Abstract
The hippocampus plays a central role in spatial and contextual learning and memory, however relatively little is known about the specific contributions of parahippocampal structures that interface with the hippocampus. The postsubiculum (PoSub) is reciprocally connected with a number of hippocampal, parahippocampal and subcortical structures that are involved in spatial learning and memory. In addition, behavioral data suggest that PoSub is needed for optimal performance during tests of spatial memory. Together, these data suggest that PoSub plays a prominent role in spatial navigation. Currently it is unknown whether the PoSub is needed for other forms of learning and memory that also require the formation of associations among multiple environmental stimuli. To address this gap in the literature we investigated the role of PoSub in Pavlovian fear conditioning. In Experiment 1 male rats received either lesions of PoSub or Sham surgery prior to training in a classical fear conditioning procedure. On the training day a tone was paired with foot shock three times. Conditioned fear to the training context was evaluated 24 hr later by placing rats back into the conditioning chamber without presenting any tones or shocks. Auditory fear was assessed on the third day by presenting the auditory stimulus in a novel environment (no shock). PoSub-lesioned rats exhibited impaired acquisition of the conditioned fear response as well as impaired expression of contextual and auditory fear conditioning. In Experiment 2, PoSub lesions were made 1 day after training to specifically assess the role of PoSub in fear memory. No deficits in the expression of contextual fear were observed, but freezing to the tone was significantly reduced in PoSub-lesioned rats compared to shams. Together, these results indicate that PoSub is necessary for normal acquisition of conditioned fear, and that PoSub contributes to the expression of auditory but not contextual fear memory.
Keywords: medial temporal lobe, postsubiculum, fear conditioning, context, auditory stimuli
Introduction
Contextual learning involves the formation of associations between diverse, multimodal sensory stimuli which are thought to provide a cohesive representation of an environment (Anagnostaras, Gale, and Fanselow, 2001; Holland and Bouton, 1999). Pavlovian fear conditioning has been a particularly useful paradigm for studying the neural circuitry that underlies contextual and auditory learning and memory. In a typical fear conditioning procedure in rodents, a neutral stimulus (e.g., a tone) is paired with an aversive unconditioned stimulus in a novel environment (the training context) during an acquisition session. Acquisition of the fear response is typically measured by examining postshock freezing during the training session. Subsequently, conditioned fear to the training environment (contextual fear memory) is assessed by returning the subject to the training context in the absence of the tone or the aversive stimulus. Similarly, auditory fear memory is assessed by placing the rat in a different environment and measuring freezing behavior in response to presentations of the auditory conditioned stimulus (Fanselow, 1980). Reduced freezing during acquisition can indicate weak associative learning during conditioning or a reduction in the ability to process sensory stimuli, whereas reduced freezing during the re-exposure sessions is often interpreted as a mnemonic deficit (if acquisition is unimpaired).
Decades of research have established that the hippocampus proper (CA1, CA3 and dentate gyrus), the fimbria and fornix, the entorhinal cortex (ENTO) and the subiculum are centrally involved in the expression of contextual fear memory (Anagnostaras, Maren, and Fanselow, 1999; Biedenkapp and Rudy, 2009; Hunsaker and Kesner, 2008; Lehmann, Lacanilao, and Sutherland, 2007; Majchrzak, Ferry, Marchand, Herbeaux, Seillier, and Barbelivien, 2006; Maren, Aharonov, and Fanselow, 1997; Maren, Anagnostaras, and Fanselow, 1998; Maren and Fanselow, 1997; Phillips and LeDoux, 1992; Phillips and LeDoux, 1994; Phillips and LeDoux, 1995; Wiltgen, Sanders, Anagnostaras, Sage, and Fanselow, 2006; Young, Bohenek, and Fanselow, 1994). More recent studies have extended this work by investigating the contributions of surrounding parahippocampal structures to contextual memory. For example, the retrosplenial (RSP) and postrhinal cortices (POR), but not posterior parietal cortex (PPC), are required for the expression of contextual fear memory (Bucci, Phillips, and Burwell, 2000; Burwell, Bucci, Sanborn, and Jutras, 2004; Keene and Bucci, 2008a; Keene and Bucci, 2008b). Importantly, many studies have demonstrated that damage to hippocampal and parahippocampal structures does not typically impair acquisition of the fear response during training or the expression of auditory conditioning, thus highlighting a role for these structures in contextual fear memory (Anagnostaras et al., 1999; Hunsaker and Kesner, 2008; Maren and Holt, 2004; Phillips and LeDoux, 1995; Selden, Everitt, Jarrard, and Robbins, 1991), but see (Hunsaker and Kesner, 2008; Maren and Holt, 2004). In contrast, both acquisition and expression of auditory (and contextual) fear conditioning involves structures within a cortico-amygdalo-thalamic loop that includes the basolateral amygdala (LeDoux, Cicchetti, Xagoraris, and Romanski, 1990; Quirk, Repa, and LeDoux, 1995; Rogan, Staubli, and LeDoux, 1997), anterior thalamus (Celerier, Ognard, Decorte, and Beracochea, 2000) and the medial geniculate nucleus of the thalamus (Heldt and Falls, 1998; LeDoux, Sakaguchi, and Reis, 1984). Combined, these findings indicate that individual brain regions have varying temporal and modality-specific roles during contextual and auditory fear learning and memory.
The current experiments were designed to expand our understanding of the parahippocampal circuitry that underlies fear conditioning by investigating the contribution of the postsubiculum (PoSub; also identified as Brodmann area 48 and as dorsal presubiculum) to contextual and auditory learning and memory. The few published studies that have investigated the behavioral contributions of PoSub indicate that this region contains a population of direction-selective cells that fire when the animal’s head is oriented in a particular direction within an environment, suggesting a prominent role for PoSub in spatial navigation (Ranck, 1984; Sharp, 1996; Taube, Muller, and Ranck, 1990a). Similarly, lesion studies have shown that PoSub is needed for optimal performance on tests of spatial memory (Taube, Kesslak, and Cotman, 1992). Based on these findings, it was predicted that damage to PoSub would impair contextual fear memory. Indeed, anatomical studies have revealed that PoSub has strong reciprocal connections with several regions that support contextual fear memory (see Figure 1) including the hippocampus and the subiculum, as well as with parahippocampal areas such as ENTO, RSP and perirhinal cortex (PER) (Sorensen and Shipley, 1979; Van Groen and Wyss, 1990; Van Groen and Wyss, 1995; Vogt and Miller, 1983; Witter and Groenewegen, 1990). At the same time, other connections of the PoSub suggest that it may also contribute to auditory fear conditioning. Specifically, the PoSub is reciprocally connected with anterior thalamus and thalamo-amaygdalar circuits which play a prominent role in the acquisition and expression of auditory (and contextual) fear conditioning. The contributions of PoSub to fear conditioning were examined in Experiment 1 in rats that sustained bilateral electrolytic PoSub lesions prior to training. In Experiment 2, contextual and auditory fear memory was assessed in rats that were lesioned one day after fear conditioning. Because we were specifically interested in the effects of damage 24 hr after fear conditioning, electrolytic lesions were selected for the precise temporal control afforded by this method.
