foxl2. Granulosa cells that populate the cortical primordial follicles activated in adult life derive from the surface epithelium perinatally, and enter mitotic arrest at that stage. Ingression from the surface epithelium dropped to undetectable levels by Postnatal Day 7, when most surviving oocytes were individually encapsulated by granulosa cells. These findings add complexity to the standard model of sex determination in which the Sertoli and granulosa cells of the adult testis and ovary directly stem from the supporting cell precursors of the bipotential gonad.
Keywords: Foxl2, granulosa cell, mitotic arrest, ovarian surface epithelium, ovary
Granulosa cells arise from mitotically arrested supporting cell precursors and the ovarian surface epithelium during fetal and perinatal mouse development.
INTRODUCTION
Granulosa cells play a critical role in supporting the growth and development of oocytes in immature and adult ovarian follicles. When a follicle is activated, the handful of granulosa cells surrounding the oocyte undergoes at least 10 clonal divisions to produce the more than 2000 cells of the mature antral follicle [1]. Some primordial follicles begin to grow shortly after assembly, but most lie dormant until they are activated during adult estrus cycles. The embryonic origin of granulosa cells is still not entirely clear. In mice, granulosa cells and Sertoli cells, the analogous supporting cell type in the testis, are thought to stem from a common progenitor population present in the bipotential gonad before the commitment to ovary or testis fate occurs [2, 3]. Sex determination in mammals is governed by an early fate decision in this cell population [2]. In the presence of a Y chromosome, these cells express the male sex-determining gene Sry, adopt Sertoli cell fate, and instruct the subsequent development of the testis. Although it is believed that these bipotential precursor cells give rise to the granulosa cell population in adult XX individuals, this theory has not been confirmed.
Ultrastructural studies of diverse mammalian ovary models originally posited three potential sources of granulosa cells: the aforementioned supporting cell precursors in the bipotential gonad, the ovarian surface epithelium, and the rete ovarii at the ovary-mesonephros border (reviewed in [4, 5]). The first two cell types are likely directly related, as a previous cell labeling study showed that at least some of the supporting cell precursors in the early male gonad derive from the surface epithelium [6]. No study has definitively shown that cells actively enter the ovary from the rete ovarii. It is possible that granulosa cells derive from more than one of these populations or that their origins differ between species. In rodents, although perhaps not in all mammals, it is thought that a restricted population specified during development accounts for all of the granulosa cells in the definitive pool of primordial follicles that is assembled shortly after birth [1, 7]. This model would be parallel to the situation in males, where pre-Sertoli cells specified during fetal life populate testis cords and give rise to the entire population of adult Sertoli cells [8].
One line of evidence that granulosa and Sertoli cells arise from a common bipotential progenitor came from the observation that granulosa cells can transdifferentiate into Sertoli-like cells and vice versa in several different rodent models, most of which involved a loss of oocytes or estrogen deficiency [2, 9–11]. A second piece of evidence for the common origin hypothesis was provided by Albrecht and Eicher [3], who generated a transgenic mouse line in which EGFP was driven by a partial Sry promoter (Sry-EGFP) [3]. In XY transgenic embryos, the EGFP expression pattern resembled endogenous Sry expression, but XX gonads also expressed EGFP in a subpopulation of somatic cells, indicating that these cells were competent to activate the Sry promoter. Based on these results, the authors proposed that cells capable of activating the Sry promoter represent a common bipotential precursor population for the supporting cell lineage from which both Sertoli and granulosa cells are derived. They noted that a few granulosa cells retained reporter activity perinatally [3], but it was unclear whether these cells were the direct descendants of the earlier EGFP-expressing precursors or had activated the promoter de novo. To address this issue, Ito et al. [12] traced the developmental outcome of XX cells that activated an Sry-Cre (constructed with different promoter regions), and detected positive reporter activity 2 wk after birth in a small subset of granulosa cells, but no other cell types [12]. Although these results are more definitive, the fact that only a few granulosa cells were labeled left open the possibility that other cell types contribute to the granulosa cell population in the adult ovary before or after birth.
Foxl2 is a forkhead transcription factor expressed in somatic cells of the early XX gonad [13]. In goats, a female-to-male sex-reversal phenotype associated with polled intersex syndrome has been attributed to misregulation of FOXL2 expression [14, 15]. These findings led to the speculation that FOXL2 is an ovary-determining gene, parallel to SRY in males. However, in humans, mutations in FOXL2 do not lead to sex reversal, but rather to blepharophimosis-ptosis-epicanthus inversus syndrome, which results in eyelid malformations and premature ovarian failure [16]. Foxl2 is likewise not required for the initial determination of ovarian fate in mice, as null mutants do develop ovaries [13, 17], but these mutant ovaries upregulate components of the testis pathway during late embryonic development [18], and postnatal follicle activation is severely impaired [13, 17]. Moreover, deletion of Foxl2 in the adult mouse ovary led to a loss of granulosa cell identity and transdifferentiation of granulosa cells into Sertoli-like cells [19, 20].
Here we investigated the relationship between the early supporting cell lineage in the bipotential gonad and the postnatal granulosa cell population. Using the Foxl2tm1(GFP/cre/ERT2)Pzg (Foxl2-GCE) mouse line to lineage trace the fate of the early supporting cell precursors, we show that these cells specifically contribute to the population of follicles activated immediately after birth. In contrast, those primordial follicles that grow during adult life are comprised of granulosa cells specified long after sex determination, just prior to and throughout the postnatal follicle assembly period.
MATERIALS AND METHODS
Mice
The Tg(Sry-EGFP)92Ei transgenic line, in which EGFP expression is driven by a 5′ fragment of the Sry promoter, was kindly provided by K.H. Albrecht and E.M. Eicher, and maintained on the C57BL/6 background. Gt(ROSA)26Sortm1Sor (R26R [21]) mice were maintained on the C57BL/6 background. The Tg(Acta2-EYFP) transgenic mouse line, in which EYFP expression is regulated by a fragment of the αSma promoter, was provided by J. Lessard (Children's Hospital Medical Center, Cincinnati, OH). The Foxl2tm1(GFP/cre/ERT2)Pzg strain (Foxl2-GCE), which carries a GFP-CreERT2 cassette knocked into the Foxl2 locus, was constructed by the GUDMAP consortium and maintained on a C57BL/6 background [22, 23]. Outbred CD1 animals were used to establish the time course of cell cycle arrest and for 5-bromo-2′-deoxyuridine (BrdU) and MitoTracker lineage-tracing experiments. All mice were housed in accordance with National Institutes of Health guidelines, and experiments were conducted with the approval of the Duke University Medical Center Institutional Animal Care and Use Committee.
