Abstract
Background
Auditory mismatch negativity (MMN) and P300 event related potentials (ERP) are reduced in schizophrenia patients, and healthy volunteers administered the N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, ketamine. In rodents, N-acetylcysteine (NAC), a stimulator of the cystine-glutamate exchanger, attenuates the cognitive and behavioral effects of NMDA receptor antagonists. Based on these findings, we tested whether NAC would reduce ketamine effects on behavior, MMN, and P300 in healthy humans.
Methods
This randomized, double-blind, placebo-controlled study consisted of two test days during which subjects (N=16) were administered oral NAC (3000 mg in divided doses) or matching placebo 165 minutes prior to the infusion of saline and then ketamine (as a bolus of 0.23 mg/kg over one minute followed by 0.58 mg/kg for 30 min, and then 0.29 mg/kg for 40 min) in a fixed order. Behavioral and ERP data including auditory MMN and P300 were collected during each test day.
Results
Ketamine produced psychotic-like positive symptoms, reductions in working memory and sustained attention performance, and amplitude reductions for the frequency- and intensity-deviant MMNs and P300. NAC pretreatment did not reduce the behavioral or ERP effects of ketamine. In addition, NAC reduced frequency-deviant MMN amplitude and increased target and novelty P3 amplitudes. The decrements in frequency-deviant MMN amplitude produced by ketamine and NAC were not additive.
Conclusions
In contrast to previous studies in animals, NAC did not attenuate the effects of ketamine in humans. NAC merits further investigation as a cognitive enhancing agent due to its ability to increase the P300 amplitude.
Keywords: NMDA, glutamate, N-acetylcysteine, P300, MMN, ketamine
Introduction
The noncompetitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist ketamine produces cognitive and behavioral effects that bear resemblance to the features of schizophrenia (1–3). Several event related potentials (ERP) that are reduced in schizophrenia, including mismatch negativity (MMN) (4, 5) and P300 (6–11) appear sensitive to the effects of ketamine (12–15).
ERPs provide a quantitative assessment of neural activity. MMN is a negative voltage deflection in the auditory ERP that peaks around 100–150 ms following any discriminable deviant sound occurring during a series of repeated standard sounds (16). The MMN is automatically elicited by deviant sounds, even when attention is directed away from the auditory channel.
The P300 is a positive voltage deflection that peaks around 300 ms after the presentation of an infrequent target, novel, or otherwise salient stimulus. P300 amplitude is thought to reflect attentional resource allocation (17, 18), phasic attentional shifts (19), working memory updating of stimulus context (20, 21) or stimulus salience (22, 23). Its latency is thought to reflect processing speed or efficiency during stimulus evaluation (24). P3b is the P300 elicited by infrequent task-relevant target stimuli and reflects top-down allocation of attentional resources with a parietal scalp maximum. P3a is the P300 elicited by infrequent task-irrelevant deviant stimuli, which are either novel or otherwise salient (25–27). It reflects “bottom-up” orienting of attentional resources with a fronto-central scalp maximum (28).
