Abstract
EGF-like growth factors control tumor progression, as well as evasion from the toxic effects of chemotherapy. Accordingly, antibodies targeting the cognate receptors, such as EGFR/ErbB-1 and the co-receptor HER2/ErbB-2, are widely used to treat cancer patients, but agents that target the EGF-like growth factors are not available. To circumvent the existence of 11 distinct ErbB ligands, we constructed a soluble fusion protein (hereinafter: TRAP-Fc) comprising truncated extracellular domains of EGFR/ErbB-1 and ErbB-4. The recombinant TRAP-Fc retained high affinity ligand binding to EGF-like growth factors and partially inhibited growth of a variety of cultured tumor cells. Consistently, TRAP-Fc displayed an inhibitory effect in xenograft models of human cancer, as well as synergy with chemotherapy. Additionally, TRAP-Fc inhibited invasive growth of mammary tumor cells and reduced their metastatic seeding in the lungs of animals. Taken together, the activities displayed by TRAP-Fc reinforce critical roles of EGF-like growth factors in tumor progression, and they warrant further tests of TRAP-Fc in pre-clinical models.
Keywords: cancer therapy, EGF, growth factor, tyrosine kinase, signal transduction
INTRODUCTION
The ErbB family of receptors and cognate growth factors, all sharing an epidermal growth factor (EGF) module, play important roles in embryonic development and in tissue remodeling throughout adulthood. The signaling cascade downstream of the ligand-ErbB complex initiates upon dimerization of occupied receptors, auto-phosphorylation, and activation of various cellular processes, including proliferation and migration (Yarden and Sliwkowski, 2001). The family includes four receptors: ErbB-1 (EGFR), which binds EGF, transforming growth factor α (TGFα), the heparin-binding EGF like growth factor (HB-EGF), amphiregulin (AR), betacellulin (BTC), epiregulin (EPR) and epigen, ErbB-2 (also called HER2), which has no known ligand, and two neuregulin (NRG) receptors, ErbB-3 and ErbB-4. The EGF-like module of 50–60 amino acids is shared by all ErbB ligands, as it confers specific receptor binding (Jorissen et al., 2003). The growth factors are synthesized as type I transmembrane precursors, which comprise an EGF-like domain. Once processed, the soluble ligand may bind and activate receptors on distant cells, neighboring cells, or on the cells of its origin, mechanisms termed endocrine, paracrine, and autocrine, respectively (Sporn and Todaro, 1980).
Several clinical studies indicate that overexpression of one or more EGF-like ligands correlates with decreased patient survival. For example, in colorectal tumors enhanced expression of TGFα is associated with over 50-fold increased risk of developing liver metastases, and TGFα levels in liver metastases associate with poor patient outcome (Barozzi et al., 2002; De Jong et al., 1998). Likewise, increased expression of TGFα in head and neck tumors correlates with decreased patient survival (Grandis et al., 1998). In bladder cancer, the elevated expression of a number of ligands is linked to decreased patient survival (Thogersen et al., 2001). Moreover, in vivo studies have shown that overexpression of neuregulins (NRGs) in mammary tissue accelerates adenocarcinoma development (Krane and Leder, 1996), and favors metastatic spread of breast cancer cells (Atlas et al., 2003). Likewise, it has recently been proposed that an autocrine loop involving NRG1 and an activated ErbB-3 drives progression of a subset of ovarian tumors (Sheng et al., 2010). Importantly, ErbB receptors and their ligands are also involved in resistance to endocrine and cytotoxic therapy, as well as to radiotherapy (Bijman et al., 2009; Freeman et al., 2009).
The currently approved drugs for the treatment of tumors driven by the ErbB family are either monoclonal antibodies directed at ErbB-1/EGFR (for example, cetuximab) or at ErbB-2/HER2 (such as trastuzumab), or small-molecule tyrosine kinase inhibitors (TKIs; for example erlotinib) (Baselga, 2006; Britten, 2004; Weiner and Borghaei, 2006). Whereas these agents can induce therapeutic responses in specific subsets of patients, acquired resistance to these drugs inevitably emerges. Mechanistically, up-regulation of ErbB receptors (Bianchi et al., 2006; Engelman et al., 2007; Karamouzis et al., 2007; Ritter et al., 2007) and EGF family ligands have been proposed as mediators of acquired resistance (Ishikawa et al., 2005; Valabrega et al., 2005; Wheeler et al., 2008; Zhou et al., 2006). In the same vein, human breast cancer cells selected in vivo for resistance to trastuzumab remarkably overexpress EGFR and specific ErbB ligands (Ritter et al., 2007). Likewise, EGFR- and ErbB-2-targeting monoclonal antibodies increase the antitumor effects of docetaxel by blocking functional receptors and drug evasion mechanisms (Bijman et al., 2009; Freeman et al., 2009).
Taken together, the aforementioned lines of evidence predict that drugs directly intercepting EGF-like growth factors would likely improve response to chemotherapy and to molecular targeted therapies. We recently described a “tailored” approach that determines which autocrine loops are operating in a specific tumor, and then combines monoclonal antibodies specific to the respective growth factors (Lindzen et al., 2010). Because of the multiplicity of EGF-like ligands and the autocrine/paracrine variation displayed by human tumors, we describe herein an alternative strategy that makes use of a tripartite soluble receptor, denoted TRAP-Fc, comprising portions of the extracellular domains of ErbB-1/EGFR and ErbB-4.
RESULTS
Construction and expression of TRAP-His fusion proteins
Our working hypothesis assumes that by combining the ligand-binding specificities of ErbB-1/EGFR and either ErbB-3 or ErbB-4 one would generate proteins able to sequester the majority, or all EGF-like ligands, respectively, thereby intercept essential autocrine/paracrine loops. As an initial step, we designed four molecular configurations by variably combining the ligand-binding domains of ErbB-1/EGFR, namely domains I, II, III and a part of domain IV, with the respective domains of ErbB-3 or ErbB-4 (Supplementary Fig. S1A). The resulting construct was fused to a six-histidine tag and stably expressed in HEK-293 human kidney cells. Immunoblot analyses detected three of the four fusion proteins in the medium of transfected cells (Supplementary Fig. S1B), and similar analysis of cell lysates confirmed that the TRAP-His not detected in the medium, namely TRAP-His 1–4, was expressed but remained confined to the cytoplasm. Covalent cross-linking of radioactive EGF or NRG1-beta to the TRAP-His molecules validated retention of ligand binding specificities. As expected, all TRAP-His molecules, as well as IgB-1, a molecule comprising the full-length extracellular domain of ErbB-1/EGFR fused to the Fc portion of human immunoglobulin (Chen et al., 1996), could bind EGF, and unlabeled EGF prevented this binding (Fig. S1C). Likewise, TRAP-His proteins 1–3 and 3-1 underwent specific cross-linking to NRG1. Binding of EGF to the TRAP-His molecules was further supported by another experiment that assayed cellular uptake of the radioactive ligand (Fig. S1D); HeLa cells were incubated with 125I-EGF, washed, lysed and the amount of ligand that underwent uptake by the cells was determined. The results we obtained clearly indicated that all secreted TRAP-His molecules decreased uptake of 125I-EGF, in line with an ability to sequester ligands.