To lineage trace Foxl2-expressing cells throughout development, male mice heterozygous for the Foxl2-GCE allele were crossed with females carrying the R26R reporter, and pregnant females were injected intraperitoneally with 2 mg tamoxifen (20 mg/ml) per 40 g body weight at Embryonic Day (E) 12.5 or E14.5. Embryos were allowed to develop to E14.5 or Postnatal Day (P) 7, P9, or P14 before dissection. At the indicated stages, pregnant females and pups were euthanized, and gonads were carefully removed and fixed for 30–45 min at room temperature or overnight at 4°C.
MitoTracker Labeling
Gonads were dissected from embryos/pups at stages E11.5–E14.5, P1, P3, or P7 and cultured in grooves cut in 1.5% agar blocks. The blocks were placed in 35-mm culture dishes and bathed in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS) and 50 μg/ml ampicillin. MitoTracker Orange CMTMRos (Invitrogen) was diluted in culture medium to a final concentration of 1 μM and then applied to the gonadal surface with a pipette. The dye was washed off after 30 min at 37°C, and samples were cultured for 2–96 h at 37°C with 5% CO2, and then fixed in 4% paraformaldehyde for 45 min at room temperature.
BrdU Tracing and Quantitation
Pregnant females were injected intraperitoneally with 1.5 mg BrdU (Sigma) dissolved in 7 mM NaOH/PBS at stages E11.5–E14.5. Pups were subcutaneously injected at P1 or P4 with 50 μg BrdU/g body weight. At 2 h postinjection, embryos/pups were either dissected or injected with excess thymidine (25 mg) and allowed to develop for 24–48 h (embryos) or 3–6 days (pups) before dissection. Gonads were fixed in 30% 50 mM glycine/70% ethanol or 4% paraformaldehyde for 1 h at room temperature. Samples were washed once in PBS, treated with 2 M HCl for 30 min at room temperature, washed again, and then subjected to immunocytochemistry as described below.
To estimate the proportion of BrdU/FOXL2 double-positive cells in the total FOXL2-positive population, gonads were immunostained with antibodies against BrdU and FOXL2 and imaged at 40× magnification on an LSM710 Meta confocal microscope (Carl Zeiss, Inc.). Images (two to three per sample) were taken near the center of each gonad, with two to four gonads imaged per time point. For each image, the numbers of FOXL2-positive and BrdU/FOXL2 double-positive cells were carefully counted in Adobe Photoshop and expressed as a percentage. For each injection stage, we compared the proportions of BrdU/FOXL2 double-positive cells after 24- and 48-h traces using a two-tailed Student t-test. The data are presented as the mean ± SEM.
Immunocytochemistry
Embryonic tissues were processed for whole-mount immunocytochemistry as follows. Fixed gonads were washed twice in PBS with 0.01% Tween-20 and incubated for 1 h at room temperature in a blocking solution consisting of 10% FBS, 3% bovine serum albumin (BSA), and 0.2% Triton-X-100 in PBS. Samples were then incubated with primary antibodies (listed below) in fresh blocking solution overnight at 4°C. The next day, samples were washed three times for 30 min each in a washing solution made with 1% FBS, 3% BSA, and 0.2% Triton-X-100 in PBS and then blocked for 1 h before secondary antibodies (listed below) were applied. Samples were again incubated overnight at 4°C, washed three times the next day, and mounted in DABCO. Gonads were imaged in the longitudinal plane with an LSM710 Meta confocal microscope and the affiliated Zen software (Carl Zeiss, Inc.).
Postnatal samples were transferred through a sucrose gradient, embedded in OCT, and cryosectioned at 20–25 μm. The sections were then rehydrated in PBS, blocked for 1 h, incubated with primary antibodies overnight at 4°C, washed three times for 10 min each, incubated with secondary antibodies for 1 h at room temperature, washed three more times, and then mounted in DABCO and imaged as above.
The primary antibodies used in this analysis included anti-GFP (Aves lab GFP1020, 1:500), anti-FOXL2 (goat anti-FOXL2 [NB100-1277; 1:200; Novus Biologicals] or rabbit anti-FOXL2 [1:500; a kind gift from D. Wilhelm, University of Queensland]), anti-β-galactosidase (55976; 1:10 000; MP Biomedicals), anti-Ki67 (MKI67) (RM-9106-S; 1:500; NeoMarkers), anti-p27 (sc-528; 1:500; Santa Cruz Biotechnology), phospho-Histone H3 (9710S; 1:500; Cell Signaling), anti-BrdU (OBT0030G; 1:200; Accurate Chemical), and anti-laminin (1:500; a kind gift from H. Erickson, Duke University). Primary antibody staining was revealed by Cy3 donkey anti-rabbit, DyLight 488 donkey anti-chicken, Cy3 donkey anti-rat (Jackson Immunoresearch), Alexa Fluor 488 donkey anti-rat, and Alexa Fluor 647 donkey anti-goat (Invitrogen) secondary antibodies. Nuclei were stained with syto13 (Invitrogen) as needed.
RESULTS
FOXL2 Marks a Granulosa Cell Precursor Lineage in the Fetal Ovary
In the mouse, FOXL2 protein expression is first apparent around E11.5 in a small number of somatic cells near the ovary/mesonephros boundary [24]. At later embryonic stages, FOXL2 is strongly expressed in the somatic cells lining germ cell nests, weakly expressed in the interstitial cells surrounding the ovarian vasculature, and excluded from the surface epithelium. In the early postnatal ovary, FOXL2 expression is apparent in both granulosa and interstitial cells, although it declines in the latter population with age [13, 25, 26].
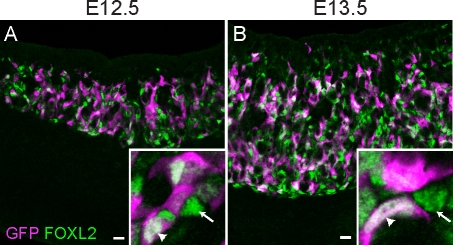
To determine whether FOXL2 specifically marks the descendants of the bipotential supporting cell precursors in the early mouse ovary, we immunostained XX Sry-EGFP transgenic gonads with a FOXL2 antibody and assessed colocalization at E12.5 and E13.5. Nearly all EGFP-positive cells colabeled with FOXL2 (Fig. 1), indicating that XX cells capable of activating the Sry promoter upregulate Foxl2 after committing to the ovarian pathway. However, only a subset of the FOXL2-positive cells expressed EGFP, indicating possible heterogeneity in transgene expression or in the origins or differentiation status of FOXL2-positive cells.
FIG. 1.
FOXL2 is expressed by supporting cell precursors that are competent to express Sry. Ovaries from E12.5 (A) and E13.5 (B) XX Sry-EGFP transgenic embryos immunostained with antibodies against FOXL2 (green, nuclear) and EGFP (magenta, nuclear and cytoplasmic) show clear overlap (white) between the two markers (arrowheads in insets), although some FOXL2-positive cells were not EGFP positive (arrows in insets). Original magnification ×20; bars = 20 μm.