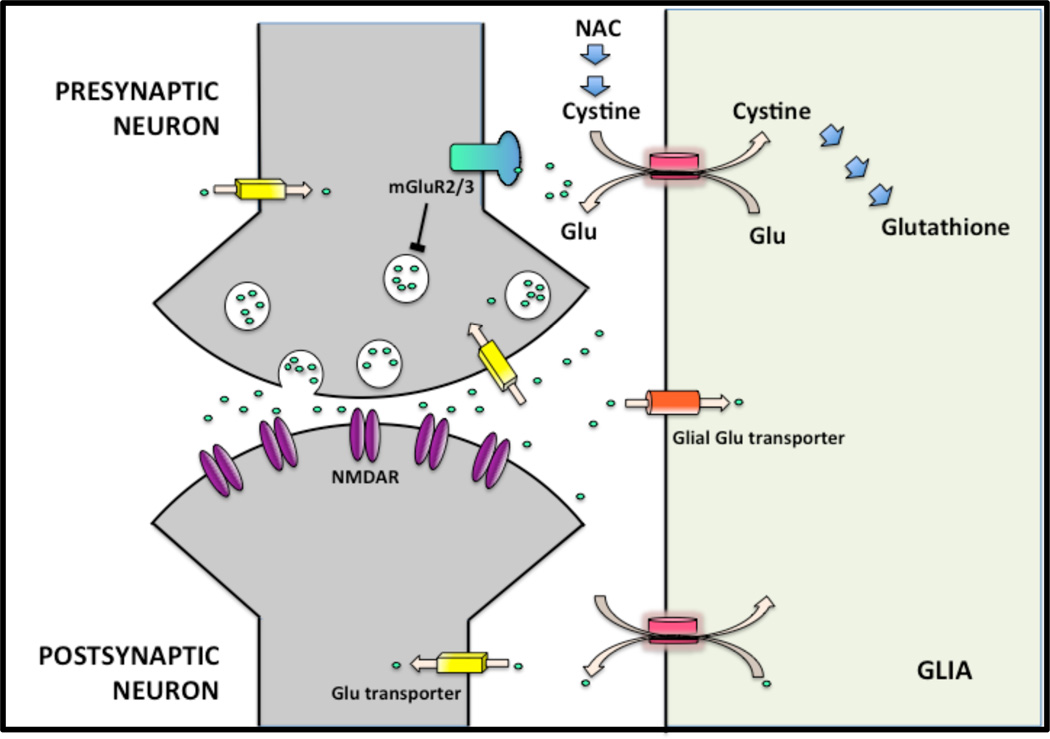
Because ketamine induces symptoms, cognitive and electrophysiological abnormalities that are similar to those observed in schizophrenia, agents that attenuate the effects of ketamine in humans are of interest for drug development (29). Drugs enhancing the activity of the cystine-glutamate exchanger have been proposed as an exemplar of this approach (30). The cystine-glutamate exchanger is expressed primarily in glial cells, but also in neurons (31, 32) where it exchanges intracellular glutamate (Glu) for extracellular cystine (Figure 1). This non-vesicular release of Glu into the extracellular space stimulates the presynaptic metabotropic Glu receptors (mGluR2/3) (33, 34) that function as autoreceptors and inhibit Glu release (35, 36).
Figure 1.
The interaction between the glial cystine–glutamate exchanger and presynaptic mGluR2/3. NAC by supplying cystine, activates the exchanger, which leads to increased Glu in the extracellular space. This stimulates the mGluR2/3 and reduces synaptic release of Glu (Baker et al., 2008). In addition, by enhancing cystine uptake, NAC promotes the synthesis of glutathione, which is a major antioxidant (Himi et al., 2003). Note that the cystine-glutamate exchanger is also expressed on cortical neurons although subcellular localization of the exchanger has not been well characterized (Burdo et al., 2006). Blue arrows, chemical reaction/effect; line with bar, inhibition; pink arrows, transport.
Baker and colleagues (2008) reported that stimulation of the cystine-glutamate exchanger by N-acetylcysteine (NAC) attenuated the behavioral and cognitive effects of phencyclidine (PCP), a potent noncompetitive NMDA receptor antagonist. NAC delivers cysteine that is oxidized to cystine in the extracellular space. The supply of cystine to the cells is a rate-limiting step for the synthesis of glutathione (GSH), a major antioxidant. Given reduced GSH concentrations in the cerebrospinal fluid and prefrontal cortex in schizophrenia (37), stimulation of the cystine-glutamate exchanger by NAC may be beneficial in this disorder (38). In a clinical trial, NAC augmentation reduced symptoms (30) and another study reported increase in MMN amplitude (39) in schizophrenia patients.
Our goal was to determine whether NAC pretreatment would attenuate the effects of ketamine on behavior, cognitive function, and ERPs in healthy humans. We predicted that ketamine would increase positive and negative symptoms, reduce working memory and sustained attention performance, decrease MMN and P300. Based on the preclinical findings above, we also predicted that NAC pretreatment would attenuate ketamine’s effects.
