Abstract
Tissue stromal cells interact with leukemia cells and profoundly affect their viability and drug sensitivity. Here we show a biochemical mechanism by which bone marrow stromal cells modulate the redox status of chronic lymphocytic leukemia (CLL) cells and promote cellular survival and drug resistance. Primary CLL cells from patients exhibit limited ability to transport cystine for glutathione (GSH) synthesis due to a low expression of Xc- transporter, while bone marrow stromal cells effectively import cystine and convert it to cysteine, which is then released into the microenvironment for uptake by CLL cells to promote GSH synthesis. The elevated GSH enhances leukemia cell survival and protects them from drug-induced cytotoxicity. Furthermore, disabling this protective mechanism significantly sensitizes CLL cells to drug treatment in stromal environment. This stromal-leukemia interaction is critical for CLL cell survival and represents a key biochemical pathway for effectively targeting leukemia cells to overcome drug resistance in vivo.
Keywords: Leukemia, Stromal cells, Microenvironment, Glutathione, Drug resistance
Introduction
CLL is the most common adult leukemia in the western countries, characterized by accumulation of functionally defective B-lymphocytes in the blood, bone marrow, spleen, lymphoid notes, and other organs, leading to functional failure and patient death1–3. In the recent years, effective therapy such as fludarabine-based regimens have significantly improved the treatment outcomes for CLL patients4. However, failure to eliminate the residual leukemia cells that are drug-resistant and the eventual reemergence of the leukemia cells continue to be a major clinical challenge, and CLL remains as an incurable disease4, 5. The stromal protection of leukemia cells is an important factor that contributes to drug resistance in vivo.
The fact that CLL cells exhibit a high level of spontaneous apoptosis when cultured in vitro6 but have a prolonged survival time in vivo suggests that the tissue microenvironment plays a critical role in promoting CLL cell survival3, 7. Although several molecules including integrins8, CD40L9, IL-410, INF-α11, INF γ12, bFGF13, SDF-114, BAFF, APRIL15, and hedgehog-related molecules16, are known to be involved in stromal-CLL interaction, the biochemical mechanisms for stromal protection of CLL cells and microenvironment-induced drug resistance remain poorly understood. The persistence of residual CLL cells after chemotherapy often leads to disease relapse. Thus, it is important to understand the mechanisms by which stromal cells protect leukemia cells in order to develop effective therapeutic strategies to eliminate leukemia cells in vivo.
Recent technological developments have allowed global analyses of biochemical alterations in cancer, and enabled the discovery of potential roles of metabolites such as sarcosine and 2-hydroxyglutarate in cancer development17–19. Among the small molecules involved in cell survival and drug resistance, GSH is particularly important for CLL cells due to the unique biological properties of this leukemia. CLL cells intrinsically have higher levels of reactive oxygen species (ROS) compared to normal lymphocytes20–22, and are highly sensitive to agents that induce further ROS stress20, 23. The elevated ROS renders CLL cells more dependent on cellular antioxidants such as GSH to maintain redox balance. However, CLL cells are unable to maintain GSH once they are isolated from patients and cultured in vitro, exhibiting high spontaneous apoptosis6, 24. Here we show that the bone marrow stromal cells promote GSH metabolism in CLL cells and enhance the leukemia cell survival and drug resistance. We also identified a potential therapeutic strategy to overcome this stromal protection of CLL cells by abrogating the GSH system.
Results
Bone marrow stromal cells promoted GSH synthesis in CLL cells and relieved their ROS stress
Previous studies suggest that CLL cells have intrinsic ROS stress20, 25 and exhibit high spontaneous apoptosis with a loss of GSH when cultured in vitro, but have a prolonged survival time in vivo 6, 24. To investigate the interaction of bone marrow stromal cells and CLL cells, we employed a co-culture system using several bone marrow stromal cell lines and primary leukemia cells isolated from CLL patients. As shown in Fig 1A, CLL cells cultured alone exhibited substantial spontaneous cell death within 3 days, while bone marrow stromal cells (HS5) significantly enhanced CLL cell viability. Similar protection was also observed in a long-term (3 weeks) co-culture (Supplementary Fig S1A). The ability of stromal cells to enhance CLL cell viability was consistently observed when the cells were cultured under ambient oxygen (21% O2) or reduced oxygen (2–5%) that resembles the in vivo oxygen conditions in 4 CLL samples tested (Supplementary Fig S1B–1C), suggesting that this protective effect was the consequence of stromal-CLL cell interaction, not due to the artificial effect of the oxygen environment.
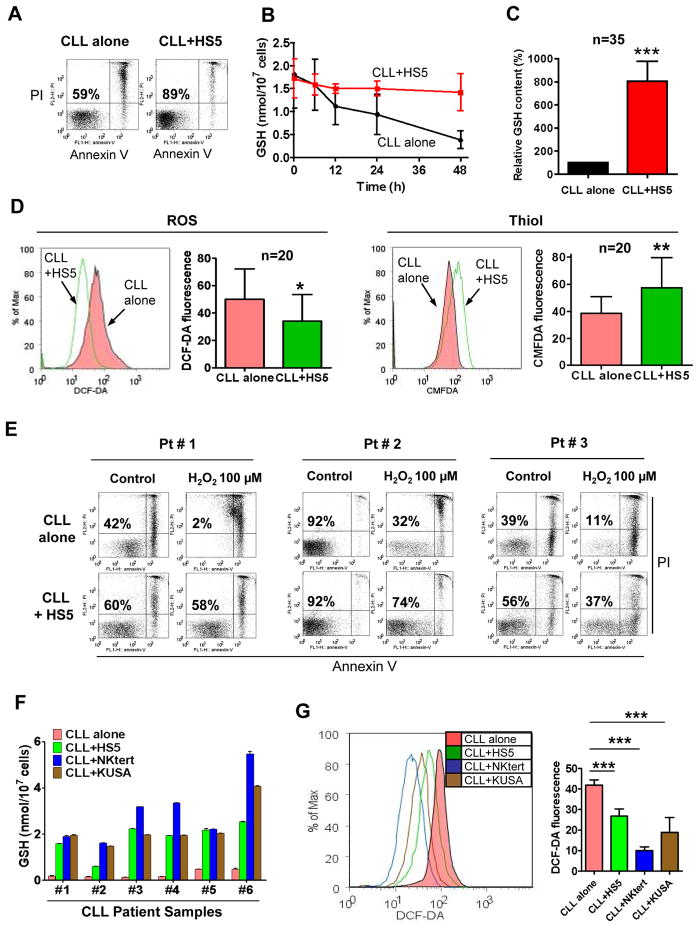
Figure 1. Bone marrow stromal cells enhanced GSH synthesis in CLL cells and relieved their ROS stress.
