Abstract
A two-year-old, female, simian immunodeficiency virus E543-infected rhesus macaque (Macaca mulatta) was presented for necropsy following euthanasia due to a history of diarrhea, weight loss, and a small, round ulcer along the left labial commisure. Histopathologic examination of the ulcer revealed infiltration by large numbers of degenerate and non-degenerate neutrophils and macrophages admixed with syncytial epithelial cells. Rare epithelial cells contained herpetic inclusion bodies. These cells stained positive for Human herpesvirus 1 via immunohistochemistry and DNA sequencing confirmed the presence of closely related Macacine herpesvirus 1 (B virus).
Keywords: Cercopithecine herpesvirus 1, cheilitis, Macaca mulatta
A two-year-old female rhesus macaque was imported into the New England Primate Research Center conventional colony following routine quarantine where it tested seropositive for simian T-lymphotropic virus, but negative for simian retrovirus and Macacine herpesvirus 1 (B virus). The animal was inoculated intravenously with simian immunodeficiency virus (SIV) E543 one year prior to presentation as part of a viral titration study. Excepting routine phlebotomy, no other experimental manipulations were performed. During the course of one year, the animal developed diarrhea and weight loss, consistent with chronic SIV infection. Three days prior to euthanasia, a small, round ulcer was noted at the left labial commisure. At necropsy, the ulcer measured 5 mm in diameter and spanned the mucocutaneous junction of the lip. Apart from soft stool and splenic follicular hyperplasia, common findings with chronic SIV infection, no significant gross lesions were observed.
Microscopic Findings