Construction, expression and functional tests of a TRAP-Fc protein
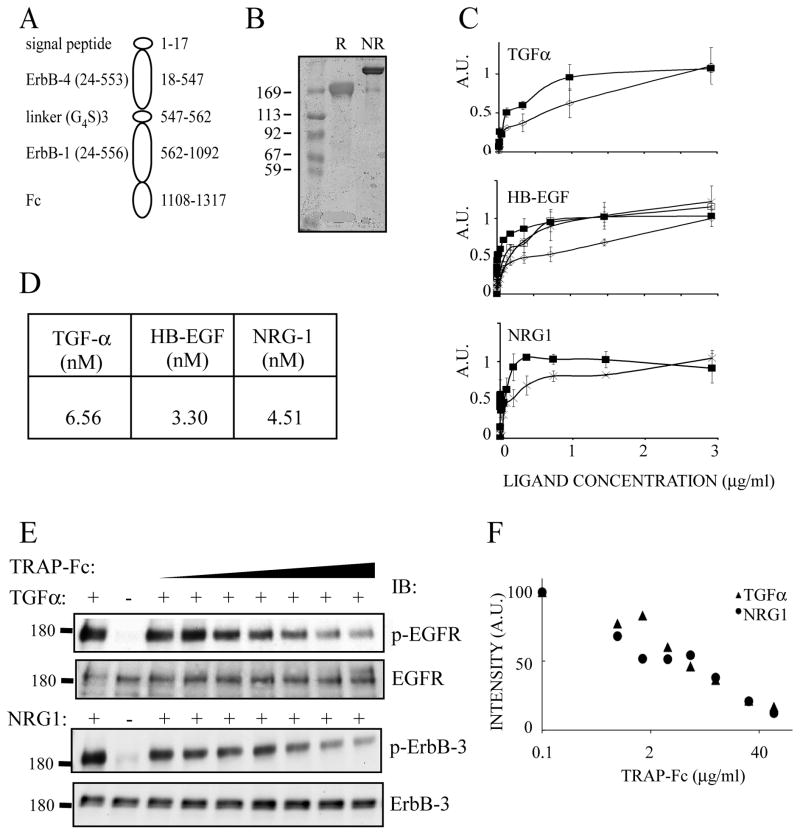
To enhance immunologic compatibility, we replaced the His tag with the Fc domain of human immunoglobulin G. In addition, as ErbB-4 binds more ligands than ErbB-3 and with higher affinity (Riese and Stern, 1998), we used the successfully secreted ErbB-4-ErbB-1 configuration in subsequent studies. The resulting structure combined a signal peptide, flanked by domains I–III, along with a portion of domain IV of ErbB-4, followed by a (Gly4Ser)3 linker domain, and the corresponding part of EGFR (Fig. 1A). First, we verified secretion of TRAP-Fc from transfected HEK-293 human kidney cells. Under reducing conditions, a single immunoreactive species was detectable in the medium (Fig. 1B). By contrast, under non-reducing conditions a larger, major immunoreactive species was detected, confirming that the secreted TRAP-Fc represents a disulfide linked dimer molecule.
Figure 1. Construction, expression and functional tests of TRAP-Fc.
(A) A scheme of the recombinant TRAP-Fc protein, including a signal peptide, the three N-terminal extracellular sub-domains of ErbB-4 called LI (domain I), SI (domain II), LII (domain III) and a portion of SII (domain IV), a linker, followed by the corresponding portion of ErbB-1 linked to human immunoglobulin lambda’s Fc portion. Residue numbers corresponding to ErbB-4 and ErbB-1 appear in parentheses. Other numbers refer to the TRAP’s full sequence. (B) Coomassie blue staining of an acrylamide gel showing the purified TRAP-Fc protein (2μg) following electrophoresis under non-reducing (NR) or reducing (R) conditions. (C) ELISA plates were coated with IgB proteins or with TRAP-Fc (each at 8 μg/ml). Ligand binding and detection using streptavidin-HRP were performed as detailed under Methods. TRAP-Fc, full squares; IgB-1, diamonds; IgB-3, empty squares and IgB-4, crosses. (D) Solutions containing the TRAP-Fc (1–100 nM) were passed over surfaces coated with the indicated ligands, to derive the indicated dissociation constants using plasmon resonance measurements. (E) HeLa (upper panel) and T47D cells (lower panel) were incubated for 10 minutes with the indicated ligands (5 ng/ml) and increasing concentrations of TRAP-Fc (0, 0.8, 1.6, 3.2, 6, 12, 30, 60 μg/ml). Cleared cell extracts were immunoblotted (IB) with the indicated antibodies, including antibodies specific to the tyrosine phosphorylated forms of ErbB-1/EGFR (Tyr-1068) or ErbB-3 (Tyr-1289). (F) Densitometric analyses corresponding to the immunoblots presented in E.
Using TGFα, HB-EGF and NRG1 along with the three IgB molecules that can bind ligands (namely IgB-1, IgB-3 and IgB-4), we confirmed that the IgBs can bind the cognate ligands with affinities similar to those of the intact receptors (Chen et al., 1996), but TRAP-Fc was able to bind all three ligands with apparent affinities similar to or better than the respective IgB molecules (Fig. 1C). Direct determination of binding affinities by surface plasmon resonance (SPR) demonstrated high affinity interactions of the TRAP-Fc with ligands that bind EGFR/ErbB-1 and ErbB-3/4 (Fig. 1D). Notably, the affinities we observed were in agreement with previous reports (Domagala et al., 2000; Fitzpatrick et al., 1998; Jones et al., 1999). Next, we examined the ability of TRAP-Fc to inhibit ligand-induced receptor phosphorylation using either HeLa cells (and TGFα) or, T47D mammary cells (and NRG1). As shown in Figures 1E and 1F, increasing concentrations of TRAP-Fc gradually reduced ligand-induced phosphorylation levels. This observation was extended to additional EGF-like ligands from human and rodent origins (Fig. S2). Altogether, the results we obtained confirmed the ability of TRAP-Fc to effectively sequester multiple growth factors with efficiencies and specificities similar to those displayed by the full-length ectodomains of ErbB-1/EGFR and ErbB-4.
