Abstract
Background
MK-2206 is a small molecule allosteric inhibitor of Akt/PKB that is undergoing clinical trials for treatment of cancer.
Procedures
MK-2206 was tested against the PPTP in vitro panel using a 96 hour exposure (1.0 nM-10 μM), and in vivo using thrice weekly dosing for a planned 4 weeks at its maximum tolerated dose (MTD) of 180 mg/kg.
Results
In vitro, the median relative IC50 value for MK-2206 was 2.2μM. Four cell lines with IC50 values < 200 nM included two ALL cell lines (COG-LL-317 and RS4;11), an AML cell line with an activating KIT mutation (Kasumi-1), and a Ewing sarcoma cell line (CHLA-10). In vivo, MK-2206 induced significant differences in EFS distribution compared to control in 12 of 29 (41%) of the evaluable solid tumor xenografts and in 2 of 8 (25%) of the evaluable ALL xenografts. Significant differences in EFS distribution were most frequently noted in the osteosarcoma panel (6 of 6). A single solid tumor xenograft (OS-31) had a greater than two-fold increase in time to event compared to control animals, with all other solid tumor xenografts showing lesser degrees of tumor growth inhibition. Objective responses were not observed for either the solid tumor or ALL xenografts.
Conclusions
MK-2206 showed its most consistent activity in vitro against ALL cell lines and in vivo against osteosarcoma xenografts. However, no objective responses were observed in solid tumor or ALL xenografts. Further preclinical work evaluating MK-2206 in pediatric models in the combination therapy setting may contribute to its pediatric development.
Keywords: Preclinical Testing, Developmental Therapeutics, MK-2206
INTRODUCTION
The PI3K/Akt signaling pathway appears to be activated in many adult carcinomas, either through enhanced growth factor receptor signaling, constitutive activation of PI3K, or loss of the negative regulator PTEN. There is some evidence that this pathway is activated in pediatric cancers [1]. Akt kinases (or protein kinase B, PKB) are three serine-threonine kinase isoforms in a signaling pathway that senses growth factor stimulation and mediates cell proliferation, metabolism and death (reviewed in [2,3]). Akt phosphorylation is detectable in a number of pediatric solid tumors, including neuroblastoma [4], rhabdomyosarcoma [5], and Ewing sarcoma [6]. In neuroblastoma clinical specimens, Akt was highly phosphorylated at Ser473 in 61.2% (71 of 116) and at Thr308 in 62.9% (73 of 116) of cases, with 66 (56.9%) cases positive at both sites [4]. Akt activation is a marker of poor prognosis in neuroblastoma [4], in which both IGF-1R signaling [7,8] and BDNF/TrkB signaling [9–11] have been suggested as initiators of activation of PI3K and Akt. For rhabdomyosarcoma clinical specimens, pAktThr308 was elevated in 42% and 35% of alveolar and embryonal rhabdomyosarcoma cases, respectively, while pAktSer473 was increased in 43% of alveolar rhabdomyosarcoma and 55% of embryonal rhabdomyosarcoma. The latter phosphorylation was associated with lower overall survival (p< 0.001) and recurrence-free survival (p< 0.0009) [5]. Similarly, Akt activation has been demonstrated in several hematological malignancies [12].
Phosphorylation at threonine residue 308 (pAktThr308) is a requirement for kinase activity and is mediated by PDK1, while further phosphorylation at serine 473 (pAktSer473) by mTORC2 increases enzymatic activity about 10-fold [13]. Akt kinase activity regulates approximately sixty downstream targets including FOXO transcription factors, glycogen synthase 3 isoforms, the pro-apoptotic protein Bad, mTORC1, as well as apoptosis inhibitors such as mdm2 and XIAP [1]. Consistent with these data, constitutively active Akt has been shown to reduce cell sensitivity to pro-apoptotic drugs [14,15].