Figure 1.
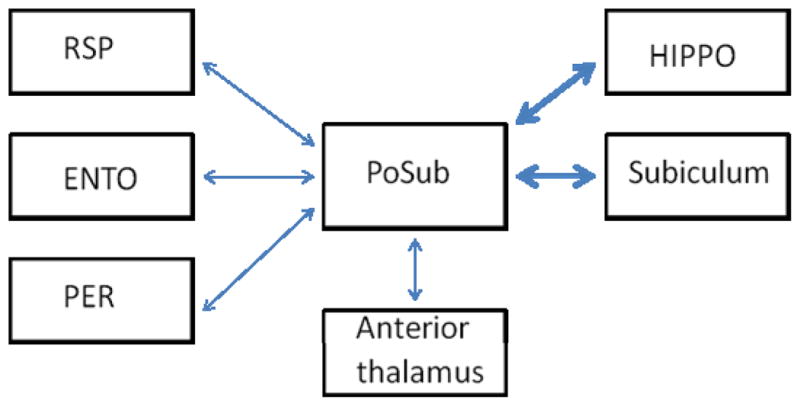
Schematic of corticohippocampal circuitry depicting multiple cortical and subcortical connections between PoSub and brain regions involved in contextual and auditory fear conditioning. Thicker arrows denote more dense connections. PoSub, postsubiculum; RSP, retrosplenial cortex; ENTO, entorhinal cortex; PER, perirhinal cortex; HIPPO, hippocampus.
EXPERIMENT 1
Materials and Methods
Subjects
Naïve male Long-Evans rats (weighing 225 – 260 g at surgery) were obtained from Harlan Laboratories (Indianapolis, IN) and housed singly in a temperature-controlled room with a 12 hour light/dark cycle with lights on at 7:00 am. Rats had ad libitum access to food (Purina standard rat chow: Nestle Purina, St. Louis, MO) and water. Rats were handled for 3 days prior to behavioral testing. All efforts were made to minimize discomfort and to limit the number of animals used. The Institutional Animal Care and Use Committee at Dartmouth College approved the use of rats in these studies and all procedures were conducted in accordance with NIH guidelines.
Surgery
Rats were anesthetized with 1.5 – 3% isofluorane gas in oxygen, placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA) and the head was leveled using bregma and lambda as landmarks. To make bilateral electrolytic lesions of the PoSub (n=24), small holes were drilled at the following 2 sites (in mm): AP, −6.8, −7.8; ML, ±2.6, ±3.3; DV (from skull), −4.0, −3.8. These coordinates were based on previous reports that targeted the PoSub (Calton, Stackman, Goodridge, Archey, Dudchenko, and Taube, 2003; Sharp, 1996; Taube et al., 1992) and on the rat brain atlas of (Paxinos and Watson, 2007)). An insect pin that was epoxy-coated except for the tip was lowered into each coordinate and a 2.5mA current was passed through the tip for durations of 10 – 15 sec per lesion site. The needle was slowly retracted 15 sec after the current was delivered and the skin was stapled together with wound clips. Rats were permitted to recover for 10 – 12 days following surgery. For the Sham-operated surgeries (n=20), rats underwent the same procedure as the PoSub-lesioned animals except that the insect pin was not lowered into the brain.
Behavioral Apparatus
Fear conditioning chambers
Experiments were conducted in standard operant conditioning chambers (24 cm × 30.5 cm × 29 cm; Med Associates, Inc., St. Albans, VT) connected to a computer and enclosed in sound-attenuating chambers (62 cm × 56 cm × 56 cm) outfitted with an exhaust fan to provide airflow and background noise (~68 dB). The operant chambers consisted of aluminum front and back walls, clear acrylic sides and top and grid floors. A dimly illuminated food cup was recessed in the center of the front wall, and a 2.8-W jeweled panel light was located 5 cm above the opening of the recessed food cup, neither of which were used in this experiment. A house light providing background illumination was mounted 15 cm above the food cup. A speaker was located 15 cm above and to the right of the food cup and was used to present the discrete auditory cue (1.5 kHz, 78 dB). Delivery of a 1.0-mA, 1 sec constant current shock through the grid floor of the operant chamber served as the unconditioned stimulus. Surveillance cameras located inside the surrounding shell were used to video record the rats’ behavior.
Activity chambers
Locomotor activity was assessed in an open field apparatus (43.2 × 43.2 × 30.5 cm) composed of Plexiglas walls and floor (Med Associates, Inc.). The chambers were equipped with 16 infrared photobeams that were arrayed an average of 5.5 cm apart. Photobeam interruptions were recorded by a computer running custom Open Field Activity Monitoring software (Med Associates Inc.) that calculated the total distance traveled.
Behavioral Procedures
Fear Conditioning
All rats were trained in a widely used fear conditioning task described previously (Arenos, Musty, and Bucci, 2006; Keene and Bucci, 2008a; Maren et al., 1997). The training session consisted of three 10-sec presentations of the tone co-terminating with a 1sec, 1.0mA foot shock (intertrial interval of 64 sec). The first trial began 3 min after the rat was placed in the chamber. The next day, rats were re-exposed to the original training chamber for a context test during which no tones or shocks were presented. Twenty-four hours later, the tone test was carried out by placing the rats in a novel context and presenting the tone 20 times beginning 30 sec after the rat was placed in the chamber. Again, no shock was delivered during this test. The novel context consisted of the original training chambers outfitted with plain white paper on the walls of the chamber to hide the recessed food cup and other stimuli present on the aluminum walls. Cardboard was also placed on top of the grid floor to provide a different tactile stimulus, and a cup containing Vicks VapoRub and vinegar was placed in each sound-attenuating chamber to provide different olfactory cues. It has been shown previously that rats exhibit very little freezing behavior to the new context itself (Arenos et al., 2006). All rats received the context test first followed by the tone test since this has previously been shown to be the optimal method for obtaining the most independent assessment of both auditory and contextual fear conditioning in the same rats (Maren et al., 1997). Nevertheless, our lab has previously examined whether the order of testing impacts levels of freezing to the context and tone during the tests and we have found identical results when the tone test was conducted prior to the context test (Arenos et al., 2006).
Open-Field Activity
After the completion of the tone test, 12 Sham-operated and 11 lesioned rats were placed individually in a novel activity chamber for 6 min to assess any activity changes induced by PoSub lesions.