(A) Comparison of CLL cell viability cultured alone or with bone marrow stromal cells (HS5) for 3 days. Cell viability was measured by annexin-V/PI staining. The numbers indicate % of viable cells (annexin-V/PI double negative). The data are representative of 3 experiments using different CLL samples. (B) Time course of GSH contents in CLL cells cultured alone or with HS5 cells. Values are mean ± SD of 3 separate experiments using 3 CLL samples. (C) Comparison of GSH levels in CLL cells cultured alone or with HS5 cells for 72 h. The bar graph shows mean ± SEM of 35 different CLL samples, each measured in triplicate. (***, p<0.001). (D) Determination of cellular ROS and thiol contents in CLL cells cultured alone or with HS5 cells (72 h), detected by flow cytometry analysis. Representative histograms and quantitative comparison of mean values ± SD from 20 CLL samples are shown (*, p< 0.05; **, p<0.01). (E) HS5 stromal cells protected CLL cells from spontaneous apoptosis and cell death induced by H2O2 (100 μM). Cell viability was measured by annexin-V/PI staining. The number in each dot blot indicates the average % of viable cells (annexin-V/PI double negative) from 3 different CLL samples. (F) Comparison of GSH levels in CLL cells after a 3-day culture alone or with different bone marrow stromal cells (HS5, StromaNKtert, KUSA-H1). Each bar shows the mean ± SD of the GSH contents (n= 6 CLL samples). (G) Decrease of ROS in CLL cells after co-culture with stromal cells (HS5, StromaNKtert, and KUSA-H1). Cellular ROS were detected by flow cytometry using 1 μM DCF-DA. Representative histograms and the means ± SD of 4 separate experiments with 4 CLL patient samples are shown (***, p<0.001).
When cellular GSH was measured, we observed a striking difference in CLL cells cultured with or without stromal cells. CLL cells cultured alone showed a time-dependent decrease in GSH, whereas GSH was maintained at high levels when co-cultured with HS5 stromal cells (Fig 1B). Comparison of 35 CLL samples cultured for 3 days with or without stromal cells showed that GSH was significantly higher in CLL cells co-cultured with the bone marrow stromal cells (Fig 1C). Thirty-three of the 35 CLL samples exhibited more than 100% increase in co-culture, with GSH in the range of 1.5–4 nmole/107 cells in the majority of the samples, while most CLL cells cultured alone had less than 0.5 nmole/107 cells on day 3 (Supplementary Fig S2).
We then tested whether the bone marrow stromal cells could relieve the intrinsic oxidative stress in CLL cells by enhancing GSH. Fig 1D showed that CLL cells co-cultured with HS5 cells had lower ROS and higher cellular thiols (mainly GSH). Moreover, this redox change rendered CLL cells more resistant to exogenous ROS stress by H2O2 (Fig 1E). We also evaluated two other bone marrow stromal cell lines (StromaNKtert and KUSA-H1)26 for their effect on GSH in CLL cells, and showed that these stromal cells consistently enhanced GSH in all 6 CLL patient samples tested (Fig 1F), leading to a decrease in ROS (Fig 1G).
Critical role of GSH in mediating stromal protection of CLL cells
The role of GSH in mediating stromal protection of CLL cells was then tested in the co-culture system with or without drug treatment. As shown in Fig 2A and Supplementary Fig S3A, HS5 stromal cells significantly reduced CLL cell death that occurred either spontaneously or induced by F-ara-A (active form of fludarabine) or oxaliplatin, two drugs used in clinical treatment of CLL. Two other bone marrow stromal lines (StromaNKtert and KUSA-H1) also exhibited similar protective effect (Supplementary Fig S3B). Addition of N-acetylcysteine (NAC, a GSH precursor) or glutathione to the medium enhanced CLL cell viability without stromal cells (Figs 2B–2C), suggesting that increasing GSH by chemical supplement was sufficient to promote cell survival. These data also suggest that CLL cells were able to utilize exogenous GSH for glutathione synthesis, consistent with the report that γ-glutamyl transpeptidase and dipeptidase on the cell surface can cleave GSH to provide cysteine for GSH synthesis27. Indeed, exogenous GSH enhanced GSH in CLL cells in a concentration-dependent manner (Fig 2C, right panel).
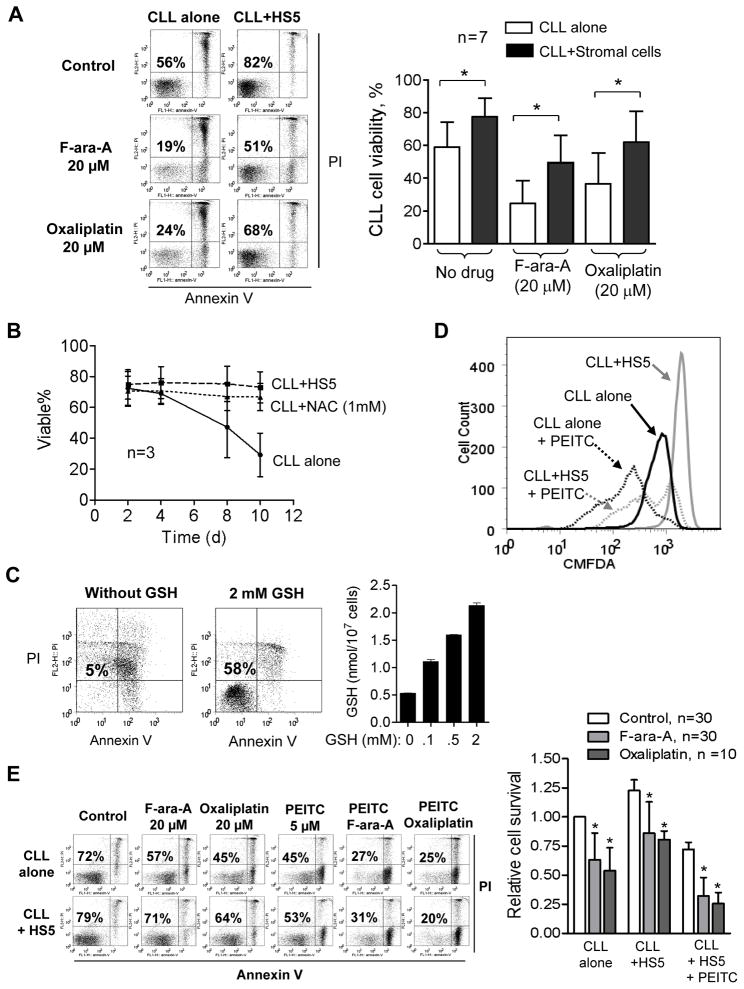
Figure 2. Critical role of GSH in mediating stromal protection of CLL cells from spontaneous and drug-induced cell death.
(A) Protection of CLL cells by bone marrow stromal cells (HS5) in the presence and absence of F-ara-A (20 μM) or oxaliplatin (20 μM). CLL cells were pre-cultured with HS5 cells for 24 h, followed by drug exposure for 48 h. Cell viability was measured by annexin-V/PI double staining. Representative dot plots of a CLL sample are shown on the left panel; the numbers indicates % of viable cells (annexin-V/PI double negative). Data of additional 6 patient samples are shown in Supplementary Fig S3A. The right panel shows the mean ± SD of the data from the 7 CLL samples. *, p<0.05. (B) Increase of CLL cell viability by HS5 stromal cells or by exogenous N-acetylcysteine (NAC). CLL cells were cultured alone, with HS5 cells, or with 1 mM NAC as indicated. Cell viability was measured by flow cytometry analysis (mean ± SD, n= 3 CLL samples for each condition). (C) Protection of CLL cells by exogenous GSH in culture medium without stromal cells (7 days). The data are representative of 3 experiments. The right panel shows the glutathione contents in CLL cells incubated with the indicated concentrations of GSH in the culture medium. (D) Determination of total thiol levels in CLL cells cultured alone or with HS5 cells in the presence or absence of PEITC (5 μM, 5 h). Cellular thiol was measured by flow cytometry using the thiol-reactive dye CMFDA. Representative histographs of 3 separate experiments are shown. (E) Annexin-V/PI assay of cell viability after CLL cells were cultured alone or with HS5 cells in the presence or absence of F-ara-A (20 μM, 48 h), oxaliplatin (20 μM, 48 h), PEITC (5 μM, 5 h), or their combination. The number in each dot blot indicates % of viable cells. The bar graph on the right shows the mean ± SD of viable cells from multiple experiments using 10–30 different CLL patient samples as indicated. *, p<0.05.