Constitutive Akt activation has been reported in a number of cancer types, and its activity has been linked to both oncogenesis and poorer prognosis [1,16]. Consequently, Akt appears as a very attractive target for pharmacological intervention in cancer [17,18]. MK-2206 is a highly selective non-ATP competitive allosteric Akt inhibitor that is equally potent towards purified recombinant human Akt1 and Akt2 and approximately 5-fold less potent against human Akt3 (IC50 = 8, 12, and 65 nM, respectively) in enzyme assays. MK-2206 is orally active and has entered clinical evaluation. As Akt has been proposed as a therapeutic target for both childhood hematologic malignancies and solid tumors, MK-2206 was evaluated in the PPTP in vitro and in vivo screens.
MATERIALS AND METHODS
In vitro testing
In vitro testing was performed using DIMSCAN, a semiautomatic fluorescence-based digital image microscopy system that quantifies viable (using fluorescein diacetate [FDA]) cell numbers in tissue culture multiwell plates [19]. Cells were incubated in the presence of MK-2206 for 96 hours at concentrations from 1.0 nM to 10 μM and analyzed as previously described [20]. The relative IC50 is the concentration of agent that gives a half-maximal response, while the absolute IC50 values represent the concentration at which the agent reduces cell survival to 50% of the control value [21]. To compare activity between cell lines, the ratio of the median relative IC50 to individual cell line’s relative IC50 value is used (larger values connote greater sensitivity). The lowest T/C% value is the Ymin. Relative In/Out (I/O)% values represent the percentage difference between the Ymin value and the estimated starting cell number and either the control cell number (for agents with Ymin > starting cell number) or 0 (for agents with Ymin < estimated starting cell number). Relative I/O% values range between 100% (no treatment effect) to -100% (complete cytotoxic effect), with a Relative I/O% value of 0 being observed for a completely effective cytostatic agent.
In vivo tumor growth inhibition studies
CB17SC-F scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [22]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [23]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Ten mice (solid tumors) or eight mice (leukemias) were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [hCD45] cells [ALL xenografts] were determined as previously described [24] and responses were determined using three activity measures as previously described [24]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies. The Mann–Whitney test was used to test the difference of medians of EC50 values between the groups of lines with similar tumor types to the remaining lines of the panel.
Drugs and Formulation
MK-2206 was provided to the PPTP by Merck & Co. Inc., through the Cancer Therapy Evaluation Program (NCI). MK-2206 was dissolved in 30% Captisol in sterile water, and sonicated 5 min before each administration. MK-2206 was administered at 180 mg/kg, the maximum tolerated dose in non-tumored mice, P.O. 3 days per week (Monday, Wednesday, Friday), determined to be an optimal schedule recommended by the drug supplier, for a planned 4 weeks. Drug was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
MK-2206 in vitro testing
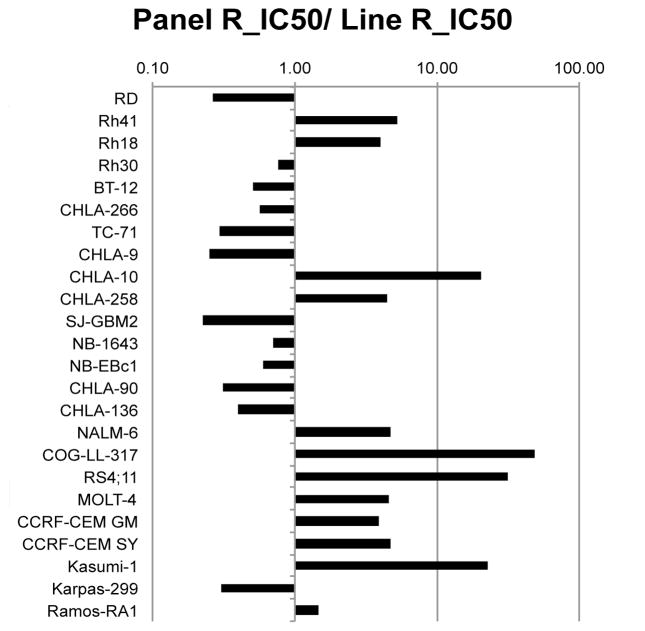
MK-2206 was tested against the PPTP’s in vitro cell line panel at concentrations ranging from 1.0 nM to 10 μM using the PPTP’s standard 96 hour exposure period. The median relative IC50 value for the PPTP cell lines was 2.2 μM, with a range from 0.05 μM for the T-cell ALL line COG-LL-317 to greater than 10 μM for the glioblastoma cell line SJ-GBM2 (Table I). Maximum achievable plasma concentrations in humans are < 200 nM [25], and there were four cell lines with IC50 values < 200 nM: two ALL cell lines (COG-LL-317 and RS4;11), an AML cell line with an activating KIT mutation (Kasumi-1), and a Ewing sarcoma cell line (CHLA-10). The median relative IC50 for the ALL cell lines (0.52 μM) was significantly lower than that of the remaining cell lines (3.86 μM, p=0.007). This relative sensitivity of the ALL cell lines can be visualized in Figure 1. MK-2206 demonstrated evidence of cytotoxic activity against some cell lines, with T/C% values approaching 0% and relative I/O% values approaching -100% (e.g., the T-cell ALL lines COG-LL-317 and MOLT-4 and the rhabdomyosarcoma cell line Rh41) (Table I).
