Abstract
We elucidated the gender and condylar effects on distal femur morphology (DFM) while evaluating a newly developed computational framework that enables fully automated analyses of DFM in an objectively defined sagittal plane. Ninety high-resolution CT-acquired distal femur models from 51 males and 39 females were analyzed. The models were accurately characterized (mean least-squares fitting residual < 0.16 mm), and re-oriented to a unified sagittal plane; three morphometric measures were extracted from each model: the semi-major (a) and semi-minor (b) axis lengths of the best-fitted ellipse, and the radius (r) of the smallest flexion facet—a circle with the smallest radius best-fitted to the posterior articulating surface. Statistical analyses employing non-parametric repeated-measures ANOVA found: no significance difference between condyles or between limbs in any of the morphometric measures; significant gender effects on a, b, and r, but no gender effect on the aspect ratio (a/b). An inspection of statistical distributions of medial-lateral condyle size differences also revealed a gender difference. The findings promote a better understanding of DFM and its relation to knee mechanics and have implications on computer-aided surgery of the knee and gender-specific implant design.
Keywords: femur, morphometry, unified sagittal plane, non-parametric statistics
INTRODUCTION
We aimed to pursue quantitative and unequivocal understanding of the distal femur morphology (DFM) for clinical applications such as ligament reconstruction and total knee arthroplasty (TKA), particularly as these applications become more contemporized with computer or robotic technology. For example, a growing body of scientific evidence suggests that anatomic ligament reconstruction, performed by creating bone tunnels and placing the substituting graft where the native ligament was attached, can better restore the joint function and deter the development of OA.1–5 Consistent anatomical tunnel placements require accurate and efficient characterization of distal femur morphometry in relation to an objectively definable bone-based reference frame. Other clinical questions include whether gender-specific implants can improve outcome6–9 and whether the femoral condyles have morphological differences10,11 that may relate to differences in contact mechanics and propensity for injury or degenerative disease.
With regard to the DFM differences between genders and condyles, collective understanding gained from prior studies remains inconclusive and incomplete. Studies focused mainly on the condyle size, shape, and landmark-based dimensions. Nuno and Ahmed11 compared measures of the sizes of the condyles from their own study to those from other studies12,13 and found the ratio of the radius of the medial condyle flexion facet (the posterior articulating profile in the sagittal plane) to that of the lateral condyle could range from 0.9311 to 1.05.12 The continuing debates over whether there are significant gender differences in DFM and whether such differences justify gender-specific femur component design are reflected in recent publications. A review by Merchant et al.14 suggested that the commonly cited gender-differences in Q-angle, anterior condyle height, and mediolateral (ML) to anteroposterior (AP) aspect ratio did not exist and did not exhibit a significant clinical effect. Lonner et al.8 measured the distal femurs of 100 men and 100 women and identified significant differences in dimension and shape. Based on a review of 1000 TKA cases and classification of morphotypes, Bellemans et al.15 also confirmed the gender effect on DFM. Mahfouz and associates conducted investigations examining gender differences;16–18 significant gender differences in an inclusive set of landmark-based dimensions were demonstrated,16,17 but flexion and extension facet radii did not indicate a clear gender or condyle effect.18