Methods and Materials
Subjects
The study was approved by the Institutional Review Boards of Yale Medical School and the VA Connecticut Healthcare System. Healthy volunteers were recruited by advertisements. All subjects gave written informed consent. They had no personal or family history of psychiatric or substance abuse disorders as determined by Structured Clinical Interview for DSM-IV, non-patient edition. Additionally, a family member or friend was contacted to verify the information about the participant. Subjects were instructed to abstain from psychoactive substance use for the duration of the study, including one week before and after. Majority of the subjects were nonsmokers (14/16). The 2 smokers (1–2 cigarettes/day) did not smoke on the test days, and showed no signs of withdrawal. Urine toxicology and pregnancy tests were performed on each study day. Females were studied during the follicular phase of their menstrual cycle (40, 41).
Study Design
The study was a double-blind, placebo-controlled study, consisting of two test days, where subjects were randomized to active NAC on one and placebo NAC on the other test day. Due to the potent effects of ketamine, blinding of ketamine was not possible. The test days were at least 3 days apart (median: 7 days, 3 min, 65 max). NAC and placebo capsules were administered orally in divided doses; 2000 mg followed by 1000 mg two hours later. Each morning, 165 min after NAC or placebo administration, subjects received a one-minute bolus of normal saline, followed by a 70 min long saline infusion during which behavioral, cognitive and ERP data were collected. The order of tests were fixed and included the following: Spatial working memory (SWM), Rapid visual processing (RVP), P300, MMN, Positive and Negative Syndrome Scale, PANSS (42–44) general and positive and negative subscales, the Clinical Administered Dissociative States Scale, CADSS (45), and a Visual Analog Scale of mood states, VAS. The modified PANSS (general) was administered at baseline and end of the test day (exit interview). Ketamine was administered intravenously as a bolus of 0.23 mg/kg over one minute followed by 0.58 mg/kg for 30 min (SPM and RVP), and then 0.29 mg/kg for 40 min (P300 and MMN). PANSS subscales, CADSS and VAS were collected immediately following ketamine infusion.
Cognitive measures
Cognitive performance was assessed using Spatial Working Memory (SWM) and Rapid Visual Processing (RVP) tasks administered using a computerized cognitive assessment battery (CANTAB) (46). Working memory and attention have been consistently shown to be impaired in schizophrenia (47, 48) and in healthy volunteers administered ketamine (43). SWM is a test of spatial working memory and strategy performance. RVP is a test of visual sustained attention with a small working memory component. Details on these tests are available in the Supplement.
ERP tasks
Subjects were seated in comfortable chairs in front of an LCD video display in an acoustically shielded, dimly lit, testing chamber. They were monitored by video and could interact with the research assistant. The subjects’ responses were continuously monitored on a screen outside the chamber for drowsiness and task performance. EEG data were recorded with Neuroscan Synamps amplifiers using a 1000 Hz sampling rate and a bandpass filter of .05 to 100 Hz. For further information, please see Supplement.
The MMN paradigm, which was adapted from Näätanen and colleagues (49) comprised three runs, each including frequent (50 % probability) standard tones and three types of infrequent deviant tones (16.7 % probability for each type) presented every 500 ms. Details of the MMN paradigm are available in the Supplement.
The auditory oddball (P300) paradigm included three runs, each containing a pseudo-random sequence of 150 stimuli comprising 120 standards (80%), 15 targets (10%) and 15 novels (10%) presented with a stimulus onset asynchrony (SOA) of 1250 ms. For more information on the P300 paradigm, please see the Supplement. The ERP data and signal processing methods are detailed in the Supplement.
Physiological Measures/Adverse Events
Blood pressure and heart rate were monitored at regular intervals. Adverse events were monitored before and after each test session. Ketamine levels were collected 10 minutes into each infusion. Long-term safety assessments were completed at 1 week, 3 and 6 months following study completion.