Behavioral Observations
Freezing served as the index of conditioned fear and was operationally defined as total motor immobility except for breathing (Blanchard and Blanchard, 1969; Fanselow, 1980). On the training day, the incidence of freezing behavior was recorded during the 64-sec period prior to the first trial (baseline freezing) and the during the 64-sec period following each trial (postshock freezing). The rats’ behavior was recorded every 8 sec during the 64-sec epochs. The context test session was divided into 64-sec bins and freezing was observed every 8 sec. For the tone test session, freezing was recorded every 2 sec during each 10-sec presentation of the tone. The frequency of freezing behavior was converted to a percentage of total observations. A primary observer scored the behavioral data, while two additional observers scored a subset of the data to assess objectivity. All observers were blind to treatment condition and their observations were highly correlated (r = 0.9; p<0.0001).
Lesion Verification
After the behavioral procedures were completed, rats were deeply anesthetized with an overdose of pentobarbital sodium and phenytoin sodium (Euthasol, Virbac Animal Health, Fort Worth, TX) and transcardially perfused with 0.9% saline for 5 min, followed by 10% buffered formalin. Brains were sectioned on a freezing microtome (60μm) and Nissl-stained using thionin. For each animal, coronal sections at 3 AP locations (from Bregma: −6.84, −7.32, & −7.80, see Figure 2C) along the rostrocaudal extent of the PoSub were used to assess the amount of tissue damage. In addition, the number of animals with and without damage to the posterior forceps of the corpus callosum (PFCC) was counted. This fiber bundle, which carries information from ENTO to the hippocampus, is dorsal to the PoSub. Using StereoInvestigator software (version 9; Microbrightfield, Inc., Williston, VT) and a compound microscope (Axioskop I, Zeiss, Inc.), we identified gross tissue damage as necrosis, missing tissue, or marked thinning of tissue. For each coronal section, areal measurements were obtained using the StereoInvestigator Cavalieri estimator probe with 50μm grid spacing. The percent damage to PoSub was calculated and any gross tissue damage to the area surrounding the target region was noted.
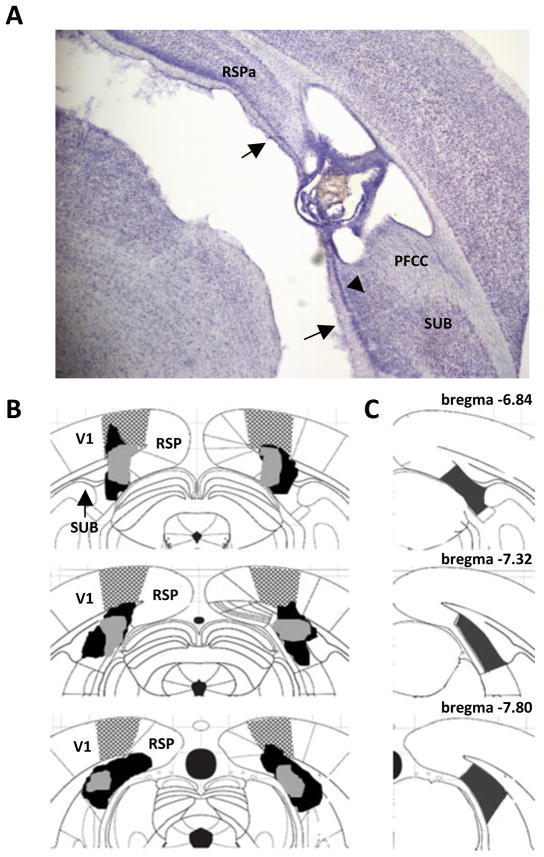
Figure 2.
(A) Photomicrograph of a postsubiculum (PoSub) lesion. Arrows delineate the dorsal and ventral borders of the PoSub. Arrowhead denotes the ventral portion of the PoSub that was spared in some rats. (B) Schematic diagram indicating the largest (black) and smallest (gray) lesions of the PoSub in Experiment 1. A range of damage to primary visual cortex (V1) was observed in all PoSub lesioned rats. The stippled area represents the extent of damage to the lesioned rat with the most V1 damage) (C) Schematic diagram showing coronal sections 6.84, 7.32 and 7.80 mm posterior to bregma. The shaded area in each diagram depicts the boundaries of PoSub. RSPA, retrosplenial cortex; SUB, subiculum; PFCC, posterior forceps of the corpus callosum.
Data Analysis
Fear conditioning
Analyses of freezing behavior that occurred during training and the tone test were conducted using repeated measures analysis of variance (ANOVA) with Group (Sham-operated, PoSub lesion) as the between-subjects variable and Trial as the within-subjects variable. For the context test data, repeated measures ANOVA was conducted using Group as the between-subjects variable and Block (64-sec epoch) as the within-subjects variable.
Locomotor behavior
To examine overall locomotor activity as well as habituation to the open field, one-way repeated measures ANOVA that compared the distance traveled per min with Group (Sham-operated, lesion) as the between-subjects variable and Block (distance traveled per minute) as the within-subjects variable, was conducted. Further, two activity-freezing analyses were conducted. First, within-subjects correlation analyses that compared the total distance traveled during the 6-min open field test with the average freezing behavior observed during each phase of fear conditioning were conducted. If hyperactivity accounted for a substantial amount of the variance observed in the freezing data, then a strong, negative within-subject activity-freezing correlation would be expected. In other words, animals with pronounced hyperactivity would exhibit low freezing behavior. For the second analysis, the mean activity level for the lesioned group was calculated and each lesioned rat was assigned to either the PoSub-Low (below the mean) or the PoSub-High (above the mean) lesion group (Anagnostaras et al., 2001). Subsequently, one-way ANOVAs were conducted on the activity behavior and on the freezing behavior of Sham-operated, PoSub-Low and PoSub-High groups. The purpose of this second analysis was to ascertain whether the freezing behavior of animals in the PoSub-High lesioned group (i.e. animals with a significant amount of hyperactivity) significantly differed from the freezing behavior observed from PoSub-Low lesioned animals which have “normal” locomotor activity profiles. An alpha level of 0.05 was adopted for all analyses and Tukey-Kramer’s post hoc paired comparisons test was used when appropriate.
Results
Histology
A total of 10 rats from the PoSub surgery group were removed from the analysis due to either unilateral PoSub damage (n = 6), significant excess damage to other cortical areas (n = 3) or insufficient PoSub damage (n = 1, less than 10% of the PoSub was damaged). The number of rats remaining in each group and included in the histological and behavioral analyses was 20 Sham-operated rats and 14 PoSub lesioned rats. The average area of damage to PoSub from each of 3 sections analyzed was 69 ± 0.04% (range 59 – 83%). As observed by Taube et al (1992), in some animals the most ventral portion of the PoSub was spared (see arrowhead in Figure 2A). Electrolytic damage to PoSub is displayed in the photomicrograph in Figure 2A, and the rostrocaudal extent of damage to PoSub is depicted in the schematic diagram in Figure 2B. Minor unilateral damage to the granular-A subdivision of RSP and minor unilateral damage to the colliculi was observed in some lesioned rats. A range of damage to primary visual cortex (V1) was observed in all PoSub lesioned rats (see stippled region in Figure 2B; the stippling represents the extent of damage to the lesioned rat with the most V1 damage). Damage to the dorsal portion of PFCC was observed in 12 of 14 rats. The dorsal subiculum and the medial geniculate nucleus sustained little to no damage.