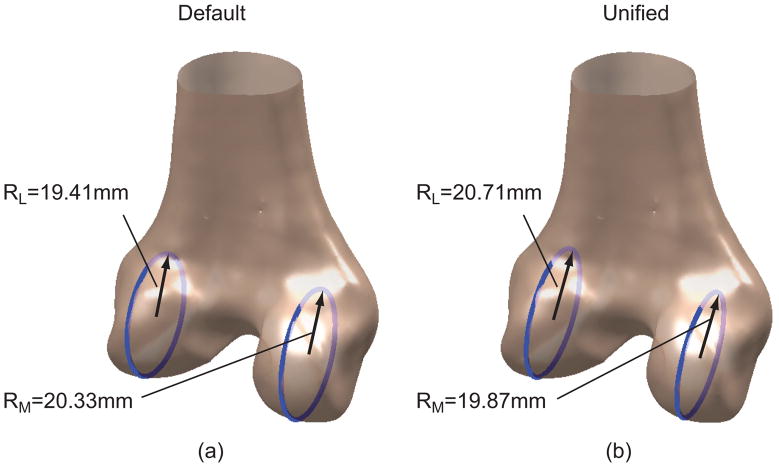
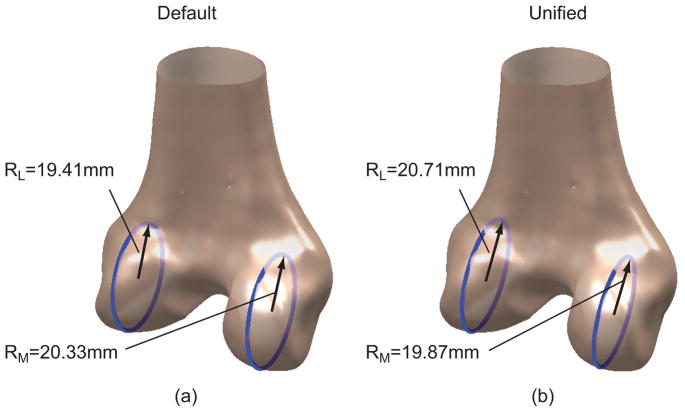
We believe the inconsistent or contradictory findings may be attributable to the lack of a unified objective basis for defining DFM. Previous studies largely relied on manual analyses to extract morphometric measures from image data, requiring visual inspections and subjective judgments. For instance, the analyses involved visually identifying cylinders19 or circles12 deemed to best fit the articulating surfaces. The process was not only laborious, but also subject to inter- and intra-observer variability. The variability within and across analyses and uncertainty in the resulting measures could easily mask the true difference or uniformity between genders or between condyles (Fig. 1). Nuno and Ahmed11 speculated that different criteria used for segmenting the flexion facet and for defining the sagittal planes could contribute to the inconsistency. A simulation-based analysis by our group20 revealed that the portion of the posterior articulating surface to which to fit a cylinder or circle and the choice of the sagittal plane could significantly affect the estimated flexion facet radius on the lateral condyle. Intuitively, the choice of sagittal plane should also affect basic definitions of linear or planar anatomical measures and the Q-angle. Automated analyses of DFM developed previously were limited to linear or angular dimensions definable based on bony landmarks.16 It is arguable whether bony landmarks as morphological features themselves should be used as references. For example, a substantial difference and a variable relationship between trans-epicondylar axis and cylindrical (or geometric center) axis was reported;19,21 the latter is established by fitting a cylinder to the articulating surfaces and considered a surrogate of the flexion-extension axis.19 We have developed a computational framework incorporating 3 algorithms that can automate DFM analyses in an objectively defined sagittal plane and without intra- or inter-observer variability. In this study, we applied the framework to a large previously acquired database of high-resolution CT models of the distal femur. Our primary goal was to characterize gender and condylar effects on selected DFM measures. These morphological measures were assessed utilizing an automated process that defined the sagittal plane in an objective, consistent, and potentially standardizable way. A secondary goal was to evaluate the robustness of this computational methodology before extending it to more dimensions and applications.
Figure 1.
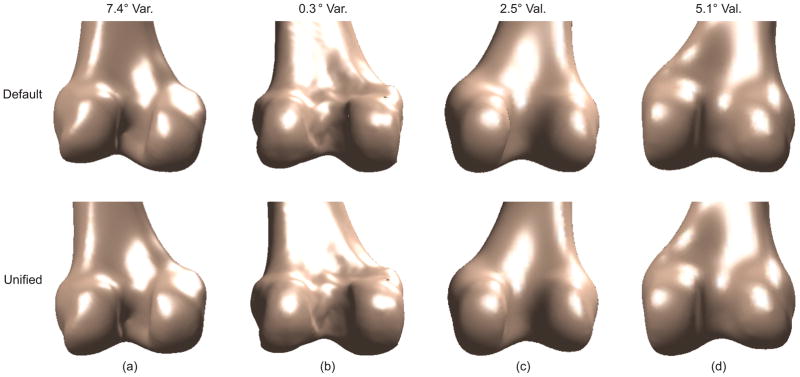
Condyle size, measured as the radius of the flexion facet, is sensitive to choice of sagittal plane. (a) The radius of the lateral condyle is smaller than that of the medial condyle in a default sagittal plane used in CT. (b) The radius of the lateral condyle is larger than that of the medial condyle in a different sagittal plane (unified sagittal plane) for the same bone model.
