Abstract
We have previously described an alternatively spliced isoform of IL-4 mRNA that omits exon 2 and is termed IL-4δ2. However, the natural production of IL-4δ2 protein and its association with disease have not been previously assessed due to unavailability of an antibody that interacts with IL-4δ2 without cross-reactivity with full length IL-4. We used a unique monoclonal antibody (mAb) that reacts with IL-4δ2, but not with IL-4, and observed that IL-4δ2 is naturally produced by T cells from patients with asthma, but not from healthy controls. The kinetics of IL-4δ2 and IL-4 production by phorbol myristate acetate (PMA)/ionomycin-activated cells differed, with IL-4δ2 increasing at 48 – 72 h and IL-4 peaking at 24 h. The steady-state levels of IL-4δ2 mRNA varied significantly among the donors and were discordant with the corresponding protein levels, suggesting post-transcriptional regulation of protein production. Polarized Th1 or Th2 lymphocytes were not a major source of IL-4δ2. Stimulation of cultured T lymphocytes with IL- 4δ2 caused elevated production of IFN-γ, IL-10, IL-6, MCP-1, and TNF-α, with notable differences between patients and controls in the production of IFN-γ, IL-10, and IL-6. Thus, IL- 4δ2 is natively produced not only as mRNA but also as a protein by cells other than Th1 or Th2. It is regulated post-transcriptionally, is associated with allergic asthma, and regulates production of other cytokines by primary T lymphocytes. Alternatively spliced interleukin-4 may be a new biomarker, a pathophysiological player, and possibly a molecular target for future therapies in asthma.
Keywords: interleukin-4, cytokines, alternative splicing, lymphocytes, asthma
Introduction
Interleukin-4 (IL-4), a pleiotropic cytokine produced predominantly by Th2 lymphocytes, regulates a plethora of functions in hematopoietic and non-hematopoietic cells. The IL-4 gene is expressed in two mRNA isoforms, a full-length variant containing four exons and an alternatively spliced variant, known as interleukin-4delta2 (IL-4δ2), in which exon 2 is omitted (1–5). Of these two isoforms, IL-4δ2 has been much less studied. Expression of IL-4δ2 mRNA in peripheral blood mononuclear cells (PBMC), thymocytes, and bronchoalveolar lavage cells was described previously (1–15). The combined expression levels of IL-4 and IL-4δ2 mRNAs, as well as the IL-4/IL-4δ2 mRNA ratio, have been measured in healthy volunteers and in patients with systemic sclerosis (6,7), asthma (8,9), acute cardiac transplant rejection (10), pulmonary tuberculosis (11,12), severe sepsis (13), Helicobacter pylori infection (14), and in calves experimentally infected with Fasciola hepatica (15). However, it remains unknown whether IL- 4δ2 is expressed as a protein in vivo.
The studies of IL-4δ2 protein thus far have been limited to testing of recombinant human (rh) IL-4δ2 in vitro and in vivo (1–5). In cell culture, rhIL-4δ2 had no independent effect on the proliferation of T cells, B cells, or Mϕ and competed with rhIL-4 effects on T cell proliferation (2,3). Similarly, rhIL-4δ2 acted as an antagonist of the rhIL-4-induced synthesis of IgE and expression of CD23 in B cells and blocked the inhibitory action of hIL-4 on LPS-induced cyclooxygenase-2 expression and subsequent prostaglandin E2 secretion in monocytes (3). These observations in vitro suggested that IL-4δ2 may be a natural functional antagonist of IL-4. However, in contrast to these in vitro results, recent in vivo observations revealed potent proinflammatory regulation by IL-4δ2 in a different fashion than regulation by IL-4 (4,5). The reason for this discrepancy between observations in cell culture and in vivo remains unclear. Nevertheless, the extensive body of literature summarized above suggests that IL-4δ2, should it exist naturally as a protein, is centrally involved in regulation of immunity and inflammation.
Until now, the natural production of IL-4δ2 protein was difficult to assess, due to unavailability of an antibody that recognizes IL-4δ2 without cross-reacting with IL-4. These two splice isoforms are 100% homologous and differ only in 16 amino acids that are absent in the alternatively spliced isoform. Therefore, commercial anti-IL-4 antibodies either react with both isoforms indiscriminately or recognize only full length IL-4. The purpose of this study was to investigate, using a unique anti-IL-4δ2 antibody that does not react with full length IL-4 (16–19), whether natural IL-4δ2 protein is produced and secreted by primary human T cells, whether the IL-4δ2 mRNA steady-state levels correlate with the corresponding protein levels, whether Th1 or Th2 cells are a source of IL-4δ2 protein, and whether IL-4δ2 has a regulatory effect on cultured primary T cells from patients with asthma and healthy control volunteers.
Patients and Methods
Patients and controls
This study was approved by the University of Maryland Institutional Review Board, and written informed consent was obtained from all participants. Patients with documented allergic asthma and healthy volunteers donated blood for the assays. The diagnosis of asthma was made following The National Asthma Education and Prevention Program (NAEPP) guidelines. Patients were recruited from the University of Maryland Medical Center through patient care visits. None of the patients received systemic steroids at the time of blood draw. Healthy controls were defined as current non-smokers who had not smoked in the past 3 years, had no known allergies or asthma, and were older than 21 years. Peripheral blood from patients and controls was obtained by venipuncture. A total of 36 adult patients with asthma and 21 healthy volunteers were included in the entire study; subsets of these volunteers were included in separate experiments as described in Results.