We then extended our analysis forward in developmental time to determine whether this early population of Foxl2-expressing cells specifically gives rise to granulosa cells in the postnatal ovary or whether it contributes to multiple cell types. To lineage trace these cells, male mice expressing GFP-CreERT2 under the endogenous Foxl2 promoter [22, 23] were crossed to females carrying the R26R reporter [21], and pregnant females were injected with tamoxifen at E12.5. Embryos were allowed to develop to E14.5 or P14 before dissection. The E14.5 XX Foxl2-GCE; R26R samples confirmed the specificity of Cre activation in FOXL2-positive cells and also revealed that the GFP-CreERT2 fusion protein was expressed in most, but not all, cells labeled by the FOXL2 antibody at E14.5 (Supplemental Fig. S1 [all Supplemental Data are available online at www.biolreprod.org]).
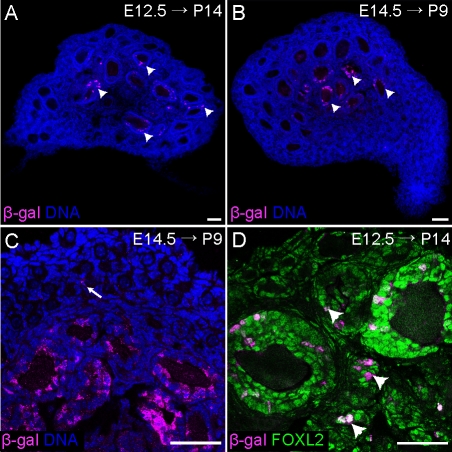
Ovaries from P14 Foxl2-GCE; R26R animals were stained with an antibody against β-galactosidase to visualize the postnatal distribution of lineage-traced cells (Fig. 2A). As predicted, the vast majority of cells expressing β-galactosidase were granulosa cells; a few β-galactosidase-positive cells could not be definitively identified and might have been stromal cells. Notably, β-galactosidase staining was not observed in the nascent theca cells surrounding each growing follicle (Fig. 2). Collectively, these results validate FOXL2 as a good marker of granulosa cell precursors in the embryonic ovary as well as the descendants of the bipotential supporting cell precursors (Figs. 1 and 2).
FIG. 2.
Foxl2-expressing cells in the fetal ovary give rise to granulosa cells in medullary follicles. A–C) Foxl2-GCE; R26R mice were exposed to tamoxifen at E12.5 and E14.5, dissected at P14 (A) or P9 (B and C), and stained with an antibody against β-galactosidase (magenta). Positive staining was nearly exclusive to granulosa cells in the large follicles in the medulla of the ovary. Arrow in C indicates a very rare lineage-traced granulosa cell in a primordial follicle. D) High-magnification image of a P14 Foxl2-GCE; R26R ovary from a mouse exposed to tamoxifen at E12.5, stained with antibodies against β-galactosidase (magenta) and FOXL2 (green). Lineage-labeled cells were observed in large secondary follicles as well as smaller primary follicles, but not primordial follicles. Arrowheads indicate growing follicles containing lineage-labeled cells that were sectioned through the edge rather than the middle of the follicle. Nuclei (blue) were stained with syto13. Original magnification ×10 (A and B) or ×40 (C and D); bars = 50 μm.
Surprisingly, lineage-traced granulosa cells were only observed in the activated (growing) follicles in the center of the ovary, not in the primordial follicles concentrated in the cortex (Fig. 2). Because our protocol targeted the first cells to express Foxl2, this result suggested a possible correlation between the stage at which a cell upregulates Foxl2 and the time at which its host follicle is activated. We hypothesized that this temporal relationship would be masked if Cre activation were delayed until later in development (e.g., E14.5). In this case, we expected that the much larger population of FOXL2-positive cells present at E14.5 would contribute to follicles in the cortex as well as the medulla. However, the distribution of lineage-traced cells in the ovaries of P9 mice exposed to tamoxifen at E14.5 (Fig. 2, B and C) was nearly indistinguishable from those injected at E12.5 (Fig. 2, A and D). Similar results were obtained with a higher dose of tamoxifen (data not shown). Therefore, the granulosa cells that populate the primordial follicles of the cortex must derive from cells that do not express the Foxl2-GCE cassette at E12.5 or E14.5. Assuming that the Foxl2-GCE cassette is activated in a random subset of cells expressing Foxl2 at E12.5–E14.5, these results suggest that granulosa cells are produced in two waves: the first corresponding to the FOXL2-positive cells derived from bipotential supporting cell precursors, which furnish growing follicles of the medullary zone, and a second pool of unknown origin that contributes to primordial follicles assembled in the cortical region of the ovary and activated throughout adult life.
FOXL2-Positive Cells Are Arrested During Embryonic Development
A previous study of the proliferation dynamics of somatic cells in the perinatal rat ovary distinguished two populations of ovarian granulosa cells based on location (cortex vs. medulla) and proliferation history [27]. Granulosa cells in cortical follicles were derived from cells that were actively proliferating during mid- to late gestation, whereas those in medullary follicles derived from cells that had been quiescent [27, 28]. Because our lineage-tracing results indicated that the medullary follicles of the postnatal mouse ovary derived from fetal Foxl2-expressing cells, we investigated whether these cells were quiescent during fetal life.
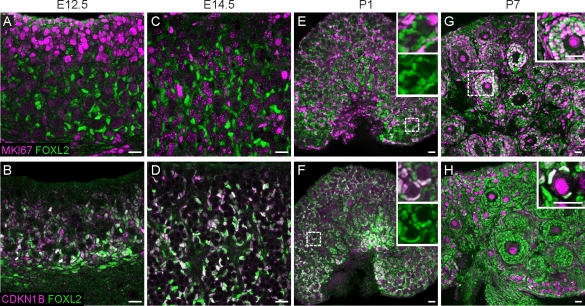
Previous microarray and immunohistochemical studies showed enrichment of Cip/Kip cyclin-dependent kinase inhibitors (p27, p21, and p57) in the somatic compartment of the embryonic ovary [29–32]. To specifically assess the proliferation status of fetal FOXL2-positive cells, we colabeled wild-type E12.5 and E14.5 ovaries with FOXL2 and MKI67 (a marker of active cell cycle) or phospho-Histone H3 (pHH3; a marker of mitosis) (Fig. 3, A and C, and Supplemental Fig. S2). As hypothesized, all FOXL2-positive cells were MKI67- and pHH3-negative. Instead, they strongly expressed the cyclin-dependent kinase inhibitor p27 (CDKN1B), and appeared to have entered cell cycle arrest (Fig. 3, B and D). Mitotic arrest of this population persisted until after birth, when granulosa cells in activated medullary follicles resumed cycling and became MKI67-positive and CDKN1B-negative (Fig. 3, E–H). Granulosa cells in primordial follicles remained arrested (Fig. 3H).