Statistical Analysis
All variables were examined for normality using normal probability plots and Kolmogorov-Smirnov test statistics. Because of the skewed distributions of the SWM task performance and other behavioral data, nonparametric analyses were performed (50). The raw behavioral data were first converted into ranks and then were entered into a mixed model with NAC (active vs placebo) and time (baseline, saline, ketamine, exit) as within-subject factors and subject as the clustering factor. The variance-covariance structure was unconstrained. Of main interest in all analyses was the NAC (NAC vs placebo) × ketamine (saline vs ketamine) interaction. Contrasts were used to parse any significant interactions or main effects. The overall alpha level for each scale (PANSS, CADSS, VAS) was fixed at p = 0.05. We used Bonferroni corrections for testing subscales, (e.g., PANSS positive and negative symptom subscales). Because this is an entirely within-subject design, we have not controlled for between-subject factors (such as education and gender). All other outcome measures conformed to normality, so they were analyzed without the use of any transformations, using linear mixed models with NAC (active vs placebo) and ketamine (saline vs ketamine) as within-subject factors, subject as a random effect and an unstructured variance-covariance matrix for condition within subject. The same post-hoc testing procedure as described above was used to parse any observed significant interactions and main effects. Bonferroni correction for six RVP measures was applied. Order effects were considered, but they were dropped from the models because they were not significant. Alternative correlation structures were also considered but dismissed because they did not fit the data as well according to Schwartz Bayesian Criterion (BIC).
ERP data analysis
The ERP data from the MMN and P300 paradigms were normally distributed. For the MMN data, a mixed model was fitted to examine the effects of NAC, ketamine and their interaction on MMN amplitude. The fixed factors were NAC (NAC vs placebo), ketamine (ketamine vs saline), stimulus type (intensity, frequency, duration) and electrode (Fz, Cz, Pz). For the P300 paradigm, a separate mixed model was fitted to examine the effects of NAC, ketamine, and their interaction on P300 amplitude to targets and novels. The fixed factors were NAC (active vs placebo), ketamine (ketamine vs saline), stimulus type (target vs novel) and electrode (Fz, Cz, Pz). In both models, all possible interactions among the fixed factors were considered and backward elimination procedure was used to drop non-significant effects under the constraint that at each step the model had to be hierarchically well formulated. Because the NAC × ketamine interaction was of utmost interest, it was always kept in the models regardless of significance. Both models included a random effect for subject, a NAC × ketamine within-subjects effect, and a structured variance-covariance matrix across electrodes and stimulus types. The best fitting variance-covariance structure was selected based on BIC. To explain significant interactions in the model, post-hoc contrasts were performed.
Results
The subjects were healthy volunteers with a mean age of 27 ± 5.6 years, 13 males and 3 females, all right handed, with mean education of 16.8 ± 2.2 years and estimated IQ of 118.9 ± 12.1 as measured by the National Adult Reading Test (NART). Fourteen of the subjects were Caucasian, one was Native American and one was Hispanic. A total of 43 subjects were consented; 21 of them never initiated the study due to ineligibility or scheduling conflicts, and 6 subjects dropped out. Sixteen subjects completed the study procedures. There were no serious adverse events. Four subjects reported mild nausea following the ketamine bolus, but were no longer nauseated by the time data collection was initiated. Plasma ketamine levels did not differ significantly between the active (mean 75.7 ± 32 ng/ml) and placebo NAC (mean 66.4 ± 23.8 ng/ml) days [F(1,12)=3.33, p=0.10]. Ketamine led to significant increases in blood pressure and heart rate (all p<0.001). Ketamine’s effect on the vital sign changes did not differ significantly between the placebo and active NAC test days (all p>0.5).
