Abstract
Background
Asthma morbidity and mortality rates are high among young inner-city children. Lack of routine primary care provider (PCP) visits, poor access to care, and poor patient-physician communication may be contributing factors.
Objective
This study evaluated the effects of providing Breathmobile services only, a Facilitated Asthma Communication Intervention (FACI) only, or both Breathmobile+FACI on asthma outcomes, relative to standard care.
Methods
Children with asthma (n=322, mean age=4 years, 53% male, 97% African American) were recruited from Head Start programs in Baltimore City and randomized into four groups. Outcome measures included symptom-free days, urgent care use (emergency department visits and hospitalizations) and medication use (courses of oral steroids and proportion on an asthma controller medication) as reported by caregivers at baseline, 6-, and 12-month assessments. Generalized Estimating Equations models were conducted to examine the differential treatment effects of Breathmobile and FACI compared to standard care.
Results
Children in the combined treatment group (Breathmobile+FACI) had an increase of 1.7 (6.6%) symptom-free days (SFD) that was not maintained at 12 months. In intent-to-treat analyses, the FACI-only group had an increase in the number of ED visits at 6 months, which was not present at 12 months or in the post hoc as-treated analyses. No significant differences were found between the intervention groups as compared to standard care on all other outcome measures.
Conclusions
Other than a slight improvement in SFD at 6 months in the Breatmobile+FACI group, the intervention components did not result in any significant improvements in asthma management or asthma morbidity.
Keywords: asthma, intervention, communication, quality of life, interaction, barriers
Low income, minority children have disproportionately higher prevalence, morbidity and mortality from asthma.(1–3) Research suggests that inappropriate asthma management practices and barriers in accessing appropriate asthma care, including over-reliance on emergency care, poor adherence to therapy, lack of routine primary care provider (PCP) visits, inadequate access to specialty asthma medical care, and poor patient-physician communication are important contributing behavioral factors.(4–6) Asthma prevalence and morbidity rates are notably high among preschool children in Head Start (HS), suggesting that HS may be an appropriate venue for targeted asthma interventions.(7;8)
Intervention programs for families of children with asthma have produced modest positive results, with some reporting improved overall management of asthma and reductions in asthma morbidity, including decreased frequency and severity of asthma flares and decreased ED visits, hospitalizations, and school absences.(9–11) Many low-income and minority families have inadequate access to such programs. Several intervention studies have examined the efficacy of delivering asthma education directly into high-risk communities. Integrating asthma education into asthma care visits and delivering asthma education at community schools show promise as strategies to deliver asthma education interventions.(9;11)
One strategy for improving access to asthma care has been the Breathmobile®, which is a mobile medical clinic that brings preventive asthma care and education directly to children in high-risk communities to reduce the barrier of accessing care.(12) Previous research using a single group pre-post design has shown that the Breathmobile can significantly reduce children’s asthma morbidity, including reductions in asthma symptoms and improvements in quality of life.(13–16)
Improving provider-patient communication has been found to contribute to reducing health care disparities for minority patients with asthma.(17) Previous studies have shown that children with asthma and their parents can be educated to communicate this information more effectively with their providers following home-based education interventions.(3;18) Moreover, a stronger partnership and improved communication between patients and providers is associated with better asthma control, fewer exacerbations, better quality of life, fewer sleep disturbances, and fewer patient-reported symptoms.(5;19)
The aim of this randomized controlled trial was to test the effectiveness of two community-based interventions designed to address barriers to appropriate asthma health care among high-risk minority preschool children enrolled in HS. We evaluated the effectiveness of Breathmobile as well as a Facilitated Asthma Communication Intervention (FACI), a home-based asthma education and parent-provider communication intervention. Our modified 2×2 cluster-randomized study design included four groups: Breathmobile alone, FACI alone, and Breathmobile+FACI, versus standard care. Our primary study outcome was symptom-free days (SFD). Secondary outcomes included acute health care utilization (ED visits and hospitalizations), number of oral steroid bursts, proportion of children on an asthma controller medication, and caregiver’s asthma-related quality of life.
We hypothesized that children receiving intervention (Breathmobile only, FACI only, and Breathmobile+ FACI) would have the greatest increase in the number of SFD, compared to those children assigned to the standard care group. We further hypothesized that children receiving intervention would report significantly decreased urgent healthcare visits, decreased number of oral steroid bursts, increased use of asthma controller medications, and improved caregiver quality of life compared to children in the standard care group.
Methods
Study Design
The Johns Hopkins Medical Institution and University of Maryland School of Medicine Institutional Review Boards approved the study. Written informed consent was obtained from the child’s primary caregiver. Overall, 336 children with persistent asthma were consented and 322 were randomized into one of four groups. HS sites were the units of randomization for the Breathmobile intervention, while families were the units of randomization for the FACI intervention. Since the Breathmobile was present only at those HS sites assigned the Breathmobile, staff and families could not be blinded to assignment. However, as is standard practice, all children attending the HS site were offered Breathmobile services and the Breathmobile staff was unaware if children scheduled and treated were enrolled in the research study.