Table I.
In vitroactivity of MK-2206
| Cell Line | Histotype | Relative IC50 (μM) | Panel R_IC50/Line R_IC50 | Observed Ymin (%) | Relative In/Out% |
|---|---|---|---|---|---|
| RD | RMS | 9.30 | 0.23 | 48.0 | 45.0 |
| Rh41 | RMS | 0.47 | 4.64 | 0.7 | −97.0 |
| Rh18 | RMS | 0.62 | 3.52 | 14.1 | −68.3 |
| Rh30 | RMS | 2.66 | 0.82 | 27.2 | 12.7 |
| BT-12 | Rhabdoid | 3.32 | 0.66 | 9.3 | 1.2 |
| CHLA-266 | Rhabdoid | 4.43 | 0.49 | 35.2 | 12.3 |
| CHLA-9 | Ewing | 9.81 | 0.22 | 45.0 | 42.9 |
| CHLA-10 | Ewing | 0.12 | 17.82 | 10.6 | 4.6 |
| CHLA-258 | Ewing | 0.69 | 3.16 | 24.7 | −36.7 |
| TC-71 | Ewing | 8.42 | 0.26 | 42.4 | 41.6 |
| SJ-GBM2 | GBM | > 10.00 | 0.20 | 57.7 | 53.1 |
| NB-1643 | NBL | 3.54 | 0.62 | 36.3 | 19.2 |
| NB-EBc1 | NBL | 4.17 | 0.52 | 37.1 | 18.5 |
| CHLA-90 | NBL | 8.10 | 0.27 | 44.1 | 22.5 |
| CHLA-136 | NBL | 6.14 | 0.36 | 34.8 | 8.5 |
| NALM-6 | ALL | 0.52 | 4.16 | 8.8 | 6.0 |
| COG-LL-317 | ALL | 0.05 | 43.63 | 0.1 | −97.8 |
| RS4;11 | ALL | 0.08 | 27.62 | 3.6 | −75.9 |
| MOLT-4 | ALL | 0.54 | 4.06 | 1.3 | −87.4 |
| CCRF-CEM #1 | ALL | 0.63 | 3.47 | 7.5 | 1.3 |
| CCRF-CEM #1 | ALL | 0.52 | 4.20 | 7.0 | 0.8 |
| Kasumi-1 | AML | 0.11 | 19.76 | 11.7 | −59.4 |
| Karpas-299 | ALCL | 8.07 | 0.27 | 32.8 | 27.1 |
| Ramos-RA1 | NHL | 1.70 | 1.28 | 0.0 | −97.5 |
| Median | 2.18 | 1.05 | 19.4 | 5.3 | |
| Minimum | 0.05 | 0.20 | 0.0 | −97.8 | |
| Maximum | > 10.00 | 43.63 | 57.7 | 53.1 |
Figure 1.
MK-2206 in vitro activity. The figure shows the ratio of the median relative IC50 of the entire panel to that of each cell line. Higher ratios are indicative of greater sensitivity to MK-2206 and are shown in the figure by bars to the right of the midpoint line. The cell lines of the ALL panel (NALM6 through CCRF-CEM near bottom of figure) are relatively sensitive to MK-2206 with each cell line having a relative IC50 value less than the median for the entire panel.