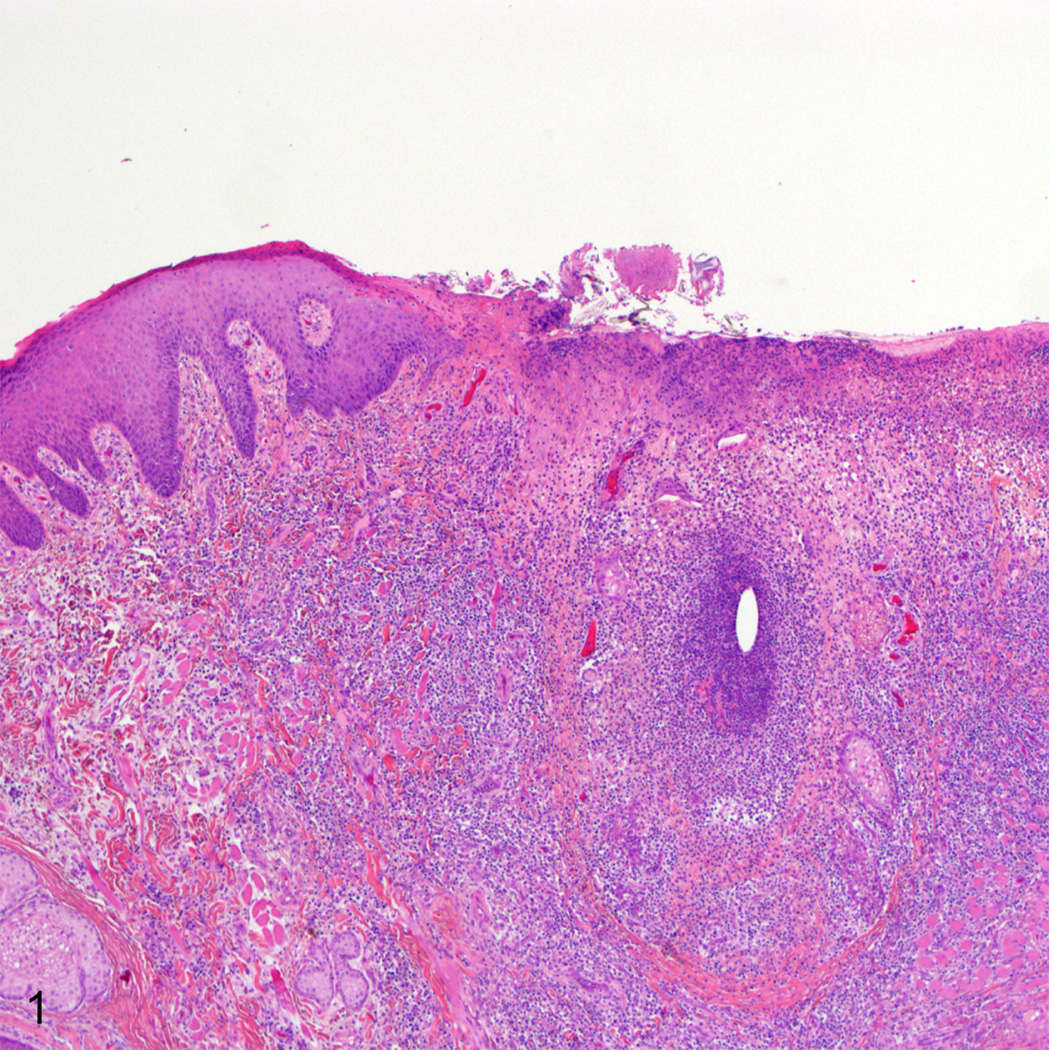
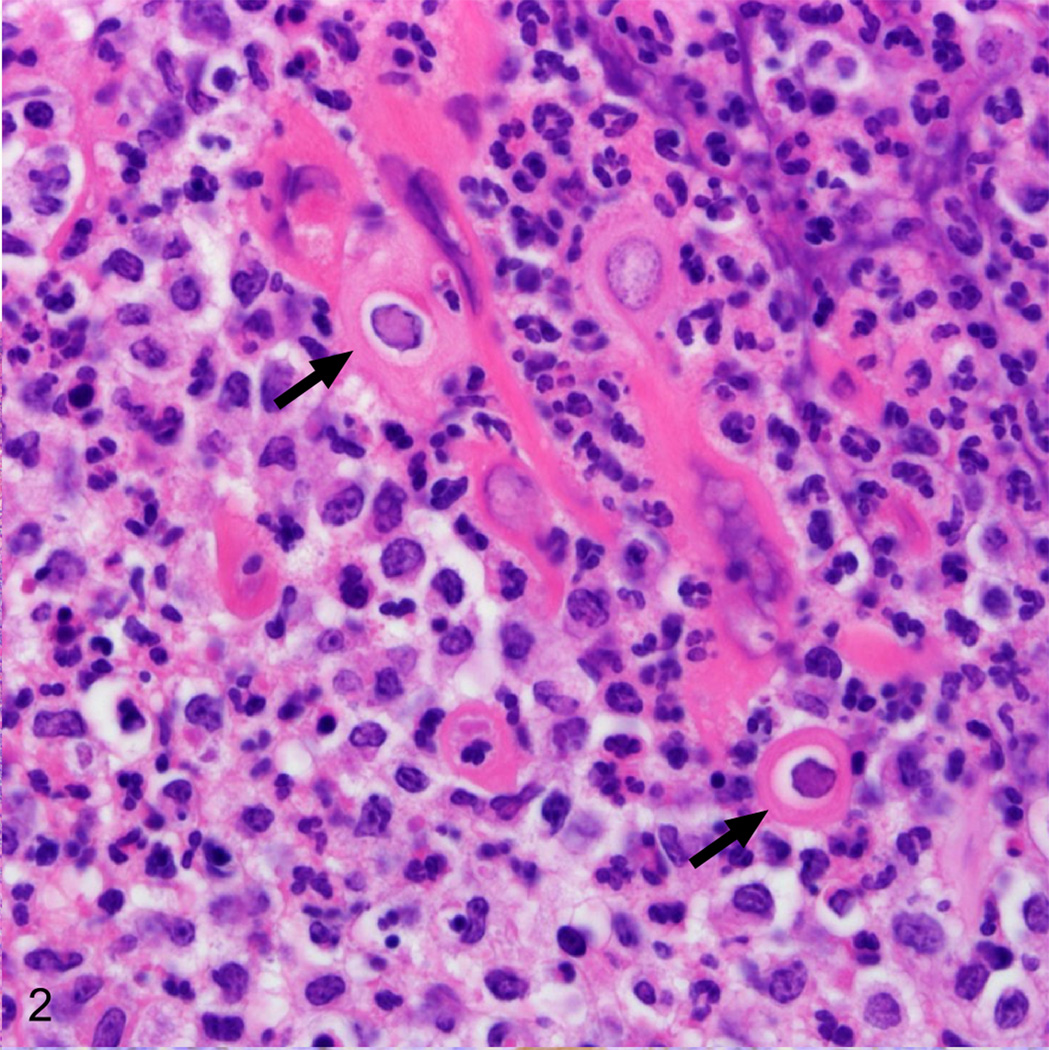
Histopathologic examination revealed a crateriform defect spanning the mucocutaneous junction of the left lip that was covered with a thick mat of fibrin, cellular debris, and scattered colonies of coccal bacteria. The border between the adjacent viable epithelium and the necrotic epithelium was sharply demarcated and the underlying lamina propria was infiltrated by large numbers of neutrophils and occasional macrophages (Fig. 1). Rare epithelial cells bordering the ulcer and associated with remnants of hair follicles contained ill-defined, glassy, eosinophilic, intranuclear inclusion bodies (Fig. 2). Syncytial cells containing between two to seven nuclei were present deep within the lesion.
Fig. 1.
Lip, rhesus macaque. There is a large ulcer along the lip border that contains large numbers of degenerate neutrophils and scattered remnant hair follicles. HE.
Fig. 2.
Lip, rhesus macaque. Within the nuclei of epithelial cells of the hair follicle remnants there are large intranuclear inclusions (arrows). HE.
Differential Diagnoses
Ulcerative cheilitis and stomatitis in macaques is usually traumatic or viral in origin. The histology, however, confirms a viral etiology. Salient features of this case include 1) localized presentation, 2) primarily ulcerative lesion, 3) viral tropism for cutaneous and mucous epithelia, 4) formation of intranuclear inclusion bodies, and 5) the presence of syncytial epithelial cells. The primary differential diagnosis is Macacine herpesvirus 1. To the authors’ knowledge, clinical cases of Human herpesvirus 1 (herpes simplex virus-1/HSV-1) have not been reported in macaques but HSV-1 causes similar lesions in a variety of other primate species and was considered as a possible diagnosis.8 Both of these viruses will cause localized ulcerative oral lesions with syncytia and intranuclear inclusions.