MATERIALS AND METHODS
We employed a database of distal femur surface models of 90 subjects (51 males; 44 left and 46 right knees), accumulated from knee-related injury studies. These studies all required the participating subjects to have injury only in one knee and have an intact contra-lateral knee free of abnormalities or pathology. The CT-based models were of subjects’ intact knees. The mean (±SD) age, body weight, and height were 40.3±18.4 yrs, 78.4±17.1 kg, and 173.6±9.8 cm, respectively. The CT scans were collected with slice spacing of 1.25 mm, 28 cm field of view, and 512×512 pixels per image (0.547 mm/pixel in-plane resolution). The distal femur was segmented using thresholding and manual segmentation. The resulting slices were reconstructed into 3D surface models using a regularized marching tetrahedra algorithm.22 The models were then smoothed by a heat diffusion technique.23
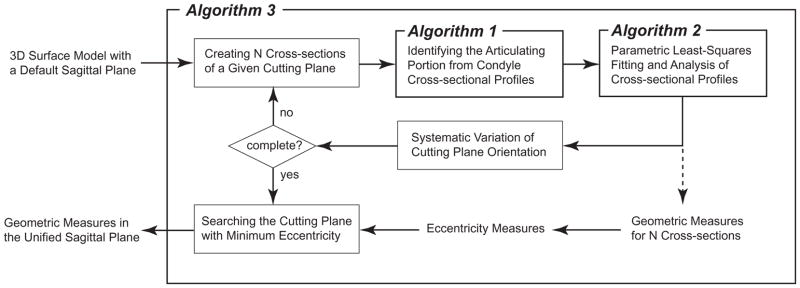
These 3D surface models were analyzed using a computational framework that applied 3 algorithms to each model to establish the unified sagittal plane and extract morphometric measures for each condyle (Fig. 2). The input to these algorithms is a 3D surface model with a default sagittal plane defined by the position of the knee at the time the CT scan was obtained, referred to also as the nominal or original sagittal plane. The output from these algorithms includes a unified (corrected) sagittal plane, along with morphometric measures in that plane.
Figure 2.
The logic diagram for the computational framework consisting of 3 algorithms for identifying the unified sagittal plane and extracting the geometric or morphometric measures.
A detailed description of the computational framework and its 3 component algorithms is featured in Li et al.20 Briefly, Algorithm 1 identifies the articulating portion from a sagittal cross-sectional condyle profile. This is an ‘intelligent’ pattern recognition algorithm based on the articulating and non-articulating surfaces exhibiting distinctive curvature characteristics that can be reliably separated. Algorithm 2 extracts morphometric measures from the articulating portion of cross-sectional profiles by fitting an ellipse to the entire profile or a series of circles to the posterior articulating surface such that the residual errors are minimized. Algorithm 3 corrects the original or nominal sagittal planes, either embedded in the data acquisition (e.g., inherited from a CT-based coordinate system and defined as orthogonal to the axial plane) or subjectively determined in pre-processing the data. It includes a mathematical optimization routine to search for the unified sagittal plane that minimizes an eccentricity measure; this eccentricity measure quantifies the extent to which contour profiles from a series of parallel cross-sections (“cuts”) have a common central axis (e.g., for circular profiles, the extent to which they are concentric). 16 cross-sections are generated, 8 on a condyle, centered at the most prominent aspect, identified as the most posterior point on a transverse plane, with 1-mm intercross-section distance. The algorithm systematically varies the orientation of these cross-sections, compares the resulting eccentricity measures, and determines the orientation or plane with the smallest eccentricity.
3 morphometric measures in the unified sagittal planes at the most prominent aspects of both condyles were obtained (Fig. 3): the semi-major (a) and semi-minor (b) axis lengths of the best-fitted ellipse, and the radius (r) of the smallest flexion facet (a circle of the smallest radius best-fitted to the posterior articulating surface).
Figure 3.
3 DFM measures automatically generated by the computer algorithms: the semi-major (a) and semi-minor (b) axis lengths of the best-fitted ellipse, and the radius (r) of the circle best-fitted to the posterior articulating surface (flexion facet).
To determine appropriate statistical methods for examining gender and condyle effects, we first inspected the distributions of the morphometric measures to verify whether they satisfied the distribution assumptions for parametric methods. We performed Hartigan’s Dip tests24 on the 3 measures stratified by gender to check whether their distributions were unimodal. Since paired data on medial and lateral condyles within each subject were analyzed, these tests were also conducted on the differences in Δa, Δb, and Δr between condyles. The differences were calculated by subtracting the measures of the lateral condyles from those of the medial condyles.