Behavior
Fear conditioning
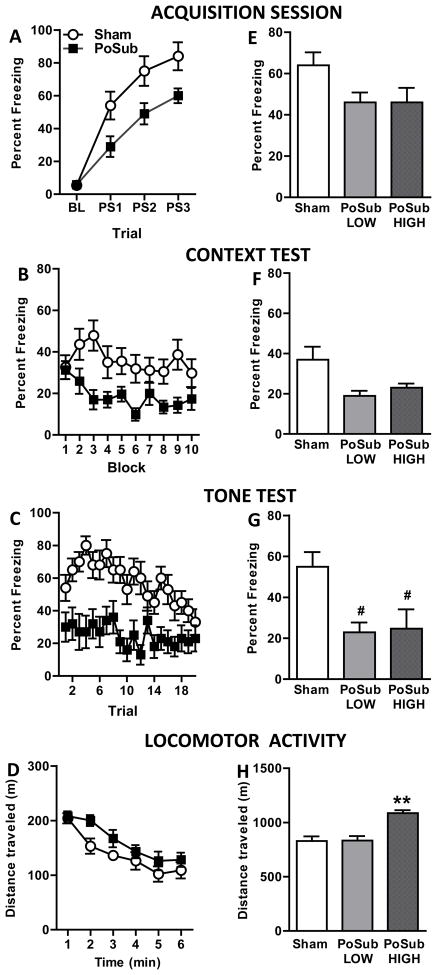
Freezing behavior observed during the acquisition session is shown in Figure 3A. Repeated measures ANOVA revealed significant main effects of Trial [F(3, 96)=64.2, p<0.0001] and Group [F(1,32)=7.3, p<0.05] but no Group X Trial interaction [F(3, 96)=0.96, p>0.05]. During acquisition, Sham-operated rats more fully acquired the conditioned fear response compared to bilateral PoSub-lesioned rats, which demonstrated less postshock freezing. A similar analysis of the context test data revealed significant main effects of Group [F(1,32)=72.1, p<0.0001] and Block [F(9,288)=2.0, p<0.05] but no Group X Block interaction [F(9,288)=1.5, p>0.05]. As shown in Figure 3B, rats in the lesion group exhibited low levels of context-specific freezing compared to Sham-operated rats whereas both groups exhibited decreased freezing over time during the extinction session. Data from the tone test is presented in Figure 3C. Repeated measures ANOVAs revealed significant main effects of Group [F(1,32)=16.2, p<0.0001] and Trial [F(19,608)=3.5, p<0.0001] and a significant Group X Trial interaction [F(19,608)=1.7, p<0.05]. The initially high level of freezing in the Sham-operated group gradually diminished over the course of the extinction session whereas lesioned rats exhibited low levels of freezing in response to all 20 presentations of the tone.
Figure 3.
Experiment 1 – Freezing behavior and locomotor activity. Freezing behavior: Effects of pre-training postsubiculum (PoSub) lesions on freezing behavior of lesioned (n = 14) and Sham-operated (n = 20) groups during the Acquisition session (A) and during the Context (B) and Tone (C) tests. BL, baseline; PS1 – 3, postshock 1 – 3. Bilateral lesions of the PoSub significantly decreased postshock freezing during training as well as freezing behavior during the Context and Tone tests (ps < 0.001). Locomotor activity: Average open field activity depicting that PoSub lesioned-rats (n = 11) were hyperactive compared to Sham-operated rats (n = 12) and that both groups habituated to the open-field over time (D). Relationship between freezing behavior and locomotor activity. Postshock freezing data from PoSub-lesioned rats was divided into normo-active (PoSub-Low, n = 5) and hyperactive (PoSub-High, n = 6) groups and replotted for the Acquisition session (E) and the Context (F) and Tone (G) tests. Replotted locomotor activity of normo-active and hyperactive groups (H). Data represent means ± standard errors. **p <05 compared to Sham-operated and PoSub-Low groups. # indicates that PoSub-lesioned rats showed a trend toward less freezing compared with Sham-operated rats (ps=0.06).
Locomotor behavior
Figure 3D depicts the activity profiles of a subset of control (n = 12) and lesioned (n = 11) rats. A repeated measures ANOVA on the locomotor data revealed significant main effects of Group [F(1,21)=5.2, p<0.05] and Block [F(5,105)=19.0, p<0.0001], but no Group × Block interaction [F(5,105)=0.8, p>0.05]. Inspection of the data reveals that PoSub-lesioned rats exhibited greater activity levels than Sham-operated rats and that both groups habituated to the open field over time.
Relationship between freezing behavior and locomotor activity
Correlation analyses that compared the total distance traveled with the mean amount of freezing during acquisition, the context test and the tone test revealed no significant correlations for either the Sham-operated or lesioned animals suggesting that hyperactivity did not account for impaired freezing behavior exhibited by PoSub lesioned rats (Sham-operated r-values = −0.31 to −0.47, Sham-operated ps = 0.1 to 0.3; lesion r-values = −0.17 to 0.18, lesion ps = 0.58 to 0.61, data not shown). The results of a second analysis that examined whether hyperactivity and freezing behavior were directly related are presented in Figure 3E–H. One-way ANOVA that compared the total distance traveled between Sham-operated, PoSub-Low and PoSub-High groups revealed a main effect of group [F(2,22)=10.1, p<0.001]. As shown in Figure 3H, animals in the PoSub-High group were significantly more active compared to either the PoSub-Low group (p<0.01) or the Sham-operated group (p<0.001). These data identifed a group of PoSub-lesioned rats that were normo-active and a group of PoSub rats that were hyperactive. Three additional analyses compared the average freezing behavior of Sham-operated, PoSub-Low and PoSub-High groups during 1) acquisition, 2) the context test and 3) the tone test (Figure 3E, F & G). The results revealed no main effects of group for the acquisition and context test data [for both, F(2,20)=2.6, ps>0.05] whereas a significant main effect of Group for the tone test data [F(2,22)=4.5, p<0.05] was observed; post hoc comparisons revealed that the Sham-operated group showed a trend toward more freezing behavior than either lesion group (ps = 0.06, Figure 3G). These data bring to light that even though locomotor behavior differed significantly between the PoSub-Low and PoSub-High lesion groups (Figure 3H), the average freezing behavior displayed by both PoSub-lesioned groups during the tone test was significantly reduced compared to Sham-operated rats. Of note, although analysis of the acquisition and context test data from the PoSub-Low, PoSub-High and Sham-operated groups did not reach statistical significance, the mean freezing behavior displayed by both the PoSub-Low and PoSub-High lesioned groups was lower than the average freezing behavior of the Sham-operated group for both test sessions (Figure 3E, F).