FIG. 3.
FOXL2-positive cells are arrested throughout embryonic development. Cells expressing FOXL2 (green) were negative for MKI67 (magenta; A and C) and positive for CDKN1B (magenta; B and D) at E12.5 and E14.5. At birth (E and F), the FOXL2-positive cells in close proximity to developing oocytes remained arrested, although cells more distant from oocytes were positive for MKI67 and negative for CDKN1B. Insets in E and F show FOXL2 expression in primordial follicles and surrounding interstitial cells, with or without MKI67 (E) or CDKN1B (F) overlay. By P7 (G and H), a subset of follicles had progressed into primary and secondary stages. FOXL2-positive cells in these activated follicles were MKI67-positive (inset in G) and CDKN1B-negative, although granulosa cells in primordial follicles in the cortical region of the gonad remained arrested (inset in H). Oocytes upregulated CDKN1B shortly after birth (F and H), as previously reported [32]. White color indicates overlap between the markers. A–D are images of whole-mount immunostained gonads taken at 40×. E–H are images of cryosectioned gonads (original magnification ×20). Bars = 20 μm.
The second population of rat granulosa cells, which were incorporated into cortical primordial follicles, derived from cells that were actively dividing during fetal development [27]. We determined that cells in the surface epithelium, which never express FOXL2 protein, and interstitial cells, which weakly express FOXL2 after E15.5, were actively cycling (MKI67-positive and CDKN1B-negative) at all stages examined (Supplemental Fig. S2, E–H, and data not shown), marking them as possible sources of the second wave of granulosa cells.
During Fetal Life, New FOXL2-Positive Cells Arise from Cycling Progenitors in the Surface Epithelium
A clear increase in the number of FOXL2-positive cells in the ovary was observed between E12.5 and E14.5 (Figs. 1 and 3). Because FOXL2-positive cells are mitotically arrested, this increase must be attributed to recruitment rather than proliferation. This also means that the original supporting cell precursors specified during the bipotential period cannot account for the full population of FOXL2-positive cells present in the embryo. Previous work in the testis revealed that proliferation in the surface epithelium gives rise to Sertoli cell precursors during the bipotential period [6, 33]. By E11.5, the population of Sertoli cells that will form testis cords is fully allocated, and further divisions in the surface epithelium produce interstitial cells rather than new Sertoli cells [6]. However, it remained possible that, in the ovary, the surface epithelium [34] continues to contribute to the FOXL2-positive population throughout fetal development.
To test this idea, we labeled the surfaces of XX gonads with MitoTracker, a mitochondrial dye, and then cultured the samples for 2–48 h. In E11.5 gonads cultured for 2 h, the dye was clearly restricted to the surface epithelium of the gonad (Fig. 4A). After 6 h, the label was present in more cell layers, and after 48 h, labeled cells had ingressed deep into the ovary (Fig. 4, B–D). To confirm that female supporting cell precursors derive from the surface epithelium, we labeled the surface of E11.5 Sry-EGFP ovaries with MitoTracker and cultured them for 48 h. Many EGFP-expressing cells in deeper layers of the gonad were labeled with MitoTracker, indicating that they were supporting cell precursors that had emerged from the surface epithelium of XX gonads after E11.5 (Fig. 4C). Consistent with this finding, in ovaries labeled at either E11.5 or E12.5 and cultured for 48 h, many ingressing cells expressed FOXL2 (Fig. 4, D and E). Less inward movement and rare colocalization between MitoTracker and FOXL2 were observed in cultures labeled at E14.5 (Fig. 4F). These findings indicate that bipotential supporting cell precursors and embryonic FOXL2-positive cells do indeed derive from the surface epithelium, but that this activity slows down after E12.5.
FIG. 4.
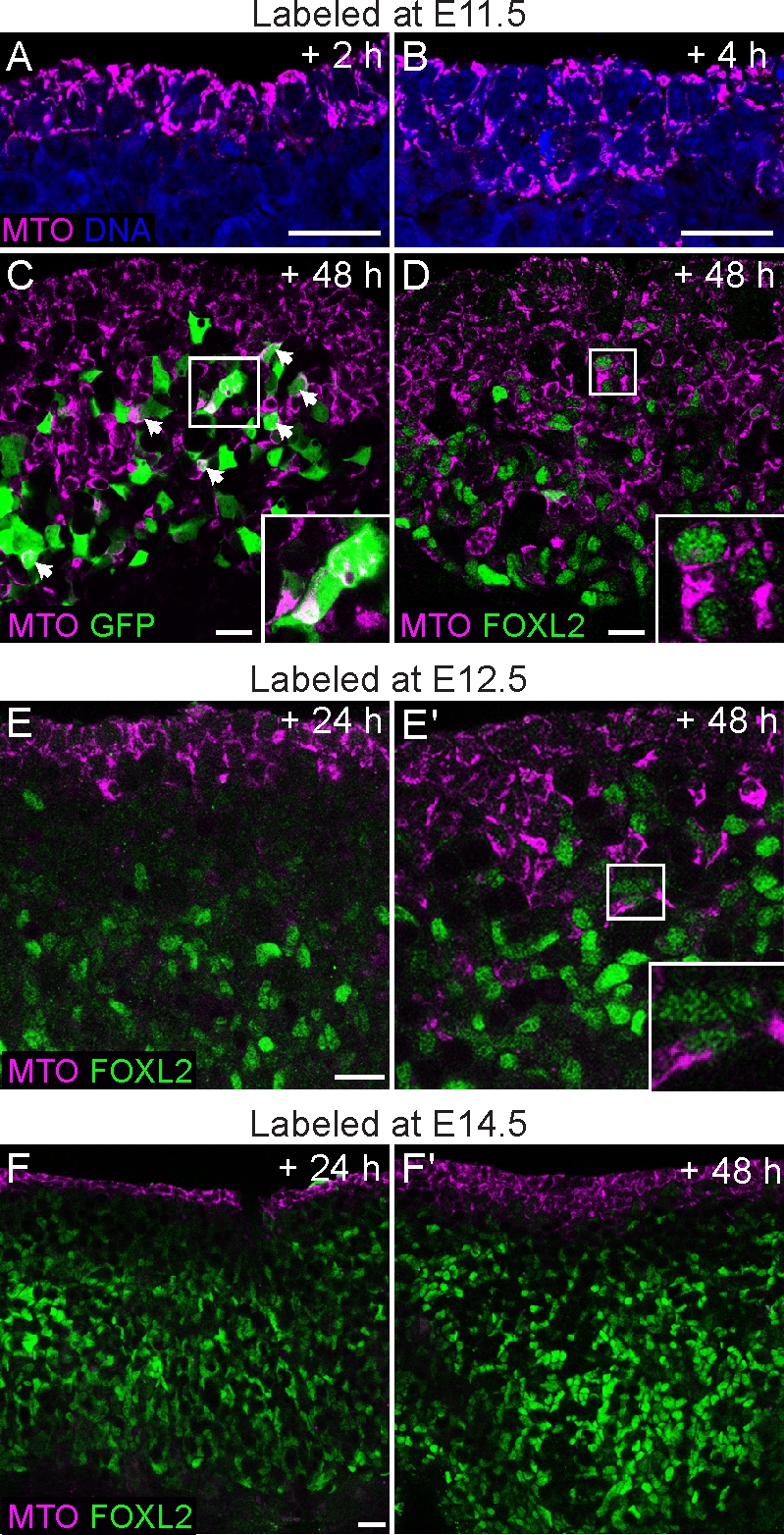
The surface epithelium is a source of new Sry-EGFP- and Foxl2-expressing cells in the fetal ovary. The ovarian surface was labeled with the cytoplasmic MitoTracker dye (MTO; magenta) at various stages of ovary development, and the samples were cultured for 2–72 h. A) Gonads from E11.5 embryos fixed after 2 h show that the dye is confined to the outermost cell layers. B) After 6 h, cell divisions in the coelomic epithelium generate labeled cells that move deeper inside the gonad. C) Ovary from an Sry-EGFP transgenic embryo labeled with MitoTracker at E11.5. Many EGFP-positive cells contained the label after 48 h of culture (overlap is white; arrowheads and inset). D) Similarly, in an E11.5 wild-type ovary cultured for 48 h, MitoTracker-labeled cells had ingressed to very deep layers, and many began to present nuclear FOXL2 staining (green; inset). E and E′) Gonads labeled at E12.5 displayed fewer ingressing cells that colabeled with FOXL2 after 24–48 h. F and F′) In samples labeled at E14.5, no ingression was observed after 24 h. Multiple cell layers were labeled after 48 h, but few ingressing cells were positive for FOXL2. Original magnification ×40 (A–E) or ×20 (F); bars = 20 μm.