If a morphometric measure (or difference thereof) did not to follow a unimodal distribution, we employed non-parametric repeated-measures ANOVA25 to examine the effects of condyle, limb (left versus right), and gender. The condyle was a within-subject factor, while limb and gender were both between-subject factors.
If a significant gender effect on a morphometric measure was indicated, additional analyses were performed to examine whether the effect could be attributed to anthropometric differences between males and females or to the effect of scale.26 We first calculated the correlation between the measure and subject height to verify the presence of effect of scale. If the correlation was more than moderate (R>0.5), we normalized the measures to height to remove the effect of scale. The Hartigan’s Dip tests were again employed to inspect whether the distributions of the normalized morphometric measures were unimodal. If a non-unimodal distribution was indicated, non-parametric repeated-measures ANOVA were used to re-examine the gender effects on the normalized measures.
RESULTS
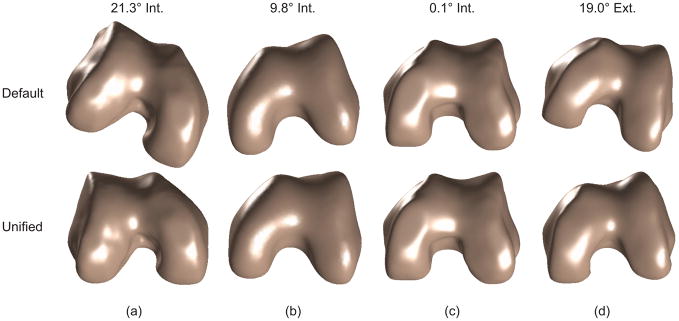
The fully automated analyses successfully identified a unified sagittal plane for each of the 90 distal femur models, resulting in consistent orientations of the models. Orientations in the original CT coordinate systems varied considerably (Figs. 4 and 5). The analyses could correct or re-orient even severely misaligned 3D distal femur models in the nominal CT coordinate system. The rotational corrections needed to reach the unified sagittal plane ranged from −5° (valgus) to 5° (varus) and from −21.3° (internal) to 19° (external). The mean (±SD) correction angles were −0.5°±2.7° in varus/valgus, and −5.7°±7.5° in internal-external. The process of correcting and unifying the sagittal planes reduced the eccentricity measure of dispersion of the posterior geometric center locations by >80% on average (Table 1). This reduction was much greater than a reduction of 61% found in our previous study20 in which the CT-based bone models had been manually pre-aligned prior to computerized analyses.
Figure 4.
Bone models before (top row) and after (bottom row) sagittal plane correction. The models are viewed from the bottom to show the internal-external rotational correction. The 4 cases are representative of the models with (a) maximum internal correction, (b) rotation comparable to the mean value, (c) near zero internal-external correction, and (d) maximum external correction, respectively.
Figure 5.
Bone models before (top row) and after (bottom row) sagittal plane correction. The models are viewed from the posterior to show the varus-valgus rotational correction. The 4 cases are representative samples of the models with (a) maximum varus correction, (b) near zero varus/valgus correction, (c) correction comparable to the mean value, and (d) maximum valgus correction, respectively.
Table 1.
Statistical Summary of the Eccentricity Measure, Fitting Residual Errors and Extracted Morphometric Measures, Comparing the Default and Unified Sagittal Planes
| Default Sagittal Cross-sections |
Unified Sagittal Cross-sections |
||
|---|---|---|---|
| Eccentricity (mm) | 3.64±1.89 | 0.69±0.26 | |
|
Ellipse Fitting Residual (mm) |
Medial | 0.17±0.08 | 0.17±0.08 |
| Lateral | 0.17±0.08 | 0.16±0.09 | |
| a (mm) | Medial | 29.84±3.86 | 30.79±3.58 |
| Lateral | 29.95±3.73 | 30.89±3.71 | |
| b (mm) | Medial | 23.44±2.69 | 23.11±2.29 |
| Lateral | 23.64±3.12 | 23.23±2.37 | |
| a/b | Medial | 1.28±0.13 | 1.33±0.08 |
| Lateral | 1.28±0.15 | 1.33±0.08 | |
|
Circle Fitting Residual (mm) |
Medial | 0.10±0.06 | 0.11±0.06 |
| Lateral | 0.10±0.06 | 0.09±0.06 | |
| r (mm) | Medial | 19.33±2.08 | 19.26±1.88 |
| Lateral | 19.32±2.37 | 19.35±2.32 | |
Distal femur sagittal contours were accurately characterized by the curve-fitting procedures in Algorithm 2. The mean residual errors from fitting ellipses to the entire articulating portion of distal femur profiles were 0.17 mm and 0.16 mm, respectively, for the default and corrected sagittal cross-sections. The mean residual errors from fitting circles to smallest flexion facets were 0.10 mm for both the default and correct sagittal cross-sections.