The TRAP-Fc inhibits proliferation of cancer cell lines of different tissue origins
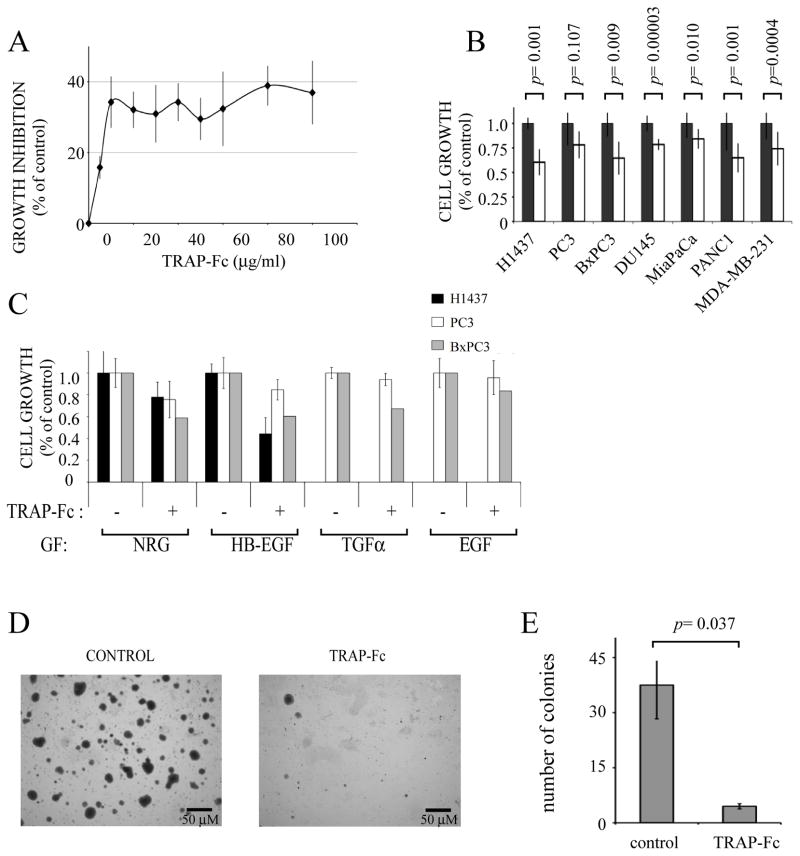
Owing to its presumed ability to sequester all known ErbB ligands, we reasoned that TRAP-Fc would reduce the growth of human cancer cell lines of epithelial origin, which often maintain ErbB-mediated autocrine loops (Witsch et al., 2010). Indeed, dose-dependent inhibition of proliferation, up to 40%, was observed when BxPC3 human pancreatic cells were incubated with increasing concentrations of TRAP-Fc (Fig. 2A). Similar or smaller inhibitory effects were observed with six additional cancer cell lines of mammary, prostate, lung and pancreatic origins (Fig. 2B), but no statistically consistent inhibition of the PC3 prostate cell line was observed. Likewise, TRAP-Fc inhibited proliferation of lung (H1437), pancreatic (BxPC3) and PC3 cells, which were exposed to exogenously added ligands (i.e., NRG1, HB-EGF, TGFα and EGF; Fig. 2C). Similarly, anchorage-independent growth of H1437 lung cancer cells was dramatically decreased in the presence of TRAP-Fc (Figs. 2D and 2E). In conclusion, the recombinant TRAP-Fc molecule we constructed can partially inhibit growth of several carcinoma cells under both autocrine and paracrine settings.
Figure 2. The TRAP-Fc inhibits proliferation of human tumor cells.
(A) BxPC3 pancreatic tumor cells (2×104) were incubated with increasing concentrations of TRAP-Fc. Cell proliferation was determined in hexaplicates after 5 days, using the MTT assay. Averages ± S.D. (bars) are indicated. (B) The indicated cancer cells (2×104) were incubated for 5 days with TRAP-Fc (20 μg/ml) and cell proliferation was determined as in A. (C) H1437 lung tumor cells, PC3 prostate tumor cells and BxPC3 pancreatic tumor cells (each at 2×104 cells per well) were incubated with the indicated ligands (5 ng/ml), along with TRAP-Fc (20 μg/ml), and cell proliferation assayed as in A. (D and E) H1437 cells were seeded in agarose (top layer: 0.3% agar; bottom layer: 0.6% agar) in 6-well plates (1×104 cells per well) and overlaid with medium. TRAP-Fc was added to both the soft agar and to the medium (at 100 μg/ml). Photographs were captured and colony numbers were determined in five randomly chosen high-powered fields. The data represent mean±S.D. The data sets were analyzed for statistically significant differences by using the two-tailed Student t test.
Anti-tumorigenic activities of TRAP-Fc in animal models
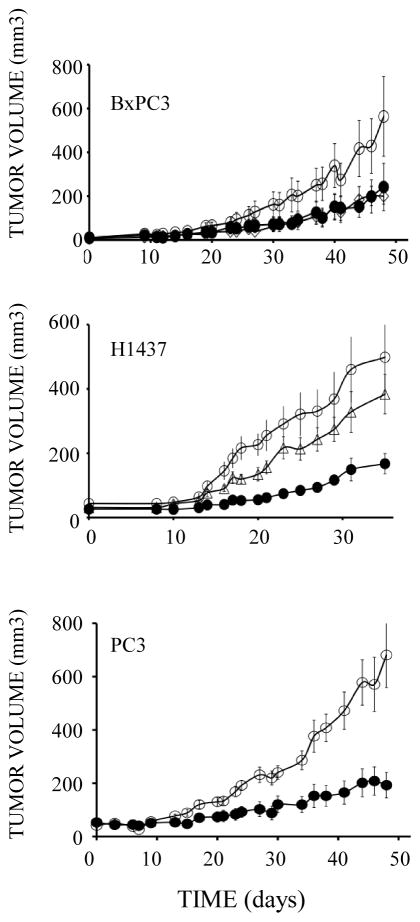
The ability of TRAP-Fc to inhibit growth of a wide spectrum of cultured carcinoma cells predicted an anti-tumorigenic activity in animals. This was assessed using human pancreatic (BxPC3), lung (H1437) and prostate (PC3) xenograft models. Treatment with TRAP-Fc (100 μg per mouse, twice per week) was started only after subcutaneous lesions became palpable. Partial inhibition of all three xenograft models was induced by the decoy receptor (Fig. 3). Moreover, two of the eight BxPC3-treated mice and two of the ten treated PC3 mice completely lost their tumors, but these cases of total regression were excluded from the statistical analyses. It is notable that no significant weight loss was observed in these experiments. For reference, we compared the TRAP-Fc’s inhibitory activity with that of a combination of monoclonal antibodies to TGFα and HB-EGF, growth factors frequently expressed by pancreatic and other tumors. As we previously reported (Lindzen et al., 2010), treatment with the combination of antibodies partially inhibited tumorigenic growth of BxPC3 and H1437 cells, and in the latter model the inhibitory effect of the decoy was even greater. In conclusion, the TRAP-Fc protein is endowed with an anti-tumorigenic activity towards several different human xenograft models.
Figure 3. TRAP-Fc inhibits tumorigenic growth of human cancer cells in mice.