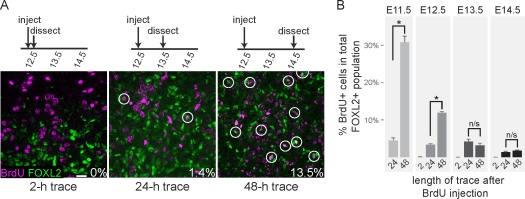
Because the surface epithelium is proliferative and FOXL2-positive cells are arrested, cycling progenitors should enter mitotic arrest after exiting the surface epithelium, but before upregulating Foxl2. To estimate the duration of this process, we performed a series of BrdU lineage-tracing experiments (Fig. 5A). We confirmed that FOXL2-positive cells were not in S-phase (i.e., BrdU-negative) by exposing E12.5, E13.5, and E14.5 embryos to BrdU for 2 h and then staining their gonads with antibodies against FOXL2 and BrdU (Fig. 5, A and B). In subsequent experiments, embryos were pulsed with BrdU at E11.5, E12.5, E13.5, or E14.5, injected with excess thymidine 2 h later, and then allowed to develop for 24–48 h before dissection. We then estimated the percentage of BrdU/FOXL2 double-positive cells within the total population of FOXL2-positive cells. At each stage, very few BrdU/FOXL2 double-positive cells were observed 24 h after injection. Conversely, in embryos pulsed at E11.5 or E12.5 and allowed to develop for 48 h, a significant increase in the proportion of double-positive cells was seen (P < 0.001; Fig. 5, A and B), suggesting that there is typically a delay of more than 24 h between a progenitor cell's last S-phase and upregulation of Foxl2. This spike was not observed in samples pulsed at E13.5 or E14.5 (Fig. 5B), suggesting that the number of new cells entering the population was proportionally much lower at these later stages. When embryos were pulsed at E15.5 or E16.5 and traced for 24–48 h, BrdU/FOXL2 colocalization was detected in the weakly FOXL2-positive interstitial cells as well as a few other FOXL2-expressing cells near the surface epithelium, indicating that these two domains were still proliferative (data not shown).
FIG. 5.
Cycling progenitor cells give rise to new Foxl2-expressing cells. A) Example of BrdU lineage tracing and data collection. In this series, pregnant females were injected with BrdU at E12.5, chased with thymidine 2 h later, and dissected after the indicated trace durations. Gonads were stained with antibodies against BrdU (magenta) and FOXL2 (green); white color indicates overlap between the markers. BrdU/FOXL2 double-positive cells (circles) were counted relative to the total number of FOXL2-positive cells and expressed as a percentage. Original magnification ×40; bar = 20 μm. B) Quantification of BrdU results. Embryonic stages listed above the bars indicate when BrdU was injected (n = 4–12 images per time point, from two to four independent gonad samples). Error bars indicate SEM. Embryos pulsed at E11.5 or E12.5 showed significantly higher proportions of BrdU/FOXL2 double-positive cells in 48-h traces relative to 24-h traces, but those injected at E13.5–E14.5 did not, suggesting that the number of new cells entering the population was proportionally much lower at later stages. *P < 0.001; n/s, not significant.
New Granulosa Cells Are Generated in the Ovarian Cortex Throughout the Follicle Assembly Period
In the outbred mouse strain used for these experiments (CD1), follicle assembly begins at birth and is nearly complete by P5. At this time, almost all surviving oocytes are individually encapsulated within primordial follicles by a dedicated set of granulosa cells [35]. Many follicles in the medullary domain are activated and begin to grow immediately upon assembly [1], whereas cortical primordial follicles remain arrested (Fig. 3). The mechanism by which some follicles are selected for activation remains unclear [36].
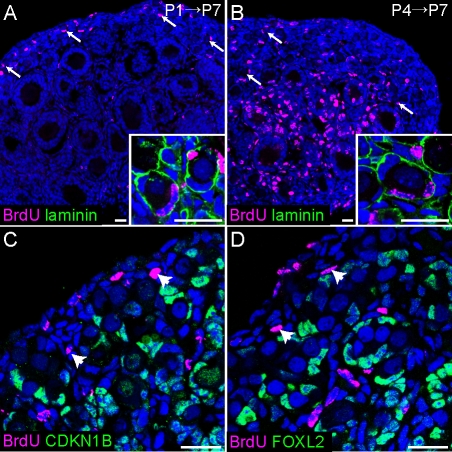
If the population of arrested FOXL2-positive cells specified before birth were sufficient to endow all surviving oocytes with a set of granulosa cells, no BrdU-positive granulosa cells should be detected in the primordial follicles of ovaries pulsed with BrdU during follicle assembly and traced until P7. Conversely, if a pool of cycling progenitors contributed to the granulosa cell population throughout this period, primordial follicles containing BrdU-positive granulosa cells should be easily detectable. To investigate these possibilities, we injected P1 and P4 female mice with BrdU and examined their ovaries at P7. Numerous BrdU-positive granulosa cells were detected in the primordial follicles of these samples (Fig. 6, A and B), indicating that these cells were still cycling when the BrdU was applied, and thus did not derive from the arrested FOXL2-positive population. This finding is in agreement with the earlier studies of the rat ovary [27].