Behavioral results
Ketamine increased PANSS positive symptom scores [ANOVA type statistic (ATS)=119.6, df=2.1, p<.0001], but did not affect PANSS negative symptoms [ATS=1.1, df=1, p=0.31]. NAC did not produce positive or negative symptoms and did not modulate ketamine effects on PANSS positive symptoms [NAC × time: ATS=0.90, df=2.1, p=0.41]. For PANSS negative symptoms, there was a significant NAC × time interaction [ATS=6.58, df=1, p=0.01], where PANSS negative symptoms were higher at exit than at baseline on the NAC day [ATS=4.15, df=1, p=0.04], but the difference between baseline and exit values did not survive correction for multiple testing.
Analysis of CADSS clinician-rated items revealed that ketamine increased dissociative symptoms [Time ATS=180.1, df=1.4, p<.0001], with significant post-hoc comparisons of ketamine to other conditions (all p<.0001); however, there were no differences in these increases due to NAC [NAC × time, ATS=0.13, df=1.0, p=0.73]. CADSS self-rated items showed a similar pattern of results with only a significant time effect [ATS=159.0, df=1.6, p<.0001], indicating higher scores during ketamine than during saline, baseline and post-ketamine assessments (all p<.0001). CADSS self-rated scores during saline were also significantly higher than during baseline and exit (both p=0.002), but there were no differences in these increases due to NAC [NAC × time, ATS=0.52, df=1.5, p=0.54].
For VAS anxiety scores, only a significant time effect was observed [ATS=9.8, df=2.0, p<.0001]. VAS anxiety scores at post-ketamine were significantly lower than VAS anxiety scores during baseline, saline and ketamine assessments (all p<.0001). There were no significant differences due to NAC [NAC × time, ATS=0.23, df=2.2, p=0.82]. VAS euphoria scores showed only a significant time effect [ATS=35.8, df=2.2, p<.0001] where higher scores were found during ketamine infusion than during baseline, saline and post-ketamine assessments (all p<.0001). There were no significant differences due to NAC [NAC × time, ATS=0.88, df=2.6, p=0.43].
Cognitive tests
For SWM “between searches error” (8 boxes, defined as occasions upon which the subject revisits a box in which a token has previously been found), a significant ketamine effect was observed [ATS=3.8, df=1, p=0.05], which did not survive correction for multiple tests. SWM between searches error scores during ketamine were higher than during saline. The NAC × ketamine interaction was not significant [ATS=2.6, df=1, p=0.11]. SWM “within search error” (8 boxes, defined as the number of errors made within a search, i.e., repeated responses to a box previously opened and shown to be empty) showed a significant ketamine effect [ATS=11.7, df=1, p=0.0006]. SWM within search error scores during ketamine were higher than SWM within search error scores during saline. The NAC × ketamine interaction was not significant [ATS=0.15, df=1, p=0.70].
The RVP A', (a signal detection measure of sensitivity to errors), showed a significant ketamine effect [F(1,41)=12.1, p=0.001] where A' was significantly lower (worse performance) on ketamine than on saline. The NAC × ketamine interaction was not significant [F(1,41)=1.4, p=0.25]. For the probability of “Hit”, a significant ketamine effect was observed as well [F(1,41)=13.0, p=0.0008], where the probability of hit was significantly lower on ketamine than on saline. The NAC × ketamine interaction was not significant [F(1,41)=1.7, p=0.20]. Similarly, for RVP number of correct rejections, a significant ketamine effect was observed [F(1,41)=4.9, p=0.04], but failed correction for multiple tests. RVP correct rejections during ketamine were decreased than during saline. The NAC × ketamine interaction was not significant [F(1,41)=0.3, p=0.58].