A block randomization schema was used by placing randomization into sealed envelopes which were opened after families completed baseline surveys. This masked research staff to group assignment during recruitment. All participants were contacted for follow-up surveys at 6 and 12 months after randomization. Research assistants conducted telephone surveys and were blinded to group assignment.
Participants
Children aged 2–6 years from all 66 HS sites in Baltimore were screened for recruitment using a caregiver questionnaire. Eligibility criteria included caregiver reported physician-diagnosed asthma or reactive airways disease and at least one of the following: 1) use of short acting beta agonist in the past 4 weeks, or 2) asthma symptoms in the past 4 weeks, or 3) treated in the ED for asthma in past 6 months. Eligible families were mailed information about the study and then contacted by phone to confirm eligibility and describe the study in more detail. Interested families were mailed a copy of the informed consent form, provided with time to raise questions, and asked to return a signed copy of the informed consent form by mail.
Intervention Description
Breathmobile Intervention
The Breathmobile is a mobile asthma clinic that delivers specialty NAEPP (20) guideline-based asthma screening, evaluation, and treatment services directly to inner city children at their schools or HS sites. A specially trained nurse practitioner, allergist, nurse and driver/patient assistant provide care on the Breathmobile. HS staff distributed a validated survey (21) to all students that identifies children with asthma or at risk for asthma. Caregivers interested in receiving services on the Breathmobile were scheduled for appointments regardless of study enrollment.
An asthma-focused history was obtained, a physical was carried out, and all children received skin testing for common asthma triggers and were prescribed controller medications based on asthma severity level. Asthma education was provided regarding trigger avoidance, appropriate use of medications, and an asthma action plan. A summary of the Breathmobile visit health information was faxed to the child’s PCP and the children continued to receive primary care services from their PCP.
Each child at an HS site who was randomized to the Breathmobile intervention had multiple opportunities to schedule an appointment on the Breathmobile during school hours. Families were encouraged to schedule a Breathmobile visit when it was at their HS site, or at another HS site or elementary school nearby. They were sent postcards with upcoming Breathmobile visit dates, and a staff member called to offer the opportunity to schedule a visit. The child’s caregiver was required to attend the Breathmobile visit with the child.
Facilitated Asthma Communication Intervention (FACI)
Families randomized to the FACI intervention received a single home session with an Asthma Educator (AE). In addition to providing basic asthma education, the session was designed to teach and model specific communication skills for caregivers to use when interacting with the child’s PCP about asthma. These skills included (1) Information Provision, or knowing what information is essential to help the PCP make an accurate diagnosis and treatment recommendations and how to provide this information effectively and efficiently; (2) Information Seeking, or asking clear, direct, and succinct questions to obtain desired information; and (3) Information Verification, or ensuring an understanding of the information the PCP has already provided, including requests for repetition and summarizing what has been said.(22) Additionally, the AE and caregiver completed the “Notes to My Child’s Doctor” tool (23) which included sections on asthma morbidity and setting goals for communication and asthma treatment. Finally, the AE joined the family at a PCP visit to encourage the sharing of the “Notes to My Child Doctor” and facilitate family-PCP communication. After the visit, the AE provided the family with constructive feedback and suggestions to improve interactions, along with assistance in scheduling the next PCP visit for asthma care.
Standard Care Group
Caregivers assigned to this group received standard asthma care from their primary care provider and follow-up assessments but did not attend an HS site assigned to Breathmobile or receive the FACI intervention.
Measures
Symptom-free days
SFD, the primary outcome, was calculated by subtracting the number of days and or/nights with asthma symptoms (i.e., cough, wheeze, shortness of breath) in the past 30 days, as reported by caregivers.(24;25)
Acute Care and Medication Use
This measure included caregiver report of ED visits, hospitalizations, prescribed asthma controller medication regimen (e.g., inhaled corticosteroid and leukotriene modifiers), and courses of oral corticosteroids in the previous 6 months.
Caregiver Quality of Life
The Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ) (26) was used to measure caregiver quality of life. The PACQLQ is a 13- item measure of activity limitations (4 items) and emotional function (9 items) experienced by caregivers of asthmatic children. Responses on the PACQLQ are given on a 7-point scale where 1 represents severe impairment and 7 represents non-impairment. Higher scores indicate higher quality of life.