Furthermore, the stromal protection of CLL cells could be abrogated by depletion of GSH using β-phenylethyl isothiocyanate (PEITC), a natural compound capable of rapidly depriving cellular glutathione23, 28. As shown in Fig 2D, 5 μM PEITC significantly decreased GSH in CLL cells co-cultured with stromal cells. Depletion of GSH by PEITC was toxic to CLL cells and enhanced the cytotoxic effect of F-ara-A or oxaliplatin in the presence of stromal cells (Fig 2E). This was observed in multiple leukemia samples from 10–30 CLL patients (Fig 2E, right panel).
The low-molecular-weight fraction of the stromal medium enhanced GSH synthesis in CLL cells and promoted cell survival
Using a trans-well co-culture system with a membrane to prevent direct contact between stromal and CLL cells, we showed that HS5 cells conferred a significant protection to CLL cells treated with F-ara-A, oxaliplatin, or H2O2 (Fig 3A). Similar protection was consistently observed using two other stromal lines (StromaNKtert and KUSA-H1) (Supplementary Fig S3B), suggesting stromal protection was likely mediated by a diffusible factor. This was further confirmed by using stromal conditioned medium to enhance GSH synthesis in CLL cells (Fig 3B) and promote CLL cell survival without stromal cells (Fig 3C). The expression of γ-glutamylcysteine liganse (GCLC), a rate-limiting enzyme in GSH synthesis29, was not enhanced by stromal co-culture in 16 CLL samples tested (Supplementary Fig S4), suggesting that the soluble factor that promoted GSH synthesis in CLL cells did not up-regulate this rate-limiting enzyme.
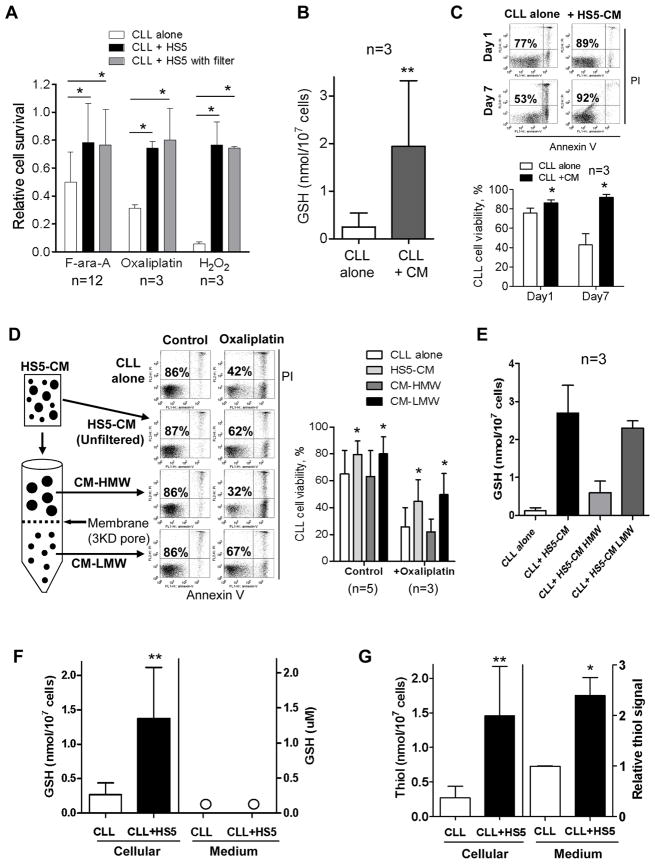
Figure 3. The low-molecular-weight fraction of the stromal medium enhanced GSH synthesis in CLL cells and promoted cell survival.
(A) Comparison of drug-induced loss of cell viability in CLL cells cultured alone or with HS5 cells in the presence or absence of a trans-well membrane. F-ara-A: 20 μM, 48 h; oxaliplatin: 20 μM, 48 h; H2O2: 100 μM, 24 h. Cell viability was measured by annexin-V/PI staining (mean ± SD; n=3; *, p<0.05). (B) Comparison of GSH levels in CLL cells after cultured in regular medium or in HS5-conditioned medium (HS5-CM) for 72 h. **, p<0.01 (mean±SD; n=3 different CLL samples). (C) Annexin V-PI assay of CLL cell viability after culture in regular medium or in HS5-conditioned medium for 1 or 7 days. The number in each dot blot indicates % of viable cells (annexin-V/PI double negative). The bar graph shows the mean ± SD of 3 separate experiments using 3 CLL samples, *, p<0.05. (D) Separation of HS5-conditioned medium into high-molecular-weight (HMW) and low-molecular-weight (LMW) fractions and their effect on the viability CLL cells exposed to oxaliplatin (20 μM, 48 h). Cell viability was measured by annexin-V/PI staining. The bar graph on the right shows the mean ± SD of multiple experiments using 3–5 CLL patient samples as indicated. *, p<0.05. (E) Enhancement of GSH levels in CLL cells by HS5-conditioned medium or its LMW fraction. Each bar shows mean ± SD of 3 experiments using 3 CLL samples. (F) Comparison of GSH levels in CLL cells or in the medium cultured with or without HS5 cells for 72h. Bar graphs of mean ± SD from 3 experiments with 3 CLL samples are shown (**, p < 0.01). (G) Comparison of thiol levels in CLL cells or in the medium cultured with or without HS5 cells for 72 h. The end-point method was used to measure thiol levels as described in Methods. Bar graphs of mean ± SD from 3 experiments with 3 CLL patient samples are shown (*, p<0.05; **, p< 0.01).
When the stromal conditioned medium was separated into high-molecular-weight (HMW) and low-molecular-weight (LMW) fractions using a 3-kD cut-off, we found that it was the LMW fraction that protected CLL cells against drug-induced cytotoxicity, whereas the HMW fraction showed little protection (Fig 3D). Furthermore, incubation of CLL cells with the LMW fraction led to an increase in GSH, similar to the GSH content in CLL cells cultured with unfiltered conditioned medium (Fig 3E). Thus, a LMW component of less than 3 kDa might play a key role in mediating the protective effect. Surprisingly, quantitative analysis showed no detectable increase of GSH in the stromal medium, while the cellular GSH in CLL cells increased significantly in co-culture (Fig 3F). Using Ellman’s reagent to detect total thiols, we found a significant increase of thiols in the co-culture medium (Fig 3G). These data together suggest that the stromal cells might release a thiol-containing compound into the medium, but this compound was not GSH.