Cell culture
T lymphocytes were isolated from whole blood by negative selection using RosetteSep® Human T Cell Enrichment Cocktail according to the protocol of the manufacturer (StemCell Technologies Inc., Vancouver, British Columbia, Canada). Purified T cells were cultured in RPMI1640 culture medium supplemented with 10% dialyzed fetal bovine serum, 2 mM glutamine, 2 mM sodium pyruvate, and antibiotic-antimycotic solution (all from Invitrogen, Carlsbad, CA). Some primary T cell cultures were activated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) with 1 μM ionomycin (both from Sigma, St. Louis, MO). To induce Th1 and Th2 polarization in cell culture, naïve primary CD4+CD45RA+ cells were purified using Human Naïve CD4+ T Cell Isolation Kit from Miltenyi Biotec (Auburn, CA). Cells were then suspended in AIM-V serum-free cell expansion medium (Gibco Invitrogen) combined with anti-CD3/anti- CD28 coated beads (Dynal Invitrogen) and 3 ng/ml recombinant human (rh) IL-2. For Th1 polarization, the medium also contained 5 ng/ml rhIL-12 and 10 μg/ml anti-hIL-4 antibody (Ab), whereas for Th2 polarization, the medium contained 10 ng/ml rhIL-4, 5 μg/ml anti-IL-12 Ab, and 5 μg/ml anti-hIFN-γ Ab. All cytokines were from R&D Systems (Minneapolis, MN), whereas all neutralizing anti-cytokine Abs were from eBioscience (San Diego, CA). Cultures were expanded for 7 days, cells were then washed three times with fresh medium, cultured for an additional 48 h without beads, cytokines, or Abs, and production of IFN-γ, IL-4, and IL-4δ2 proteins was measured by ELISA.
Recombinant human IL-4δ2 and IL-4
Recombinant human (rh) IL-4δ2, rhIL-4, and the corresponding NULL control preparation were expressed and purified as previously described (4,5). Briefly, adenoviral constructs encoding IL- 4δ2, IL-4, or not encoding a cytokine (NULL) were used to infect monolayers of HEK293 cells cultured in Hyclone (Logan, UT) non-serum medium. The supernates were cleared by centrifugation, passed through a 300-kDa-cutoff Macrosep filter (PALL Life Sciences, East Hills, NY) to remove any possibility of contamination with viral particles and concentrated tenfold using a 3-kDa-cutoff Macrosep filter. The concentrates were filter-sterilized using a low-protein-binding, 0.2-μm syringe filter, followed by ELISA assays (see below) to measure the concentration of the recombinant proteins. The concentrated stocks were stored at −80° C. For experiments, these preparations were diluted with cell culture medium to the required final concentration of IL-4 or IL-4δ2. Similarly processed supernates of HEK293 cells infected with the NULL adenoviral construct were used as a negative control.
ELISA
Monoclonal Ab against hIL-4d2 with no cross-reactivity with hIL-4 was produced, validated, and used in ELISA assays as previously described (16–19). An ELISA for hIL-4 with no cross-reactivity with hIL-4δ2, as well as an ELISA for hIFN-γ, were obtained from R&D Systems (Minneapolis, MN).
Multiplex analyses of cytokines
Primary T cells from 11 asthma patients (8 mild and 3 severe) and 9 healthy donors were purified and activated with 300 ng/ml rhIL-4 or rhIL-4δ2 for 48 h. Then, the supernates were harvested and eotaxin, IFN-γ, IL-10, IL-13, IL-17, IL-5, IL-6, MCP-1, and TNF-α were measured using the Luminex 100 System (Austin, TX). Fold changes were calculated for each donor by dividing the cytokine concentration from rhIL-4- or rhIL-4δ2-treated groups by the cytokine concentration from control NULL-treated groups.
Reverse transcriptase-quantitative polymerase chain reaction (RT-QPCR)
Peripheral blood mononuclear cells (PBMC) were isolated, and total RNA purified and reverse transcribed as previously described (2). The RT² qPCR Primer Assays (SABiosciences, Frederick, MD) were used to measure expression of human IL-4, IL-4δ2, and refernce 18S rRNA, following the manufacturer’s recommendations. All RT-Q-PCR experiments were performed in duplicate using Applied Biosystems Step-One Plus system (Foster City, CA).
Statistical analyses
Patients with asthma were compared with healthy controls using the two-tailed Student’s t-test, and the effects of stimulation with IL-4δ2 and IL-4 were assessed with the Wilcoxon signed rank test. The differences between asthma patient and healthy control groups in the fraction of subjects responding to stimulation with IL-4δ2 and IL-4 were evaluated using the chi-square test. For all statistical analysis, the level of significance was set at p<0.05.