Patent infections with the β-herpesvirus rhesus cytomegalovirus (rhCMV) in rhesus macaques may also result in ulcerative oral lesions. This occurs most commonly through neutrophilic facial neuritis leading to inadvertent self-trauma to the affected area. rhCMV will also form intranuclear inclusion bodies, but it rarely causes epithelial syncytia, nor does it exhibit tropism for keratinocytes or oral mucosal epithelium.1 The opportunistic γ-herpesvirus rhesus lymphocryptovirus (rhLCV) has a predilection for oral and esophageal mucosa and forms intranuclear inclusions, but the lesions consist of plaques of proliferative, parakeratotic epithelium rather than ulcers.2
Another α-herpesvirus, simian varicella virus (SVV), can cause both syncytia and intranuclear inclusions, but cutaneous lesions are more common than oral involvement and the disease is systemic rather than localized.3 Measles virus (MV) can cause syncytia and intranuclear (as well as intracytoplasmic) inclusions and infection can result in small white lesions of the oral mucosa known as Koplik’s spots. Koplik’s spots, however, are papular eruptions characterized by parakeratosis rather than ulceration.10 Also, similar to simian varicella, measles presents as a systemic illness. Likewise, adenoviruses (AV) and polyomaviruses (PV) can cause intranuclear inclusions, but these viruses do not target skin or oral mucosa. A summary of potential etiologies and their associated characteristics is provided in Table 1.
Table 1.