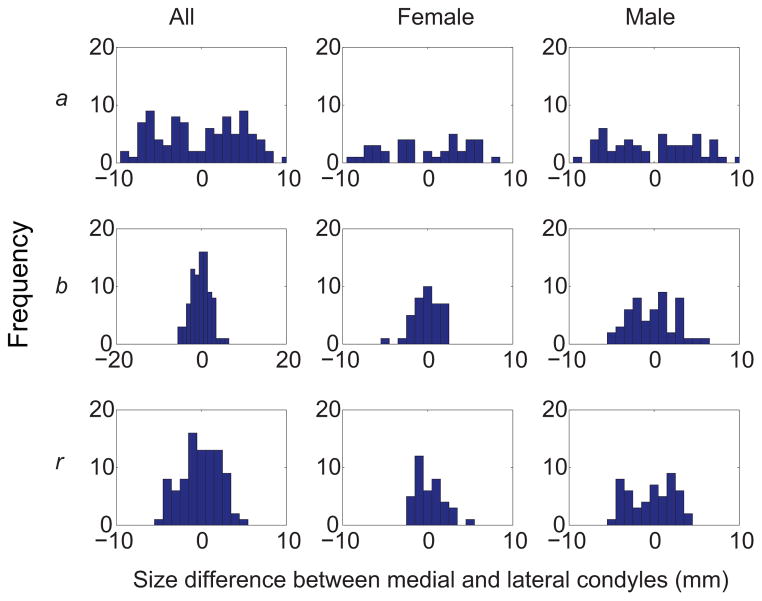
Most DFM measures in the unified sagittal plane for each condyle were unimodally distributed, as the p-values from the Hartigan’s Dip tests for all the three measures ranged from 0.17 to 0.93. Differences between medial and lateral condyles in a, b, and r of the smallest flexion facet also displayed unimodal distributions (p = 0.32 for a, 0.78 for b, and 0.78 for r) when the bone models from both genders were combined (Fig. 6). However, the medial-lateral differences for males exhibited a trend towards multimodal distributions (p = 0.12 for a, 0.15 for b and 0.05 for r), whereas the differences for females seemed to follow unimodal distributions (p = 0.51 for a, 0.85 for b, and 0.73 for r). Since the data did not consistently follow normal distributions, non-parametric repeated-measures ANOVA were employed.
Figure 6.
Histograms of the differences between medial and lateral condyles in semi-major axis (a) and semi-minor axis (b) lengths of the ellipse, and radius (r) of the smallest flexion facet. The differences were calculated by subtracting the measures of lateral condyles from those of medial condyles.
No significant difference was found between limbs or between condyles for any morphometric measure. Significant gender effects were found on a (p < .0001), b (p < .0001), and r (p < .0001); an average male condyle size was 12 to 13% greater than an average female condyle size (Table 2). Correlation tests confirmed the existence of effects of scale on all 3 morphometric measures. Body weight had moderately high correlations with a (R = 0.78) and b (R = 0.79) of the ellipse and with r of the smallest flexion facet (R = 0.74). The aspect ratio (a/b) of the best-fit ellipses remained constant across condyle side and gender (Table 2) and was not associated with subject height. After normalization to height, a trend remained towards non-unimodal distributions of the differences between condyles in the morphometric measures for males (p = 0.267 for a, 0.174 for b, and 0.029 for r). The gender effects remained significant when the 3 measures were normalized by height (p = .002 for a; p < .0001 for b; and p = .004 for r). The interaction effect between gender and condyle side on b, which was only marginally significant prior to the normalization, became significant (p = 0.05). Neither before nor after the normalization was an interaction effect detected, suggesting the nonparametric test was more discriminating.
Table 2.