Results
IL-4 and IL-4d2 mRNAs are expressed by PBMC obtained from asthma patients and healthy controls
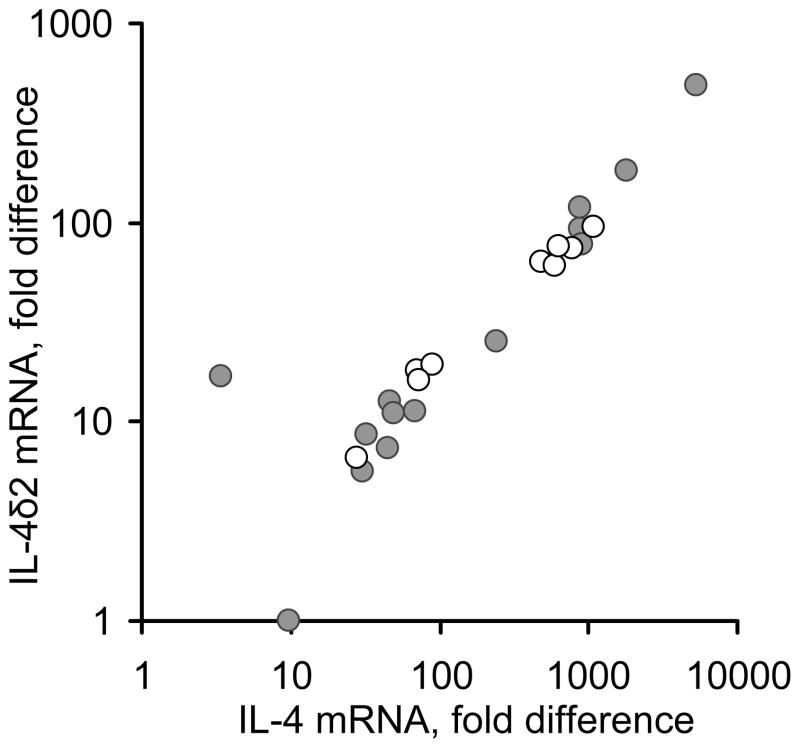
Initial experiments assessed the steady-state levels of IL-4 and IL-4δ2 mRNAs in freshly purified non-stimulated peripheral blood mononuclear cells (PBMC) from 14 patients with asthma (6 mild, 5 moderate, and 3 severe) and 9 healthy age- and gender-matched individuals (Figure 1), by Q-PCR. After RNA isolation and reverse transcription to cDNA, IL-4 and IL-4δ2 mRNA expression levels were measured by Q-PCR. The expression levels of these mRNAs in asthma patients and healthy controls varied significantly and did not, in this small cohort, correlate with disease or its severity, therapies, levels of IgE, or clinical status at the time of blood draw. The levels of IL-4 mRNA were 3.5- to 11-fold higher than those of IL-4δ2 mRNA. Consistent with a previous report by others (compare with Figure 1 in ref. 9), there was a notable correlation between the levels of IL-4 and IL-4δ2, in both patient and control groups. The relative levels of IL-4δ2 mRNA were lower than those of IL-4 mRNA in each of the tested volunteers with one exception (the outlying data point in Figure 1). However, expression of IL-4δ2 mRNA in some individuals was as high or even higher than expression of IL-4 mRNA in other individuals across the cohort. For example, the levels of IL-4δ2 in patients represented by the data points in the upper right corner of the plot in Figure 1 are comparable with the levels of IL-4 in volunteers represented by the points in the middle of the plot. These observations confirm that IL-4δ2 mRNA is expressed in health and disease and that, although expressed in lower concentration than IL-4 mRNA in most individuals, the levels of IL-4δ2 mRNA are comparable to those of IL- 4 mRNA in other individuals, suggesting a likely contribution to immune regulation by IL-4δ2.
Figure 1.
The relationship between IL-4 and IL-4δ2 basal steady-state mRNA levels, as determined by RT-Q-PCR, in PBMC from asthma patients and healthy controls. Each dot represents an individual volunteer, either a healthy control (open circles) or an asthma patient (closed circles).
Activation of purified T cells variably affects the levels of IL-4 and IL- 4δ2 mRNAs
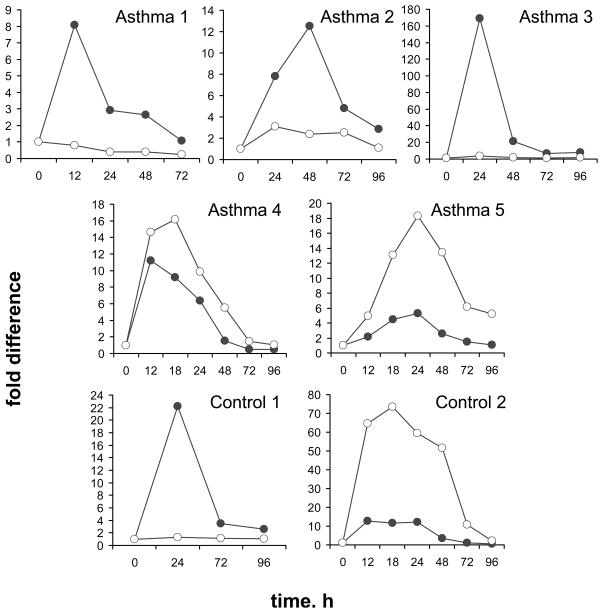
To address the possibility that steady-state IL-4δ2 mRNA levels are affected by cell stimulation, T lymphocytes were freshly purified from blood samples of 5 patients (1 mild, 3 moderate, and 1 severe) and 2 controls and activated with PMA/ionomycin as described in Methods. The cells were then harvested at various times and analyzed for IL-4 and IL-4δ2 mRNA levels. Significant variability in response to such stimulation was observed (Figure 2). For example, the levels of IL-4δ2 mRNA were not affected by activation in T cells obtained from 2 patients with asthma and a healthy control volunteer (Asthma 1, Asthma 3, and Control 1 in Figure 2), whereas IL-4δ2 mRNA levels were affected in other patients with asthma or in a different control volunteer (Asthma 2, Asthma 4, Asthma 5, and Control 2 in Figure 2). Combined with the data shown in Figure 1, the results presented in Figure 2 suggest that significant variability exists in IL-4 and IL-4δ2 mRNA levels in asthma patients and controls under basal conditions and upon cell activation.
Figure 2.