MK-2206 in vivo testing
MK-2206 was tested against the PPTP solid tumor xenografts using a dose of 180 mg/kg administered M-W-F by oral gavage. The total planned treatment period was 4 weeks with an additional 2 weeks observation. MK-2206 was generally well-tolerated with 16 of 688 deaths, 2 in control groups (0.6%) and 14 of 348 mice in treatment groups (4.0%). Thirty-seven of 37 tested xenograft models were considered evaluable for efficacy. Complete details of testing are provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
MK-2206 induced significant differences in EFS distribution compared to control in 12 of 29 (41%) of the evaluable solid tumor xenografts and in 2 of 8 (25%) of the evaluable ALL xenografts. Significant differences in EFS distribution were most frequently noted in the osteosarcoma panel (6 of 6). The neuroblastoma panel was the only other solid tumor panel for which more than 1 xenograft showed a significant difference in EFS distribution. For those xenografts with a significant difference in EFS distribution between treated and control groups, the EFS T/C activity measure additionally requires an EFS T/C value of > 2.0 for intermediate activity and indicates a substantial agent effect in slowing tumor growth. High activity further requires a reduction in final tumor volume compared to the starting tumor volume. MK-2206 induced tumor growth inhibition meeting criteria for intermediate EFS T/C activity in 1 of 29 (3.4%) evaluable solid tumor xenografts, the osteosarcoma xenograft OS-31. For the ALL panel, 2 of 8 (25%) xenografts met criteria for intermediate activity.
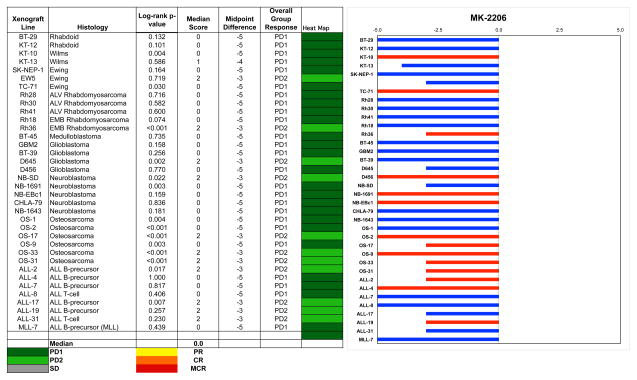
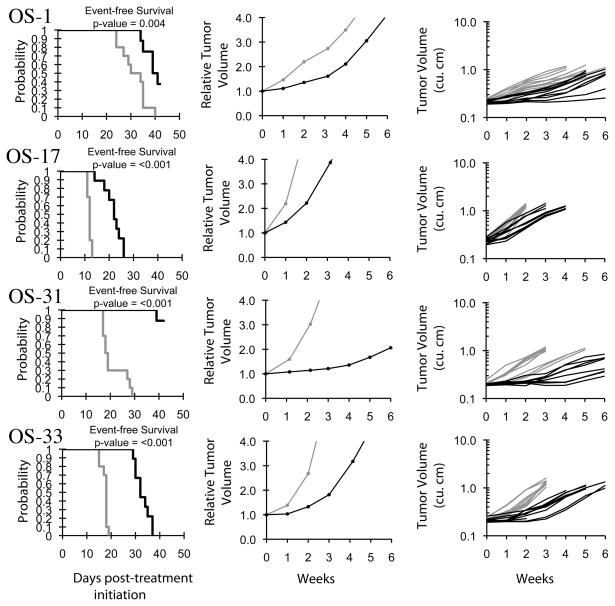
Objective responses were not observed for either the solid tumor or ALL xenografts. The best response was PD2 (progressive disease with growth delay > 1.5-fold), which was observed in 8 of 29 solid tumor and 4 of 8 ALL xenografts. The osteosarcoma panel had 3 of 6 xenografts with a PD2 response, and no other panel had more than a single xenograft with a PD2 response. The in vivo testing results for the objective response measure of activity are presented in Figure 2 in a ‘heat-map’ format as well as a ‘COMPARE’-like format, based on the scoring criteria described in the Material and Methods and the Supplemental Response Definitions section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of 5 (stable disease). Examples of relative tumor volume growth curves for osteosarcoma xenografts with EFS T/C values among the highest for the xenografts tested are shown in Figure 3.
