Summary
Controlled cortical impact injury was used to examine relationships between focal posttraumatic cortical damage and mossy fiber sprouting (MFS) in the dentate gyrus in three mouse strains. Posttraumatic MFS was more robust when cortical injury impinged upon the hippocampus, versus contusions restricted to neocortex, and was qualitatively similar among CD-1, C57BL/6, and FVB/N background strains. Impact parameters influencing injury severity may be critical in reproducing epilepsy-related changes in neurotrauma models.
1. Introduction
Mossy fiber sprouting (MFS) into the inner molecular layer of the dentate gyrus is a consistent marker of the epileptic dentate gyrus after traumatic brain injury (TBI) in humans (Swartz et al., 2006) and animals (Kharatishvili et al., 2006; Hunt et al., 2009; 2010; 2011). MFS is generally more robust after severe versus mild TBI (Santhakumar et al., 2001; Kharatashivilli et al., 2006, 2007; Hunt et al., 2009), but responses in posttraumatic animals can be highly variable. We recently described the development of posttraumatic epilepsy (PTE) and localized, robust MFS and synaptic reorganization 6–12 weeks after controlled cortical impact (CCI) injury in mice (Hunt et al., 2009; 2010). However, background strain may influence cellular events and seizure thresholds in mice after TBI (Chrzaszcz et al., 2010). Other studies detected only mild mossy fiber reorganization in posttraumatic mice at similar time points after injury (Hanell et al., 2010). These findings could be due to considerable technical differences among laboratories or high variability in the degree of cortical damage in individual animals. Tissue responses produced after CCI injury depend greatly on external injury parameters (i.e., impact depth and velocity, impactor shape and size, and number of craniotomies) (Mao et al., 2010; Pleasant et al., 2011). While CCI is increasingly used to model epilepsy-related changes after TBI, the parameters of focal cortical damage necessary to consistently reproduce MFS in posttraumatic animals is unknown.
2. Methods
All procedures were approved by the University of Kentucky Animal Care and Use Committee and adhered to NIH guidelines for the care and use of laboratory animals. Six to ten week old CD-1 (Harlan), C57BL/6 (The Jackson Laboratory), or FVB/N (The Jackson Laboratory) mice were subjected to a unilateral cortical contusion by CCI injury as previously described (Hunt et al., 2009; 2010; 2011). We chose these strains because they display different cellular responses in status epilepticus models (Schauwecker and Steward, 1997), and/or are often used in transgenic studies. Severe brain injury was delivered using an electronically-controlled, pneumatically-driven impactor fitted with a stainless steel tip 3mm in diameter (Precision Systems and Instrumentation, Fairfax, VA) to compress the cortex to a depth of 1.0mm (or, for C57BL/6 mice only, 1.2mm), at 3.5m/sec and 400–500ms duration. Mice were injured with two differently-shaped impactor tips, beveled or rounded, to achieve a variable degree of cortical damage. Impactor shape is an important determinant for CCI-induced cortical damage (Mao et al., 2010; Pleasant et al., 2011) and has been a common variation among studies examining epilepsy-related changes after CCI. A subset of injured CD-1 mice was monitored for injury-induced behavioral seizures during a 90 min interval beginning 90 min post-injury. Seizure severity was scored from 1–5, according to a modified Racine scale (Hunt et al., 2009; 2010).
Mice were perfused with 0.37% sodium sulfide in 0.1M NaHPO4 followed by 4% paraformaldehyde in 0.15 M phosphate buffer. Brains were cryoprotected with 30% sucrose in 0.01M phosphate-buffered saline; 20μm coronal brain sections were cut on a cryostat and collected at 400μm intervals. Timm’s and Nissl staining was performed as previously described to visualize mossy fibers and cell bodies (Shibley and Smith, 2002) ipsilateral and contralateral to the injury. Timm’s scores were plotted with respect to the distance of each section from the injury epicenter, which was qualitatively defined as the section with the most extensive cortical damage. Scores for sprouting were assigned from 0–3 based on the rating scale of Tauck and Nadler (1985). If Timm's staining between the blades of the granule cell layer was variable, an averaged score was used (e.g., if the lower blade was scored 1 while the upper blade was scored 2, the section was given an overall grade of 1.5). MFS was defined as at least one section with a Timm’s score >1 (Hunt et al., 2009; 2010; 2011).
Data were analyzed using Microsoft Excel and Instat3 programs. Numerical data are presented as the mean±SD. The nonparametric Chi square or Kruskal–Wallis test with Dunn's post hoc tests were used to analyze Timm score differences between groups. Mann-Whitney U was used to examine differences between pairs. Significance was set at P<0.05.