Salient diagnostic characteristics of selected viral agents of rhesus macaques.
| Virusa | Tropism | Lesion Type | Single Lesion |
Inclusions | Syncytia |
|---|---|---|---|---|---|
| B virus | ganglia, oral mucosa, skin | vesicular, ulcerative | common | eosinophilic, intranuclear | common |
| HSV-1 | ganglia, oral mucosa, skin | vesicular, ulcerative | common | eosinophilic, intranuclear | common |
| rhCMV | nerves, ganglia, gut, lung, kidney | necrotizing, neutrophilic | common | eosinophilic, intranuclear | rare |
| rhLCV | oral mucosa, esophagus, B-cells | plaque-like, parakeratotic | common | amphophilic, intranuclear (epithelial only) | never |
| SVV | skin, lymphoid, lung, liver, oral mucosa (uncommon) | vesicular | never | eosinophilic, intranuclear | common |
| MV | skin, lymphoid, lung, brain, oral mucosa | papular, parakeratotic | never | eosinophilic, intranuclear and intracytoplasmic | common |
| AV | gut | none to erosive | common | basophilic, intranuclear | never |
| PV | brain, kidney | necrotizing, lymphoplasmacytic | common | amphophilic, intranuclear | rare |
Abbreviations: HSV-1: Herpes simplex virus-1, rhCMV: rhesus cytomegalovirus, rhLCV: rhesus lymphocryptovirus, SVV: simian varicella virus, MV: measles virus, AV: adenovirus, PV: polyomavirus
Immunohistochemistry and DNA Sequencing
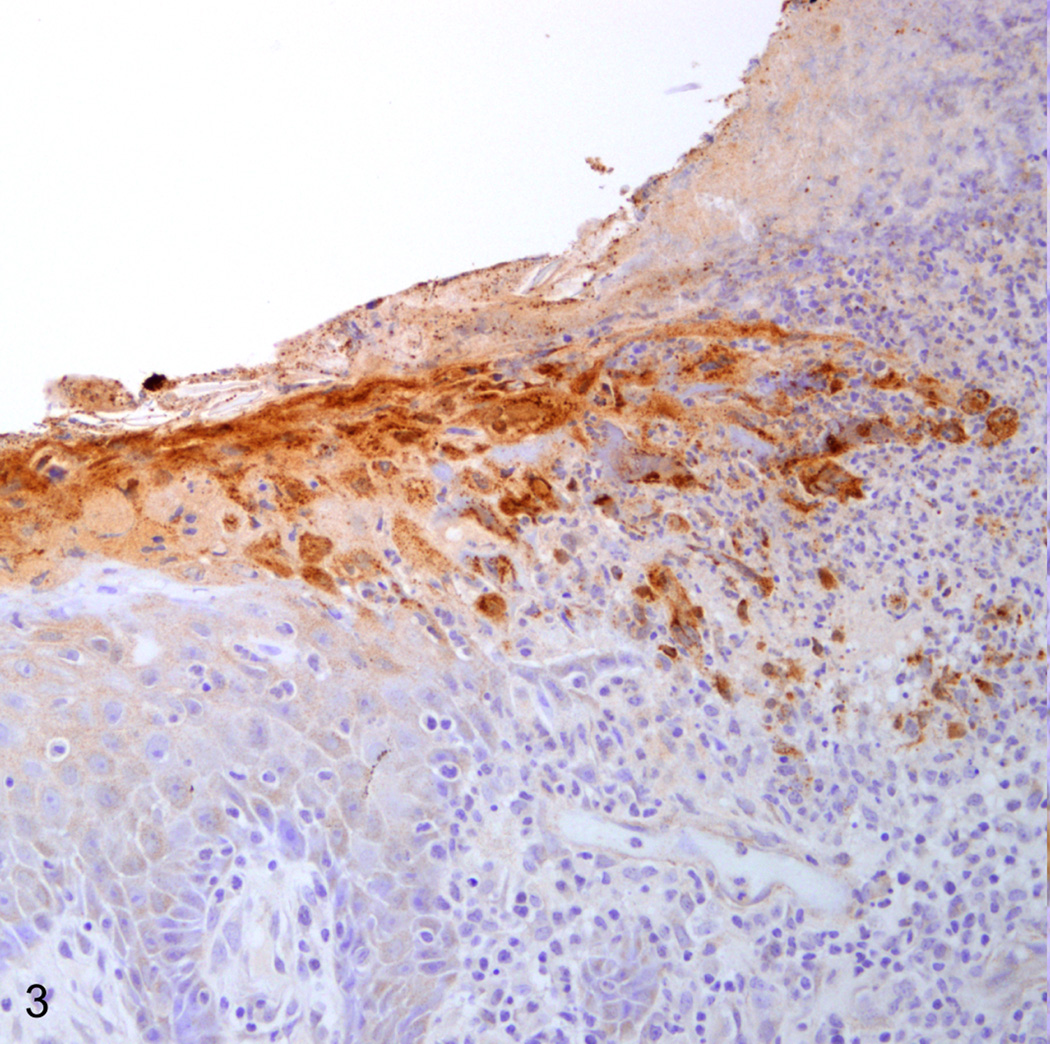
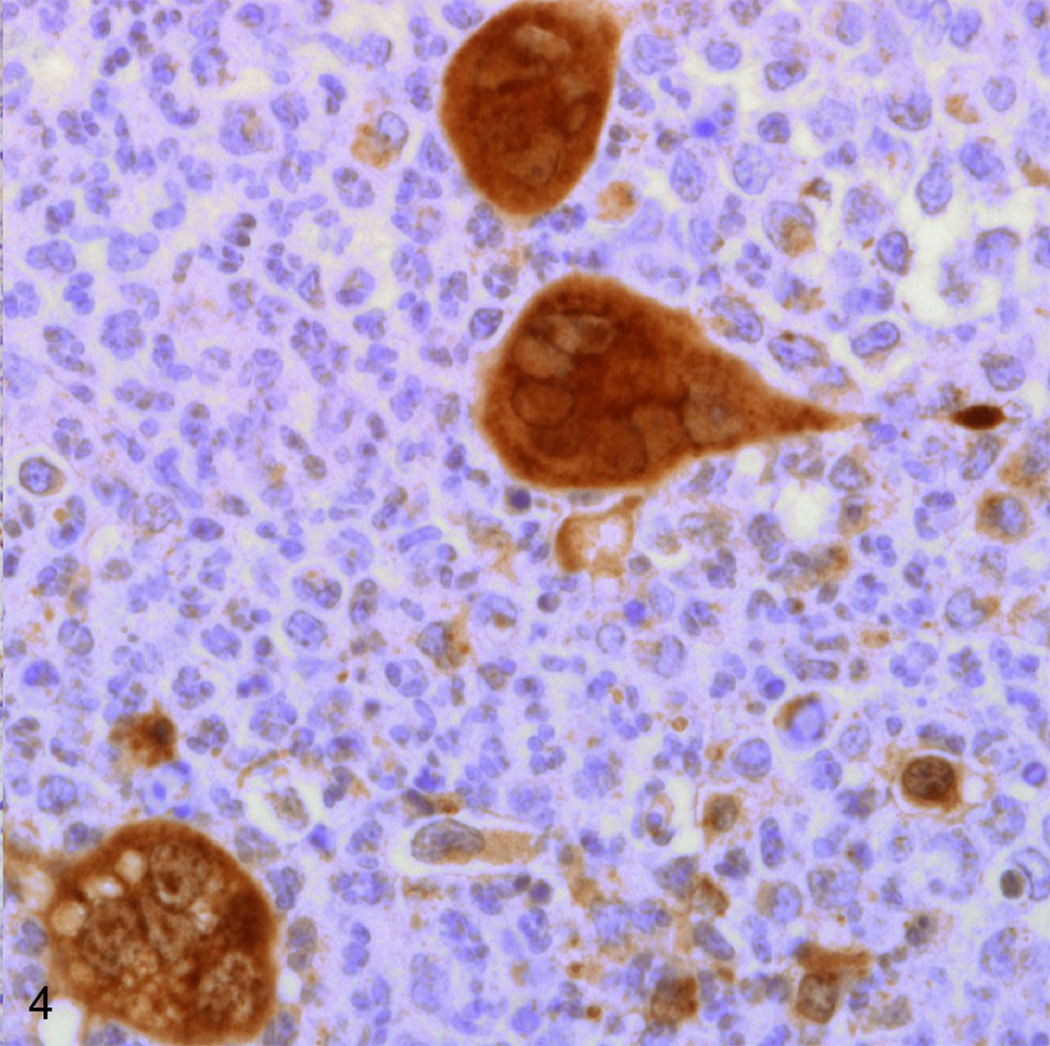
Immunohistochemical staining for Simplexviruses was carried out using a rabbit polyclonal antibody to HSV-1 lysate (Dako B0114, Dako Corporation, Carpinteria, CA) at a 1:1000 dilution. Epithelium bordering the lesion and syncytia deeper within the lesion were both strongly positive (Fig. 3, 4). To differentiate between B virus and HSV-1, DNA was isolated from the paraffin block containing the lip lesion by means of the QIAGEN QIAamp DNA FFPE Tissue Kit (Valencia, CA). Polymerase chain reaction (PCR) was carried out using primers 5’-CAA GCT CAC CGA CGT CTA CA-3’ and 5’-CCG GTA GTT GAG GTC CTT CTT-3’ designed to amplify a 244 base pair fragment from B virus UL30. A single band of the expected size was isolated by agarose gel electrophoresis and submitted for DNA sequencing. 