Figure 2.
MK-2206 in vivo objective response activity, left: The colored heat map depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of > 2 but < 6, and low activity by a score of < 2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different.
Figure 3.
MK-2206 activity against individual osteosarcoma xenografts (OS-1, OS-17, OS-31, OS-33). Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Grey lines correspond to control animals and black lines to drug treated animals.
DISCUSSION
The in vitro response to MK-2206 has similarities to the in vitro response to the ATP-competitive Akt inhibitor GSK690693 previously studied by the PPTP [26]. For both agents the ALL cell line panel was the most sensitive to Akt inhibition, whereas the neuroblastoma cell lines all had IC50 values greater than the panel median. For both agents, COG-LL-317 (T-cell ALL) is the most sensitive cell line, with 40- to 100-fold greater sensitivity to the two Akt inhibitors than the median sensitivity for all PPTP cell lines. Other cell lines showing enhanced sensitivity to MK-2206 and GSK690693 included the Ewing sarcoma cell line CHLA-10, the rhabdomyosarcoma cell line Rh41, the AML cell line Kasumi-1, and several ALL cell lines including RS4;11, MOLT-4, CCRF-CEM, and NALM-6. Potential explanations for the increased sensitivity are available for some of these cell lines. Some of the lines are known to be driven by activated receptor tyrosine kinases. For example, Rh41 is the most sensitive PPTP cell line to IGF-1R inhibition [27,28], and Kasumi-1 has an activating KIT mutation [29,30]. The sensitivity of these cell lines to MK-2206 mirrors the sensitivity of adult cancer cell lines with HER2 amplification to allosteric Akt inhibitors [31]. Akt has also been reported to be a particularly relevant target in T-ALL [32,33], which may explain the sensitivity of the three T-cell ALL cell lines to MK-2206 and GSK690693. It should be noted that osteosarcoma, which demonstrates consistent tumor growth delay in the in vivo panel, is not included in the in vitro panel.
The clinical experience with MK-2206 in adults provides relevant information in interpreting the in vitro data. In a phase 1 trial in adults utilizing an every-other-day (QOD) schedule, maximum plasma levels were in the 100 – 150 nM range, and these were achieved at doses exceeding the maximum tolerated dose (MTD) [25]. This concentration range is one at which Akt inhibition is near maximal in the in vitro setting [34,35]. Thus, most of the PPTP cell lines show little or no effect on growth and proliferation at MK-2206 concentrations achievable in the clinic and at concentrations that potently inhibit Akt in vitro.
The in vivo responses to MK-2206 were limited to tumor growth inhibition, as no objective responses (tumor regressions) were observed for either the solid tumor or ALL xenografts. A single dose of MK-2206 at 120 mg/kg inhibited Akt phosphorylation in NCI-H292 lung carcinoma xenografts by approximately 50% at 12 hours post-dosing [36], suggesting that at the dose used in the current study (180 mg/kg) substantial target inhibition should be achieved following each dose. However, the depth and duration of Akt inhibition may not be adequate for high level in vivo activity. The most consistent level of tumor growth inhibition was noted for the osteosarcoma xenografts, with all of the xenografts in this panel showing significant differences in EFS distribution between treated and control animals. The magnitude of tumor growth inhibition was modest, with a single xenograft (OS-31) showing a more than two-fold prolongation of time to event compared to control animals. The in vivo results for MK-2206 are very similar to those described for GSK690693, which also showed specificity for the osteosarcoma panel and modest tumor growth inhibition as the best response [26]. The consistency of this response in osteosarcoma utilizing two different inhibitors of the same pathway may be meaningful and suggests relevance of inhibition of this pathway to osteosarcoma therapy.