Statistical Summary of the Morphometric Measures Obtained in the Largest (and Most Prominent) Distal Femur Cross-Section Along the Unified Sagittal Plane, Comparing Genders, Limbs, and Condyles
| a (mm) | b (mm) | a/b | r (mm) | ||
|---|---|---|---|---|---|
| Gender | Male | 32.38±3.24 | 24.41±2.05 | 1.33±0.10 | 20.29±2.02 |
| Female | 28.82±3.10 | 21.55±1.53 | 1.34±0.06 | 18.02±1.41 | |
|
| |||||
| Limb | Left | 31.10±2.55 | 23.40±1.95 | 1.33±0.05 | 19.47±1.78 |
| Right | 30.59±2.80 | 22.96±2.11 | 1.33±0.05 | 19.14±1.80 | |
|
| |||||
| Condyle | Medial | 30.79±3.58 | 23.11±2.29 | 1.33±0.08 | 19.26±1.89 |
| Lateral | 30.89±3.71 | 23.23±2.37 | 1.33±0.08 | 19.35±2.32 | |
|
| |||||
| Overall | 30.84±2.68 | 23.17±2.04 | 1.33±0.05 | 19.31±1.79 | |
DISCUSSION
Although distal femur morphometry has been studied for centuries,12 the DFM differences between genders and between condyles continue to perplex clinicians and basic science researchers. We completed a fully automated DFM analysis with a newly developed computational framework in an attempt to clarify the effects of gender and condyle side. This was also a continued endeavor to test and refine the framework. The fact that it could generate consistent, plausible morphometric measures for 90 distal femur models without any analyst’s intervention provides a testimony for its robustness and reliability.
An important feature and distinct advantage of our study compared to previous DFM studies was the ability to objectively and consistently determine the sagittal plane for analyses and comparisons. We showed that a 3D bone model with a default sagittal plane defined by the knee position during CT imaging could be misaligned substantially from the unified sagittal plane. In fact, pre-alignement of distal femur models using a conventional manual method could still result in a significantly deviated plane.20 Such misalignment could distort the analysis, as illustrated in Fig. 1 and shown in our previous study.20 We speculate the misalignment and the variability in alignment may have been major causes for controversy regarding the size difference between condyles in the sagittal plane.11 Our computer algorithms can eliminate the inter- and intra-observer variability in identifying the sagittal plane and extracting the morphometric measures, and establish consistently orientated distal femur models for further analyses. Our algorithms greatly expand the morphometric features that can be automatically extracted or measured.16
We also demonstrated how a prudent statistical approach can make a difference in quantitative morphologic studies with clinical implications. Improper choices of statistical methods are found in many published clinical studies and can result in incorrect conclusions.27 We chose a non-parametric repeated-measures ANOVA based on a careful inspection of the distributions of the dependent variables, and were able to detect a significant interaction between gender and condyle side. If classical parametric statistics were used, this interaction would not have been found, giving rise to the concern that inappropriate statistical tests may have contributed to contradictory findings regarding the size difference between condyles reported in previous studies.
The automated computer analysis enabled inclusion of a sizable sample of 90 knees. Previous morphometry studies, as reviewed by Nuno and Ahmend,11 were based on labor-intensive manual analyses with much smaller sample sizes, making it difficult to gain insight into the distributions of morphologic measures stratified by gender. Furthermore, the automated computer analysis offered a powerful means for evaluating the effect of sagittal plane variation.20 Such a systematic “what-if” evaluation would be impractical with a manual method.
Our study supports the conclusion that no difference exists in condyle size as measured in the sagittal plane. When the distal femur articulating surface was characterized using an ellipse, the semi-major and semi-minor axis lengths did not show any difference between condyles; nor did the radius of the smallest flexion facet exhibit a condylar difference when the posterior distal femur articulating surface was characterized using a circle. These are not in agreement with findings from a recent study based on manual measurements,28 which showed a significant and large condylar difference in anterior circle (extension facet) radius and a small yet significant difference in posterior circle (flexion facet) radius for males, but no difference in females. Our study did confirm the existence of gender difference in distal femur size.28–30 We also showed that the gender difference could not be explained by stature differences between genders, as suggested by Malek et al.29 and Dargel et al.30. In addition, we identified an interaction effect between gender and condyle factors, and a gender difference in the distributions of select morphometric measures, both of which were novel findings.