Female nude mice (6 week old) were inoculated subcutaneously with the indicated human tumor cells (2×106 per animal). Once tumors became palpable, mice were randomized into three groups and injected intraperitoneally with TRAP-Fc (100 μg; closed circles), or with a combination of anti-TGFα and anti HB-EGF mAbs (each at 250 μg/mouse, open triangle; BxPC3 and H1437 cells only). Mice bearing BxPC3 xenografts were injected on days: 9, 16, 20, 23, 26, 30, 33, 37, 40 and 44. Both the control and the treatment group included 8 mice. Note: two mice of the treatment group completely lost their tumors and were not included in the results. The mAb group included 3 mice. Mice bearing H1437 xerografts were injected on days 6, 10, 14, 18 and 21. The control group included 15 mice, the TRAP-Fc-treated group included 7 mice and the mAb group included 11 mice. Mice bearing PC3 xenografts were injected on days 1, 3, 6, 9, 13, 17, 20, 23, 27 and 30. The control group included 11 mice and the TRAP-Fc-treated group included 10 mice (two of them lost their tumors and are not represented).
TRAP-Fc can sensitize pancreatic tumor cells to both molecular targeted drugs and chemotherapeutic agents
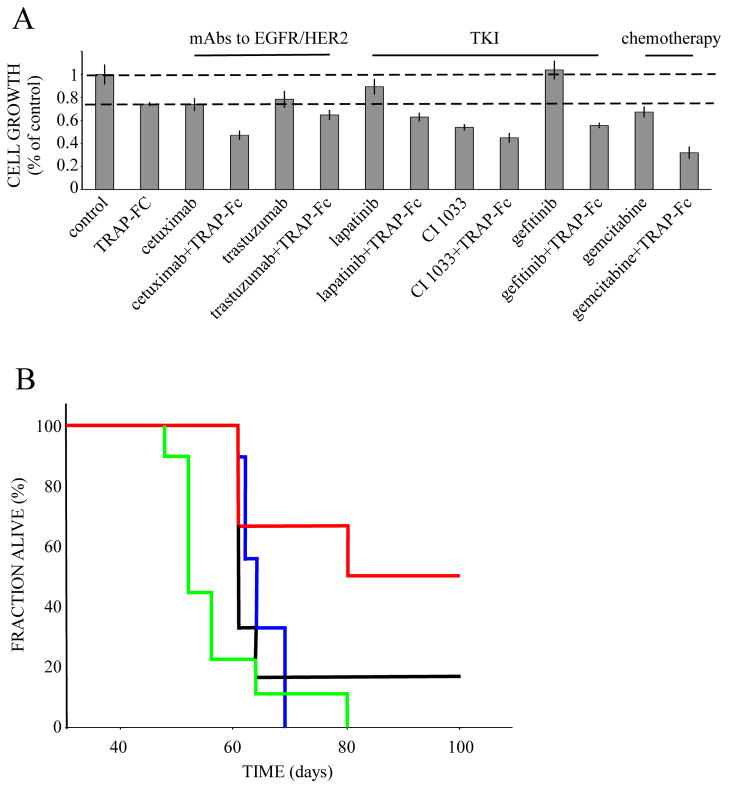
Self-produced growth factors may play essential roles in acquired resistance to chemo-and targeted therapies (Bianchi et al., 2006; Engelman et al., 2007; Karamouzis et al., 2007; Ritter et al., 2007). Hence, blocking such autocrine loops may re-sensitize tumors to specific drugs. As an initial test of this scenario, we examined in vitro the combined effect on pancreatic tumor cells of TRAP-Fc and each of the following agents: mAbs specific to EGFR or to HER2, ErbB-specific TKIs, and gemcitabine. The results presented in Figure 4A verified the ability of the TRAP-Fc molecule to augment the inhibitory effects of the EGFR- and HER2- targeting mAbs, namely, cetuximab and trastuzumab, respectively. Combining TRAP-Fc with the TKIs lapatinib, CI-1033 and gefitinib, also resulted in statistically significant additive effects.
Figure 4. Combinations of TRAP-Fc and therapeutic agents effectively inhibit growth of human pancreatic cancer cells in vitro and extend survival of tumor-bearing mice.
(A) BxPC3 pancreatic tumor cells (2×104) were incubated with TRAP-Fc (30 μg/ml), either alone or in combination with cetuximab (20 μg/ml), trastuzumab (20 μg/ml), lapatinib (0.05 nM), erlotinib (0.2 nM), CI-1033 (0.5 nM), AG1478 (0.2 nM), gefitinib (0.004 nM), or gemcitabine (at 0.5 ng/ml). Cellular proliferation was measured in hexaplicates after 5 days, using the MTT assay. The experiment was repeated twice. (B) Female nude mice (6-week old) were inoculated subcutaneously with BxPC3 pancreatic cancer cells (2×106). Once tumors became palpable, mice were randomized into four groups. The control group (9 mice; blue) is shown. The TRAP-Fc-treated group (6 mice; black line) was injected intraperitoneally with TRAP-Fc (100 μg). A third group (9 mice) was treated with gemcitabine (intraperitoneal injection, 25 mg/kg; green line), and a fourth group (6 mice; red) was treated with a combination of gemcitabine and TRAP-Fc. Mice were injected with TRAP-Fc on days: 19, 28, 32, 35, 39, 42, and 45. Gemcitabine injections were given on the same days and repeated three more times on days: 49, 52 and 55. Body weights were measured once a week, but no consistent differences were observed (data not shown).
Notably, the largest effect was observed when we combined TRAP-Fc with gemcitabine, the mainstay single-agent drug of advanced pancreatic tumors (Mackenzie and McCollum, 2009). Hence, we addressed the effect of the combined treatment in an animal model of pancreatic cancer. BxPC3 cells were injected subcutaneously and allowed to grow until palpable tumors appeared. Thereafter, mice were randomized into four groups, which received no treatment, TRAP-Fc alone (administered for the first 3 weeks only), gemcitabine alone, or the combination. Kaplan-Meier analysis of animal survival showed that the combined treatment led to a statistically significant prolongation of survival: the median (95% CI) survival of the control group was 52±1.5 days (blue line). Treatment with TRAP-Fc (black line) or gemcitabine alone (green line) moderately prolonged survival to 61 (p=0.071) and 64 (p=0.103) days, respectively, and the combination resulted in the longest prolongation of median survival, 80 days (p=0.006) (red line). In conclusion, the TRAP-Fc molecule exhibits anti-tumor activity when tested in animals, and this activity can be enhanced when combined with an established chemotherapeutic agent.