Statistical Analyses
Baseline characteristics were compared across the groups. Initial Intent-to-treat (ITT) analyses compared each group (FACI only, Breathmobile only, and Breathmobile+FACI) to the standard care group at each time-point for outcome measures. Generalized estimating equations (GEE) was used to estimate the group population average for each outcome over time to control for the correlation among longitudinal measures within an individual, while adjusting for baseline level of each outcome. For each outcome, time (baseline = time 1, 6-months= time 2, and 12- months = time 3) and intervention group (Standard Care, Breathmobile only, FACI only, and Breathmobile+FACI) were modeled as categorical fixed effects of outcomes. Using GEE, we examined the interaction of treatment * time to determine an intervention effect at each time point. For binary, count, and continuous outcomes, a Poisson with log-link function, Bernoulli, and normal models were specified, respectively. An exchangeable correlation structure was specified for each model. Due to low completion rates for the intervention, post hoc analyses were conducted for as- treated groups for each outcome that was significant during the ITT analyses. As-treated analyses compared participants who completed all of their assigned intervention within each treatment group to children who did not complete their assigned intervention (standard care group and intervention non-completers). Stata 11 (College Station, TX) was used for all data analyses.
Results
Sample characteristics
Of the 336 families who consented to participate, 322 (96%) completed the baseline questionnaire, and 321 were randomized (Figure 1); one participant was excluded due to lack of an asthma diagnosis at baseline. Intervention completion rates are shown in Table 1. Over 375 appointment slots were made available specifically to study participants who attended an HS site randomized to Breathmobile and staff also offered appointments at nearby locations. Of all the eligible participants, 73 (45%) scheduled a visit and 30 (18%) attended a Breathmobile visit. Furthermore, only 14 (18%) in the Breathmbile+FACI group completed all FACI sessions and had a visit on the Breathmobile. There was some crossover as 6 children who were not randomized to the Breathmobile were seen on the Breathmobile. This occurred when children transferred HS sites during the study time and were now eligible for the Breathmobile services at the new site. Most children -- 303 (94%) -- reported at baseline that they had seen a doctor for a regular appointment in the past six months. Participants who completed their assigned intervention visits reported fewer baseline SFD (p < 0.01) and more urgent care visits to PCP (p<0.04) than those who did not complete their assigned intervention, but were similar on all other baseline characteristics. There were no differences in baseline demographics and asthma morbidity measures between participants who completed their follow-up surveys and those that did not.
Figure 1.

Consort Figure
Table 1.
Intervention Completion Rates by group
| Standard Care (n=77) N(%) |
Breathmobile only (n=83) N (%) |
FACI only (n=84) N (%) |
Breathmobile +FACI (n=77) N (%) |
|
|---|---|---|---|---|
| Completed Breathmobile visit | 4 (5) | 16 (19) | 2 (2) | 17 (22) |
| Completed FACI visit 1 | 0 (0) | ------0 (0) | 75 (89) | 68 (88) |
| Completed FACI visit 2 | 0 (0) | ------0 (0) | 54 (64) | 50 (65) |
Table 2 presents the baseline demographic characteristics. As shown in Table 3, asthma morbidity was high as indicated by means of asthma outcomes at all three time points. Overall, children experienced 25.2 SFD a month at baseline and had a mean of 1 ED visit in the past 6 months; only 160 (50%) were prescribed an asthma controller medication. There were no differences in any baseline demographic or health outcome by group status.
Table 2.
Baseline Child Demographic Characteristics by Group
| Demographic variables | Standard Care (n=77) N (%) |
Breathmobile only (n=83) N (%) |
FACI only (n=84) N (%) |
Breathmobile +FACI (n=77) N (%) |
|---|---|---|---|---|
| Age*, Mean (SD) | 4.0 (0.6) | 4.1 (0.7) | 4.0 (0.7) | 4.1 (0.7) |
| Female | 36 (47) | 44 (53) | 42 (50) | 28 (36) |
| African-American | 74 (96) | 81 (98) | 82 (98) | 75 (97) |
| Health Insurance Medical assistance Private insurance/HMO Self pay/cash Missing |
67 (87) 6 (8) 3 (4) 1 (1) |
77 (93) 5 (6) 1 (1) 0 (0) |
78 (93) 2 (2) 3 (4) 1 (1) |
68 (88) 7 (9) 2 (3) 0 (0) |
| Family Income <$10,000 $10–19,999 $20–29,999 $30–39,999 Above $40,000 Missing |
31 (40) 23 (30) 15 (18) 2 (3) 6 (8) 0 (0) |
39 (47) 17 (20) 15 (18) 5 (6) 6 (7) 1 (1) |
32 (38) 19 (23) 20 (24) 9 (11) 2 (2) 2 (2) |
34 (44) 17 (22) 13 (17) 7 (9) 4 (5) 2 (3) |
| Attended PCP appointment in past 6 months | 74 (96) | 82 (98) | 77 (92) | 70 (91) |
age is reported in mean (SD) years; all other variables are n (%)
Table 3.