3. Results
Gross damage 8–12wks after CCI consisted of a cortical cavity 3 mm in diameter extending through the thickness of the neocortex at the injury epicenter, located midway between lambda and bregma, 5mm lateral to midline. In all mice injured with a rounded-tip impactor, the cortical cavity at the injury site was restricted to the neocortex (n=5 CD-1; n=9 FVB; n=12 C57BL/6). In most mice injured with a beveled tip, a variably-sized cavity extended into the hippocampus (260–1070 µm3), accompanied by hippocampal distortion extending 300–1600 µm from the injury epicenter (n=18 of 20 CD-1; n=18 of 23 FVB; n=7 of 10 C57BL/6). These results are consistent with recent studies demonstrating greater hippocampal damage after injuries administered using beveled versus rounded-tip impactors (Mao et al., 2010; Pleasant et al., 2011).
MFS was detected ipsilateral to the injury in all three strains of posttraumatic mice. In contrast, none of the hippocampi contralateral to the injury had abnormal mossy fiber organization (i.e., all Timm scores were ≤ 1). The degree of hippocampal distortion and pattern of MFS were variable (Figure 1). The most robust Timm’s staining was always found within 800μm of the injury epicenter toward the ventral pole (Figure 2).
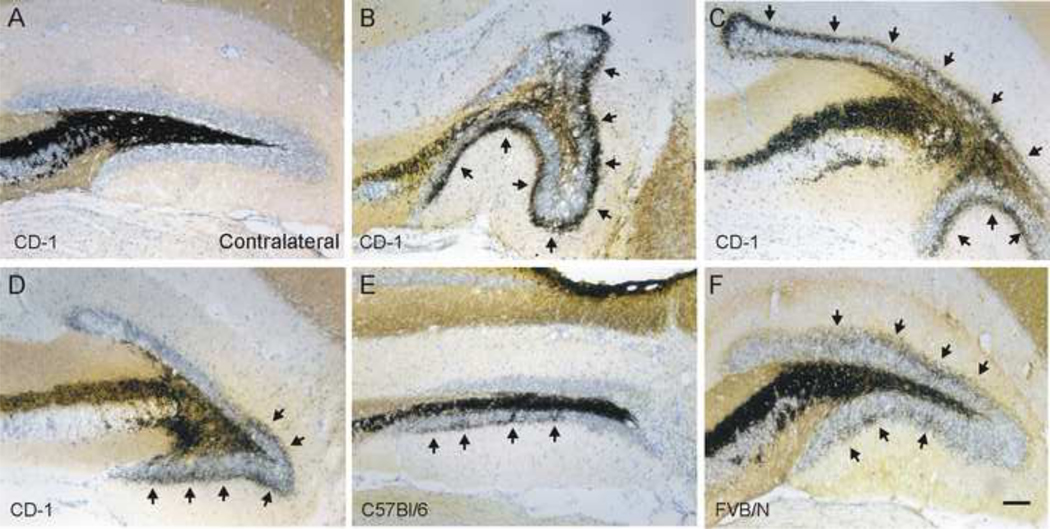
Figure 1.
MFS after cortical contusion injury is not uniform. Example Timm’s and Nissl stained sections of the dentate gyrus 8–12wk after CCI injury A. Representative image of hippocampus contralateral to the injury shows the absence of mossy fiber sprouting in the inner molecular layer (Timm score = 0). B–F. Representative images of Timm’s staining in the ipsilateral dentate gyrus near the injury epicenter. Note that the pattern of MFS and distortion of the granule cell layer is different in each section. MFS into the inner molecular layer is indicated by arrows. B–D. Sections obtained from CD-1 mice. Timm scores for these sections are B, 2; C, 3; D, 2. E. Section from a C57BL/6 mouse (Timm score = 1.5). F. Section from an FVB mouse (Timm score = 2). Scale bar is 100 μm.
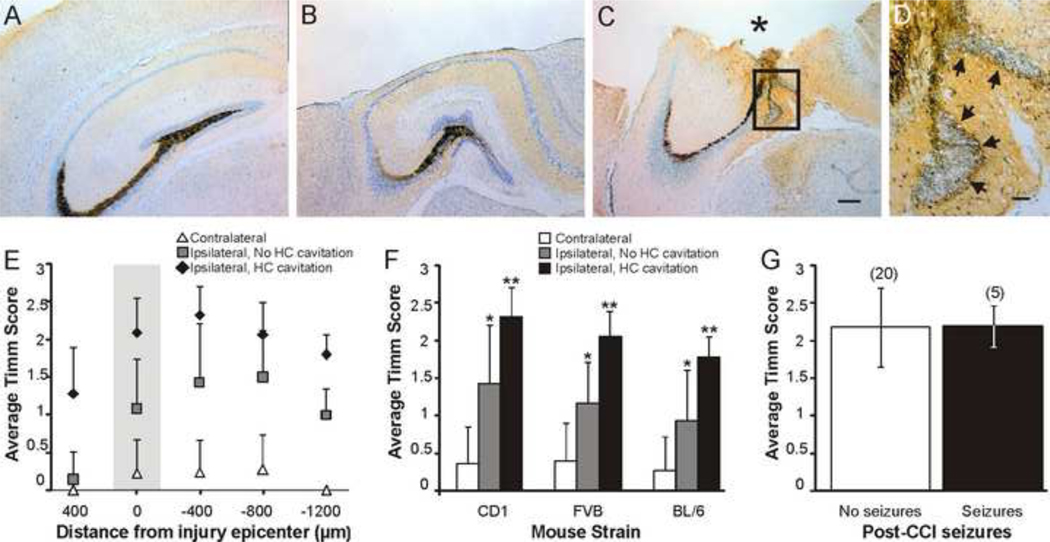
Figure 2.