Discussion
One possible outcome of this experiment was that damage to PoSub prior to training would not affect acquisition of the conditioned fear response, but instead, would produce selective impairments in the expression of contextual fear memory. This prediction was based on the few published studies that have investigated functional contributions of PoSub (Taube et al., 1992) and the strong interconnections with regions such as hippocampus and RSP, damage to which have selective effects on contextual fear memory. Contrary to this hypothesis, PoSub damage impaired acquisition of the conditioned fear response during the training session in addition to producing reduced expression of contextual and auditory fear. These data indicate that the PoSub is instead involved in the normal acquisition of conditioned fear, which is more reminiscent of the role of the amygdala than the hippocampus during fear conditioning and retrieval.
Although PoSub-lesioned rats did exhibit reduced freezing during both the context and tone tests compared to Sham-operated rats, these deficits could have been secondary to an encoding impairment during training rather than reflect a mnemonic deficit. Therefore, based on the results of Experiment 1, it was not possible to conclude whether the PoSub is involved in contextual and auditory fear memory per se. Thus, Experiment 2 tested whether fear memory would be impaired in rats that sustained bilateral PoSub lesions 1 day after the training session.
EXPERIMENT 2
Materials and Methods
Subjects
Male Long-Evans rats (weighing 225 – 260 g at surgery) were obtained from Harlan Laboratories and maintained and handled as described in Experiment 1.
Behavioral Procedures and Surgery
All rats received the fear conditioning training session as described in Experiment 1. Freezing data were analyzed and rats were assigned to two equivalent groups based on the average postshock freezing observed across the three postshock intervals (see Fig 4A). Subjects underwent surgery one day after the training session. PoSub lesions were made in 14 rats as described in Experiment 1 with the inclusion of two changes to the surgical procedure. First, in an effort to reduce the number of rats with unilateral damage, an adjustment to the coordinates was made such that the following 2 sites were targeted (in mm): AP, −7.0, −7.8; ML, ±3.2, ±3.3; DV (from skull) −4.0, −3.8. Compared to Experiment 1, the first AP coordinate was moved 0.2 mm posterior and the first ML coordinate was moved 0.6 mm lateral. The DV coordinate was not changed. The second adjustment, which was made in an effort to reduce the amount of damage to extra cortical damage, entailed reducing the duration of current from 10–15 sec per site in Experiment 1 to 5 sec per site in Experiment 2. Pilot surgeries (n = 8) were conducted to verify that damage to the PoSub was equivalent to that observed in Experiment 1. Two types of Sham-operated surgeries were conducted in Experiment 2. Eleven Sham-operated surgeries were conducted as described for Experiment 1. Four additional Sham-operated surgeries were conducted in which the insect pin was lowered into all 4 coordinates but current was not passed through the tip. Operated rats recovered for 10–12 days prior to the start of behavioral testing.
Figure 4.
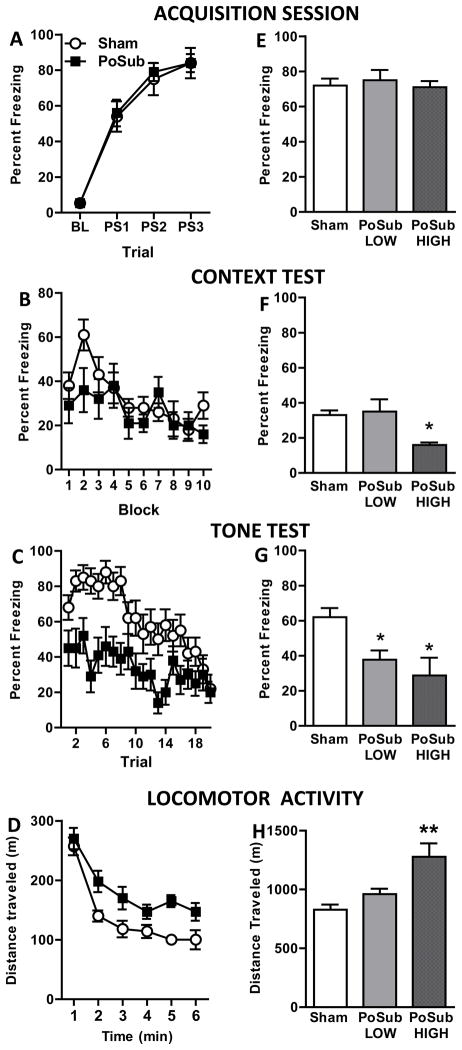
Experiment 2 – Freezing behavior and locomotor activity. Freezing behavior: Effects of post-training postsubiculum (PoSub) lesions on freezing behavior of lesioned (n = 14) and Sham-operated (n = 15) groups during the Acquisition session (A) and during the Context (B) and Tone (C) tests. BL, baseline; PS1 – 3, postshock 1 – 3. Bilateral lesions of the PoSub significantly decreased freezing during the Tone test, but had no effect on freezing during the Context test. Locomotor activity: Average open field activity demonstrating that PoSub lesioned-rats (n = 14) were hyperactive compared to Sham-operated rats (n = 15) and that both groups habituated to the open-field over time (D). Relationship between freezing behavior and locomotor activity: Postshock freezing data from PoSub-lesioned rats was divided into normo-active (PoSub-Low, n = 8) and hyperactive (PoSub-High, n = 6) groups and replotted for the Acquisition session (E) and the Context (F) and Tone (G) tests. Replotted locomotor activity of sham, normo-active and hyperactive groups (H). Data represent means ± standard errors. *p <05 compared to Sham-operated group. **p <05 compared to Sham-operated and PoSub-Low groups.
Behavioral Apparatus and Observations, Lesion Verification, and Data Analysis
The fear conditioning and open-field chambers were the same ones used in Experiment 1. The lesions were verified and freezing and locomotor behavior was analyzed as described for Experiment 1.
Results
Histology
All PoSub-lesioned rats (n = 14) and all Sham-operated rats (n = 15) were included in the histological and behavioral analyses. The average area of damage to PoSub from each of 3 sections analyzed was 65 ± 0.03% (range 50 – 82%), which was comparable to the amount of damage observed in Experiment 1. Minor unilateral damage to the granular-A subdivision of RSP and minor unilateral damage to the colliculi was observed in some lesioned rats. Damage to the PFCC was observed in most cases (10 of 14 rats). Damage to visual cortex was minimal in rats in Experiment 2. The dorsal subiculum and the medial geniculate nucleus sustained little to no damage. A qualitative comparison of the lesion damage from Experiment 1 and Experiment 2 showed that, overall, the extent of electrolytic damage was equivalent across experiments. One exception to this statement is that V1 damage was evident in PoSub lesioned rats in Experiment 1, but not in Experiment 2. This difference is highlighted in Figure 2B; damage was observed within the stippled area in Experiment 1, but not in Experiment 2. The improvements in lesion specificity (i.e. zero unilateral lesions, zero animals removed for excessive cortical damage and minimal V1 damage in Experiment 2) are likely attributable in part, to the adjustments made to the surgical procedure.