Collectively, our findings with regard to gender may shed new light on gender-specific knee implant design, which remains the subject of debate.9,14,15,30 Some studies suggested female-specific knee implants may improve TKA for females as clinical outcomes were reportedly worse in females.31,32 Others contended the notion of female-specific designs,9 pointing out the “flaws” in evidence or arguments supporting the notion.14,33 Our findings support gender-specific femur design from a new perspective. Since the aspect ratio (a/b) as a sagittal plane shape surrogate measure was consistent across individuals of varied heights and between genders, there appears to be no compelling need to differentiate male and female implants in terms of shape and size. However, the bimodal distribution of the medial-lateral condyle differences for males, in contrast to the unimodal distribution for females, seems to justify two implant designs for males. We theorize that the gender-neutral knee implant might contribute to poorer clinical outcomes in males. Merchant et al.14 indeed found that females had equal or better clinical outcomes than males after traditional TKA with gender-neutral implants.15
The success of our computational framework in fully automated CT-based DFM analyses marks an important stride forward in advancing computer-aided orthopaedic surgical innovation. Real-time, accurate, and consistent characterization of DFM is a key to implementing computer-aided individually-optimized surgical procedures including TKA and ligament reconstruction. Such characterization has been a formidable challenge with manual methods. Applications of the framework and “intelligent” algorithms have been limited to sagittal plane measures and bone models of morphologically normal knees acquired with uniform imaging quality by a clinical CT. Further efforts are warranted to extend the methodology to 3D space and to models of varied imaging quality or from a differed modality (e.g., MRI and surface digitization).
Acknowledgments
This work was supported in part by a grant from NIH/NIAMS (3R01AR046387-10S1) under the American Recovery and Reinvestment Act (ARRA) of 2009.
References
- 1.Yasuda K, van Eck CF, Hoshino Y, et al. Anatomic Single- and Double-Bundle Anterior Cruciate Ligament Reconstruction, Part 1: Basic Science. Am J Sports Med. 2011 doi: 10.1177/0363546511402659. [DOI] [PubMed] [Google Scholar]
- 2.Sadoghi P, Kropfl A, Jansson V, et al. Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:355–364. doi: 10.1016/j.arthro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 3.van Eck CF, Schreiber VM, Mejia HA, et al. “Anatomic” anterior cruciate ligament reconstruction: a systematic review of surgical techniques and reporting of surgical data. Arthroscopy. 2010;26:S2–12. doi: 10.1016/j.arthro.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Forsythe B, Kopf S, Wong AK, et al. The location of femoral and tibial tunnels in anatomic double-bundle anterior cruciate ligament reconstruction analyzed by three-dimensional computed tomography models. J Bone Joint Surg Am. 2010;92:1418–1426. doi: 10.2106/JBJS.I.00654. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Hsu WH, Woo SL, et al. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32:1825–1832. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]
- 6.Barrett WP. The need for gender-specific prostheses in TKA: does size make a difference? Orthopedics. 2006;29:S53–55. [PubMed] [Google Scholar]
- 7.Blaha JD, Mancinelli CA, Overgaard KA. Failure of sex to predict the size and shape of the knee. J Bone Joint Surg Am. 2009;91(Suppl 6):19–22. doi: 10.2106/JBJS.I.00563. [DOI] [PubMed] [Google Scholar]
- 8.Lonner JH, Jasko JG, Thomas BS. Anthropomorphic differences between the distal femora of men and women. Clin Orthop Relat Res. 2008;466:2724–2729. doi: 10.1007/s11999-008-0415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald SJ, Charron KD, Bourne RB, et al. The John Insall Award: gender-specific total knee replacement: prospectively collected clinical outcomes. Clin Orthop Relat Res. 2008;466:2612–2616. doi: 10.1007/s11999-008-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuno N, Ahmed AM. Sagittal profile of the femoral condyles and its application to femorotibial contact analysis. J Biomech Eng. 2001;123:18–26. doi: 10.1115/1.1339819. [DOI] [PubMed] [Google Scholar]
- 11.Nuno N, Ahmed AM. Three-dimensional morphometry of the femoral condyles. Clin Biomech. 2003;18:924–932. doi: 10.1016/s0268-0033(03)00172-4. [DOI] [PubMed] [Google Scholar]