FIG. 6.
New granulosa cells arise in the ovarian cortex after birth. A and B) Ovaries of P7 pups exposed to BrdU at P1 (A) or P4 (B) and stained with antibodies against BrdU (magenta) and αlaminin (green) show BrdU-positive granulosa cells inside primordial follicles (arrows, insets). As expected, granulosa cells in actively dividing medullary follicles were heavily labeled in samples pulsed at P4, but not in samples pulsed at P1. We speculate that the label was titrated out during the week-long chase in the latter case. C and D) Somatic cells in and near the surface epithelium remain proliferative after birth. Ovaries from P1 mice were injected with BrdU 2 h prior to dissection and stained with antibodies against BrdU (magenta) and CDKN1B (C) or FOXL2 (D) (green). Arrowheads point to clusters of proliferative (CDKN1B-negative, BrdU-positive) FOXL2-negative somatic cells under the surface epithelium. Nuclei (blue) were stained with syto13. Original magnification ×20 (A and B) or ×40 (C and D); bars = 20 μm.
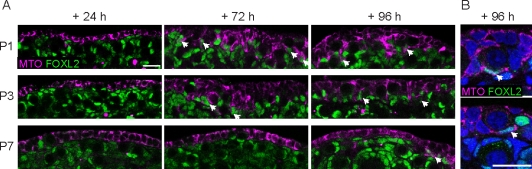
We predicted that these cycling progenitors would reside in the surface epithelium, which was still proliferative at P1 (Fig. 6, C and D). BrdU staining was also observed in small clusters of CDKN1B-negative, FOXL2-negative somatic cells residing just below the surface (Fig. 6, C and D). To determine whether new granulosa cells arise from the surface epithelium during the postnatal period, we labeled P1, P3, and P7 ovaries with MitoTracker and cultured the samples for 24–96 h. In all samples, the surface epithelium was very strongly labeled after 24–48 h, but almost no inward movement was observed (Fig. 7A and data not shown). When P1 and P3 ovaries were cultured for 72–96 h, the labeled domain clearly expanded to multiple cell layers. In contrast, in ovaries labeled at P7, when follicle assembly is nearly complete, labeled cells remained almost completely confined to the surface epithelium, even after 96 h of culture (Fig. 7A).
FIG. 7.
The ovarian surface epithelium gives rise to new granulosa cells during the follicle assembly period. A) Ovaries from P1, P3, and P7 pups were labeled with the cytoplasmic MitoTracker dye (MTO, magenta), cultured for 24–96 h, then fixed and stained with an antibody against FOXL2 (green, nuclear). Only the surface epithelium was labeled after 24 h, but obvious ingression of labeled cells was observed after 72–96 h in cultures started at P1 or P3, but not P7. Arrowheads point to FOXL2-positive cells labeled with MitoTracker. B) A few squamous MitoTracker-labeled FOXL2-positive cells (arrowheads) were incorporated into primordial follicles after 96 h in ovaries placed into culture at P3. Nuclei (blue) were stained with syto13. Original magnification ×20 (A) or ×40 (B); bars = 20 μm.
Most ingressing cells did not express FOXL2 at these time points. However, we were able to detect several MitoTracker-labeled FOXL2-positive cells in primordial follicles (Fig. 7B) and in other less well-defined positions under the surface epithelium (Fig. 7A). To determine whether the FOXL2-negative ingressing cells were contributing to the stromal population, we used a transgenic line in which EYFP is expressed under control of the α smooth muscle actin promoter (Acta2-EYFP). This reporter is generally excluded from follicles (Supplemental Fig. S3, A and B), but highly expressed in the stromal cells of the ovary, including many of the somatic cells proximal to the surface epithelium (Supplemental Fig. S3B). To test the idea that many ingressing cells belong to the Acta2-EYFP population, we labeled the surface epithelium of P1 and P3 transgenic Acta2-EYFP ovaries and cultured them for 72–96 h. However, only a small number of ingressing MitoTracker-labeled cells expressed the EYFP reporter (Supplemental Fig. S3C). Therefore, cells ingressing from the surface epithelium of the perinatal ovary appear to contribute to three cell populations: FOXL2-positive granulosa cells, Acta2-EYFP-expressing stromal cells, and cells that express neither marker. However, given sufficient time, we expect that most ingressing cells would eventually express FOXL2 and/or Acta2-EYFP, because the vast majority of nonendothelial somatic cells in the early postnatal ovary express one or both of these markers (see Fig. 3H and Supplemental Fig. S3A). Longer cultures with MitoTracker were impractical, as the dye is progressively diluted at each cell division. Collectively, these results show that the proliferative ovarian surface epithelium generates granulosa cell precursors at two stages of ovary development, from at least E11.5–E14.5 and during the postnatal follicle assembly period.
DISCUSSION
The idea that supporting cell precursors in the murine bipotential gonad give rise either to adult Sertoli or adult granulosa cells is inaccurate, at least in its simplest form. Fetal FOXL2-positive cells derived from this population do not give rise to the granulosa cells present in the adult mouse. Instead, they specifically contribute to the subset of follicles in the medulla of the ovary that are activated immediately after birth and reach antral stages before puberty (Figs. 2 and 8 and [36]). Without the required stimulation from pituitary-derived FSH, the vast majority of these follicles are destined to be lost to atresia or converted into interstitial tissue [36, 37]. In contrast, the granulosa cells that equip the cortical primordial follicles and will be activated in adult life arise at more advanced stages, as late as the end of the postnatal follicle assembly period, a few days after birth (Figs. 6–8).
FIG. 8.
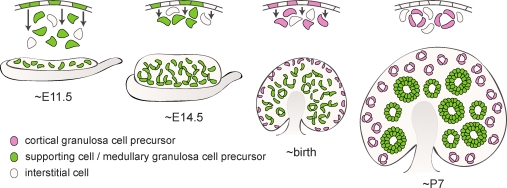
Origins of ovarian granulosa cells. Divisions in the surface epithelium of the bipotential gonad (E11.5) produce both interstitial cells (beige) and supporting cell precursors (green) that differentiate as Sertoli cells in the presence of a Y chromosome; in XX individuals, these cells move deep into the ovary and give rise to the granulosa cells in the medullary follicles activated immediately after their assembly at birth (green). As the ovary differentiates (∼E14.5), further divisions in the surface epithelium contribute more cells to the granulosa population, although they remain closer to the ovarian surface. The granulosa cells that populate the primordial follicles in the cortex (pink), which are activated in adult life, arise from the surface epithelium around birth. By P7, follicle assembly is mostly complete, and surface cells no longer move into the ovary.