Conversion of cystine to cysteine by bone marrow stromal cells was essential to enhance GSH synthesis in CLL cells and promote their survival
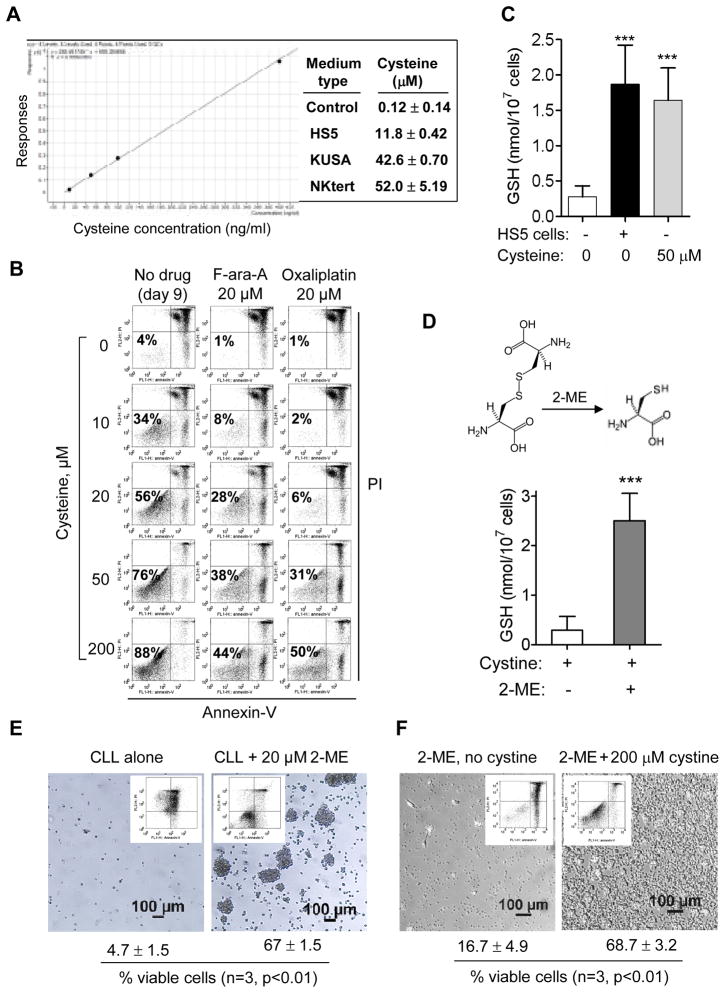
Since cysteine is a thiol-containing compound and a rate-limiting substrate for GSH synthesis29, we speculated that the LMW component in the stromal medium might be cysteine. Using LC-MS/MS analysis, we indeed detected 10–50 μM of cysteine in the conditioned medium from 3 stromal cell lines, while the control medium contained little cysteine (0.12 μM) (Fig 4A). The LC-MS/MS spectra of authentic cysteine in comparison with the cysteine in stromal medium are shown in Supplementary Fig S5. Because human plasma contains 10–20 μM cysteine30, 31, we tested if these concentrations of cysteine could protect CLL cells without stromal co-culture. Daily addition of cysteine promoted CLL viability and suppressed drug-induced cell death in a concentration-dependent manner (Fig 4B), and 50 μM cysteine enhanced GSH in CLL cells to the same level observed in stromal co-culture (Fig 4C). Moreover, chemical conversion of the cystine in RPMI medium to cysteine by a reducing agent 2-mercaptoethanol (2-ME, 20 μM) effectively increased GSH in CLL cells (Fig 4D), confirming the importance of cysteine. Consistently, CLL cells lost their viability in regular medium (containing cystine) without 2-ME, while addition of 2-ME conferred significant protection and kept most CLL cells viable (Fig 4E). In a separate experiment, 2-ME failed to protect CLL cells when cystine was omitted from the medium (Fig 4F), indicating that cystine was required to generate cysteine by 2-ME in order to provide the protective effect.
Figure 4. Generation of cysteine by bone marrow stromal cells was essential to enhance GSH synthesis in CLL cells and promote their survival.
(A) Quantitation of cysteine in the conditioned medium of three bone marrow stromal cell lines using triple quadrupole mass spectrometer LC-MS/MS (Agilent 6460). Media were collected freshly and analyzed immediately. Culture medium without stromal cells was used as a control. All samples were analyzed in triplicates. The left panel is the standard curve showing the linearity of this assay. The LC-MS/MS spectra are shown in Supplementary Fig S5. (B) Effect of cysteine on CLL cell survival and drug sensitivity cultured without stromal cells. The indicated concentrations of cysteine were added daily to the culture medium. On day 7, F-ara-A or oxaliplatin was added and incubated for additional 48 h. Cell viability was measured by flow cytometry analysis on day 9. Representative dot plots of 3 separate experiments are shown. (C) Extracellular cysteine (50 μM, added daily for 3 days) enhanced CLL cellular GSH contents. CLL cells cultured alone or with HS5 cells were used as controls for comparison. Each bar represents mean ± SD of 4 separate experiments (***, p<0.001). (D) Conversion of extracellular cystine to cysteine by 2-mercaptolethanol (2-ME, 20 μM) enhanced GSH synthesis in CLL cells (mean ± SD; n= 3 different CLL samples; ***, p<0.001). (E) Conversion of cystine to cysteine in the culture medium by 2-ME (20 μM) promoted CLL cell long-term survival in culture with regular RPMI medium (containing 200 μM cystine). Photographs and flow cytometry analysis of cell viability using annexin-V/PI double staining were performed on day 20. The mean ± SD (% viable cells) of 3 separate experiments are indicated below each panel. (F) 2-ME could not protect CLL cells when cystine was withdrew from the medium. Cell viability was measured using annexin-V/PI double staining, and the mean ± SD (% viable cells) of 3 separate experiments are indicated below each panel.