Timm scores are greater in mice with cortical cavitation that enters the hippocampus (HC). A. Image of Timm’s and Nissl stained contralateral dentate gyrus. B. Image of Timm’s and Nissl stained ipsilateral dentate gyrus at the injury epicenter. Note that the cortical cavity does not include portions of the hippocampus. C. Image of Timm’s and Nissl stained ipsilateral dentate gyrus at the injury epicenter in an animal where the cortical cavity extended into the hippocampus (asterisk). The contralateral hippocampus from this mouse is shown in A. D. Enlarged image of the boxed area in C shows MFS into the inner molecular layer (arrows). Scale bar is 200μm in A–C and 50μm in D; sections from CD-1 mice shown in A–D. E. In CD-1 mice, average Timm score in relation to the distance from the injury epicenter (zero on the x-axis), septal (400μm) to temporal (−1200μm). F. Average “peak” Timm score at −400μm for each group in CD-1, FVB, and C57BL/6 (BL/6) mice. Asterisk indicates significant difference from slices contralateral to the injury. Double asterisk indicates significant difference from both contralateral hippocampi and ipsilateral hippocampi without hippocampal cavity. G. Timm scores 8–12 weeks after injury are not greater in mice observed to have behavioral seizures in the first 90min post-TBI, versus mice in which seizures were not observed. Number of mice in each category is shown in parentheses.
All mice, regardless of strain, in which the cortical cavity impinged upon the hippocampus had MFS into the inner molecular layer ipsilateral to the injury (CD-1, n=18 of 18; FVB, n=18 of 18; C57BL/6, n=7 of 7). In mice where the cavity was restricted to the neocortex, MFS was observed ipsilateral to the injury in 57% of CD-1 (n=4 of 7), 29% of FVB (n=4 of 14), and 27% of C57BL/6 (n=4 of 15) mice, with no detectable difference between strains (X2=2.241, d.f.=2, P=0.33). We evaluated “peak” Timm scores in sections that were 400μm ventral to the injury epicenter to examine whether damage to the hippocampus was associated with greater MFS. For this analysis, we compared hippocampi ipsilateral to the injury, in mice with and without a cavity into the hippocampus, with contralateral hippocampi. A Kruskal–Wallis test detected a significant difference in Timm score ranges among groups for each strain (CD-1: H(2, 49)=38.58, P<0.001; FVB: H(2, 61)=47.21, P<0.001; C57BL/6: H(2, 43)=24.79, P<0.001; Figure 2E). Post-hoc analysis revealed that ipsilateral hippocampi had higher Timm scores than contralateral hippocampi for all strains, regardless of the extent of cortical damage. However, Timm scores were greater in mice in which the cortical cavity included portions of the hippocampus versus mice in which the cavity was restricted to the neocortex, regardless of strain. No difference was detected in the time post-TBI in which MFS was evaluated between mice with (9.86 ± 1.1 wks) and without (9.22 ± 0.8 wks) a cavity into the hippocampus for any strain (P>0.05).
The development of MFS after pilocarpine administration in mice relates to seizure number induced during status epilepticus (Shibley and Smith, 2002). Therefore, we evaluated whether Timm’s scores were greater in CD-1 mice that displayed immediate injury-induced behavioral seizures versus mice that did not have seizures. Five mice displayed immediate seizures after TBI (one to four seizures/mouse; category 2–5) and had an average Timm’s score of 2.2 ± 0.27. Mice that did not have immediate seizures had an average Timm’s score of 2.2 ± 0.53 (n=20; P>0.05). Immediate seizures after TBI did not predict the development of posttraumatic MFS.