Descriptive Statistics for the Health Outcomes at Each Assessment by Group
| Outcome Follow-Up (in months) |
Standard Care (N=77) |
Breathmobile Only (n=83) |
FACI only (n=84) |
Breathmobile+ FACI (n=77) |
|---|---|---|---|---|
| Symptom-Free Days per month, Mean (SD) | ||||
| 0 | 26.2 (5.8) | 25.0 (7.2) | 24.1 (7.3) | 25.5 (4.6) |
| 6 | 24.8 (6.2) | 25.3 (6.2) | 24.8 (7.0) | 27.2 (3.3) |
| 12 | 26.2 (5.8) | 24.9 (7.2) | 24.1 (7.3) | 25.5 (4.6) |
| ED visits in the last 6 months, Mean (SD) | ||||
| 0 | 0.72 (1.3) | 0.69 (1.1) | 0.69 (1.2) | 0.62 (1.1) |
| 6 | 0.70 (1.4) | 0.52 (0.9) | 1.10 (2.4) | 0.71 (1.5) |
| 12 | 0.73 (1.3) | 0.69 (1.1) | 0.69 (1.2) | 0.62 (1.1) |
| Hospitalizations in the last 6 months, Mean (SD) | ||||
| 0 | 0.19 (0.8) | 0.21 (0.7) | 0.16 (0.9) | 0.18 (0.5) |
| 6 | 0.14 (0.7) | 0.08 (0.4) | 0.12 (0.4) | 0.03 (0.2) |
| 12 | 0.07 (0.26) | 0.09 (0.38) | 0.09 (0.5) | 0.03 (0.2) |
| Courses of oral steroids in the last 6 months, Mean (SD) | ||||
| 0 | 0.42 (0.7) | 0.64 (1.1) | 0.61 (1.1) | 0.36 (0.8) |
| 6 | 0.37 (0.7) | 0.32 (0.6) | 0.46 (0.8) | 0.29 (0.6) |
| 12 | 0.35 (0.9) | 0.34 (0.6) | 0.31 (0.62) | 0.24 (0.58) |
| Number of Children on Asthma Controller Medication, n (%) | ||||
| 0 | 24 (31) | 29 (35) | 43 (51) | 34 (44) |
| 6 | 34 (44) | 37 (44) | 42 (50) | 45 (58) |
| 12 | 29 (38) | 36 (43) | 37 (44) | 38 (49) |
| Caregiver Quality of Life, Activity Limitations Mean (SD) | ||||
| 0 | 4.6 (0.5) | 4.5 (0.5) | 4.4 (0.7) | 4.4 (0.7) |
| 6 | 4.7 (0.4) | 4.7 (0.5) | 4.6 (0.7) | 4.6 (0.7) |
| 12 | 4.8 (0.5) | 4.7 (0.4) | 4.6 (0.6) | 4.6 (0.5) |
| Caregiver Quality of Life, Emotional Symptoms Mean (SD) | ||||
| 0 | 4.5 (0.6) | 4.2 (0.7) | 4.3 (0.6) | 4.2 (0.7) |
| 6 | 4.6 (0.5) | 4.5 (0.6) | 4.5 (0.7) | 4.5 (0.7) |
| 12 | 4.8 (0.4) | 4.7 (0.6) | 4.6 (0.6) | 4.6 (0.6) |
Symptom-Free days
We observed a time * treatment interaction for the Breathmobile+FACI group at 6 months compared to standard care in ITT analysis. The Breathmobile+FACI group (N = 77) had an increase of 1.7 (6.6%) SFD per month at 6 months follow-up (b = 3.43 p = 0.01) that was not maintained at 12 months (b = 0.35 p = 0.80; Table 4 for GEE results and Figure 2a). There were no significant time * treatment interactions for the other three groups. Post hoc as- treated analyses also demonstrated a significant time* treatment interaction for the Breathmobile+FACI group (N = 14) at 6 months (b = 1.23 p = 0.03) and a trend at 12 months (b = 1.21 p = 0.05).
Table 4.