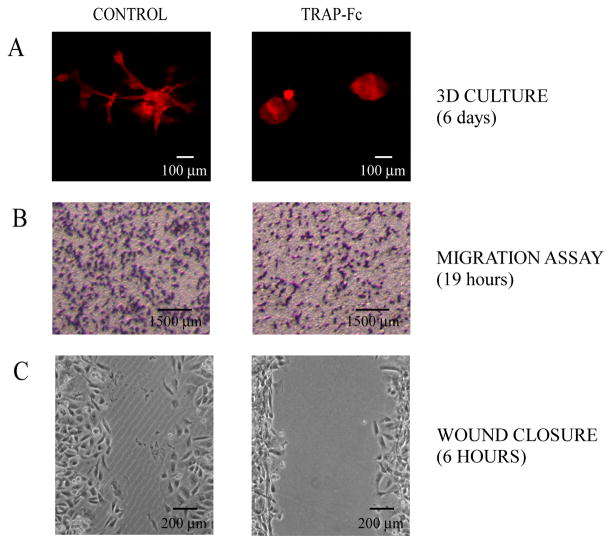
TRAP-Fc inhibits invasive growth of mammary tumor cells
ErbB signaling has been implicated in invasion and metastasis of cancer cells (De Luca et al., 2008). To test the TRAP-Fc’s effect on the ability of cancer cells to invade through tissue barriers, we used the highly metastatic MDA-MB-231 human breast cancer cell line. When plated in a natural preparation of extracellular matrix (Matrigel™), MDA-MB-231 cells tend to invade into the surrounding matrix by growing long and branched extensions (Fig. 5A, left panel). However, when the TRAP-Fc was added to their medium, the cells exhibited a round, non-invasive morphology (Fig. 5A, right panel). We further confirmed the inhibitory effect of TRAP-Fc on cell migration using a dual chamber Transwell tray. Cells were allowed to migrate through pores within a nitrocellulose filter, which separates the chambers. Thereafter, cells that migrated and adhered to the bottom of the filter were photographed, and the percentage of the area they covered was quantified (Fig. 5B). The results we obtained in two different experiments indicated that migration of TRAP-Fc-treated cells was inhibited by 27±7%.
Figure 5. TRAP-Fc inhibits invasive growth of MDA-MB-231 mammary tumor cells in vitro.
(A) Eight-chambered plates were coated with an extracellular matrix (Matrigel™). MDA-MB-231 cells (2,000 cells/well) were mixed with medium containing Matrigel™ and then added to the chambers. Cells were incubated without or with TRAP-Fc (30 μg/ml), and phase contrast photomicrographs were captured six days later. (B) MDA-MB-231 cells (1.5×105) were incubated in Transwell chambers in the absence or presence of TRAP-Fc (30 μg/ml). Cell migration across the filter separating the two compartments was measured 19 hours later and representative photographs of the lower faces of the filters were taken. Migration was normalized to the input number of cells. (C) MDA-MB-231 cells were plated on wound-healing inserts. Twenty-four hours later, plugs were removed, cells were treated with TRAP-Fc (100 μg/ml) and allowed to migrate. Snapshots captured after 6 hours are presented.
The “wound closure” (scratch) assay was used to further test the TRAP-Fc’s ability to inhibit collective cell migration of MDA-MB-231 cells. The cells were plated into two compartments separated by an insert, resulting within 24 hours in a confluent, but split monolayer. Thereafter, the inserts were removed, TRAP-Fc was added, and cells were allowed to migrate. Snapshots taken after 6 hours are presented in Figure 5C. By this time, the residual wound areas differed significantly: control cells covered approximately 71.21±0.93% of the gap area, while TRAP-Fc-treated cells covered 44.73±2.60% (Fig. 5C), in line with an ability of TRAP-Fc to inhibit collective cell migration.
TRAP-Fc exhibits anti-metastasis activities in an animal model
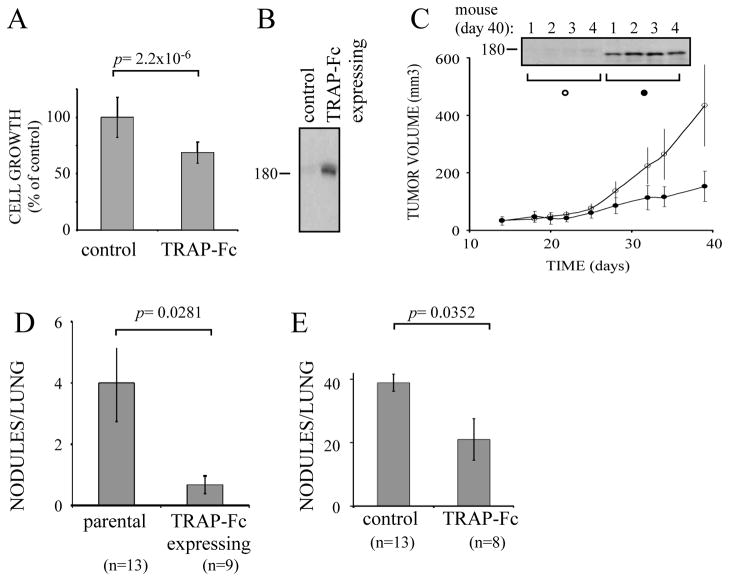
To examine the effect of the TRAP-Fc on metastasis, we employed MDA-MB-231 cells, which were initially tested in vitro using a cell proliferation assay. As expected, when incubated with TRAP-Fc, the cells exhibited a partial growth inhibitory effect (Fig. 6A). Hence, we transfected a TRAP-Fc-encoding plasmid into the MDA-MB-231 cells and confirmed expression of the decoy by immunoblotting (Fig. 6B). In the next step, these cells were implanted subcutaneously in the flanks of mice, and both TRAP-Fc concentrations and tumor size were monitored. As expected, persistently high concentrations of tumor’s TRAP-Fc correlated with effective growth inhibition (Fig. 6C), which led us to examine the ability of the ectopically expressed decoy to modulate metastasis to the lungs.
Figure 6. TRAP-Fc inhibits lung metastasis of MDA-MB-231 mammary cancer cell.
(A) MDA-MB-231 cells (2×104) were treated with TRAP-Fc (30 μg/ml) and proliferation was determined in hexaplicates after 5 days, using the MTT assay. (B) Conditioned media collected from parental cells and from MDA-MB-231 cells stably expressing the TRAP-Fc protein were immunoblotted (IB) with an anti-EGFR antibody. (C) Parental MDA-MB-231 and TRAP-Fc-expressing cells (2.5×106) were inoculated into the mammary fat pad of scid mice (6 and 7 mice, respectively). Tumor size was monitored twice a week. Average tumor sizes and standard deviations (bars) are shown. In the end of the experiment (day 40) tumors were removed from 4 mice of each group, homogenized, electrophoresed and immunoblotted with an anti-EGFR antibody. Representative blots are shown. (D) Parental MDA-MB-231 and TRAP-Fc-expressing cells (2.5×106) were injected into the tail vein of scid mice. Nodules in the lungs were counted at day 60 and their averages presented. The control group included 13 mice, and the TRAP-expressing group included 9 mice. (E) MDA-MB-231 cells (1.5×105) were injected into the tail vein of scid mice. TRAP-Fc (100 μg per injection) was injected intraperitoneally (8 mice) on days 1, 3, 6 and 9. Nodules in the lungs were counted at day 60. The control group included 13 mice.
Parental and TRAP-Fc-expressing MDA-MB-231 cells were injected into the tail vein of SCID mice, and two months later lung metastases were assessed by counting the number of nodules. Compared to the parental cells, the TRAP-Fc-secreting MDA-MB-231 derivatives displayed an 80% reduction in lung metastasis (Fig. 6D). This anti-metastasis effect was verified by using a recombinant TRAP-Fc and the parental MDA-MB-231 cells. Cells were injected into the tail vein of SCID mice, and half of the mice were additionally injected intraperitoneally with the TRAP-Fc protein (100μg per injection) on days 0, 3, 6 and 9. Two months later, we assessed metastasis by counting nodules in the lung. Consistent with the results obtained with the stably expressing clones of MDA-MB-231 cells, the injected TRAP-Fc reduced metastasis to the lung by 46% (Fig. 6E).