IL-4 (closed circles) and IL-4δ2 (open circles) mRNA levels, as determined by RTQ- PCR, in purified primary T cells from asthma patients and healthy controls following stimulation with PMA/ionomycin.
Primary human T lymphocytes from asthma patients but not healthy controls secrete IL-4δ2 protein
Whether IL-4δ2 protein is naturally expressed is unknown. We previously reported that HEK293 cells infected with IL-4δ2-encoding adenoviral constructs or transfected with a corresponding plasmid construct secrete IL-4δ2 in cell culture (4,5). Similar experiments revealed that primary human T cells transfected with IL-4δ2-encoding plasmid under the control of the CMV promoter also secrete IL-4δ2 into the cell culture supernate at ~150 – 250 pg/ml. Although these observations confirm that IL-4δ2 protein can be secreted by cells expressing IL-4δ2 mRNA, the expression of IL-4δ2 was forced by delivery of artificial genetic constructs. In the present study, we hypothesized that primary T cells naturally produce IL-4δ2 protein and tested whether such production takes place in health and disease. To determine whether primary T cells secreted IL- 4δ2, an ELISA approach was utilized to detect either IL-4 with no cross-reactivity to IL-4δ2 or IL-4δ2 with no cross-reactivity to IL-4, as described in Methods.
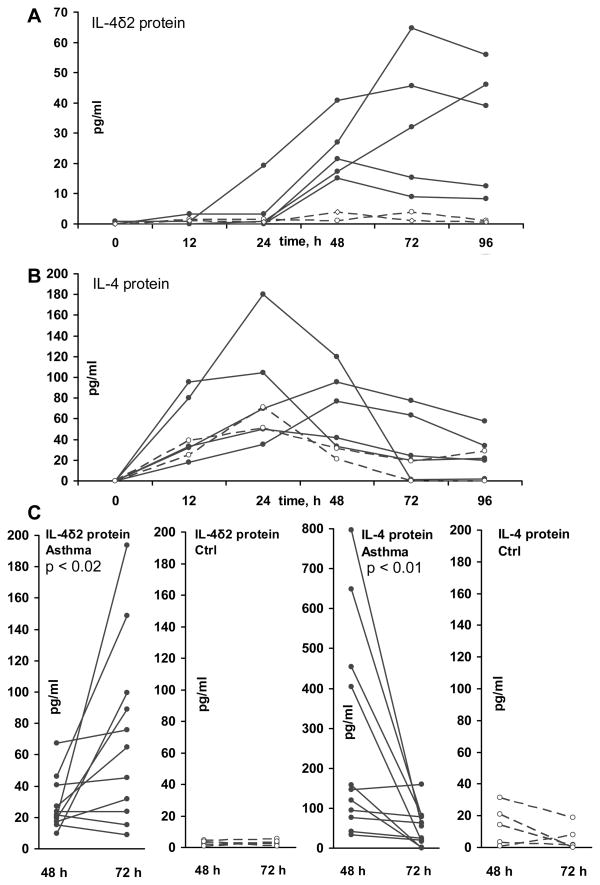
Following activation with PMA/ionomycin, T lymphocytes from patients with asthma, but not from healthy controls, secreted IL-4δ2 into the culture supernate (Figure 3A) in a time-dependent fashion, which was different from the kinetics of the IL-4 level in the same supernates (Figure 3B). Levels of IL-4 tended to peak after 12 – 24 h of cell stimulation, declined by 48 h, and declined further at 72 h. In contrast, levels of IL-4δ2 became detectable only at 12 – 24 h, increased at 48 h, and, in some samples, further increased at 72 and 96 h. The supernates tested in this experiment were from the cultured cells whose mRNA levels for IL-4 and IL-4δ2 had been tested in Figure 2. Despite the variable mRNA patterns in Figure 2, IL-4 and IL-4δ2 proteins showed consistent patterns of upregulation, with notable difference in IL-4δ2 protein production by T cells from patients with asthma, but not from control volunteers.
Figure 3.
IL-4δ2 and IL-4 protein levels, as determined by ELISA, in purified primary T cell culture supernates from asthma patients and healthy controls following stimulation with PMA/ionomycin at indicated times (h). Cytokine levels (pg/ml) in individual cultures are shown. Levels of IL-4δ2 (A) and IL-4 (B) proteins in T cell culture supernates from the same 5 patients with asthma (solid lines) and from 2 healthy controls (dashed lines) as in Figure 2 are shown. In panel C, the levels of IL-4δ2 and IL-4 were also measured in additional primary T cell cultures at 48 h and 72 h for a total of 11 patients with asthma (1 mild, 8 moderate, and 2 severe) and 5 healthy controls. The significance of differences in the cytokine production levels at the measured times was assessed using the Wilcoxon signed rank test.
To confirm that IL-4 levels indeed decrease, while IL-4δ2 levels increase, between 48 h and 72 h, additional similarly purified T cell samples from 6 patients with asthma (5 moderate and 1 severe) and healthy controls were tested at these times, validating this observation (Figure 3C). Again, T cells from patients with asthma, but not from healthy controls, produced IL-4δ2 protein, the levels of which increased later during stimulation, whereas the levels of IL-4 simultaneously declined.
These results show, for the first time, that IL-4δ2 is naturally produced as a protein, particularly in T cells from patients with asthma, but not in T cells from healthy individuals, and that the kinetics of IL-4δ2 production are different from that of IL-4.