Behavior
Fear conditioning
There were no significant differences in average freezing behavior between the two Sham-operated surgery groups during the acquisition, context, or tone sessions and therefore data from these two groups were combined for all subsequent analyses. Figure 4A illustrates that prior to surgery, the rats assigned to each group (lesion or sham) exhibited robust postshock freezing during the acquisition session. A repeated measures ANOVA revealed a significant main effect of Trial [F(3,81)=100.7, p<0.0001] but no significant main effect of Group [F(1,27)=0.04, p>0.05] nor a significant Group X Trial interaction [F(3,81)=0.9, p>0.05]. In contrast to the results from Experiment 1, lesions of PoSub made one day after training had no effect on the expression of contextual fear (Figure 4B). These observations were supported by a repeated measures ANOVA that revealed no main effect of Group [F(1,27)=1.52, p>0.05]. A significant main effect of Block [F(9,288)=2.0, p<0.05] but no Group X Block interaction [F(9,288)=1.2, p>0.05] was observed, indicating equivalent decreases in freezing behavior over the extinction session. Data from the tone test is presented in Figure 4C. Repeated measures ANOVAs revealed significant main effects of Group [F(1,27)=14.8, p<0.001] and Trial [F(19,513)=7.5, p<0.0001] and a significant Group × Trial interaction [F(19, 513)=1.95, p<0.01], revealing a robust deficit in the expression of auditory fear memory in PoSub lesioned rats compared to controls.
Locomotor behavior
The activity profiles of Sham-operated (n = 15) and lesioned (n = 14) rats are shown in Figure 4D. Statistical analysis of locomotor activity revealed significant main effects of Group [F(1,27) = 11.3, p<0.005] and Block [F(5,135) = 42.5, p <0.0001], but no Group X Block interaction [F(5,135) = 1.3, p>0.05]. Visual inspection of the data reveals that lesioned rats had higher levels of locomotor activity and that both groups exhibited habituation to the open field over time.
Relationship between freezing behavior and locomotor activity
Correlation analyses that compared the total distance traveled with the mean amount of freezing during acquisition, the context test, and the tone test revealed that the PoSub group showed a significant negative correlation between freezing behavior and locomotor behavior during the context test [r-value = −.55, p<0.05). The correlation was not significant during the tone test [r-value = −.29, p>0.05]. There were no significant correlations between locomotor activity and freezing during any phase of the experiment for rats in the Sham-operated group [r-values = 0.10 and −0.11; ps = 0.74 and 0.69]. Correlation analysis for the acquisition session was not conducted because the lesions were made after training.
As in Experiment 1, PoSub-lesioned rats were divided into PoSub-Low and PoSub-High groups based on locomotor activity. A one-way ANOVA on the locomotor data revealed a significant main effect of group [F(2,22) = 10.1, p<0.001] and post hoc comparisons revealed that rats in the PoSub-High group were significantly more active compared with rats in either the PoSub-Low (p<0.01) or the Sham-operated group (p<0.001, Figure 4H). Two additional one-way ANOVAs that compared the average freezing behavior of Sham-operated, PoSub-Low and PoSub-High groups during the context test and during the tone test revealed significant main effects of Group for both test sessions [F(2,26)>4.5, ps<0.05]. Post hoc comparisons revealed that during the context test, PoSub-High rats froze significantly less than either the Sham-operated or the PoSub-Low group (ps<0.05, Figure 4F). Combined with the first activity-correlation analyses above, these data suggest that a causal relationship between locomotor behavior and freezing behavior may exist for the PoSub-lesioned rats during the context test in Experiment 2. In contrast, during the tone test, Sham-operated rats froze more than either lesion group (PoSub-Low, p<0.05; PoSub-High, p<0.01, Figure 4G). These data indicate that although locomotor behavior differed significantly between the PoSub-Low and PoSub-High lesion groups, the average freezing behavior displayed during the tone test by the lesion groups were equivalent. The same pattern of results was observed during the tone test in Experiment 1.
General Discussion
Based on existing anatomical and behavioral data, it was predicted that PoSub damage would result in a specific deficit in contextual fear memory. Indeed, the PoSub is well suited as a site of thalamo-cortico-hippocampal integration (Aggleton, O’Mara, Vann, Wright, Tsanov, and Erichsen, 2010; Van Groen and Wyss, 1990) and behavioral data to date suggest that the PoSub is involved in processing spatial information (Ranck, 1984; Sharp, 1996; Taube et al., 1990a). However, the present data are instead more similar to the results obtained after amygdalar damage. Specifically, damage to PoSub prior to training impaired the acquisition of conditioned fear as well as the subsequent expression of contextual and auditory fear. These findings underscore the need to further investigate possible interactions between the PoSub and the thalamo-amygdala circuitry that plays a prominent role in the acquisition and expression of auditory (and contextual) fear conditioning (LeDoux, Cicchetti et al. 1990; Celerier, Ognard et al. 2000). A second finding is that a pronounced deficit in the expression of auditory fear conditioning is present when PoSub damage occurs after training, indicative of a specific deficit in auditory fear memory.
Acquisition of conditioned fear
The finding that acquisition is impaired in PoSub-lesioned rats during Pavlovian fear conditioning is consistent with the view that PoSub may play a role in associative processes (Goodridge and Taube, 1997; Johnson, Seeland, and Redish, 2005). This suggestion is based in part on studies of “head direction” cells. These neurons were first identified in PoSub (Ranck, 1984; Taube et al., 1990a) and a defining feature is that they change their firing rate as a function of head orientation within an environment. Up to 89% of cells in the PoSub show a relationship between firing rate and an animal’s head direction (Sharp, 1996) and neural ensemble recordings have established that the PoSub contains a complete representation of an animal’s orientation (Johnson et al., 2005). Experimental manipulations that involve rotations of an external cue card within an animal’s environment have shown that the preferred directions of recorded head direction cells rotates a corresponding amount (Taube, Muller, and Ranck, 1990b). Goodridge and Taube (1997) used cue-card manipulations to examine the relationship between the PoSub and the anterior thalamus and found that when the PoSub is lesioned, head direction cells in the anterior thalamus continue to show direction selectivity, but no longer follow cue card rotation. One interpretation of these data is that the PoSub-anterothalamic pathway is involved in associative processes that link external cues (cue card) with an animal’s internal representation of its environment. By extension, it is possible that lesions of the PoSub also disrupt associative processes that link relevant external cues with internal cues that are involved in fear conditioning. In accordance with this notion, electrophysiological evidence suggests that the activity of neurons in rabbit associative cortex (a region corresponding to the PoSub in rats) may be related to eye movements involved in the processing of visual information during learned behavior (Sikes, Vogt, and Swadlow, 1985). Combined, these studies provide support for the notion that the PoSub and the PoSub-anterothalamic pathway may be involved in associative learning.