In summary, MK-2206 showed limited in vivo activity as a single agent against the PPTP solid tumor and ALL xenografts. Tumor cells can be sensitized to biological and cytotoxic apoptotic stimuli by Akt inhibition [36,37], and MK-2206 has been shown to enhance the efficacy of standard chemotherapy agents and molecularly targeted agents (e.g., erlotinib, lapatinib, and the MEK inhibitor AZD6244) in adult cancer preclinical models [36,38]. Akt inhibitors have also been shown to be additive with rapamycin in blocking hypoxia-induced VEGF secretion in vitro [39]. These observations provide rationale for further preclinical work to evaluate MK-2206 in pediatric models in the setting of combination therapy with signal transduction inhibitors available to the PPTP or with conventional cytotoxic agents.
Supplementary Material
Table II.
Summary of in Vivo Activity of MK-2206
| Line | Tumor Type | Median Time to Event | P- value | EFS T/C | Median RTV/CD45 at End of Study | Tumor Volume T/C | Median Group Response | T/C Volume Activity | EFS Activity |
|---|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | 17.7 | 0.132 | 1.2 | >4 | 0.82 | PD1 | Low | Low |
| KT-12 | Rhabdoid | 15.5 | 0.101 | 1.2 | >4 | 1.01 | PD1 | Low | Low |
| KT-10 | Wilms | 19.9 | 0.004 | 1.5 | >4 | 0.43 | PD1 | Int | Low |
| KT-13 | Wilms | 14.7 | 0.586 | 1.4 | >4 | 0.85 | PD1 | Low | Low |
| SK-NEP-1 | Ewing | 11.5 | 0.164 | 1.4 | >4 | 0.81 | PD1 | Low | Low |
| EW5 | Ewing | 10.8 | 0.719 | 1.5 | >4 | 0.81 | PD2 | Low | Low |
| TC-71 | Ewing | 10.6 | 0.030 | 0.6 | >4 | 1.19 | PD1 | Low | Low |
| Rh10 | ALV RMS | 27.4 | <0.001 | 1.9 | >4 | 0.65 | PD2 | Low | Low |
| Rh28 | ALV RMS | 16.4 | 0.716 | 1.0 | >4 | 1.07 | PD1 | Low | Low |
| Rh30 | ALV RMS | 15.8 | 0.582 | 1.0 | >4 | 0.99 | PD1 | Low | Low |
| Rh41 | ALV RMS | 12.3 | 0.600 | 0.9 | >4 | 1.01 | PD1 | Low | Low |
| Rh18 | ALV RMS | 11.7 | 0.074 | 1.3 | >4 | 0.72 | PD1 | Low | Low |
| Rh36 | ALV RMS | 13.2 | <0.001 | 2.0 | >4 | 0.47 | PD2 | Low | Low |
| BT-45 | Medulloblastoma | 4.3 | 0.735 | 0.9 | >4 | 1.34 | PD1 | Low | Low |
| GBM2 | Glioblastoma | 19.8 | 0.158 | 1.3 | >4 | 0.70 | PD1 | Low | Low |
| BT-39 | Glioblastoma | 9.3 | 0.256 | 1.1 | >4 | 0.93 | PD1 | Low | Low |
| D645 | Glioblastoma | 16.1 | 0.002 | 1.6 | >4 | 0.63 | PD2 | Low | Low |
| D456 | Glioblastoma | 6.4 | 0.770 | 1.0 | >4 | 1.05 | PD1 | Low | Low |
| NB-SD | Neuroblastoma | 20.2 | 0.022 | 1.6 | >4 | 0.36 | PD2 | Int | Low |
| NB-1691 | Neuroblastoma | 12.4 | 0.003 | 1.4 | >4 | 0.68 | PD1 | Low | Low |
| NB-EBc1 | Neuroblastoma | 4.2 | 0.159 | 1.0 | >4 | 0.76 | PD1 | Low | Low |
| CHLA-79 | Neuroblastoma | 8.7 | 0.836 | 1.2 | >4 | 0.95 | PD1 | Low | Low |
| NB-1643 | Neuroblastoma | 8.2 | 0.181 | 0.9 | >4 | 1.28 | PD1 | Low | Low |
| OS-1 | Osteosarcoma | 40.0 | 0.004 | 1.3 | >4 | 0.60 | PD1 | Low | Low |
| OS-2 | Osteosarcoma | 23.6 | <0.001 | 1.3 | >4 | 0.67 | PD1 | Low | Low |
| OS-17 | Osteosarcoma | 22.3 | <0.001 | 1.9 | >4 | 0.48 | PD2 | Low | Low |
| OS-9 | Osteosarcoma | 41.4 | 0.003 | 1.3 | >4 | 0.68 | PD1 | Low | Low |
| OS-33 | Osteosarcoma | 32.3 | <0.001 | 1.8 | >4 | 0.31 | PD2 | Int | Low |
| OS-31 | Osteosarcoma | > EP | <0.001 | > 2.3 | 2.8 | 0.30 | PD2 | Int | Int |
| ALL-2 | ALL B-precursor | 21.2 | 0.017 | 2.5 | >25 | . | PD2 | Int | |
| ALL-4 | ALL B-precursor | 5.0 | 1.000 | 1.0 | >25 | . | PD1 | Low | |
| ALL-7 | ALL B-precursor | 3.0 | 0.817 | 1.0 | >25 | . | PD1 | Low | |
| ALL-8 | ALL T-cell | 7.7 | 0.406 | 1.2 | >25 | . | PD1 | Low | |
| ALL-17 | ALL B-precursor | 14.2 | 0.007 | 4.6 | >25 | . | PD2 | Int | |