GEE estimates of selected asthma outcomes over time for Symptom Free Days, Hospitalizations and ED visits
| Parameter | Estimate† | p |
|---|---|---|
| Symptom-Free Days | ||
| Time | 2.20 | 0.03 |
| Baseline Symptom Free Days | 0.57 | 0.001 |
| Breathmobile only | −0.59 | 0.50 |
| FACI only | −0.85 | 0.33 |
| Breathmobile+FACI | −0.36 | 0.69 |
| Breathmobile only* time2 | 1.92 | 0.17 |
| FACI only* time2 | 2.14 | 0.11 |
| Breathmobile+FACI* time2 | 3.43 | 0.01 |
| Breathmobile only* time3 | 0.12 | 0.93 |
| FACI only* time3 | −0.01 | 0.99 |
| Breathmobile+FACI* time3 | 0.35 | 0.81 |
| Hospitalizations | ||
| Time | 0.35 | 0.04 |
| Baseline Hospitalizations | 1.82 | 0.001 |
| Breathmobile only | 1.07 | 0.83 |
| FACI only | 0.51 | 0.08 |
| Breathmobile+FACI | 1.23 | 0.57 |
| Breathmobile only* time2 | 0.77 | 0.68 |
| FACI only* time2 | 0.98 | 0.98 |
| Breathmobile+FACI* time2 | 0.23 | 0.08 |
| Breathmobile only* time3 | 1.81 | 0.40 |
| FACI only* time3 | 1.59 | 0.51 |
| Breathmobile+FACI* time3 | 0.45 | 0.39 |
| ED visits | ||
| Time | 0.49 | 0.001 |
| Baseline ED visits | 1.29 | 0.001 |
| Breathmobile only | 0.59 | 0.001 |
| FACI only | 0.87 | 0.29 |
| Breathmobile+FACI | 0.91 | 0.51 |
| Breathmobile only* time2 | 0.99 | 0.99 |
| FACI only* time2 | 1.69 | 0.01 |
| Breathmobile+FACI* time2 | 1.19 | 0.45 |
| Breathmobile only* time3 | 1.24 | 0.35 |
| FACI only* time3 | 1.00 | 0.99 |
| Breathmobile+FACI* time3 | 0.35 | 0.81 |
Parameter estimates for ED visits and hospitalizations are based on Incidence Rate Ratio (IRR). The estimates for symptom-free days are based on unstandardized regression coefficient (b).
Figure 2.
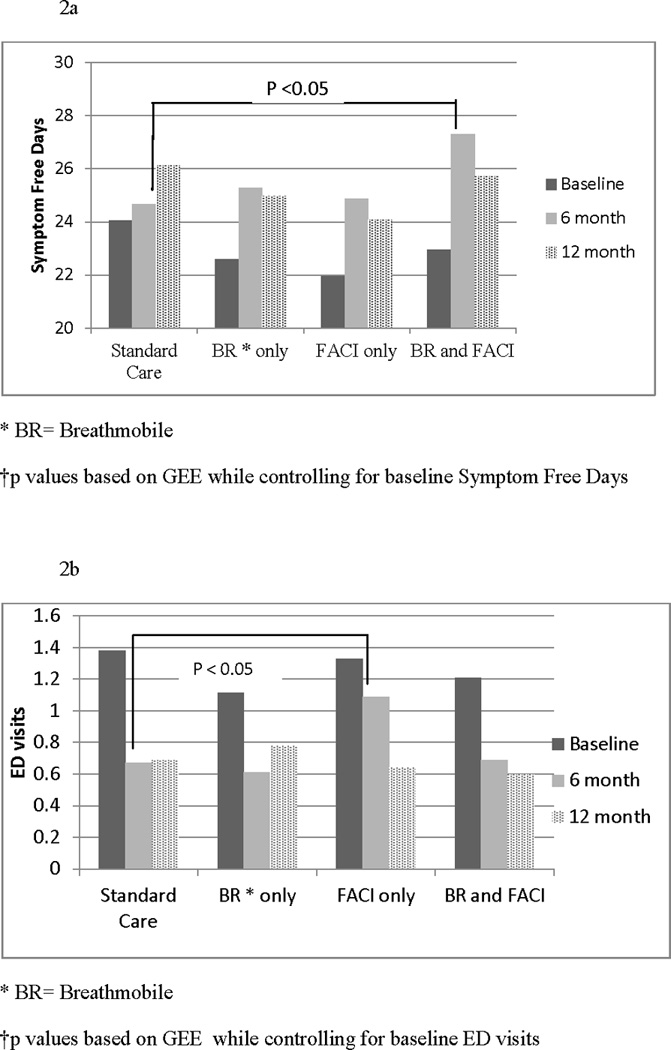
a. Symptom- free days at Baseline, 6 and 12 months by group
b. ED Visits at Baseline, 6 and 12 months by group
Urgent Care use
Our secondary outcomes included caregiver-reported ED use and hospitalizations in the past six months as measures of urgent care use. For ED visits, we observed a time * treatment interaction for the FACI-only group at 6 months in ITT analysis. Participants randomized to the FACI-only group (N = 83) had an increase of 0.61 in the mean number of ED visits (IRR = 1.69; p = 0.01) that was not present at 12 months (IRR = 1.00 p = 0.99; Table 4 and Figure 2b). This increase in ED visits was not found in post hoc as-treated analyses for the FACI-only group (N =55) at either 6 months (IRR = 1.05 p = 0.84) or 12 months (IRR = 1.08 p = 0.78). There were no time * treatment interaction for the other three groups.