Leukemia cells exhibited low ability to directly utilize cystine and were dependent on stromal cells to convert cystine to cysteine for GSH synthesis
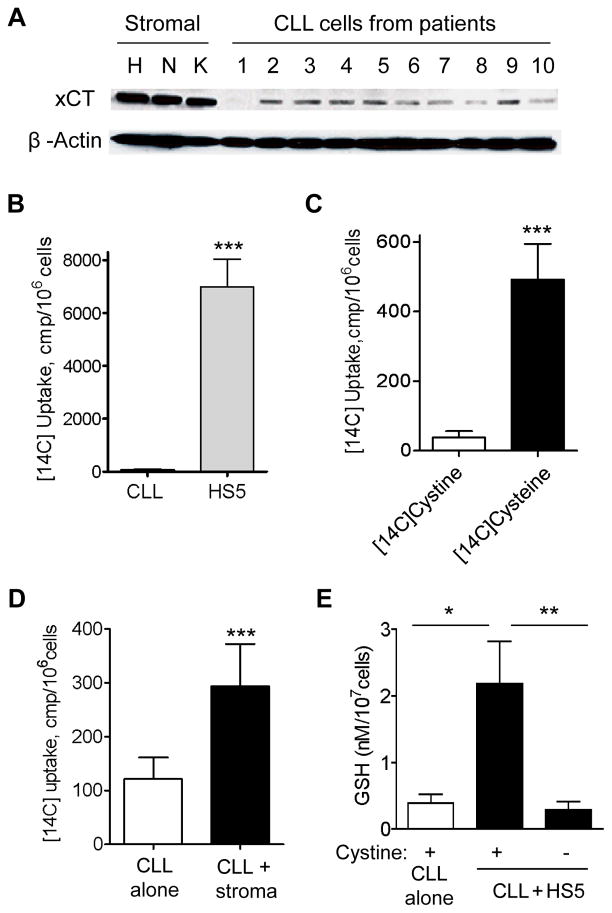
Cysteine is chemically unstable, and cells normally use the stable cystine as a precursor for GSH synthesis. We examined the expression of the cystine transporter Xc-, and found that xCT (the active subunit of Xc-) expression was very low in CLL cells. In contrast, xCT was highly expressed in bone marrow stromal cells (Fig 5A). Using the National Center for Biotechnology Information gene expression omnibus database (ID: GDS1454) that contains microarray data from CLL and normal control samples32, we also found that xCT mRNA expression in CLL cells (n=100) was significantly lower than in normal lymphocytes (n=11) (p<0.001). These data together suggest that CLL cells might have limited ability to take up cystine due to low expression of its transporter.
Figure 5. Leukemia cells (CLL) exhibited low ability to directly utilize cystine and were dependent on stromal cells to convert cystine to cysteine for GSH synthesis.
(A) Expression of the cystine transporter xCT in HS5 (H), StromaNKtert (N), and KUSA-H1 (K) stromal cells and primary CLL cells from patients (n=10). The un-cropped blots are shown as Supplementary Information. (B) Comparison of [14C]cystine uptake by HS5 stromal cells and CLL cells (4 h incubation). Bar graph of mean ± SD of 3 separate experiments is shown (***, p<0.001). (C) Effective uptake of [14C]cysteine, but not [14C]cystine by CLL cells (4 h incubation; mean ± SD; n=3 patient samples; ***, p<0.001). (D) Stromal cells (HS5) increased the uptake of radioactivity by CLL cells in culture medium containing [14C]cystine (6 h incubation; mean ± SD; n=3 patient samples; ***, p<0.001). (E) Extracellular cystine was required for stromal cells to enhance GSH synthesis in CLL cells. CLL cell were co-culture with HS5 cells in presence or absence of 200 μM cystine for 72 h, and GSH contents in CLL cell extracts were measured (mean ± SD; n=3 patient samples; *, p<0.05; **, p<0.01).
We then performed functional analysis and found that CLL cells exhibited very little uptake of [14C]-cystine, whereas HS5 stromal cells showed significantly higher uptake (Fig 5B). When [14C]-cystine was chemically converted to [14C]-cysteine by 2-ME, CLL cells could then take up a substantial amount of the radioactive substrate (Fig 5C), indicating that CLL cells were able to transport cysteine but not cystine. There was a significant increase in the uptake of radioactivity by CLL cells when co-cultured with HS5 cells (Fig 5D), suggesting that the stromal cells converted cystine to cysteine for uptake by CLL cells. Consistently, removal of cystine from the medium abrogated the ability of stromal cells to promote GSH synthesis in CLL cells (Fig 5E). We also found that stromal cells did not increase the expression of xCT in CLL cells (Supplementary Fig S4). These data together suggest that bone marrow stromal cells promoted GSH synthesis in CLL cells mainly by converting cystine to cysteine, not by enhancing the expression of cystine transporter.
Overcoming drug resistance in stromal environment by abolishing the GSH protective mechanism in vitro and in vivo
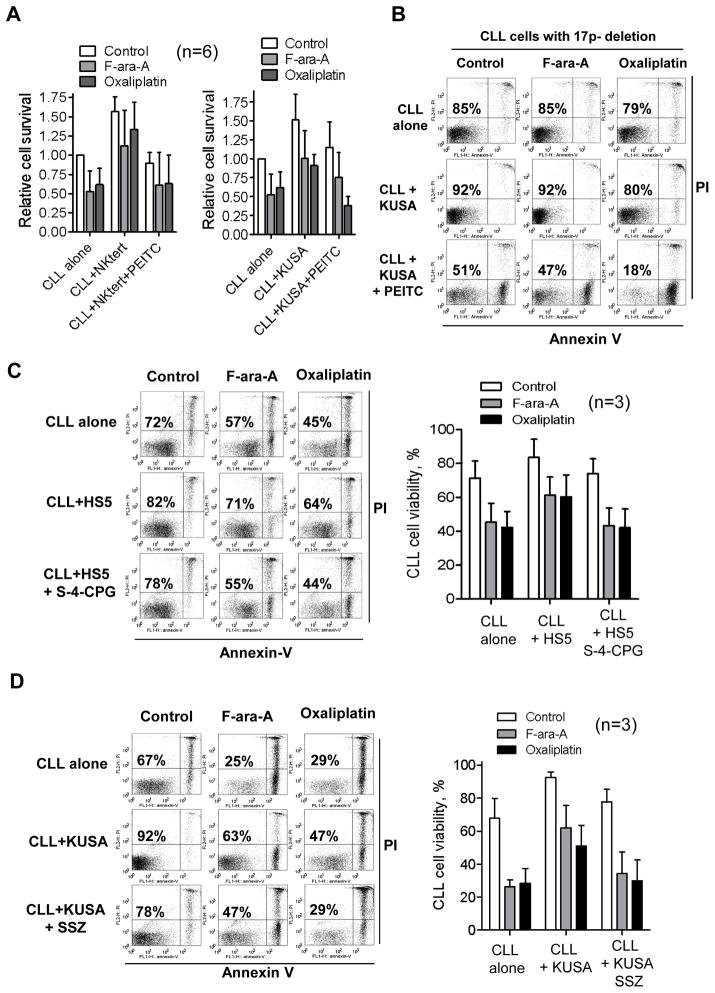
Since enhancement of GSH synthesis in CLL cells is likely a key mechanism by which stromal cells promote leukemia cell survival and drug resistance, we reasoned that abrogation of this protective mechanism would sensitize leukemia cells to drug treatment in the stromal environment. Indeed, we found that PEITC, a compound known to cause rapid depletion of GSH in CLL cells23, was able to effectively abrogate the ability of HS5 stromal cells to protect CLL cells (Fig 2E). This was consistently observed in experiments using two other bone marrow stromal cell lines (StromaNKtert and KUSA-H1) (Fig 6A). Of note, combination of 10 μM PEITC and 20μM oxaliplatin exhibited striking synergistic effect against CLL cells in the presence of stromal cells (Supplementary Fig S6A). The loss of p53 in CLL cells due to chromosome 17p deletion is known to cause drug resistance and poor prognosis33, 34. We found that combination of PEITC and oxalliplatin was highly effective in killing the CLL cells with 17p-deletion in the presence of stromal cells (Fig 6B).
Figure 6. Overcoming stromal-induced drug resistance in CLL cells by depleting GSH in leukemia cells or blocking cystine uptake by stromal cells.