In summary, by using human pancreatic, lung, prostate and breast xenografts we obtained evidence supporting the notion that sequestering multiple EGF-like ligands may bear therapeutic significance in vivo. The therapy we propose might be especially useful in terms of sensitizing tumors to cytotoxic regimens and delaying onset of chemoresistance. Our results also demonstrate an anti-metastatic activity of the TRAP-Fc molecule in animals, an observation that might be important in clinical settings.
DISCUSSION
Early studies indicated that ligand-blocking anti-receptor antibodies can sensitize tumors to chemotherapy (Aboud-Pirak et al., 1988), a finding that was later translated to combination therapies of colorectal cancer patients and head and neck cancer patients (Baselga, 2006). These considerations have lead to the prediction that sequestration of growth factors would bear therapeutic benefits. Several pre-clinical attempts to construct and employ decoy receptors have been reported. A truncated ErbB-1/EGFR sequence fused to a human immunoglobulin domain, designated sEGFR501.Fc, exhibited high affinity for a number of EGFR ligands, but administration of sEGFR501.Fc to mice bearing human xenografts resulted in weak retardation of tumor growth (Adams et al., 2009). Similarly, moderate to high tumor-inhibitory effects have been reported by a study that separately fused to Fc the full-length extracellular domains of ErbB-1/EGFR and ErbB-3 (Sarup et al., 2008). It is notable that this strategy results in a mixture of ErbB-1 homodimers, ErbB-3 homodimers, and the respective heterodimers, denoted (HER-1:HER-3)Fc.
The TRAP-Fc protein we constructed combines into a single molecular entity the binding properties of both ErbB-1 and ErbB-4. It is notable that the spectrum of EGF-like ligands that bind with ErbB-4 is wider than the ErbB-3’s spectrum, and, in addition, ErbB-4 binds NRGs with higher affinity (Riese and Stern, 1998). Hence, our TRAP-Fc is expected to exhaustively deplete all soluble growth factors of the EGF family. Notably, like sEGFR501.Fc and (HER-1:HER-3)Fc, TRAP-Fc makes use of an Fc domain. In addition, the TRAP-Fc molecule, in similarity to (HER-1:HER-3)Fc, makes use of two, rather than one receptor, but unlike the full length receptors that comprise (HER-1:HER-3)Fc, truncated forms have been used to construct both TRAP-Fc and sEGFR501.Fc. The crystal structures of the ectodomains of both ErbB-1 and ErbB-4 suggest an inhibitory contribution of the distal extracellular subdomain IV, as directly shown for ErbB-1/EGFR (Elleman et al., 2001). For these and other reasons, TRAP-Fc may open new pharmacological opportunities in terms of the challenges imposed by ligand multiplicity, tumor-specific distinct repertoires of ligands, and the roles played by EGF family growth factors in frequent emergence of resistance to chemotherapy.
Potentially, anti-ligand antibodies would deplete specific growth factors, in similarity to TRAP-Fc. Although no antibody to an EGF-like molecule has so far been approved for clinical applications, it is notable that an HB-EGF inhibitor (CRM197) is active both as a single agent and in combination with other anticancer agents (Sanui et al., 2010; Tsujioka et al., 2011), and bevacizumab, a therapeutic antibody used to treat colorectal and other tumors, can intercept the vascular endothelial growth factor (VEGF). In contrast to the VEGF family, the greater multiplicity of the EGF family ligands calls for a more general approach, such as the “tailor made” strategy we developed (Lindzen et al., 2010). This strategy employs combinations of monoclonal antibodies to two or more growth factors, which are secreted by specific tumors. Side-by-side comparison of this approach and TRAP-Fc suggests comparable, if not superior, efficacy of TRAP-Fc (Fig. 3). However, unlike the “tailoring” of distinct antibody combinations to specific tumors, application of the TRAP-Fc requires no pre-determination of the identity of secreted growth factors. In addition, this strategy may better target tumors whose spectrum of secreted ligands undergoes dynamic changes in response to the metastatic niche, or under cellular stress conditions imposed by irradiation or chemotherapy.
The binding constants of TRAP-Fc are comparable to those displayed by the full-length receptors (Domagala et al., 2000; Fitzpatrick et al., 1998; Jones et al., 1999). This may explain the in vitro growth-inhibitory activity of TRAP-Fc toward breast, lung, prostate and pancreatic tumor cells, as well as the anti-tumor activities we observed using three xenograft models of pancreas, lung and prostate cancer (Fig. 3). Along the same vein, wide-spectrum ligand sequestration explains the ability of TRAP-Fc to inhibit invasive growth of tumor cells both in vitro and in animals (Figs. 5 and 6). EGF family growth factors are considered major mediators of epithelial cell invasion across tissue barriers. For example, a comparison between primary lung tumors and the corresponding brain metastases showed significantly higher expression of EGF, amphiregulin, phosphorylated EGFR and phosphorylated ErbB-3 in the metastases (Sun et al., 2009). Likewise, constitutive activation of ErbB-3 by an autocrine NRG1 contributes to intrahepatic metastasis and to early recurrence of hepatocellular carcinoma (Hsieh et al., 2011). Our study made use of the highly metastatic MDA-MB-231 breast cancer cell line and found that TRAP-Fc inhibited migration and invasive growth, which translated to a significant reduction in lung metastasis in treated animals.
The ability of the TRAP-Fc to collaborate with currently approved cancer drugs (Fig. 4) is yet another indication that the decoy molecule would retard late stage tumors and reverse resistance to chemotherapy, as well as to molecular targeted therapies. The latter (e.g., kinase inhibitors and anti-receptor antibodies) often elicit resistance due to up-regulation of either alternative ErbB family members (Bianchi et al., 2006; Engelman et al., 2007; Karamouzis et al., 2007; Ritter et al., 2007) or certain EGF family ligands (Ishikawa et al., 2005; Valabrega et al., 2005; Wheeler et al., 2008; Zhou et al., 2006). Hence, depletion of soluble growth factors in the vicinity of heavily treated tumors and metastases may block escape pathways and delay resistance onset. Although TRAP-Fc comprises only human-derived amino acid sequences, and the animals we treated showed no remarkable adverse effects, more rigorous toxicology studies are mandatory for examination of the true therapeutic potential of TRAP-Fc in clinical settings.