There were no significant time * treatment interactions for any of the groups using hospitalizations as an outcome in ITT analysis. However, a trend was noted for hospitalizations in the Breathmobile+FACI group (N = 77) at 6 months (IRR = 0.23 p = 0.08). This group had an 83% decrease in the mean number of hospitalizations in the previous 6 months. This trend, however, was not seen at 12 months (IRR = 0.45 p = 0.39).
Medication use
There were no significant time * treatment interactions on caregiver-reported medication use among the treatment groups over time for either outcome (courses of oral steroids and prescriptions of inhaled corticoid steroids) in ITT analyses (Results not shown in Table 4).
Caregiver Quality of Life
There were no significant time * treatment interactions for any group at any time point for caregiver quality-of-life activity limitations and emotional symptoms (Results not shown in Table 4).
Discussion
This study evaluated the effects of providing Breathmobile services only, a Facilitated Asthma Communication Intervention (FACI) only, or combined Breathmobile+FACI intervention on asthma outcomes, relative to standard care in low-income minority preschool children. Both interventions tested in this study were designed to remove common barriers to asthma care. The Breathmobile was intended to overcome structural barriers such as transportation, access to care, and health insurance status. The FACI intervention was designed to empower families to communicate with their physicians more effectively and to improve the quality of medical care through better communication. Other than slight improvement in symptom-free days at 6 months in the Breathmobile+FACI group, these community-based intervention strategies did not result in any significant improvements in asthma management or asthma morbidity among low-income preschool children. This suggests that more research is needed to evaluate community interventions that may result in more meaningful and sustainable improvements.(27)
Our study design was the first to evaluate a Breathmobile intervention in a randomized, controlled clinical trial. Despite free services, multiple locations, and multiple attempts at direct outreach to schedule families for appointments, we found that less than half of participants eligible to receive the Breathmobile scheduled an appointment and only one in five attended a visit, indicating underutilization of the services. Similarly, despite offering assistance to schedule and travel to appointments for the FACI group, many families did not attend a PCP appointment. As at least 3 visits were required to achieve well-controlled asthma in a previous pre-post study of a Breathmobile intervention, (28) our modest results may be likely due to a limited exposure to the intervention. A recent pre-post study of over 7000 children age 3 to 18 years evaluated and treated on the Breathmobile found significant reductions in asthma morbidity. (16) This study, however, evaluated outcomes only of children receiving Breathmobile care and did not offer information about children with asthma who declined to attend an appointment on the Breathmobile. The preschool children in our community sample differed from the prior studies: they were much younger, had milder disease as seen in their mean number of SFD, and had a regular primary care physician. Thus, their parents may have been less motivated to utilize Breathmobile services. Furthermore, research has shown that families who enroll in studies often improve over time whether they were assigned to intervention or control groups. (29)
A process evaluation of reasons for declining to schedule appointments on the Breathmobile or the FACI intervention indicated that families who felt that their child’s asthma was asymptomatic were uninterested in scheduling appointments either at the Breathmobile or with their PCP for the FACI intervention. Studies by Smith et al.(30) and Boukour et al.(31) have highlighted that it is common for low-income minority parents to underestimate the severity and chronicity of their child’s asthma, as well as the need for preventive asthma care. Our findings highlight the fact that offering community asthma services, such as Breathmobile care or family asthma education, may not be sufficient to engage all parents of children with asthma to utilize these free services. Increasing utilization of community asthma care services likely requires multi-level, multi-modal efforts to increase knowledge and motivation regarding the importance of routine asthma care.(27)
Furthermore, interventions directly addressing health beliefs that serve as attitudinal barriers to seeking preventive care may be needed, rather than those addressing traditional structural barriers. Halm et. al. found that over half of adult low-income minority asthma patients held the health belief “ no symptoms, no asthma” which is the belief that they no longer have asthma when they do not have symptoms.(32) Another study found that negative parental beliefs towards asthma management were associated with parent-PCP discordance over the child’s prescribed treatment plan.(33) A further influence on the decision to seek preventive care may be the uncertainty of asthma diagnosis in preschool children(34) or lack of appreciation of importance of preventive care by families with young children who are new to asthma diagnosis and management.
Despite the study’s methodological strengths, our findings should be considered in light of their limitations. Although we relied on parent report of asthma symptoms, healthcare utilization and medication use, previous studies have demonstrated that parents can accurately recall the frequency of ED visits and hospitalizations.(35) In spite of our success in screening, recruiting, and retaining participants, we only enrolled a subset of all eligible patients and were unable to collect data on nonparticipants to assess the generalizability of our results to the larger population. While our intervention participation rates were low, they were comparable with other studies that target improving access to asthma care. (36;37)
In summary, our study found that caregivers of HS children with asthma who received both a patient-physician communication intervention and had access to the Breathmobile had modest short-term improvements in SFD; however, the overall impact of these two community-based intervention strategies was not significant. Further studies are needed testing multi-level and/or multi-modal intervention strategies to enhance the effectiveness, generalizability, and sustainability using cost-effectiveness analyses of community-based interventions for high-risk children with asthma.