(A) Effect of PEITC (5 μM) on CLL cell viability cultured alone or with StromaNktert (left panel) or KUSA-H1 stromal cells (right panel) in the presence or absence of F-ara-A or oxaliplatin. CLL cells in co-culture were exposed to F-ara-A (20 μM) or oxaliplatin (20 μM) for 48 h. PEITC (5 μM) was added during the last 5 h of incubation. Cell viability was analyzed by annexin-V/PI assay. The bar graph shows the mean ± SD of 6 separate experiments using 6 CLL samples. (B) Effect of PEICT on drug-resistant leukemia cells from a CLL patient with chromosome 17p deletion. CLL cells were cultured alone or with KUSA-H1 stromal cells in the presence or absence of 20 μM F-ara-A or 20 μM oxaliplatin for 48 h. PEITC (10 μM) was added during the last 5 h of incubation. The numbers show % of viable cells. (C) Sensitization of CLL cells to F-ara-A and oxaliplatin by inhibition of cystine transport with (S)-4-carboxyphenylglycine (S-4-CPG). CLL and HS5 cells in co-culture were first incubated with S-4-CPG (500 μM) for 24 h, and then exposed to F-ara-A (20 μM) or oxaliplatin (20 μM) for 48 h. Cell viability was analyzed by annexin-V/PI assay. Representative dot plots are shown with % viable cells (annexin-V/PI double negative) indicated. The right panel shows the mean ± SD of 3 separate experiments. (D) Sensitization of CLL cells to F-ara-A and oxaliplatin by inhibition of cystine transport using sulfasalazine (SSZ). CLL and stromal (KUSA-H1) cells in co-culture were first incubated with SSZ (300 μM) for 24 h to inhibit cystine transport, and then exposed to F-ara-A (20 μM) or oxaliplatin (20 μM) for 48 h. Cell viability was analyzed by flow cytometry. Representative dot plots are shown with % viable cells indicated. The right panel shows the mean ± SD of 3 separate experiments using 3 CLL patient samples.
We then tested another strategy to abolish stromal protection of CLL cells by inhibiting the cystine transporter to block the uptake of cystine by stromal cells. Two Xc- inhibitors, (S)-4-carboxyphenylglycine35 and sulfasalazine36 were used in this study. As shown in Fig 6C,D, HS5 cells reduced the sensitivity of CLL cells to F-ara-A or oxaliplatin, and a subtoxic concentration of (S)-4-carboxyphenylglycine (S-4-CPG, 500 μM) and sulfasalazine (SSZ, 300 μM) enhanced F-ara-A or oxaliplatin-induced cytotoxicity to a level comparable to that observed in CLL cells without stromal protection. Similar results were also observed in experiments using a different CLL sample and another bone marrow stromal cell line (KUSA-H1, Supplementary Fig S6B). In control experiments, we found that 300 μM SSZ and 500 μM S-4-CPG caused minimum cytotoxicity in normal bone marrow stromal cells, with 90–95% viable cells after the drug incubation (Supplementary Fig S6C). The concentrations of F-ara-A (20 μM), oxaliplatin (20 μM), and PEITC (5–10 μM) used in these experiments are clinically relevant and consistent with other reports23, 37–39.
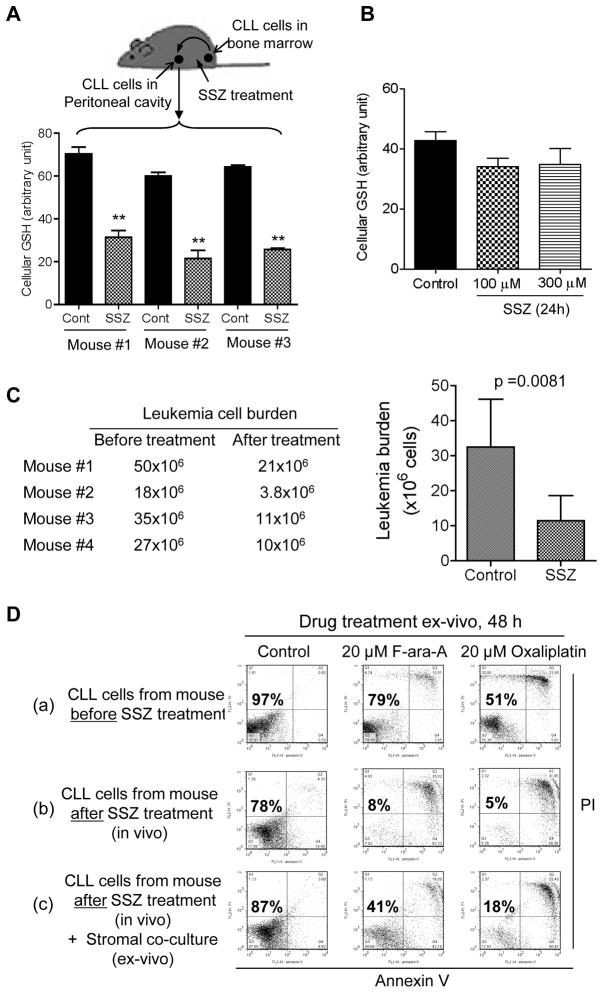
The Tcl-1 transgenic mice, a mouse model that resembles human CLL disease40, were used to further evaluate the in vivo relevance of stromal-leukemia interaction in cystine/GSH metabolism. As shown in Fig 7A, blocking cystine uptake by in vivo administration of SSZ resulted in a significant decrease of GSH in CLL cells (p<0.001) in all 3 mice tested. In contrast, incubation of primary CLL cells with SSZ in vitro without stromal cells did not cause a significant change in cellular GSH (Fig 7B). These data together suggest that the higher concentration of GSH in CLL cells in vivo was due to stromal cell uptake of cystine and its conversion to cysteine for CLL cells. This metabolic communication was blocked by SSZ in vivo. Importantly, treatment of the mice with SSZ caused a significant reduction in leukemia burden in all 4 mice tested. The leukemia cells in the peritoneal cavity were reduced from 32.5 million to 11.4 million cells during a week of treatment (p=0.0081) (Fig 7C). CLL cells isolated from a mouse treated with SSZ showed a moderate decrease in cells viability (78% viable), compared with the CLL cells isolated from the same mouse before SSZ treatment (97% viable cells, Fig 7D). In vivo SSZ treatment rendered the leukemia cells more sensitive to F-ara-A or oxaliplatin when cultured ex-vivo, and this sensitization was partially reversed by co-culture with bone marrow stromal cells (Fig 7D). These data together suggest that inhibition of cystine transporter by SSZ in vivo could significantly reduce tumor burden, decrease leukemia cell viability, and increase their drug sensitivity.
Figure 7. Effect of abolishing GSH protection on CLL cells by blocking cystine uptake by stromal cells in vivo.