4. Discussion
Our finding that MFS is increased in mice with posttraumatic hippocampal cavitation is consistent with previous reports describing greater spontaneous seizure incidence after severe CCI using a beveled impactor (36–40%; Hunt et al., 2009; 2010) versus rounded-tip impactors (9–13%; Bolkvadze et al., 2009; Statler et al., 2009). Hippocampal damage was more likely with beveled tips. Injuries without hippocampal cavitation resulted in less prevalent MFS, despite similar impact depth. In addition to impact parameters, rodent species, animal age, or injury location, might also affect MFS and seizure incidence after CCI injury.
The relatively low seizure incidence in PTE models suggests the need for surrogate biomarkers. We found that the degree of neocortical damage might be a less useful predictor of posttraumatic MFS than is hippocampal cavitation; all mice with hippocampal cavitation developed sprouting. Why MFS occurs is controversial. Among potential triggers include hilar or hippocampal cell loss, neurogenesis, and growth factor overexpression, all of which occur after CCI. Correlation of MFS with these parameters may be useful for identifying other key features of posttraumatic epileptogenesis. While MFS is qualitatively related to epileptogenesis, it does not correlate quantitatively with seizure frequency or severity in temporal lobe epilepsy models (Buckmaster and Dudek, 1997). MFS ipsilateral to TBI might be related to posttraumatic EEG spike activity (Kharatashivilli et al., 2007); MRI markers have been used to evaluate brain damage after brain injury in rodents (Kharatishvili et al., 2007; 2009; Onyszchuk et al., 2007). Perhaps the presence of hippocampal damage could serve as a biomarker for animals with the highest probability for developing epilepsy.
Acknowledgements
This research was supported by NIH NS052302 and USAMRMC W81XWH-11-1-0502 to B.N.S., NIH AG21981 to S.W.S., NIH P01 NS058484 to K.E.S., and an Epilepsy Foundation Training Fellowship to R.F.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bolkvadze T, Nissinen J, Kharatishvili I, Pitkänen A. Development of post-traumatic epilepsy in C57BL/6 mice after controlled cortical impact injury. [Abstract] J Neurotrauma. 2009 doi: 10.1089/neu.2011.1954. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kaintate treated rats. J Comp Neurol. 1997;385:404. 385. [PubMed] [Google Scholar]
- Chen J, Larionov S, Pitsch J, Hoerold N, Ullmann C, Elger CE, Schramm J, Becker AJ. Expression analysis of metabotropic glutamate receptors I and III in mouse strains with different susceptibility to experimental temporal lobe epilepsy. Neurosci Lett. 2005;375:192–197. doi: 10.1016/j.neulet.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Chrzaszcz M, Venkatesan C, Dragisic T, Watterson DM, Wainwright MS. Minozac treatment prevents increased seizure susceptibility in a mouse "two-hit" model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures. J Neurotrauma. 2010;27(7):1283–1295. doi: 10.1089/neu.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JC, Cherian L, Bryan RM, Jr, Robertson CS. Lateral cortical impact injury in rats Pathologic effects of varying cortical compression and impact velocity. J Neurotrauma. 1994;11:587–597. doi: 10.1089/neu.1994.11.587. [DOI] [PubMed] [Google Scholar]
- Hanell A, Clausen F, Biork M, Jansson K, Phillipson O, Nilsson LN, Hillered L, Weinreb PH, Lee D, McIntosh TK, Gimbel DA, Strittmatter SM, Marklund N. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J Neurotrauma. 2010;27(7):1297–1309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol. 2009;215(2):243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J Neurophysiol. 2010;103:1490–1500. doi: 10.1152/jn.00957.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Increased local excitatory input to hilar GABAergic interneurons accompanies reduced synaptic inhibition of granule cells after traumatic brain injury. J. Neuroscience. 2011;31:6880–6890. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Gröhn O, Pitkänen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain. 2007;130:3155–3158. doi: 10.1093/brain/awm268. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Sierra A, Immonen RJ, Gröhn OH, Pitkänen A. Quantitative T2 mapping as a potential marker for the initial assessment of the severity of damage after traumatic brain injury in rat. Exp Neurol. 2009;217(1):154–164. doi: 10.1016/j.expneurol.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Mao H, Yang KH, King AI, Yang K. Computational neurotrauma-design, simulation, and analysis of controlled cortical impact model. Biomech Model Mechanobiol. 2010;9(6):763–772. doi: 10.1007/s10237-010-0212-z. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160(2):187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasant JM, Carlson SW, Mao H, Scheff S, Yang KH, Saatman KE. Rate of Neurodegeneration in the Mouse Controlled Cortical Impact Model is Influenced by Impactor Tip Shape: Implications for Mechanistic and Therapeutic Studies. J Neurotrauma. 2011 doi: 10.1089/neu.2010.1499. doi:10.1089/neu.2010.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524(Pt1):117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: Implications for gene targeting approaches. Proc Nat Acad Sci. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002;49:109–120. doi: 10.1016/s0920-1211(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006;47(8):1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J. Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]