Clinical Implications.
Increasing utilization of community asthma care services likely requires multi-level and multi-modal strategies to increase knowledge and motivation regarding the importance of routine asthma care.
Acknowledgments
This research was supported by National Heart Lung Blood Institute grant HL073833
Glossary
- AE
Asthma educator
- ED
Emergency department
- FACI
Facilitated asthma communication intervention
- GEE
Generalized estimating equations
- HS
Head Start
- IRR
Incidence Rate Ratio: IRR
- ITT
Intent-to-treat
- PACQLQ
Pediatric asthma caregiver's quality of life questionnaire
- PCP
Primary care provider
- SFD
Symptom-free days
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117(5):e868–e877. doi: 10.1542/peds.2005-1721. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;381:1–24. [PubMed] [Google Scholar]
- 3.Bravata DM, Sundaram V, Lewis R, Gienger A, Gould MK, McDonald KM, et al. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Report No.: 04(07)-005 1–5 ed. [PubMed] [Google Scholar]
- 4.Rand CS, Butz AM, Kolodner K, Huss K, Eggleston P, Malveaux F. Emergency department visits by urban African American children with asthma. J Allergy Clin Immunol. 2000;105(1 Pt 1):83–90. doi: 10.1016/s0091-6749(00)90182-9. [DOI] [PubMed] [Google Scholar]
- 5.Sarver N, Murphy K. Management of asthma: new approaches to establishing control. J Am Acad Nurse Pract. 2009;21(1):54–65. doi: 10.1111/j.1745-7599.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- 6.Celano MP, Linzer JF, Demi A, Bakeman R, Smith CO, Croft S, et al. Treatment adherence among low-income, African American children with persistent asthma. J Asthma. 2010;47(3):317–322. doi: 10.3109/02770900903580850. [DOI] [PubMed] [Google Scholar]
- 7.Vargas PA, Simpson PM, Gary WJ, Goel R, Feild CR, Tilford JM, et al. Characteristics of children with asthma who are enrolled in a Head Start program. J Allergy Clin Immunol. 2004;114(3):499–504. doi: 10.1016/j.jaci.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Garwick AW, Seppelt A, Riesgraf M. Addressing asthma management challenges in a multisite, urban head start program. Public Health Nurs. 2010;27(4):329–336. doi: 10.1111/j.1525-1446.2010.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Guttmann A. Recent innovations to improve asthma outcomes in vulnerable children. Curr Opin Pediatr. 2009;21(6):783–788. doi: 10.1097/MOP.0b013e328332537d. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CE, Johnson T, Clark H, Schirwian K, Thomas O. A library-site asthma education program for inner-city communities. J Asthma. 2006;43(1):9–18. doi: 10.1080/02770900500446831. [DOI] [PubMed] [Google Scholar]
- 11.Murray NG, Low BJ, Hollis C, Cross AW, Davis SM. Coordinated school health programs and academic achievement: a systematic review of the literature. J Sch Health. 2007;77(9):589–600. doi: 10.1111/j.1746-1561.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones CA, Clement LT, Hanley-Lopez J, Morphew T, Kwong KY, Lifson F, et al. The Breathmobile Program: structure, implementation, and evolution of a large-scale, urban, pediatric asthma disease management program. Dis Manag. 2005;8(4):205–222. doi: 10.1089/dis.2005.8.205. [DOI] [PubMed] [Google Scholar]
- 13.Jones CA, Hanley-Lopez J, Kwong KY, Clement LT, Stotts CL, Maalouf NB, et al. Breathmobile™ Program: Two Year Outcomes. J Allergy Clin Immuno. 2000;105(1, Part 2):S103. [Google Scholar]
- 14.Liao O, Morphew T, Amaro S, Galant SP. The Breathmobile: a novel comprehensive school-based mobile asthma care clinic for urban underprivileged children. J Sch Health. 2006;76(6):313–319. doi: 10.1111/j.1746-1561.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 15.Bollinger ME, Morphew T, Mullins CD. The Breathmobile program: a good investment for underserved children with asthma. Ann Allergy Asthma Immunol. 2010;105(4):274–281. doi: 10.1016/j.anai.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Scott L, Morphew T, Bollinger ME, Samuelson S, Galant S, Clement L, et al. Achieving and maintaining asthma control in inner-city children. J Allergy Clin Immunol. 2011;128(1):56–63. doi: 10.1016/j.jaci.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Diette GB, Rand C. The contributing role of health-care communication to health disparities for minority patients with asthma. Chest. 2007;132 5 Suppl:802S–809S. doi: 10.1378/chest.07-1909. [DOI] [PubMed] [Google Scholar]