171 base pairs of interpretable non-primer sequence were read and showed 100% homology to Macacine herpesvirus 1 strain E2490 (Genbank AF533768.1) by BLAST search. By contrast, the closest matching human herpesviruses were various isolates of Human herpesvirus 2 (beginning with Genbank HQ123176.1) which showed only 91% homology.
Fig. 3.
Lip, rhesus macaque. Immunohistochmical staining for Herpes simplex virus-1 highlights degenerate and necrotic epithelium at the lesion border. Immunoperoxidase staining/Meyer’s hematoxylin (Dako, Carpinteria, CA).
Fig. 4.
Lip, rhesus macaque. Large syncytial cells deep within the lesion are Herpes simplex virus-1 positive via immunohistochemical staining. Immunoperoxidase staining/Meyer’s hematoxylin (Dako, Carpinteria, CA).
Diagnosis
Following histopathologic and immunohistochemical examination, the diagnosis in this case was severe, locally extensive, acute, necroulcerative cheilitis with syncytial cells and intranuclear herpetic inclusions (Macacine herpesvirus 1).
Discussion
Current taxonomic nomenclature refers to B virus as Macacine herpesvirus 1, but older reports use the names Cercopithecine herpesvirus 1 or Herpesvirus simiae. B virus is an α-herpesvirus of the Simplexvirus genus closely related to HSV-1 of humans. Disease in macaques closely parallels that of HSV-1 in infected humans. Infection is normally asymptomatic due to latent infection of the trigeminal ganglia and shedding usually occurs in the absence of any gross lesions. When gross lesions do occur they are typically mild and self-limiting, consisting of conjunctivitis, vesicles on the lips and face, or ulcers in the oral mucosa that heal without scarring. Cases of fulminate, disseminated infection have been reported but these are uncommon.7,9 Although this particular animal was immunosuppressed due to concurrent SIV infection, B virus shedding and patent infection can occur in immunocompetent animals and B virus should be considered a primary, rather than opportunistic, infection. Although macaques are the primary host and reservoir species, one report has confirmed natural transmission to capuchin monkeys suggesting the potential host range may be broader than currently appreciated.5