(A) Effect of the sulfasalazine (SSZ) on CLL cellular GSH in vivo. CLL cells were obtained by peritoneal washing from Tcl-1 transgenic mice that had developed CLL disease with leukemia cells in the peritoneal cavity. After a 7-day recovery period, the mice were treated with SSZ (8 mg/mouse, i.p., every 12 h x 3 injections). A second sample of peritoneal CLL cells was obtained 12 h after the last SSZ treatment. GSH was measured as described in Methods (mean ± SD; n=3; **, p<0.01). (B) Effect of SSZ on GSH in CLL cells cultured in vitro without stromal cells. Primary CLL cells were cultured overnight to remove stromal cells attaching to the flask surface. CLL cells in suspension were transferred to fresh flasks and incubated with or without SSZ (100–300 μM, 24 h). GSH was measured in triplicate (mean ± SD; n=3). (C) Treatment of Tcl-1 mice with SSZ (8 mg/kg, i.p., 3 times per week, M/W/F) significantly reduced the leukemia cell burden. The numbers in the left panel show the total leukemia cell counts in the peritoneal cavity of each mouse before and after SSZ treatment; the right bar graph shows the mean ± SD of the leukemia cells in all 4 mice tested. (D) Treatment of Tcl-1 mice with SSZ decreased leukemia cell viability and enhanced drug sensitivity ex-vivo. (a) Leukemia cells were isolated from a CLL mouse by peritoneal washing. Cell viability was analyzed before and after the cells were incubated ex-vivo with F-ara-A or oxaliplatin as indicated. (b) The same mouse was allowed a 7-day recovery period and then treated with SSZ (8 mg/kg, i.p., three times per week, M/W/F). At 24 h after the last drug treatment, CLL cells were isolated and cell viability was analyzed before and after the cells were incubated ex-vivo with F-ara-A or oxaliplatin as indicated. (c) The experimental conditions were the same as in (b), except that the CLL cells were co-cultured with bone marrow stromal cells (StromaNKtert).
Discussion
Our study revealed an important metabolic interaction between CLL cells and bone marrow stromal cells to enhance GSH synthesis, and thus increase the ability of CLL cells to maintain redox balance and promote cell survival and drug resistance. As illustrated in Supplementary Fig S7, this metabolic pathway involves the uptake of cystine by stromal cells, the conversion of cystine to cysteine and its release to the microenvironment, and the uptake of cysteine by CLL cells for GSH synthesis. Several important factors underscore the critical need of this biochemical pathway to protect CLL cells. The intrinsic ROS stress in CLL cells renders them highly dependent on GSH to maintain redox balance23, but they have limited ability to take up cystine for GSH synthesis due to the low expression of cystine transporter Xc-. Although CLL cells can transport cysteine, this compound is chemically unstable in extracellular environment and thus requires the constant production by stromal cells. Bone marrow stromal cells have high expression of Xc- and thus can effectively take up cystine and convert it to cysteine for use by CLL cells.
Accumulation of CLL cells in patients is due in part to a prolonged CLL cell survival in vivo41. Paradoxically, CLL cells often exhibit spontaneous apoptosis in vitro when cultured alone42. Thus the in vivo environment has a profound effect on CLL cell survival. Although several stromal factors and cytokines have been implicated in promoting CLL cell viability8–16, the exact mechanisms by which the stromal environment protects CLL cells still remain unclear. Our study revealed an important intercellular metabolic pathway between CLL and bone marrow stromal cells that promoted CLL cell viability and drug resistance by enhancing GSH synthesis. Cysteine is a rate-limiting substrate for GSH synthesis. In many cell types, the main source of cysteine is the uptake of extracellular cystine and cysteine by their respective transporters43–45. Cysteine is transported by both Na+-dependent ASC transporter and Na+-independent transporters46, while cystine is mainly transported by Xc- and converted to cysteine inside the cells35, 47–49. The plasma cystine is in 100–200 μM range, whereas cysteine is much lower (10–20 μM)50, reflecting the dynamic balance between its generation from the cells and its extracellular oxidation. Interestingly, 10 μM of cysteine could enhance CLL viability without drug treatment (Fig 4B). This may explain why CLL cells have a relatively long survival time in the blood circulation. Higher concentrations of cysteine (20–200 μM) showed further protection of CLL cells and promoted drug resistance (Fig 4B). Although stromal cells did not enhance the expression of Xc- in CLL cells to promote cystine uptake, they constantly converted cystine to cysteine for CLL cells. Thus, when CLL cells are in a close proximity to the bone marrow stromal cells in vivo, the high local concentrations of cysteine near the stromal cells may provide strong protection to the leukemia cells and promote drug resistance.
One prominent biochemical feature of CLL cells is their high ROS generation and oxidative stress20, 23, 25. Such intrinsic ROS stress renders them more dependent on the redox regulatory systems to maintain redox balance. GSH is the most abundant antioxidant in cells to maintain redox balance and significantly affect cell survival51. GSH also inhibits apoptosis through mechanisms not directly involving modulation of ROS52. Glutathionylation of the anti-apoptotic protein MCL-1 prevents its cleavage by caspase-3 and thus promotes CLL cell survival23. A recent study indicated that GSH may promote lymphoid cell survival through maintaining cellular ionic homeostasis53. With its nucleophilic property, GSH may conjugate with electrophilic drugs, promote their export, and thus decrease the efficacy of anticancer agents54. GSH can also reduce the activity of oxaliplatin by decreasing ROS stress induced by the drug55. Since CLL cells highly rely on stromal cells to provide cysteine for GSH synthesis, this intercellular metabolic pathway may represent a potentially important target for effective killing of CLL cells in vivo. Indeed, our results from the proof-of-principle study using (S)-4-CPG or SSZ to interrupt the cystine→cysteine→GSH flow or using PEITC to directly deplete GSH suggest that such therapeutic approach is effective in abrogating the stromal protection on CLL cells and enhancing their drug sensitivity. Further evaluation of this biochemical intervention strategy in preclinical and clinical settings is important for the development of effective therapy to overcome drug resistance in vivo. It is possible, however, that stromal cells may protect CLL cells by additional mechanisms such as secretion of cytokines and other stromal factors that promote cell-cell interactions and activate survival signals8–16. The possibility of stromal cells to enhance other antioxidant systems such as thioredoxins, superoxide dismutases, and catalase in CLL cells should also be considered in developing redox-modulating therapeutic strategies.
Supplementary Material
Acknowledgments
The authors thank Dr. D.H. Hawke for expert assistance in LC-MS/MS analysis of cystine and cysteine, and B. A. Hayes, A. G. Melendez, M.A. Ogasawara, L. Feng, and H. Zhang for technical assistance and helpful discussion. This work was supported in part by grants CA085563 and CA100428 from the National Institutes of Health, and a grant for the CLL Global Research Foundation.