The effects of PoSub damage on acquisition and expression of fear conditioning are similar to those observed following functional inactivation of the amygdala. For example, blockade of GABAa receptors in the amygdala before, but not after auditory fear conditioning, prevents memory formation (Wilensky, Schafe, and LeDoux, 1999). In that study, the authors conclude that synaptic activity in the amygdala is necessary during learning. Future studies, including those that selectively lesion or inactivate PoSub neurons as well as electrophysiological studies that test the capacity of PoSub for experience-dependent plasticity, will continue to identify the specific contributions of PoSub to fear learning and memory.
Expression of contextual fear conditioning
Damage to PoSub prior to training (Experiment 1) impaired the expression of contextual fear when rats were placed back in the training chambers for the context test. However, since PoSub damage also impaired acquisition of the conditioned fear response during the training session, the deficit in freezing behavior during the context test could have been secondary to an encoding impairment during training. Indeed, when lesions were instead carried out after training (Experiment 2), there was no significant effect of PoSub damage during the context test. This pattern of results is different from those observed after lesions of the dorsal hippocampus or other parahippocampal structures. Specifically, lesions of the hippocampus prior to training do not necessarily impair contextual learning whereas lesions made one day after acquisition results in robust contextual fear conditioning deficits (but see, (Hunsaker and Kesner, 2008; Lee and Kesner, 2004; Maren and Holt, 2004). The present data establish that the PoSub and the hippocampus have distinct roles in fear conditioning. These findings are consistent with recent electrophysiological data that show distinct roles for PoSub and hippocampus during replay (i.e. retrieval) of spatial information (Brandon, Bogaard, Andrews, and Hasselmo, 2011).
In addition, the pattern of behavioral results following PoSub damage are distinct compared to that of RSP or POR which have been shown to be involved in the expression, but not acquisition of contextual fear (Burwell et al., 2004; Keene and Bucci, 2008b). Thus, one interpretation of the present data is that PoSub is necessary for the normal encoding of contextual (and auditory) information, but has only a temporary (i.e. one day or less) or no role in the consolidation and expression of contextual fear memory. Given the connections between PoSub and the hippocampal formation, an alternative explanation for the observed pre-training lesion-induced deficit in contextual fear conditioning is that the PoSub serves as a conduit of sensory information to medial temporal lobe structures that are critical for hippocampal-dependent learning and memory. However, in this case, lesions made either before or after training would have been expected to disrupt the flow of sensory information, leading to impaired expression of contextual conditioning.
Expression of auditory fear conditioning
Lesions of the PoSub dramatically reduced freezing during the tone test regardless of whether damage occurred before or after training. In this regard too, the effects of PoSub damage are more similar to those following amygdalar lesions than hippocampal lesions. Indeed, evidence of both anterograde and retrograde auditory fear amnesia exists following lesions of the amygdala (LeDoux et al., 1990; Maren, Aharonov, and Fanselow, 1996; Maren and Fanselow, 1996; Nader, Majidishad, Amorapanth, and LeDoux, 2001; Sacchetti, Lorenzini, Baldi, Tassoni, and Bucherelli, 1999; Vazdarjanova and McGaugh, 1999; Wilensky et al., 1999) whereas auditory fear conditioning is usually intact in hippocampal-lesioned rats (Anagnostaras et al., 1999; Phillips and LeDoux, 1995; Selden et al., 1991).
PoSub has reciprocal connections with the anterior thalamus and electrophysiological studies have shown that behavior-related anterior thalamic activity is dependent on the integrity of PoSub, suggestive of a functional relationship between these regions (Goodridge and Taube, 1997). Thus it is plausible that PoSub participates within the thalamo-amygdalar circuitry that has been shown to play a prominent role in auditory fear conditioning. Indeed, although the anterior thalamus has traditionally been associated with spatial learning (Vann and Aggleton, 2004), recently it has been shown that lesions of this structure disrupt auditory fear conditioning (Celerier et al., 2000; Conejo, Gonzalez-Pardo, Lopez, Cantora, and Arias, 2007). Moreover, expression of the immediate early gene, c-fos, is increased in anterior thalamus after auditory fear conditioning (Conejo et al., 2007). In summary, the present findings suggest that PoSub is part of an essential circuit that processes sensory information during fear conditioning and is specifically involved in auditory fear memory processes.
Alternative explanations
An alternative explanation for the effects of PoSub lesions on freezing is that the hyperactivity exhibited by PoSub-lesioned rats interfered with their ability to freeze and therefore any decrease in freezing behavior may simply reflect a performance deficit rather than either a learning or mnemonic deficit. However, several pieces of evidence argue against this possibility. First, there was no significant correlation between locomotor behavior and freezing behavior during any of the four fear conditioning tests in which there was a significant difference between PoSub-lesioned and Sham-operated rats (i.e. Experiment 1: acquisition, context test and tone test; Experiment 2: tone test). There was a significant correlation between context freezing and locomotor activity in lesioned rats in Experiment 2, however, context freezing did not differ between the control and PoSub-lesioned rats during the context test. Therefore this significant correlation, while relevant to interpretation of the context data, has little bearing on the results and interpretation of the acquisition or tone test data. Secondly, for both Experiment 1 and Experiment 2, when PoSub-lesioned rats were re-grouped based on their locomotor activity, both normo-active (PoSub-Low) and hyperactive (PoSub-High) groups froze significantly less than Sham-operated rats. In summary, reduced expression of auditory fear conditioning was observed in two groups of normo-active PoSub-lesioned rats (Experiment 1 and Experiment 2), which substantiates the claim that hyperactivity does not account for the freezing deficit observed during the tone test.
Another possibility is that damage outside of PoSub contributed to the deficits in freezing behavior. The most consistent damage outside of PoSub was to PFCC. Of central importance to this study, axons from ENTO to the hippocampus travel in this fiber bundle (Taube et al., 1992) and thus damage here may interfere with the transfer of information to and from the hippocampus. However, it is unlikely that damage to the PFCC can account for the freezing deficits observed in this study. Indeed, the PFCC was damaged to a similar extent in Experiment 1 and 2 (12 of 14 rats and 10 of 14 rats, respectively) yet freezing during the context test was only affected in Experiment 1. In addition, there is no evidence that PFCC contains fibers from regions of the auditory thalamus or auditory cortex, thus the consistent deficits in freezing to the tone are not likely due to PFCC damage. Another possibility is that the contextual freezing deficits observed in Experiment 1 are due to visual cortex damage rather than to PoSub damage. While visual cortex damage may contribute to the deficit, it is not likely that this damage can account for the entire effect because contextual discrimination is dependent on a combination of multiple cues, not an individual sensory modality (Bucci, Saddoris, and Burwell, 2002).