Assessment of the production of IL-4δ2 by Th1, Th2, and Th17 lymphocytes
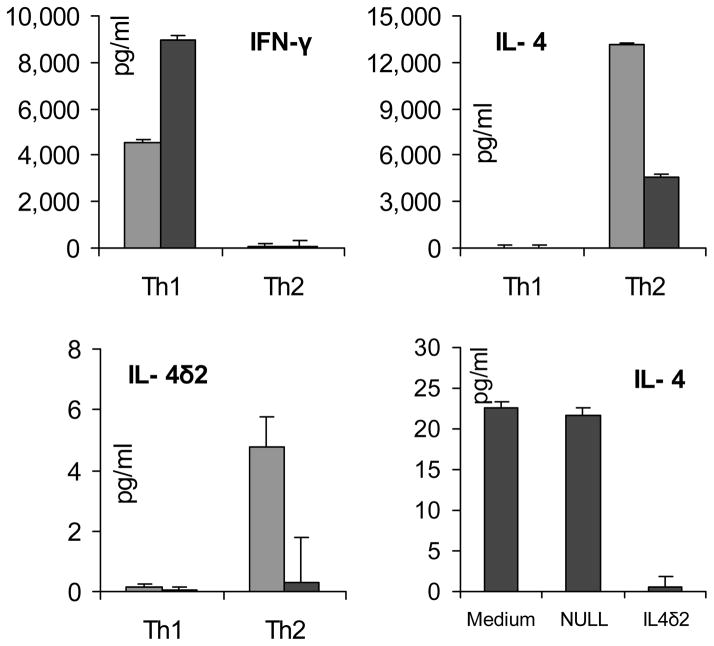
Production of IL-4 defines the Th2 pattern of lymphocyte differentiation, which is typical of atopic asthma. Whether IL-4δ2 is produced by Th2 or perhaps Th1 cells is not known. This question is particularly important in light of our previous observation that, in vivo, IL-4δ2 expression skews the pulmonary milieu toward a pro-inflammatory Th1-like pattern (4,5). To address this issue, naïve primary human T cells from a healthy volunteer were purified and expanded by CD3 stimulation and CD28 co-stimulation using pro-Th1 or pro-Th2 polarizing cocktails of cytokines and neutralizing antibodies, as described in Methods. ELISA assays confirmed successful polarization of cultures toward Th1 cells, producing IFN-γ but not IL-4, or toward Th2 cells, producing IL-4 but not IFN-γ (left and middle panels in Figure 4). ELISA assays of the supernates from the polarized Th1 and Th2 cultures revealed that IL-4δ2 protein is produced in very small, if any, amounts by either Th1 or Th2 lymphocytes (compare the scales for IL-4 and IL-4δ2 levels in Figure 4). Additional experiments assessed secretion of IL-4δ2 by Th17 lymphocytes. Differentiation of primary lymphocytes toward Th17 was driven as described in (20), resulting in 9.5 – 12 % of IL-17+ T cells by intracellular staining with subsequent flowcytometric analyses. These was no detectable IL-4δ2 in the supernates of these cultures. Thus, it appears that Th1, Th2, or Th17 cells are unlikely to be the main source of IL-4δ2 protein observed in primary cell cultures derived from patients with asthma (Figure 3).
Figure 4.
Production of cytokines by artificially polarized Th1 and Th2 cells generated from naïve (CD4+CD45RA+) T cells derived from a patient with moderate asthma (grey) and from a healthy control volunteer (black). In the right lower panel, levels of IL-4 are shown in the Page 25 of 25 supernates of rested Th2-polarized cultures that were additionally incubated with 100 ng/ml IL- 4δ2 or similarly processed NULL control. The ELISAs were repeated on two independent occasions with different volunteers and with similar results.
In a separate experiment, Th2-polarized T cell cultures were washed three times and allowed to rest for 24 h in fresh medium with no additives. Then, additional medium replacement was performed with no additives, with 100 ng/ml rhIL-4δ2, or with a similarly processed NULL control. After 48 h, ELISA assays revealed that IL-4δ2 inhibited production of IL-4 in these Th2- polarized cultures (lower right panel in Figure 4).