Footnotes
Author contributions
WZ: project planning, experimental work, data analysis, manuscript writing; DT, JL, GC, CGP, WL: experimental work, data analysis; HP, JAB, WP: project planning and data interpretation; CMC: provided Tcl-1 transgenic mice; MJK: provided CLL specimens and participated in project planning and data interpretation; PH: project planning, data analysis, and manuscript writing.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Zwiebel JA, Cheson BD. Chronic lymphocytic leukemia: staging and prognostic factors. Seminars in oncology. 1998;25:42–59. [PubMed] [Google Scholar]
- 2.Keating MJ, et al. Biology and treatment of chronic lymphocytic leukemia. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. 2003:153–175. doi: 10.1182/asheducation-2003.1.153. [DOI] [PubMed] [Google Scholar]
- 3.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 4.Tam CS, Keating MJ. Chemoimmunotherapy of chronic lymphocytic leukemia. Best practice & research. 2007;20:479–498. doi: 10.1016/j.beha.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Rai KR, Chiorazzi N. Determining the clinical course and outcome in chronic lymphocytic leukemia. The New England journal of medicine. 2003;348:1797–1799. doi: 10.1056/NEJMe030032. [DOI] [PubMed] [Google Scholar]
- 6.Collins RJ, et al. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. British journal of haematology. 1989;71:343–350. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- 7.Munk Pedersen I, Reed J. Microenvironmental interactions and survival of CLL B-cells. Leukemia & lymphoma. 2004;45:2365–2372. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- 8.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 9.Grdisa M. Influence of CD40 ligation on survival and apoptosis of B-CLL cells in vitro. Leukemia research. 2003;27:951–956. doi: 10.1016/s0145-2126(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 10.Dancescu M, et al. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. The Journal of experimental medicine. 1992;176:1319–1326. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jewell AP, et al. Interferon-alpha up-regulates bcl-2 expression and protects B-CLL cells from apoptosis in vitro and in vivo. British journal of haematology. 1994;88:268–274. doi: 10.1111/j.1365-2141.1994.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 12.Buschle M, et al. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. The Journal of experimental medicine. 1993;177:213–218. doi: 10.1084/jem.177.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konig A, et al. Basic fibroblast growth factor (bFGF) upregulates the expression of bcl-2 in B cell chronic lymphocytic leukemia cell lines resulting in delaying apoptosis. Leukemia. 1997;11:258–265. doi: 10.1038/sj.leu.2400556. [DOI] [PubMed] [Google Scholar]
- 14.Burger JA, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 15.Endo T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109:703–710. doi: 10.1182/blood-2007-04-081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegde GV, et al. Hedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic target. Mol Cancer Res. 2008;6:1928–1936. doi: 10.1158/1541-7786.MCR-08-0142. [DOI] [PubMed] [Google Scholar]
- 17.Lawton KA, et al. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–397. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- 18.Sreekumar A, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 465:966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 21.Oltra AM, Carbonell F, Tormos C, Iradi A, Saez GT. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free radical biology & medicine. 2001;30:1286–1292. doi: 10.1016/s0891-5849(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 22.Carew JS, et al. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437–1447. doi: 10.1038/sj.leu.2403043. [DOI] [PubMed] [Google Scholar]
- 23.Trachootham D, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silber R, et al. Glutathione depletion in chronic lymphocytic leukemia B lymphocytes. Blood. 1992;80:2038–2043. [PubMed] [Google Scholar]
- 25.Hileman EO, Liu J, Albitar M, Keating MJ, Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer chemotherapy and pharmacology. 2004;53:209–219. doi: 10.1007/s00280-003-0726-5. [DOI] [PubMed] [Google Scholar]
- 26.Kurtova AV, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodlock TJ, et al. Decreased L system amino acid transport and decreased gamma-glutamyl transpeptidase are independent processes in human chronic lymphocytic leukemia B-lymphocytes. J Cell Physiol. 1990;145:217–221. doi: 10.1002/jcp.1041450205. [DOI] [PubMed] [Google Scholar]
- 28.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Lu SC. Regulation of glutathione synthesis. Current topics in cellular regulation. 2000;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]
- 30.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free radical biology & medicine. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato H, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. The Journal of biological chemistry. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 32.Haslinger C, et al. Microarray gene expression profiling of B-cell chronic lymphocytic leukemia subgroups defined by genomic aberrations and VH mutation status. J Clin Oncol. 2004;22:3937–3949. doi: 10.1200/JCO.2004.12.133. [DOI] [PubMed] [Google Scholar]
- 33.Zenz T, et al. Detailed analysis of p53 pathway defects in fludarabine-refractory chronic lymphocytic leukemia (CLL): dissecting the contribution of 17p deletion, TP53 mutation, p53-p21 dysfunction, and miR34a in a prospective clinical trial. Blood. 2009;114:2589–2597. doi: 10.1182/blood-2009-05-224071. [DOI] [PubMed] [Google Scholar]
- 34.Turgut B, et al. 17p Deletion is associated with resistance of B-cell chronic lymphocytic leukemia cells to in vitro fludarabine-induced apoptosis. Leukemia & lymphoma. 2007;48:311–320. doi: 10.1080/10428190601059829. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer research. 2005;65:7446–7454. doi: 10.1158/0008-5472.CAN-04-4267. [DOI] [PubMed] [Google Scholar]
- 36.Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 37.Moufarij MA, Sampath D, Keating MJ, Plunkett W. Fludarabine increases oxaliplatin cytotoxicity in normal and chronic lymphocytic leukemia lymphocytes by suppressing interstrand DNA crosslink removal. Blood. 2006;108:4187–4193. doi: 10.1182/blood-2006-05-023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham MA, et al. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res. 2000;6:1205–1218. [PubMed] [Google Scholar]
- 39.Danhauser L, Plunkett W, Keating M, Cabanillas F. 9-beta-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate pharmacokinetics in plasma and tumor cells of patients with relapsed leukemia and lymphoma. Cancer chemotherapy and pharmacology. 1986;18:145–152. doi: 10.1007/BF00262285. [DOI] [PubMed] [Google Scholar]
- 40.Bichi R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci U S A. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed JC. Molecular biology of chronic lymphocytic leukemia. Seminars in oncology. 1998;25:11–18. [PubMed] [Google Scholar]
- 42.Caligaris-Cappio F. Biology of chronic lymphocytic leukemia. Reviews in clinical and experimental hematology. 2000;4:5–21. doi: 10.1046/j.1468-0734.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 43.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochimica et biophysica acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 44.Iglehart JK, York RM, Modest AP, Lazarus H, Livingston DM. Cystine requirement of continuous human lymphoid cell lines of normal and leukemic origin. The Journal of biological chemistry. 1977;252:7184–7191. [PubMed] [Google Scholar]
- 45.Uren JR, Lazarus H. L-cyst(e)ine requirements of malignant cells and progress toward depletion therapy. Cancer treatment reports. 1979;63:1073–1079. [PubMed] [Google Scholar]
- 46.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. The Biochemical journal. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, et al. Cystine-glutamate transporter SLC7A11 mediates resistance to geldanamycin but not to 17-(allylamino)-17-demethoxygeldanamycin. Molecular pharmacology. 2007;72:1637–1646. doi: 10.1124/mol.107.039644. [DOI] [PubMed] [Google Scholar]
- 48.Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. Journal of cellular physiology. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 49.Okuno S, et al. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. British journal of cancer. 2003;88:951–956. doi: 10.1038/sj.bjc.6600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chawla RK, et al. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology. 1984;87:770–776. [PubMed] [Google Scholar]
- 51.Lu SC. Regulation of glutathione synthesis. Molecular aspects of medicine. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franco R, Panayiotidis MI, Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. The Journal of biological chemistry. 2007;282:30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franco R, DeHaven WI, Sifre MI, Bortner CD, Cidlowski JA. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. The Journal of biological chemistry. 2008;283:36071–36087. doi: 10.1074/jbc.M807061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer research. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 55.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nature reviews. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.