It is likely that neurotoxic lesions would have produced less damage to the PFCC compared to the electrolytic approach used here. However, it was important to have the precise temporal control afforded by electrolytic lesions particularly in Experiment 2, since we were specifically interested in the effects of damage 24 hr after fear conditioning. Future studies using neurotoxins (e.g. ibotenic acid or N-methyl-D-Aspartate) that do not produce substantial cell loss in regions distal to the site of injection and that do not interfere with memories that are already stored in other structures (Anagnostaras et al., 2001; Jarrard, 1983; Jarrard, 1989; Jarrard, 2002), will continue to shed light on the role of the PoSub during fear learning and memory.
Conclusion
The present findings provide the first evidence that PoSub is involved in contextual and auditory fear conditioning. A main finding of the present study is that encoding, but not retrieval and/or expression of contextual fear conditioning requires the PoSub. A deficit in expression of contextual and auditory fear conditioning was observed when lesions were made prior to training, however, these impairments may have been secondary to a deficit in acquisition. The second main finding is that expression and/or retrieval of auditory fear memory is impaired when damage to the PoSub occurs after conditioning. Future studies, including those that selectively lesion or inactivate PoSub neurons as well as electrophysiological and neurochemical studies that examine experience-dependent plasticity, will continue to elucidate the specific contributions of PoSub to fear learning and memory.
Acknowledgments
Grant sponsor: NSF; Grant numbers: 0441934 and 0922075 (DJB).
Grant sponsor: NIMH; Grant number F32MH092991 (SR).
The authors thank Dr. Robert Leaton as well as three anonymous reviewers for valuable comments on a previous version of the manuscript and Hannah Payne for excellent technical assistance with the experiments.
References
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31(12):2292–307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19(3):1106–14. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenos JD, Musty RE, Bucci DJ. Blockade of cannabinoid CB1 receptors alters contextual learning and memory. Eur J Pharmacol. 2006;539(3):177–83. doi: 10.1016/j.ejphar.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn Mem. 2009;16(1):38–45. doi: 10.1101/lm.1099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67 (3):370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Andrews CM, Hasselmo ME. Head direction cells in the postsubiculum do not show replay of prior waking sequences during sleep. Hippocampus. 2011 doi: 10.1002/hipo.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav Neurosci. 2000;114(5):882–94. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav Neurosci. 2002;116(3):479–88. [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci. 2004;24(49):11023–8. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. Hippocampal place cell instability after lesions of the head direction cell network. J Neurosci. 2003;23(30):9719–31. doi: 10.1523/JNEUROSCI.23-30-09719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier A, Ognard R, Decorte L, Beracochea D. Deficits of spatial and non-spatial memory and of auditory fear conditioning following anterior thalamic lesions in mice: comparison with chronic alcohol consumption. Eur J Neurosci. 2000;12(7):2575–84. doi: 10.1046/j.1460-9568.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Conejo NM, Gonzalez-Pardo H, Lopez M, Cantora R, Arias JL. Induction of c-Fos expression in the mammillary bodies, anterior thalamus and dorsal hippocampus after fear conditioning. Brain Res Bull. 2007;74(1–3):172–7. doi: 10.1016/j.brainresbull.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15(4):177–82. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J Neurosci. 1997;17(23):9315–30. doi: 10.1523/JNEUROSCI.17-23-09315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Falls WA. Destruction of the auditory thalamus disrupts the production of fear but not the inhibition of fear conditioned to an auditory stimulus. Brain Res. 1998;813(2):274–82. doi: 10.1016/s0006-8993(98)01047-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9(2):195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89(1):61–9. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behav Neurosci. 1983;97(6):873–89. doi: 10.1037//0735-7044.97.6.873. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29(3):251–9. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Use of excitotoxins to lesion the hippocampus: update. Hippocampus. 2002;12:405–414. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- Johnson A, Seeland K, Redish AD. Reconstruction of the postsubiculum head direction signal from neural ensembles. Hippocampus. 2005;15(1):86–96. doi: 10.1002/hipo.20033. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 2008a;122(1):89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 2008b;122(5):1070–7. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4(3):683–98. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14(3):301–10. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Lacanilao S, Sutherland RJ. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur J Neurosci. 2007;25 (5):1278–86. doi: 10.1111/j.1460-9568.2007.05374.x. [DOI] [PubMed] [Google Scholar]
- Majchrzak M, Ferry B, Marchand AR, Herbeaux K, Seillier A, Barbelivien A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in the rat. Hippocampus. 2006;16(2):114–24. doi: 10.1002/hipo.20138. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110(4):718–26. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88(2):261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Anagnostaras SG, Fanselow MS. The startled seahorse: is the hippocampus necessary for contextual fear conditioning? Trends Cogn Sci. 1998;2(2):39–42. doi: 10.1016/s1364-6613(98)01123-1. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16(2):237–40. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67(2):142–9. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118(1):97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8(3):156–63. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; San Diego, CA: 2007. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1(1):34–44. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15(7 Pt 2):5308–15. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15 (5):1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Head-direction cells in the deep cell layers of dorsal presubiculum in freely moving rats. Society for Neuroscience Abstracts. 1984;10:599. [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19(21):9570–8. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42(2):335–50. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Multiple spatial/behavioral correlates for cells in the rat postsubiculum: multiple regression analysis and comparison to other hippocampal areas. Cereb Cortex. 1996;6(2):238–59. doi: 10.1093/cercor/6.2.238. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Vogt BA, Swadlow HA. Limbic cortex neuronal activity associated with saccadic eye movements in awake rabbits and possible underlying afferents. Soc Neurosci Abstr. 1985:1042. [Google Scholar]
- Sorensen KE, Shipley MT. Projections from the subiculum to the deep layers of the ipsilateral presubicular and entorhinal cortices in the guinea pig. J Comp Neurol. 1979;188(2):313–33. doi: 10.1002/cne.901880208. [DOI] [PubMed] [Google Scholar]
- Taube JS, Kesslak JP, Cotman CW. Lesions of the rat postsubiculum impair performance on spatial tasks. Behav Neural Biol. 1992;57(2):131–43. doi: 10.1016/0163-1047(92)90629-i. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990a;10(2):420–35. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10 (2):436–47. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Res. 1990;529(1–2):165–77. doi: 10.1016/0006-8993(90)90824-u. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol. 1995;358(4):584–604. doi: 10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5(1):35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19(15):6615–22. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol. 1983;216(2):192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19(24):RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res. 1990;83:47–58. doi: 10.1016/s0079-6123(08)61240-6. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108(1):19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]