Effect of rhIL-4δ2 on production of cytokines by primary human T cells
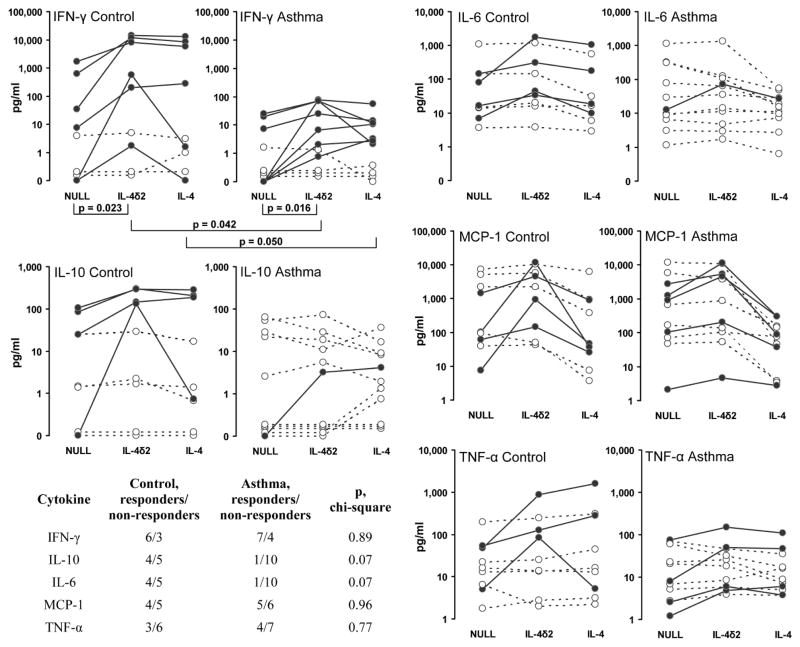
Previous studies in cell culture have suggested that rhIL-4δ2 has no independent effect on proliferation of lymphocytes and that it competes with IL-4 for binding to a shared receptor and mildly inhibits functional effects of IL-4 (2,3). In contrast, previous in vivo studies showed that IL-4δ2 induces lymphocytic inflammation independently of IL-4 (4,5). We therefore hypothesized that IL-4δ2 activates lymphocytes not by inducing proliferation but through activation of cytokine production. Multiplex assays were used to determine whether stimulation with rhIL-4δ2 or rhIL-4 changes the expression levels of cytokines in purified primary T cell cultures compared with cells treated with NULL control. T lymphocytes were purified from 11 patients with allergic asthma (4 mild, 5 moderate, and 2 severe) and 9 healthy controls. Stimulation with IL-4δ2 did not have significant effects on the expression of IL-5, IL-13, IL-17, or eotaxin in cultures from healthy controls or asthma patients. Stimulation with IL-4δ2, compared to treatment with NULL control, affected production of IFN-γ in T cell cultures from healthy controls (p = 0.023, Wilcoxon paired signed rank test) and from asthma patients (p = 0.016). Although the difference in basal (upon treatment with NULL control) levels of IFN-γ production was not significant (p = 0.153, two-tailed unpaired t-test), the differences in the expression of IFN-γ were significant between patients and controls following stimulation with IL-4δ2 (p = 0.042) or IL-4 (p = 0.050). In these assays, some primary T cell cultures did and some did not respond to stimulation with IL-4δ2 by increasing IFN-γ production in both control and patients groups, with no difference in frequency of responders and non-responders between these groups according to the chi-square test. Similarly, responders and non-responders were observed in tests for IL-10, IL-6, MCP-1, and TNF-α production, with responders defined as those cultures in which the levels of the tested cytokine increased two fold or more in response to stimulation with IL-4δ2 compared to NULL-treated cultures. There was a tendency to a higher number of healthy volunteer responders compared to asthmatics, who produced increased IL-10 and IL-6 following stimulation with IL-4δ2 (p = 0.07, chi-square test, in both cases, Figure 5), although the mean production levels of these cytokines did not differ between asthma patients and control and did not change significantly following stimulation with IL-4δ2 or IL-4 (Figure 5). Responder and non-responder T cell cultures were also observed that responded by increasing production of MCP-1 and TNF-α, although there were no statistically significant differences between cultures from patients and controls with or without stimulation with IL-4δ2 (Figure 5). These observations suggest that IL-4δ2 regulates cytokine production by primary T lymphocytes, with particularly pronounced effects on expression of IFN-γ.
Figure 5.
The levels (pg/ml) of indicated cytokines in the supernates of purified T lymphocytes from patients with asthma and from healthy controls following activation with rhIL-4δ2 or rhIL-4. Responders, defined as those cultures in which the cytokine levels changed 2 fold or more in response to stimulation with IL-4δ2 for 48 h, are shown as filled circles connected by solid lines, whereas non-responders are shown as open circles connected with dashed lines. The Wilcoxon signed rank test was used to assess the effects of stimulation within the control and asthma groups, whereas Student’s two-tailed t-test was used to compare cytokine levels in cultures from patients with asthma versus cultures from healthy controls. The table in the lower left corner shows the differences between asthma patient and control groups in the numbers of responders and non-responders and the significance of these differences according to the chi-square test.
Discussion
The goal of this study was to determine whether alternatively spliced Interleukin-4 (IL-4δ2) is naturally produced in humans, whether its production is associated with asthma, and whether it has functional effects on T lymphocytes. The number of volunteers studied was sufficient to clarify these issues, but substantially larger populations will be required to determine with statistical certainty whether an association exists between the levels of IL-4δ2 protein production and severity of asthma and whether therapeutic interventions affect such an association.
Various levels for IL-4δ2 and IL-4 mRNAs were observed in PBMC from asthma patients and healthy controls (Figure 1). The steady-state expression levels of IL-4δ2 mRNA were comparable with, although consistently lower than, those of IL-4 mRNA. However, expression of IL-4δ2 mRNA in some individuals was as high or even higher than expression of IL-4 mRNA in other individuals across the cohort (Figure 1), suggesting that IL-4δ2, if produced as a protein, may be a viable functional member of the cytokine network.
Further experiments revealed that stimulation of purified primary T cells induces the expression of IL-4δ2 and IL-4 mRNAs, but there was a significant variability among patients with asthma and healthy controls (Figure 2). Considering the substantial variability in IL-4δ2 mRNA profiles in non-stimulated (Figure 1) and stimulated (Figure 2) cells, the possibility exists that there is a post-transcriptional regulation, and that the expression of IL-4δ2 protein may be more consistent than that of IL-4δ2 mRNA. While the existence of IL-4δ2 protein has never been demonstrated in the past, we measured, for the first time, the production of IL-4δ2 protein. Following activation with PMA/ionomycin, T lymphocytes from patients with asthma, but not from healthy controls, secreted IL-4δ2 into the culture supernate in a time-dependent fashion, which was different from the kinetics of the IL-4 levels in the same supernates (Figure 3). Despite the variable mRNA levels (Figures 1 and 2), production of IL-4δ2 and IL-4 proteins was consistently observed in T cells from patients with asthma, but not in healthy controls (Figure 3). This is the first demonstration of natural production of IL-4δ2 protein in a disease context.