ERP results
MMN
We found no drug effects on the number of epochs for MMN deviants (all p>0.15). There was a significant NAC × ketamine × stimulus type interaction effect on MMN amplitude [F(2,494)=5.91, p=0.003]. Post-hoc tests revealed significant NAC × ketamine interactions for the frequency [F(3,494)=4.28, p=0.005] and intensity [F(3,494)=5.44, p=0.001], but not the duration [F(3,494)=1.1, p=0.3] deviants (Table I; Figures 2 and 4). Ketamine alone reduced MMN amplitude for the intensity deviant [F(1,494)=7.3, p=0.007]. Ketamine’s effect on the intensity deviant remained significant [F(1,494)=8.82, p=0.003] despite pretreatment with NAC. Both NAC alone [F(1,494)=5.43, p=0.02] and ketamine alone [F(1,494)=11, p=0.001] reduced MMN amplitude for the frequency deviant. For the frequency deviant, the effect of NAC and ketamine given together was no different from the effect of NAC alone [F(1,494)=0.26, p=0.6] or that of ketamine alone [F(1,494)=0.23, p=0.6].
Table I.
Medication effects on Mean Peak Amplitudes (Least squares means and standard errors) of P300 (target and novel), and MMN difference waves (intensity, frequency and duration deviants) at midline electrodes (Fz, Cz and Pz).
| Placebo+saline | Placebo+ketamine | NAC+saline | NAC+ketamine | |||
|---|---|---|---|---|---|---|
| P300 | Targets | Fz | 2.5 ± 0.8 | 3.2 ± 0.8 | 5.2 ± 0.8 | 3.4 ± 0.8 |
| Cz | 7.7 ± 1.0 | 5.5 ± 1.0 | 9.9 ± 1.0 | 5.4 ± 1.0 | ||
| Pz | 11.8 ± 1.1 | 8.1 ± 1.1 | 12.6 ± 1.1 | 8.2 ± 1.1 | ||
| Novels | Fz | 5.6 ± 0.8 | 5.2 ± 0.8 | 7.6 ± 0.8 | 5.3 ± 0.8 | |
| Cz | 10.5 ± 1.0 | 7.0 ± 1.0 | 11.5 ± 1.0 | 7.4 ± 1.0 | ||
| Pz | 10.7 ± 1.2 | 5.7 ± 1.2 | 10.8 ± 1.2 | 6.1 ± 1.2 | ||
| MMN | Intensity | Fz | −3.5 ± 0.2 | −2.7 ± 0.2 | −3.4 ± 0.2 | −2.6 ± 0.2 |
| Cz | −3.2 ± 0.2 | −2.5 ± 0.2 | −3.4 ± 0.2 | −2.6 ± 0.2 | ||
| Pz | −2.1 ± 0.2 | −2.0 ± 0.2 | −2.4 ± 0.2 | −2.1 ± 0.2 | ||
| Frequency | Fz | −3.3 ± 0.3 | −2.7 ± 0.3 | −3.1 ± 0.2 | −2.9 ± 0.2 | |
| Cz | −3.3 ± 0.3 | −2.6 ± 0.3 | −2.7 ± 0.2 | −2.5 ± 0.2 | ||
| Pz | −2.3 ± 0.2 | −1.7 ± 0.2 | −1.8 ± 0.2 | −1.9 ± 0.2 | ||
| Duration | Fz | −3.2 ± 0.3 | −3.1 ± 0.3 | −3.2 ± 0.3 | −2.8 ± 0.3 | |
| Cz | −3.1 ± 0.3 | −3.1 ± 0.3 | −3.3 ± 0.3 | −2.8 ± 0.3 | ||
| Pz | −2.4 ± 0.2 | −2.3 ± 0.2 | −2.4 ± 0.2 | −2.1 ± 0.2 |
Figure 2.
Grand average difference wave ERPs for Intensity (left) and Frequency (right) deviants are plotted from electrodes Fz, Cz, and Pz to show interactive effects of N-acetylcysteine (NaC) and ketamine on auditory mismatch negativity (MMN) amplitude. Time is shown in milliseconds (ms) on the x-axis and amplitude in microVolts (µV) on the y-axis. Scalp topographic maps of MMN amplitude are shown for the negative peak chosen from the grand average across all conditions at electrode Cz for Intensity (173ms) and Frequency (139ms) deviants.
Figure 4.
Interactive effects N-acetylcysteine and ketamine on auditory P300 (target and novel stimuli) and MMN amplitude (intensity and frequency deviants) shown as least square means and standard errors across conditions.