- 18.Butz AM, Walker J, Land CL, Vibbert C, Winkelstein M. Improving asthma communication in high-risk children. J Asthma. 2007;44(9):739–745. doi: 10.1080/02770900701595683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small M, Vickers A, Anderson P, Kay S. The patient-physician partnership in asthma: real-world observations associated with clinical and patient-reported outcomes. Adv Ther. 2010;27(9):591–599. doi: 10.1007/s12325-010-0054-1. [DOI] [PubMed] [Google Scholar]
- 20.[Anon] National asthma education and prevention program - Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma - Summary report 2007. Journal of Allergy and Clinical Immunology. 2007;120(5):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Jones CA, Morphew T, Clement LT, Kimia T, Dyer M, Li M, et al. A school-based case identification process for identifying inner city children with asthma: the Breathmobile program. Chest. 2004;125(3):924–934. doi: 10.1378/chest.125.3.924. [DOI] [PubMed] [Google Scholar]
- 22.Cegala DJ, Marinelli T, Post D. The effects of patient communication skills training on compliance. Arch Fam Med. 2000;9(1):57–64. doi: 10.1001/archfami.9.1.57. [DOI] [PubMed] [Google Scholar]
- 23.Butz AM, Walker J, Land CL, Vibbert C, Winkelstein M. Improving asthma communication in high-risk children. J Asthma. 2007;44(9):739–745. doi: 10.1080/02770900701595683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson-Pessano SR, Latini D, Starr NJ, Fish L, Loes LM, Page A, et al. Education of parents of infants and very young children with asthma:a developmental evaluation of the Wee Wheezers Program. J Asthma. 1996;33:239–254. doi: 10.3109/02770909609055365. [DOI] [PubMed] [Google Scholar]
- 25.Brown JV, Bakeman R, Celano MP, Demi AS, Kobrynski L, Wilson SR. Home-based asthma education of young low-income children and their families. J Pediatr Psychol. 2002;27(8):677–688. doi: 10.1093/jpepsy/27.8.677. [DOI] [PubMed] [Google Scholar]
- 26.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996;5(1):35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 27.Canino G, McQuaid EL, Rand CS. Addressing asthma health disparities: a multilevel challenge. J Allergy Clin Immunol. 2009;123(6):1209–1217. doi: 10.1016/j.jaci.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CA, Clement LT, Morphew T, Kwong KY, Hanley-Lopez J, Lifson F, et al. Achieving and maintaining asthma control in an urban pediatric disease management program: the Breathmobile Program. J Allergy Clin Immunol. 2007;119(6):1445–1453. doi: 10.1016/j.jaci.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, et al. Medication adherence feedback to improve asthma outcomes among inner-city children: A randomized controlled trial. Pediatrics. 2009;124(6):1513–1521. doi: 10.1542/peds.2008-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith LA, Bokhour B, Hohman KH, Miroshnik I, Kleinman KP, Cohn E, et al. Modifiable risk factors for suboptimal control and controller medication underuse among children with asthma. Pediatrics. 2008;122(4):760–769. doi: 10.1542/peds.2007-2750. [DOI] [PubMed] [Google Scholar]
- 31.Bokhour BG, Cohn ES, Cortes DE, Yinusa-Nyahkoon LS, Hook JM, Smith LA, et al. Patterns of concordance and non-concordance with clinician recommendations and parents' explanatory models in children with asthma. Patient Educ Couns. 2008;70(3):376–385. doi: 10.1016/j.pec.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halm EA, Mora P, Leventhal H. No symptoms, no asthma: The acute episodic disease belief Is associated With poor self-management among inner-vity adults with persistent asthma. Chest. 2006;129(3):573–580. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- 33.Riekert KA, Butz AM, Eggleston PA, Winkelstein M, Rand CS. Caregiver-Physician Medication Concordance and Undertreatment of Asthma Among Inner-City Children. Pediatrics. 2003;111:e214–e220. doi: 10.1542/peds.111.3.e214. [DOI] [PubMed] [Google Scholar]
- 34.Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5–34. doi: 10.1111/j.1398-9995.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 35.D'Souza-Vazirani D, Minkovitz CS, Strobino DM. Validity of maternal report of acute health care use for children younger than 3 years. Arch Pediatr Adolesc Med. 2005;159(2):167–172. doi: 10.1001/archpedi.159.2.167. [DOI] [PubMed] [Google Scholar]
- 36.Nelson KA, Highstein GR, Garbutt J, Trinkaus K, Fisher EB, Smith SR, et al. A randomized controlled trial of parental asthma coaching to improve outcomes among urban minority children. Arch Pediatr Adolesc Med. 2011;165(6):520–526. doi: 10.1001/archpediatrics.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quint DM, Teach SJ. IMPACT DC: Reconceptualizing the Role of the Emergency Department for Urban Children with Asthma. Clinical Pediatric Emergency Medicine. 2009;10(2):115–121. [Google Scholar]