Concern over B virus arises due to its high zoonotic potential. In humans, the virus causes severe encephalomyelitis that approaches an 80% mortality rate in untreated cases. B virus is thus classified as a Risk Group 4 pathogen and select agent by the United States Department of Health and Human Services. Transmission usually occurs via infected saliva. Veterinarians and animal care workers are at high risk due to the potential for bite wounds, needle sticks, and potential splash exposures to eyes. Personnel working with unfixed tissues of macaque origin must also exercise extreme caution. Symptoms in humans include painful or pruritic vesication at the site of inoculation within 1–5 days followed by paresthesia and/or paresis in the exposed extremity progressing to central nervous system involvement. In rare instances, primary exposure is never recognized and CNS symptoms develop acutely within a month. Serologic diagnosis in infected humans is difficult due false positives resulting from cross-reactive anti-HSV antibodies. Accurate serological assessments must be made with competitive ELISA and confirmed by B virus antigen-specific western blot.6 Post-exposure prophylaxis and therapy consist of treatment with thymidine analogues (the “-cyclovir” family of drugs).4
Due to the serious zoonotic risk, research colonies should attempt to maintain B virus-free animals. The animal discussed in this case tested seronegative during quarantine; however the original supplier had documented a previously positive test and ante-mortem serum collected from the animal and submitted to the National B Virus Resource Center yielded an indeterminate result. The unreliability of serologic testing underscores the need to maintain adherence to proper biosecurity protocols when working with a macaque colony.