The possibility was considered that Th2 or perhaps Th1 or Th17 lymphocytes may be the main source of IL-4δ2 protein observed in cell cultures from patients with asthma. Polarized Th2, Th1, or Th17 lymphocytes did not produced substantial levels of IL-4δ2 (Figure 4). A conclusion was made that T cell subpopulations different from classical Th2, Th1, or Th17 are likely responsible for most of the IL-4δ2 observed in T cell cultures in Figure 3.
Previous data suggested that IL-4δ2 induces immune inflammation in vivo (4,5), although IL- 4δ2 did not activate proliferation of cultured lymphocytes (2,3). The possibility was considered that IL-4δ2 regulates lymphocytes not by inducing proliferation but through activation of cytokine production. Many T cell cultures from patients with asthma and healthy controls responded to IL-4δ2 stimulation (Figure 5). Production of IFN-γ was significantly increased by IL-4δ2 stimulation in T cells from healthy controls and from patients with asthma, consistent with the previous findings of elevated IFN-γ upon IL-4δ2 gene delivery in vivo (4,5). This consistency of findings between cell culture and in vivo studies suggests that IL-4δ2 is a pro-Th1 cytokine. There was no difference in the fraction of IFN-γ responders to IL-4δ2 stimulation between healthy controls and patients with asthma, although IL-4δ2-stimulated cell cultures from healthy controls produced higher levels of IFN-γ (Figure 5). There was a tendency to a higher proportion of responders to IL-4δ2 stimulation among healthy controls that produced increased levels of IL-10 and IL-6 (Figure 5). Thus, IL-4δ2 stimulates cytokine production by primary T lymphocytes, with particularly pronounced effects on the expression of IFN-γ and notable differences between patients with asthma and healthy controls in the production of IFN-γ, IL-10, and IL-6.
In light of our data, the question of the potential biomedical relevance of IL-4δ2 must be asked. Its mRNA (Figures 1 and 2) and protein (Figure 3) expression levels appear to be comparable with, but overall lower than, those of IL-4. Moreover, IL-4 and IL-4δ2 likely share the same cell surface receptor (2–5), and the receptor affinity of IL-4 appears to be much higher than that of IL-4δ2, suggesting that IL-4 will easily outcompete IL-4δ2 for binding to the receptor (2,5). These considerations suggest that a lesser role for IL-4δ2 in immune regulation. However, it is important to consider that cytokines generally act over short distances, usually in the immediate vicinity of the producing cells where the concentration remains high. It is therefore conceivable that an IL-4δ2-secreting cell would regulate its neighboring cells irrespective of IL-4 secreted by other cells elsewhere. This mechanism is speculative and needs to be validated by demonstrating exclusive secretion of IL-4δ2 and IL-4 by individual cells. The second indication of the potential role of IL-4δ2 stems from the observation that the kinetics of IL-4 and IL-4δ2 secretion upon cell activation differ (Figure 3), and the two isoforms likely do not occur together at the same time. Secretion of IL-4δ2 occurs later, when the levels of IL-4 have declined (Figure 3). Considering that the physiological effects of IL-4δ2 are consistent with the Th1 activation pattern, it is possible that the switch from IL-4 to IL-4δ2 production represents the early stages of a regulatory mechanism by which the Th2 effects of IL-4 are inhibited through IL-4δ2/pro-Th1 regulation at the end of a physiological Th2 immune reaction. The purpose of such regulation would be to prevent an unnecessarily prolonged or exaggerated Th2 response (Figure 3, lower right panel). Finally, the results presented above suggest that IL- 4δ2 protein may at least be used as a molecular marker associated with the asthma phenotype.
In summary, IL-4δ2 is natively produced not only as mRNA but also as a protein by cells other than Th1 or Th2, its production is associated with allergic asthma, and it regulates production of other cytokines by primary T lymphocytes. Further studies are necessary to assess a possible association between severity of asthma and the levels of IL-4δ2 protein expression, which would identify IL-4δ2 as a biomarker of asthma. A more detailed mechanistic understanding of IL-4δ2-induced inflammation needs to be developed, which might identify IL- 4δ2 as a new molecular target for future asthma therapies.
Highlights.
An alternative splice variant of IL-4 protein termed IL-4δ2 is naturally produced.
IL-4δ2 protein is produced by T cells from asthma patients but not from controls.
Kinetics of induced production IL-4δ2 and IL-4 proteins differ.
IL-4δ2 mRNA and protein are discordant suggesting post-transcriptional regulation.
Recombinant IL-4δ2 regulates production of cytokines by T cells in culture.