MATERIALS AND METHODS
Reagents, cells and plasmids
Growth factors were from PeproTech Asia (Rocky Hill, NJ). ATBS (2, 2′-Azino-bis (3-ethylbenzthiozoline-6-sulfonic acid) and MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were purchased from Sigma (St. Louis, MO) and Matrigel from BD Biosciences (San Jose, CA). Trastuzumab was purchased from Roche Diagnostics GmbH (Mannheim, Germany), Cetuximab from Merck-Serono (Darmstadt, Germany) and Gemcitabine from Eli Lilly (Hampshire, England). Antibodies to specific phosphotyrosines were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase (HRP)-conjugated anti-mouse antibody was purchased from Jackson Immuno Research Laboratories (West Grove, Pennsylvania). Human cancer cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in DME (HeLa, T47D, HEK-293, Panc-1, MiaPaCa and PC3 cells) or RPMI medium (BxPC3, MDA-MB-231, DU145 and H1437) supplemented with 10% fetal calf serum. CD293 serum-free medium was purchased from GIBCO (Invitrogen, Carlsbad, CA). DNA sequences encoding the different TRAP molecules were custom synthesized (Invitrogen, Carlsbad, CA) and cloned into pIRES or pcDNA3 plasids.
Expression and purification of soluble proteins
HEK-293 and MDA-MB-231 cells were transfected with expression plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, Ca). Conditioned media from drug-selected TRAP-His expressing clones were centrifuged, filtered and imidazole (5 mM) added just before application to a NiNTA column. The column was extensively washed (20 mM Hepes, pH 7.5, 1 M NaCl, 1% Tween 20) and bound proteins eluted with 0.5 M immidazole in saline. Conditioned media from IgB and TRAP-Fc-expressing cells were similarly applied to a column packed with Protein A beads (2 ml). The column was washed and adsorbed proteins eluted with 0.1 M glycine solution (pH 2.7). TRAP-Fc- expressing cells were grown in serum free CD293 medium at 32°C.
Cross-linking with BS3
Recombinant molecules were incubated with 125I-EGF or 125I-NRG1 (each at 1.5 ng/ml) in the presence or absence of unlabeled EGF or NRG1 (each at 3.3 mg/ml), and 2 mM BS3 (from Pierce, Rockford, IL). The cross-linking reaction was stopped after a 60 min long incubation at 4°C, by adding glycine (50 mM).
Surface Plasmon Resonance (SPR)
SPR was performed on a BIAcore 3000 instrument (BIAcore, Uppsala, Sweden). Proteins were diluted in 100 mM Na-acetate (pH 4.6) to 50μg/ml and immobilized on a CM5 sensor chip. The protein solution was then run over the chip for 5 minutes at a rate of 10 ml/min. The binding assay was performed by injecting the analyte solutions at 12 different concentrations (1–100 nM) at a flow rate of 20 ml/min at 25°C. These conditions resulted in a linear relation between protein concentration and maximal (steady-state) response, indicating a pseudo first-order regime in relation to the immobilized ligand. The net signal was obtained by subtracting the blank signal (dextran matrix). The association phase for analyte binding to all ligands was followed for 4 min, and dissociation phases were monitored for 3 min. The response was monitored as a function of time (sensorgram) at 25°C. Multi-concentration data were fit using BIAevaluation 3.2 software.
Ligand binding assays
Ninety-six well plates were coated with solutions of the recombinant proteins (8 μg/ml) by incubating overnight at room temperature. Plates were washed, blocked for 1 hour with saline containing 1% albumin and 0.05% Tween-20, followed by a two-hour incubation with increasing concentrations of the indicated ligands. After washing, the plates were incubated for 2 hours with avidin-bound antibody that targeted a ligand epitope, followed by a 20-minute incubation with biotin-HRP. Subsequently, the plates were incubated with ATBS (Sigma, St. Louis, MO). Signals were determined using an ELISA reader (420 nm).
Cell proliferation and anchorage-independent growth assays
Cells were plated in hexaplicates in 96-well plates (2,000 cells/well). Cell proliferation was assayed after 24, 48 or 72 hours using the MTT method. To assay anchorage-independent growth, H1437 lung cancer cells were seeded in 6-well plates (104 cells per well), within a top layer of 0.3% agar and a bottom layer of 0.6% agar. TRAP-Fc (100 μg/ml) was added at the beginning of the incubation. During the 14 days of incubation the dishes were checked every day and media replenished. The assay was repeated twice in triplicates.
Determination of anti-tumor activities in animals
Female athymic NCr-nude mice (6-week old) were inoculated subcutaneously with 2×106 human cancer cells. Alternatively, MDA-MB-231 cells (2.5×106) and their derivatives were inoculated into the mammary fat pad of CD-1-NU mice (10 weeks old). Once tumors became palpable (5–7 days), the mice were randomized into groups and injected intraperitoneally at the indicated time points with the recombinant proteins, chemotherapy or various combinations. Tumor volumes were monitored twice a week, and body weights were measured once a week. Where indicated, tumors were removed, homogenized in SBN buffer (1 mM EDTA pH 7.5, 50 mM Tris-HCl, 150 mM NaCl, 10% Glycerol, 1% NP-40) and electrophoresed.
Morphogenesis assay in 3D culture
Trypsin-treated cells were re-suspended in DME/F12 medium supplemented with 4% horse serum, insulin, cholera toxin and hydrocortisone. Eight-chambered plates (BD Biosciences) were coated with 50 μl Matrigel™ solution (Growth Factor Reduced Matrigel from BD Biosciences, San Jose, CA) per well. MDA-MB-231 cells (2,000 per well) were mixed 1:1 with medium (4% serum) containing 25% Matrigel™ and added to chambers.
Cell migration assays
MDA-MB231 cells (1.5×105 cells per well) were plated in the upper compartment of a 24-well Transwell tray (Corning, Acton, MA). Cells were allowed to migrate through the intervening nitrocellulose membrane (8μm pore size) during 19 hours of incubation at 37°C. The filter was removed, fixed for 15 minutes in saline containing paraformaldehyde (3%), and cells were permeabilized in Triton-X-100 (0.05% in saline) and stained with methyl violet. Cells growing on the upper side of the filter were scraped using cotton swabs, whereas cells growing on the bottom side of the membrane were photographed and counted.
Wound closure assays
Wound closure assays were performed using iBidi chambers, according to the manufacture’s protocol (iBidi, München, GmbH). Briefly, MDA-MB-231 cells were trypsinized, re-suspended and plated into each well (6.0 × 105 cells/well), resulting in a confluent layer within 24 hours. Thereafter, the Culture–Insert from iBidi was removed and cells were allowed to migrate for 6 hours.
Determination of anti-metastasis activities
MDA-MB-231 cells (1.5×105) were injected into the tail vein of female Scid c.b-17/ICR mice (6 week old). TRAP-Fc injections were given twice a week in the first two weeks. Sixty days later, the mice were sacrificed, their lungs removed and nodules were counted.
Supplementary Material
Acknowledgments
We acknowledge the help of the Israel Structural Proteomics Center. Our lab is supported by the National Cancer Institute (CA072981), the Israel Cancer Research Fund and Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. WJK received a fellowship for Ph.D. track for specialist medical doctors from the Linda and Michael Jacobs Charitable Trust. Y.Y. is the incumbent of the Harold and Zelda Goldenberg Professorial Chair.