- 12.Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg. 2000;82B:1189–1195. doi: 10.1302/0301-620x.82b8.10717. [DOI] [PubMed] [Google Scholar]
- 13.Zoghi M, Hefzy MS, Fu KC, Jackson WT. A three-dimensional morphometrical study of the distal human femur. Proc Inst Mech Eng H. 1992;206:147–157. doi: 10.1243/PIME_PROC_1992_206_282_02. [DOI] [PubMed] [Google Scholar]
- 14.Merchant AC, Arendt EA, Dye SF, et al. The female knee: anatomic variations and the female-specific total knee design. Clin Orthop Relat Res. 2008;466:3059–3065. doi: 10.1007/s11999-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellemans J, Carpentier K, Vandenneucker H, et al. The John Insall Award: both morphotype and gender influence the shape of the knee in patients undergoing TKA. Clin Orthop Relat Res. 2010;468:29–36. doi: 10.1007/s11999-009-1016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahfouz MR, Merkl BC, Fatah EE, et al. Automatic methods for characterization of sexual dimorphism of adult femora: distal femur. Comput Methods Biomech Biomed Engin. 2007;10:447–456. doi: 10.1080/10255840701552093. [DOI] [PubMed] [Google Scholar]
- 17.Mahfouz MR, Abdel Fatah EE, Merkl BC, Mitchell JW. Automatic and manual methodology for three-dimensional measurements of distal femoral gender differences and femoral component placement. J Knee Surg. 2009;22:294–304. doi: 10.1055/s-0030-1247766. [DOI] [PubMed] [Google Scholar]
- 18.Leszko F, Hovinga KR, Lerner AL, et al. In vivo normal knee kinematics: is ethnicity or gender an influencing factor? Clin Orthop Relat Res. 2011;469:95–106. doi: 10.1007/s11999-010-1517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckhoff DG, Bach JM, Spitzer VM, et al. Three-dimensional mechanics, kinematics, and morphology of the knee viewed in virtual reality. J Bone Joint Surg. 2005;87A:71–80. doi: 10.2106/JBJS.E.00440. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Tashman S, Fu F, et al. Automating analyses of the distal femur articular geometry based on three-dimensional surface data. Ann Biomed Eng. 2010;38:2928–2936. doi: 10.1007/s10439-010-0064-9. [DOI] [PubMed] [Google Scholar]
- 21.Most E, Axe J, Rubash H, Li G. Sensitivity of the knee joint kinematics calculation to selection of flexion axes. J Biomech. 2004;37:1743–1748. doi: 10.1016/j.jbiomech.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Treece GM, Prager RW, Gee AH. Regularised marching tetrahedra: improved iso-surface extraction. Comput Graph. 1999;23:583–598. [Google Scholar]
- 23.Desbrun M, Meyer M, Meyer M, et al. Implicit fairing of irregular meshes using diffusion and curvature flow. Proc. ACM. SIGGRAPH; Los Angeles, USA. 1999. pp. 317–324. [Google Scholar]
- 24.Hartigan JA, Hartigan PM. The dip test of unimodality. Annals of Statistics. 1985;13:70–84. [Google Scholar]
- 25.Brunner E, Domhof S, Langer F. John Wiley & Sons. 2002. Nonparametric analysis of longitudinal data in factorial experiments. [Google Scholar]
- 26.Biewener AA. Biomechanical consequences of scaling. J Exp Biol. 2005;208:1665–1676. doi: 10.1242/jeb.01520. [DOI] [PubMed] [Google Scholar]
- 27.Song JW, Haas A, Chung KC. Applications of statistical tests in hand surgery. J Hand Surg Am. 2009;34:1872–1881. doi: 10.1016/j.jhsa.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue B, Varadarajan KM, Ai S, et al. Gender differences in the knees of Chinese population. Knee Surg Sports Traumatol Arthrosc. 2011;19:80–88. doi: 10.1007/s00167-010-1139-8. [DOI] [PubMed] [Google Scholar]
- 29.Malek IA, Moorehead JD, Abiddin Z, Montgomery SC. The correlation between femoral condyle radii and subject height. Clin Anat. 2009;22:517–522. doi: 10.1002/ca.20787. [DOI] [PubMed] [Google Scholar]
- 30.Dargel J, Michael JW, Feiser J, et al. Human Knee Joint Anatomy Revisited: Morphometry in the Light of Sex-Specific Total Knee Arthroplasty. J Arthroplasty. 2010 doi: 10.1016/j.arth.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Greene KA. Gender-specific design in total knee arthroplasty. J Arthroplasty. 2007;22:27–31. doi: 10.1016/j.arth.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Booth RE., Jr Sex and the total knee: gender-sensitive designs. Orthopedics. 2006;29:836–838. doi: 10.3928/01477447-20060901-24. [DOI] [PubMed] [Google Scholar]
- 33.Parsley BS, Bertolusso R, Harrington M, et al. Influence of gender on age of treatment with TKA and functional outcome. Clin Orthop Relat Res. 2010;468:1759–1764. doi: 10.1007/s11999-010-1348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]