| ALL-19 | ALL B-precursor | 19.0 | 0.257 | 3.9 | >25 | . | PD2 | Low | |
| 21.6 | 0.230 | 3.6 | >25 | . | PD2 | Low | |||
| 5.9 | 0.439 | 1.1 | >25 | . | PD1 | Low |
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used MK-2206 supplied by Merck & Co. Inc. In addition to the authors represents work contributed by the following: Sherry Ansher, Catherine A. Billups, Joshua Courtright, Edward Favours, Henry S. Friedman, Danuta Gasinski, Debbie Payne-Turner, Chandra Tucker, Joe Zeidner, Jianrong Wu, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and Sydney Children’s Hospital.
Footnotes
Conflict of interest statement: The authors consider that there are no actual or perceived conflicts of interest.
Reference List
- 1.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9(6):667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 2.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends in Biochemical Sciences. 2001;26(11):657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opel D, Poremba C, Simon T, et al. Activation of Akt Predicts Poor Outcome in Neuroblastoma. Cancer Res. 2007;67(2):735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]
- 5.Petricoin EF, III, Espina V, Araujo RP, et al. Phosphoprotein Pathway Mapping: Akt/Mammalian Target of Rapamycin Activation Is Negatively Associated with Childhood Rhabdomyosarcoma Survival. Cancer Res. 2007;67(7):3431–3440. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- 6.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor Activity of the Insulin-Like Growth Factor-I Receptor Kinase Inhibitor NVP-AEW541 in Musculoskeletal Tumors. Cancer Res. 2005;65(9):3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 7.Kim B, van Golen CM, Feldman EL. Insulin-Like Growth Factor I Induces Preferential Degradation of Insulin Receptor Substrate-2 through the Phosphatidylinositol 3-Kinase Pathway in Human Neuroblastoma Cells. Endocrinology. 2005;146(12):5350–5357. doi: 10.1210/en.2005-0356. [DOI] [PubMed] [Google Scholar]
- 8.Schwab TS, Madison BB, Grauman AR, et al. Insulin-like growth factor-I induces the phosphorylation and nuclear exclusion of forkhead transcription factors in human neuroblastoma cells. Apoptosis. 2005;10(4):831–840. doi: 10.1007/s10495-005-0429-y. [DOI] [PubMed] [Google Scholar]
- 9.Ho R, Eggert A, Hishiki T, et al. Resistance to Chemotherapy Mediated by TrkB in Neuroblastomas. Cancer Res. 2002;62(22):6462–6466. [PubMed] [Google Scholar]
- 10.Jaboin J, Kim CJ, Kaplan DR, et al. Brain-derived Neurotrophic Factor Activation of TrkB Protects Neuroblastoma Cells from Chemotherapy-induced Apoptosis via Phosphatidylinositol 3′-Kinase Pathway. Cancer Res. 2002;62(22):6756–6763. [PubMed] [Google Scholar]
- 11.Li Z, Jaboin J, Dennis PA, et al. Genetic and Pharmacologic Identification of Akt as a Mediator of Brain-Derived Neurotrophic Factor/TrkB Rescue of Neuroblastoma Cells from Chemotherapy-Induced Cell Death. Cancer Res. 2005;65(6):2070–2075. doi: 10.1158/0008-5472.CAN-04-3606. [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7(4):285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Cron P, Thompson V, et al. Molecular Mechanism for the Regulation of Protein Kinase B/Akt by Hydrophobic Motif Phosphorylation. Molecular Cell. 2002;9(6):1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 14.Wendel H-G, Stanchina Ed, Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428(6980):332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 16.Bellacosa A, Kumar CC, Cristofano AD, et al. Advances in Cancer Research. Vol. 94. Academic Press; 2005. Activation of AKT Kinases in Cancer: Implications for Therapeutic Targeting; pp. 29–86. [DOI] [PubMed] [Google Scholar]