Acknowledgments
Funding
This research was funded, in part, by NIH grants RR00168 and RR07000.
References
- 1.Baskin GB. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am J Pathol. 1987;129:345–352. [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin GB, Roberts ED, Kuebler D, Martin LN, Blauw B, Heeney J, Zurcher C. Squamous epithelial proliferative lesions associated with rhesus Epstein-Barr virus in simian immunodeficiency virus-infected rhesus monkeys. J Infect Dis. 1995;172:535–539. doi: 10.1093/infdis/172.2.535. [DOI] [PubMed] [Google Scholar]
- 3.Blakely GA, Lourie B, Morton WG, Evans HH, Kaufmann AF. A varicella-like disease in macaque monkeys. J Infect Dis. 1973;127:617–625. doi: 10.1093/infdis/127.6.617. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE. Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1) Clin Infect Dis. 2002;35:1191–1203. doi: 10.1086/344754. [DOI] [PubMed] [Google Scholar]
- 5.Coulibaly C, Hack R, Seidl J, Chudy M, Itter G, Plesker R. A natural asymptomatic herpes B virus infection in a colony of laboratory brown capuchin monkeys (Cebus apella) Lab Anim. 2004;38:432–438. doi: 10.1258/0023677041958891. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Hilliard J, Southers J, Murray M, Savarese B, Schmitt JM, Straus SE. A controlled seroprevalence survey of primate handlers for evidence of asymptomatic herpes B virus infection. J Infect Dis. 1995;171:1031–1034. doi: 10.1093/infdis/171.4.1031. [DOI] [PubMed] [Google Scholar]
- 7.Scharf BA, Wan CH, Bluth M, Eberle R, Videan EN, Smith E, Coplan J. Lethargy, ulcers, bronchopneumonia and death in two aged female bonnet macaques presumed to be caused by Cercopithicine herpes virus I. J Med Primatol. 2008;37 Suppl 1:60–64. doi: 10.1111/j.1600-0684.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 8.Schrenzel MD, Osborn KG, Shima A, Klieforth RB, Maalouf GA. Naturally occurring fatal herpes simplex virus 1 infection in a family of white-faced saki monkeys (Pithecia pithecia pithecia) J Med Primatol. 2003;32:7–14. doi: 10.1034/j.1600-0684.2003.01040.x. [DOI] [PubMed] [Google Scholar]
- 9.Simon MA, Daniel MD, Lee-Parritz D, King NW, Ringler DJ. Disseminated B virus infection in a cynomolgus monkey. Lab Anim Sci. 1993;43:545–550. [PubMed] [Google Scholar]
- 10.Suringa DW, Bank LJ, Ackerman AB. Role of measles virus in skin lesions and Koplik's spots. N Engl J Med. 1970;283:1139–1142. doi: 10.1056/NEJM197011192832105. [DOI] [PubMed] [Google Scholar]