Granulosa cells of dormant primordial follicles exist in stable cell cycle arrest for months, years, or even decades, waiting for the enigmatic activation signal that releases their proliferative potential and results in the final maturation of a Graafian follicle. Interestingly, the granulosa cell precursors specified early in development enter mitotic arrest concomitant with their embryonic specification, shortly before they upregulate Foxl2 (Figs. 3 and 5). Because they resume cycling upon follicle activation at birth (Fig. 3), their period of quiescence is relatively short. The cortical granulosa cells, in contrast, arise perinatally (∼E15.5–P4) from proliferating progenitors, enter mitotic arrest as they are assembled into follicles, and may remain arrested for the entirety of a female's reproductive life.
While the granulosa cells of the medulla and cortex can be classified into two separate populations (Fig. 2 and [27]), they are likely the descendants of a single progenitor source, born at different stages of development (Fig. 8). The surface epithelium of the gonad appears to be a major, if not the only, source of granulosa cell precursors, although other potential sources cannot be wholly discounted. The first cells to emerge from this epithelium (prior to E11.5) constitute the bipotential supporting cell precursor population, competent to activate the Sry promoter and differentiate as either granulosa or Sertoli cells (Fig. 4C and [6]). In the testis, the original Sry-expressing cells and their progeny account for all of the Sertoli cells present in the adult [8]: they assemble into testis cords by E12.5, and then accommodate the growing tubules by proliferating [33] rather than recruiting new cells into the population. This does not appear to be the case in the ovary, because the number of FOXL2-positive cells increases in the absence of intrinsic proliferation (Figs. 3 and 5). New FOXL2-expressing granulosa cell precursors must therefore be recruited into the population after the bipotential period has concluded.
In accord with this observation, after sex is determined at E11.5, cells continue to drop out of the ovarian surface epithelium and, in some cases, enter mitotic arrest and upregulate Foxl2 (Fig. 4). Those that do not arrest likely contribute to the interstitial population (Supplemental Fig. S3). Surprisingly, new granulosa cells were specified from cycling progenitors in the cortex as late as P4, toward the end of the follicle assembly period (Figs. 6 and 7). In contrast, no ingression from the surface epithelium was observed in ovaries labeled at P7, even after 96 h of culture, suggesting that the role of the ovarian surface epithelium as a source of new granulosa cells normally concludes coincident with the end of the follicle assembly phase. The signals that control the temporal activity of the coelomic epithelium at fetal and neonatal stages are unknown. However, cells of the ovarian surface epithelium retain the ability to move into the ovary in adult life, as this is an important part of the postovulatory repair process in the mature animal [38].
In addition to their distinct cellular origins, the first wave of follicles to be activated in the ovary exhibit several properties that distinguish them from follicles activated after puberty. They progress to antral stages almost twice as fast as adult follicles [1], present distinct morphological features [28], and show altered expression of several steroidogenic enzymes and steroid receptors relative to similarly sized follicles in the adult [39]. Differences between these two groups of follicles might be attributable to their different origins or to environmental factors, such as gonadotropin levels and diet, which differ in prepubertal and adult animals. Such differences are consistent with the distinct functions of follicles activated before puberty, which establish ovarian functionality at the hormonal level prior to the first LH surge [40], and those that grow during adult estrus cycles and are destined for ovulation/luteinization or atresia. In female rodents, which enter puberty around 30–40 days postpartum (e.g., [41, 42]), the first oocytes to be ovulated may actually derive from the abnormal follicles activated at birth [40]. However, in larger mammals that enter puberty many months or years after birth, the prepubertal period is characterized by the development of multiple waves of anovulatory follicles (e.g., [43, 44]); in some species, functionality of the hypothalamic-pituitary-gonadal axis is established early. FSH is produced, allowing such follicles to grow to antral stages, but the surge in luteinizing hormone required for ovulation is repressed by negative feedback from low levels of estrogen until the animal is sufficiently mature to bear young (reviewed by Rawlings et al. [45]).
The function and upstream regulators of the extended mitotic arrest of granulosa cell precursors remain uncertain. It is possible that mitotic arrest during the embryonic period prolongs and preserves the proliferative potential of granulosa cells for the time at which they will be tasked with the formidable demands of folliculogenesis. Alternatively, mitotic arrest may be required for the adoption of granulosa cell fate, or, more generally, supporting cell fate: preliminary work indicates that supporting cell precursors are arrested in both sexes during the bipotential period (data not shown). Mice carrying null mutations in the cyclin-dependent kinase inhibitors p27 or p21 do not have overt phenotypes in the embryonic ovary. However, female p27 single mutants have a larger endowment of primordial follicles at birth and exhibit premature ovarian failure and sterility after puberty, attributed to the failure of oocytes to remain meiotically arrested [32]. It is unknown whether embryonic granulosa cell precursors arrest normally in these mutants, in spite of the loss of the inhibitory factors, or whether their re-entry into cycle supports the assembly of an increased number of primordial follicles.
Mutant models that fail to arrest or prematurely resume cycling may provide insight into these issues. While FOXL2 was primarily used as a marker in the present work, it has also been linked to disruptions in cell cycle. A somatic mutation in its forkhead DNA-binding domain (C134W) is present in >95% of adult granulosa cell tumors [46, 47], and its expression is markedly reduced in the juvenile form of this disease [48]. In addition, a recent study showed that FOXL2 regulates the expression of several cell cycle inhibitors and promotes G1 arrest in cultured granulosa cell lines [49]. However, while FOXL2 may regulate the cell cycle in granulosa cells of the adult ovary, we do not attribute the embryonic mitotic arrest phenotype to the action of this protein, because cell cycle arrest seems to precede FOXL2 expression (Figs. 3 and 5 and data not shown) and is established normally in E14.5 Foxl2 mutant [13] ovaries (S. Jakob and R. Lovell-Badge, personal communication).
In conclusion, we show in the present work that the supporting cell precursors of the bipotential gonad specifically contribute to the rapidly depleted population of medullary follicles present in the prepubertal mouse. These precursors are mitotically arrested throughout fetal development, but increase in number as new cells exit the surface epithelium and upregulate Foxl2. Granulosa cells of follicles activated during adult life arise from the ovarian surface epithelium perinatally. These findings add complexity to the standard model of sex determination in which the Sertoli and granulosa cells of the adult testis and ovary directly stem from the supporting cell precursors of the bipotential gonad.
Supplementary Material
ACKNOWLEDGMENT
We thank members of the Capel laboratory for comments and advice on the manuscript, Susanne Jakob and Robin Lovell-Badge for assessing proliferation in Foxl2 mutant ovaries, and Drs. Dagmar Wilhelm and Harold Erickson for the FOXL2 and α laminin antibodies.
Footnotes
Work in the laboratory of B.C. was supported by a grant from the National Institutes of Health (NIH) (HD39963). Work in the laboratory of A.P.M. was supported by a grant from the NIH (DK070181).