The auditory oddball (P300) paradigm
Correct responses and reaction times
There were no drug effects on the number of epochs for the P300 paradigm (statistics not performed due to limited variability). For percent correct responses to targets, there were no significant effects of ketamine [ATS =2.3, df=1, p=0.13], NAC [ATS =0.02, df=1, p=0.9], or NAC × ketamine interaction [ATS =0.02, df=1, p=0.9]. For target reaction times, the findings were similar; there were no significant effects of ketamine [ATS =2.1, df=1, p=0.15], NAC [ATS =0.15, df=1, p=0.7], or NAC × ketamine interaction [ATS =0.14, df=1, p=0.7].
P300
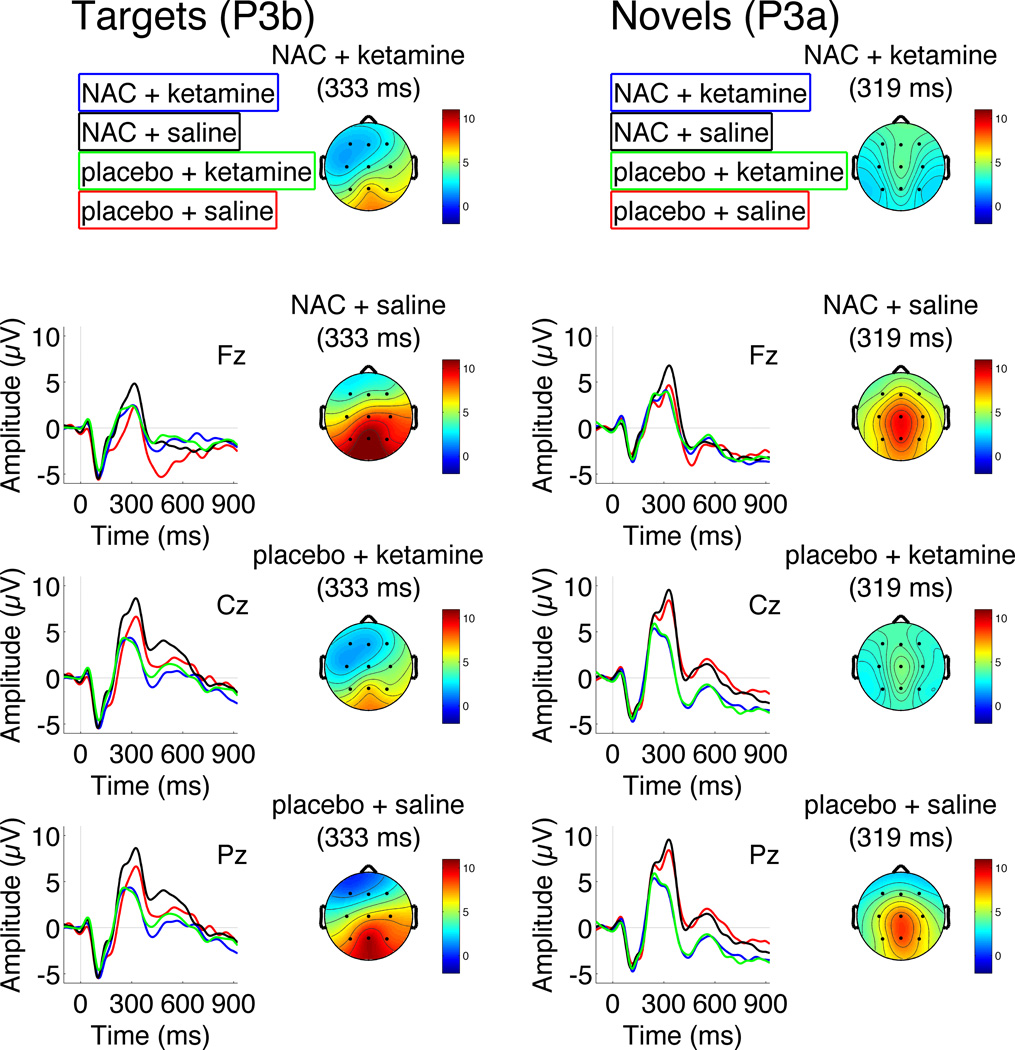
In the overall model, there was a significant ketamine effect [F(1,313)=27.6, p<0.0001], indicating that P300 amplitudes were smaller on ketamine than on saline (Table I, Figures 3 and 4). This ketamine effect significantly depended on electrode (ketamine × electrode interaction [F(2,313)=9.94, p<0.001]), with the effect evident at Cz [F(1,313)=23.4, p<0.0001] and Pz [F(1,313)=27.3, p<0.0001] but not Fz [F(1,313)=2.76, p=0.1]. However, the ketamine effect did not significantly interact with the stimulus type [F(1,310)=0.70, p=0.40]. Similarly, the ketamine × electrode interaction did not significantly depend on stimulus type [F(2,310)=0.31, p=0.73], indicating that ketamine produced similar reductions in the amplitudes of both target P3a and novelty P3b at central and parietal sites (see Figure 3). While there was no main effect of NAC [F(1,313)=1.83, p=0.18], there was a significant NAC × ketamine interaction [F(1,313)=5.58, p=0.02]; Figures 3 and 4. Post-hoc tests showed that NAC alone, relative to placebo, significantly increased P300 amplitudes [F(1,313)=5.29, p=0.02], an effect that did not significantly depend on stimulus type [F(1,300)=0.46, p=0.50], electrode [F(2,300)=0.54, p=0.58], or their interaction [F(2,300)=0.45, p=0.64], However, NAC pretreatment, relative to placebo, did not significantly modulate P300 amplitude during ketamine administration [F(1,313)=0.01, p=0.9]. Thus, despite NAC’s enhancing effect on P300, it did not prevent or attenuate ketamine’s reduction of P300 amplitude. Other significant effects included a