Acknowledgments
This study was funded by VA Merit Review Awards (IGL and SPA), and NIH R21HL106196 (SPA). We thank Dr. Igor Dubinkin for technical help in production of anti-IL-4δ2 mAb, Dr. Alexander Bocharov for technical help with some of the initial experiments, and Dr. Paul Todd for his expert editorial assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alms WJ, Atamas SP, Yurovsky VV, White B. Generation of a variant of human interleukin-4 by alternative splicing. Mol Immunol. 1996;33:361–70. doi: 10.1016/0161-5890(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 2.Atamas SP, Choi J, Yurovsky VV, White B. An alternative splice variant of human IL-4, IL-4 delta 2, inhibits IL-4-stimulated T cell proliferation. J Immunol. 1996;156:435–41. [PubMed] [Google Scholar]
- 3.Arinobu Y, Atamas SP, Otsuka T, Niiro H, Yamaoka K, Mitsuyasu H, Niho Y, Hamasaki N, White B, Izuhara K. Antagonistic effects of an alternative splice variant of human IL-4, IL- 4delta2, on IL-4 activities in human monocytes and B cells. Cell Immunol. 1999;191:161–7. doi: 10.1006/cimm.1998.1431. [DOI] [PubMed] [Google Scholar]
- 4.Luzina IG, Lockatell V, Todd NW, Keegan AD, Hasday JD, Atamas SP. Splice isoforms of human interleukin-4 are functionally active in mice in vivo. Immunology. 2011;132:385–93. doi: 10.1111/j.1365-2567.2010.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzina IG, Lockatell V, Todd NW, Highsmith K, Keegan AD, Hasday JD, Atamas SP. Alternatively spliced variants of interleukin-4 promote inflammation differentially. J Leukoc Biol. 2011;89:763–70. doi: 10.1189/jlb.0510271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, Alms WJ, White B. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42:1168–78. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Sakkas LI, Tourtellotte C, Berney S, Myers AR, Platsoucas CD. Increased levels of alternatively spliced interleukin 4 (IL-4delta2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin Diagn Lab Immunol. 1999;6:660–4. doi: 10.1128/cdli.6.5.660-664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glare EM, Divjak M, Rolland JM, Walters EH. Asthmatic airway biopsy specimens are more likely to express the IL-4 alternative splice variant IL-4delta2. J Allergy Clin Immunol. 1999;104:978–82. doi: 10.1016/s0091-6749(99)70078-3. [DOI] [PubMed] [Google Scholar]
- 9.Seah GT, Gao PS, Hopkin JM, Rook GA. Interleukin-4 and its alternatively spliced variant (IL-4delta2) in patients with atopic asthma. Am J Respir Crit Care Med. 2001;164:1016–8. doi: 10.1164/ajrccm.164.6.2012138. [DOI] [PubMed] [Google Scholar]
- 10.Bijlsma FJ, van Kuik J, van Hoffen E, de Jonge N, Tilanus MG, Gmelig-Meyling FH, de Weger RA. Acute cardiac transplant rejection is associated with low frequencies of interleukin-4 producing helper T-lymphocytes rather than with interleukin-4 promoter or splice variants. Hum Immunol. 2002;63:317–23. doi: 10.1016/s0198-8859(02)00370-1. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher HA, Owiafe P, Jeffries D, Hill P, Rook GA, Zumla A, Doherty TM, Brookes RH Vacsel Study Group. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4delta2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology. 2004;112:669–73. doi: 10.1111/j.1365-2567.2004.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dheda K, Chang JS, Breen RA, Kim LU, Haddock JA, Huggett JF, Johnson MA, Rook GA, Zumla A. In vivo and in vitro studies of a novel cytokine, interleukin 4delta2, in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:501–8. doi: 10.1164/rccm.200502-278OC. [DOI] [PubMed] [Google Scholar]
- 13.Wu HP, Wu CL, Chen CK, Chung K, Tseng JC, Liu YC, Chuang DY. The interleukin-4 expression in patients with severe sepsis. J Crit Care. 2008;23:519–24. doi: 10.1016/j.jcrc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Orsini B, Vivas JR, Ottanelli B, Amedei A, Surrenti E, Galli A, Milani S, Pinzani P, Del Prete G, Surrenti C, Baldari CT, Touati E, D’ Elios MM. Human gastric epithelium produces IL- 4 and IL-4delta2 isoform only upon Helicobacter pylori infection. Int J Immunopathol Pharmacol. 2007;20:809–18. doi: 10.1177/039463200702000417. [DOI] [PubMed] [Google Scholar]
- 15.Waldvogel AS, Lepage MF, Zakher A, Reichel MP, Eicher R, Heussler VT. Expression of interleukin 4, interleukin 4 splice variants and interferon gamma mRNA in calves experimentally infected with Fasciola hepatica. Vet Immunol Immunopathol. 2004;97:53–63. doi: 10.1016/j.vetimm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Vasiliev AM, Vasilenko RN, Kulikova NL, Andreev SM, Chikileva IO, Puchkova GY, Kosarev IV, Khodyakova AV, Khlebnikov VS, Ptitsyn LR, Shcherbakov GY, Uversky VN, DuBuske LM, Abramov VM. Structural and functional properties of IL-4delta2, an alternative splice variant of human IL-4. J Proteome Res. 2003;2:273–81. doi: 10.1021/pr025586y. [DOI] [PubMed] [Google Scholar]
- 17.Andreev SM, Dubinkin IV, Petrukhina AO, Vasiliev AM, Kosarev IV, Tokhtamysheva NV, Puchkova GY, Babakhin AA, DuBuske LM. B Epitope assay of hIL-4 delta 2, an alternative splicing variant of hIL-4. Clin Immunol. 2005;115:S57. [Google Scholar]
- 18.Andreev S, Petrukhina A, Bashkatova Y, Dubinkin I, Vasiliev A, Babakhin A, DuBuske L. Development of an Assay to Detect IL-4δ2. Clin Immunol. 2006;119:124. [Google Scholar]
- 19.Andreev SM, Bashkatova YN, Petrukhina AO, Dubinkin IV, Vasiliev AM, Abramov VM, Khlebnikov VS, Kulikova NL, Khaitov MR. Human IL-4delta2: structure and Quantitative assay. Immunologiya (Moscow, Russia) 2010;(1):18–24. [Google Scholar]
- 20.Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, Rückert B, Meiler F, Akdis M, Littman DR, Akdis CA. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–15. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]