REFERENCES
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124: 43 101 [DOI] [PubMed] [Google Scholar]
- McLaren A. Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays 1991; 13: 151 156 [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol 2001; 240: 92 107 [DOI] [PubMed] [Google Scholar]
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 2001; 22: 255 288 [DOI] [PubMed] [Google Scholar]
- Liu CF, Liu C, Yao HH. Building pathways for ovary organogenesis in the mouse embryo. Curr Top Dev Biol 2010; 90: 263 290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol 1998; 203: 323 333 [DOI] [PubMed] [Google Scholar]
- Chang HL, MacLaughlin DT, Donahoe PK. Somatic stem cells of the ovary and their relationship to human ovarian cancers (April 30, 2009). : StemBook 2008–2009. Cambridge, MA: The Stem Cell Research Community; World Wide Web (URL: http://www.stembook.org). (September 27, 2010) [PubMed] [Google Scholar]
- Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 2004; 274: 271 279 [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science 1999; 286: 2328 2331 [DOI] [PubMed] [Google Scholar]
- Britt KL, Kerr J, O'Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. Faseb J 2002; 16: 1389 1397 [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011; 476: 101 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yokouchi K, Yoshida K, Kano K, Naito K, Miyazaki J, Tojo H. Investigation of the fate of Sry-expressing cells using an in vivo Cre/loxP system. Dev Growth Differ 2006; 48: 41 47 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004; 131: 933 942 [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Vigier B, Schibler L, Cribiu EP, Cotinot C, Vaiman D. Positional cloning of the PIS mutation in goats and its impact on understanding mammalian sex-differentiation. Genet Sel Evol 2005; 37 (suppl 1): S55 S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger L, Kocer A, Daniel N, Pannetier M, Chesne P, Heyman Y, Renault L, Mandon-Pepin B, Chavatte-Palmer P, Vignon X, Vilotte JL, Cotinot C, et al. Attempt to rescue sex-reversal by transgenic expression of the PISRT1 gene in XX PIS−/− goats. Sex Dev 2008; 2: 142 151 [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 2001; 27: 159 166 [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 2004; 13: 1171 1181 [DOI] [PubMed] [Google Scholar]
- Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, Schlessinger D, Ottolenghi C. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev Biol 2009; 9: 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 2005; 14: 2053 2062 [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009; 139: 1130 1142 [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21: 70 71 [DOI] [PubMed] [Google Scholar]
- McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P. GUDMAP: the Genitourinary Developmental Molecular Anatomy Project. J Am Soc Nephrol 2008; 19: 667 671 [DOI] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-Macgilp S, Pi X, Roochun Y, Sharghi M, Tindal C, et al. The GUDMAP database—an online resource for genitourinary research. Development 2011; 138: 2845 2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech Dev 2009; 126: 324 336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M, Veitia RA. Evolution and expression of FOXL2. J Med Genet 2002; 39: 916 921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigon CJ, Coudouel N, Mazaud-Guittot S, Forest MG, Magre S. Follicular cells acquire sertoli cell characteristics after oocyte loss. Endocrinology 2005; 146: 2992 3004 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol Reprod 1992; 47: 466 472 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN, DeSanti AM. Patterns of ovarian cell proliferation in rats during the embryonic period and the first three weeks postpartum. Biol Reprod 1995; 53: 1208 1221 [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol 2005; 287: 361 377 [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Affourtit JP, Bult CJ, Eicher EM. Transcriptional profile of mouse pre-granulosa and Sertoli cells isolated from early-differentiated fetal gonads. Gene Expr Patterns 2007; 7: 113 123 [DOI] [PubMed] [Google Scholar]
- Cory AT, Boyer A, Pilon N, Lussier JG, Silversides DW. Presumptive pre-Sertoli cells express genes involved in cell proliferation and cell signalling during a critical window in early testis differentiation. Mol Reprod Dev 2007; 74: 1491 1504 [DOI] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 2007; 21: 2189 2202 [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development 2000; 127: 65 73 [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod 2002; 66: 1134 1150 [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001; 234: 339 351 [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 2009; 30: 438 464 [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsu SY, Kaipia A, Hsueh AJ. Cell death and survival during ovarian follicle development. Mol Cell Endocrinol 1998; 140: 15 18 [DOI] [PubMed] [Google Scholar]
- Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol 2007; 213: 581 588 [DOI] [PubMed] [Google Scholar]
- Galas J, Slomczynska M, Knapczyk-Stwora K, Durlej M, Starowicz A, Tabarowski Z, Rutka K, Szoltys M. Steroid levels and the spatiotemporal expression of steroidogenic enzymes and androgen receptor in developing ovaries of immature rats. Acta Histochem 2011; (in press) published online ahead of print 25 May 2011; DOI 10.1016/j.acthis.2011.04.006 [DOI] [PubMed]
- Guigon CJ, Mazaud S, Forest MG, Brailly-Tabard S, Coudouel N, Magre S. Unaltered development of the initial follicular waves and normal pubertal onset in female rats after neonatal deletion of the follicular reserve. Endocrinology 2003; 144: 3651 3662 [DOI] [PubMed] [Google Scholar]
- Safranski TJ, Lamberson WR, Keisler DH. Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol Reprod 1993; 48: 669 673 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 1997; 99: 391 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GP, Evans AC, Rawlings NC. Follicular waves and circulating gonadotrophins in 8-month-old prepubertal heifers. J Reprod Fertil 1994; 100: 27 33 [DOI] [PubMed] [Google Scholar]
- Mahdi D, Khallili K. Relationship between follicle growth and circulating gonadotrophin levels during postnatal development of sheep. Anim Reprod Sci 2008; 106: 100 112 [DOI] [PubMed] [Google Scholar]
- Rawlings NC, Evans AC, Honaramooz A, Bartlewski PM. Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats. Anim Reprod Sci 2003; 78: 259 270 [DOI] [PubMed] [Google Scholar]
- Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med 2009; 360: 2719 2729 [DOI] [PubMed] [Google Scholar]
- Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ, Anttonen M. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Mod Pathol 2010; 23: 1477 1485 [DOI] [PubMed] [Google Scholar]
- Kalfa N, Philibert P, Patte C, Ecochard A, Duvillard P, Baldet P, Jaubert F, Fellous M, Sultan C. Extinction of FOXL2 expression in aggressive ovarian granulosa cell tumors in children. Fertil Steril 2007; 87: 896 901 [DOI] [PubMed] [Google Scholar]
- Benayoun BA, Georges AB, L'Hote D, Andersson N, Dipietromaria A, Todeschini AL, Caburet S, Bazin C, Anttonen M, Veitia RA. Transcription factor FOXL2 protects granulosa cells from stress and delays cell cycle: role of its regulation by the SIRT1 deacetylase. Hum Mol Genet 2011; 20: 1673 1686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.