- 17.Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powis G, Ihle N, Kirkpatrick DL. Practicalities of Drugging the Phosphatidylinositol-3-Kinase/Akt Cell Survival Signaling Pathway. Clinical Cancer Research. 2006;12(10):2964–2966. doi: 10.1158/1078-0432.CCR-06-0617. [DOI] [PubMed] [Google Scholar]
- 19.Frgala T, Kalous O, Proffitt RT, et al. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 20.Kang MH, Smith MA, Morton CL, et al. National Cancer Institute Pediatric Preclinical Testing Program: Model description for in vitro cytotoxicity testing. Pediatr Blood Cancer. 2011;56(2):239–249. doi: 10.1002/pbc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharmaceut Statist. 2010 doi: 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- 22.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48(15):4189–4195. [PubMed] [Google Scholar]
- 23.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 24.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 25.Tolcher AW, Yap TA, Fearen I, et al. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST) J Clin Oncol. 2009;15s(suppl):abstr 3503. [Google Scholar]
- 26.Carol H, Morton CL, Gorlick R, et al. Initial testing (stage 1) of the Akt inhibitor GSK690693 by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55(7):1329–1337. doi: 10.1002/pbc.22710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(6):1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 28.Houghton PJ, Maris JM, Courtright J, et al. Pediatric Preclinical Testing Program (PPTP) stage 1 evaluation of BMS-754807 IGF-1 receptor inhibitor. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research ; 2010. p. Abstr #5265. [Google Scholar]
- 29.Larizza L, Magnani I, Beghini A. The Kasumi-1 cell line: a t(8;21)-kit mutant model for acute myeloid leukemia. Leukemia & lymphoma. 2005;46(2):247–255. doi: 10.1080/10428190400007565. [DOI] [PubMed] [Google Scholar]
- 30.Beghini A, Magnani I, Ripamonti CB, et al. Amplification of a novel c-Kit activating mutation Asn(822)-Lys in the Kasumi-1 cell line: a t(8;21)-Kit mutant model for acute myeloid leukemia. Hematol J. 2002;3(3):157–163. doi: 10.1038/sj.thj.6200168. [DOI] [PubMed] [Google Scholar]
- 31.She QB, Chandarlapaty S, Ye Q, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008;3(8):e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118(11):3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez A, Sanda T, Grebliunaite R, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114(3):647–650. doi: 10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan L. MK-2206: A potent oral allosteric AKT inhibitor. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009. [Google Scholar]
- 35.Lu W, Defeo-Jones D, Davis L, et al. In vitro and in vivo antitumor activities of MK-2206, a new allosteric Akt inhibitor. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009. p. Abstr #3714. [Google Scholar]
- 36.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular cancer therapeutics. 2010;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 37.DeFeo-Jones D, Barnett SF, Fu S, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4(2):271–279. [PubMed] [Google Scholar]
- 38.Meng J, Dai B, Fang B, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PloS one. 2010;5(11):e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurmasheva RT, Harwood FC, Houghton PJ. Differential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumors. Molecular cancer therapeutics. 2007;6(5):1620–1628. doi: 10.1158/1535-7163.MCT-06-0646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.