References
- Aboud-Pirak E, Hurwitz E, Pirak ME, Bellot F, Schlessinger J, Sela M. Efficacy of antibodies to epidermal growth factor receptor against KB carcinoma in vitro and in nude mice. J Natl Cancer Inst. 1988;80:1605–11. doi: 10.1093/jnci/80.20.1605. [DOI] [PubMed] [Google Scholar]
- Adams TE, Koziolek EJ, Hoyne PH, Bentley JD, Lu L, Lovrecz G, et al. A truncated soluble epidermal growth factor receptor-Fc fusion ligand trap displays anti-tumour activity in vivo. Growth Factors. 2009;27:141–54. doi: 10.1080/08977190902843565. [DOI] [PubMed] [Google Scholar]
- Atlas E, Cardillo M, Mehmi I, Zahedkargaran H, Tang C, Lupu R. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–75. [PubMed] [Google Scholar]
- Barozzi C, Ravaioli M, D’Errico A, Grazi GL, Poggioli G, Cavrini G, et al. Relevance of biologic markers in colorectal carcinoma: a comparative study of a broad panel. Cancer. 2002;94:647–57. doi: 10.1002/cncr.10278. [DOI] [PubMed] [Google Scholar]
- Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–8. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Palli D, Falchetti M, Saieva C, Masala G, Mancini B, et al. ErbB-receptors expression and survival in breast carcinoma: a 15-year follow-up study. J Cell Physiol. 2006;206:702–8. doi: 10.1002/jcp.20535. [DOI] [PubMed] [Google Scholar]
- Bijman MN, van Berkel MP, Kok M, Janmaat ML, Boven E. Inhibition of functional HER family members increases the sensitivity to docetaxel in human ovarian cancer cell lines. Anticancer Drugs. 2009;20:450–60. doi: 10.1097/CAD.0b013e32832afc24. [DOI] [PubMed] [Google Scholar]
- Britten CD. Targeting ErbB receptor signaling: a pan-ErbB approach to cancer. Mol Cancer Ther. 2004;3:1335–42. [PubMed] [Google Scholar]
- Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, et al. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–9. [PubMed] [Google Scholar]
- De Jong KP, Stellema R, Karrenbeld A, Koudstaal J, Gouw AS, Sluiter WJ, et al. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology. 1998;28:971–9. doi: 10.1002/hep.510280411. [DOI] [PubMed] [Google Scholar]
- De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- Domagala T, Konstantopoulos N, Smyth F, Jorissen RN, Fabri L, Geleick D, et al. Stoichiometry, kinetic and binding analysis of the interaction between epidermal growth factor (EGF) and the extracellular domain of the EGF receptor. Growth Factors. 2000;18:11–29. doi: 10.3109/08977190009003231. [DOI] [PubMed] [Google Scholar]
- Elleman TC, Domagala T, McKern NM, Nerrie M, Lonnqvist B, Adams TE, et al. Identification of a determinant of epidermal growth factor receptor ligand-binding specificity using a truncated, high-affinity form of the ectodomain. Biochemistry. 2001;40:8930–9. doi: 10.1021/bi010037b. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick VD, Pisacane PI, Vandlen RL, Sliwkowski MX. Formation of a high affinity heregulin binding site using the soluble extracellular domains of ErbB2 with ErbB3 or ErbB4. FEBS Lett. 1998;431:102–6. doi: 10.1016/s0014-5793(98)00737-6. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Bush T, Ogbagabriel S, Belmontes B, Juan T, Plewa C, et al. Activity of panitumumab alone or with chemotherapy in non-small cell lung carcinoma cell lines expressing mutant epidermal growth factor receptor. Mol Cancer Ther. 2009;8:1536–46. doi: 10.1158/1535-7163.MCT-08-0978. [DOI] [PubMed] [Google Scholar]
- Grandis JR, Chakraborty A, Zeng Q, Melhem MF, Tweardy DJ. Downmodulation of TGF-alpha protein expression with antisense oligonucleotides inhibits proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. J Cell Biochem. 1998;69:55–62. [PubMed] [Google Scholar]
- Hsieh SY, He JR, Hsu CY, Chen WJ, Bera R, Lin KY, et al. Neuregulin/erythroblastic leukemia viral oncogene homolog 3 autocrine loop contributes to invasion and early recurrence of human hepatoma. Hepatology. 2011;53:504–16. doi: 10.1002/hep.24083. [DOI] [PubMed] [Google Scholar]
- Ishikawa N, Daigo Y, Takano A, Taniwaki M, Kato T, Hayama S, et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005;65:9176–84. doi: 10.1158/0008-5472.CAN-05-1556. [DOI] [PubMed] [Google Scholar]
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447:227–31. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Karamouzis MV, Badra FA, Papavassiliou AG. Breast cancer: the upgraded role of HER-3 and HER-4. Int J Biochem Cell Biol. 2007;39:851–6. doi: 10.1016/j.biocel.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Krane IM, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996;12:1781–8. [PubMed] [Google Scholar]
- Lindzen M, Lavi S, Leitner O, Yarden Y. Tailored cancer immunotherapy using combinations of chemotherapy and a mixture of antibodies against EGF-receptor ligands. Proc Natl Acad Sci U S A. 2010;107:12559–63. doi: 10.1073/pnas.1006218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Rev Anticancer Ther. 2009;9:1473–85. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–8. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Sanui A, Yotsumoto F, Tsujioka H, Fukami T, Horiuchi S, Shirota K, et al. HB-EGF inhibition in combination with various anticancer agents enhances its antitumor effects in gastric cancer. Anticancer Res. 2010;30:3143–9. [PubMed] [Google Scholar]
- Sarup J, Jin P, Turin L, Bai X, Beryt M, Brdlik C, et al. Human epidermal growth factor receptor (HER-1:HER-3) Fc-mediated heterodimer has broad antiproliferative activity in vitro and in human tumor xenografts. Mol Cancer Ther. 2008;7:3223–36. doi: 10.1158/1535-7163.MCT-07-2151. [DOI] [PubMed] [Google Scholar]
- Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–80. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res. 2009;15:4829–37. doi: 10.1158/1078-0432.CCR-08-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thogersen VB, Sorensen BS, Poulsen SS, Orntoft TF, Wolf H, Nexo E. A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res. 2001;61:6227–33. [PubMed] [Google Scholar]
- Tsujioka H, Yotsumoto F, Hikita S, Ueda T, Kuroki M, Miyamoto S. Targeting the heparin-binding epidermal growth factor-like growth factor in ovarian cancer therapy. Curr Opin Obstet Gynecol. 2011;23:24–30. doi: 10.1097/GCO.0b013e3283409c91. [DOI] [PubMed] [Google Scholar]
- Valabrega G, Montemurro F, Sarotto I, Petrelli A, Rubini P, Tacchetti C, et al. TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene. 2005;24:3002–10. doi: 10.1038/sj.onc.1208478. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Borghaei H. Targeted therapies in solid tumors: monoclonal antibodies and small molecules. Hum Antibodies. 2006;15:103–11. [PubMed] [Google Scholar]
- Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–56. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.