stimulus type × electrode interaction [F(2,313)=57.72, p<.0001]; Figures 3 and 4, confirming the expected parietal distribution of the target P3b and the more centro-frontal distribution of the novelty P3a. There were significant differences between target and novel stimuli values for all electrodes (all p<0.001) where novel stimuli values were higher than targets for Fz and Cz and lower for Pz.
Figure 3.
Grand average ERPs for Targets (left) and Novels (right) are plotted from electrodes Fz, Cz, and Pz to show interactive effects of N-acetylcysteine (NaC) and ketamine on auditory P300 amplitude. Time is shown in milliseconds (ms) on the x-axis and amplitude in microVolts (µV) on the y-axis. Scalp topographic maps of P300 amplitude are shown for the positive peak chosen from the grand average across all conditions at electrode Pz for Targets (333ms) and Cz for Novels (319ms).
Discussion
As hypothesized, ketamine produced significant increase in PANSS positive symptoms, reduction in working memory and sustained attention performance, and MMN and P300 amplitudes. Ketamine’s reduction of MMN was only evident for frequency and intensity deviants, sparing the duration deviant. This underscores that the MMNs elicited by different deviant types are not uniform in their underlying generators (51–53). Notably, the duration deviant MMN has been shown to have the greatest sensitivity to the schizophrenia effect (5, 54). Ketamine produced similar reductions of both the target P3b and novelty P3a, suggesting that it impacted the generators and/or neurophysiological mechanisms common to both of these P300 sub-components.
NAC alone did not have significant effects on behavioral or cognitive performance, but it reduced frequency-deviant MMN amplitude and significantly increased P300 amplitude. However, unlike the findings in rodents, NAC pretreatment did not attenuate the behavioral/cognitive and ERP effects of ketamine in healthy volunteers.
Neurochemical mechanisms activated by acute systemic ketamine administration have been reviewed elsewhere (55–57). While in vitro, ketamine has affinity not only for NMDA but several other receptors including dopamine (58), selective D2 agonist bromocriptine, dopamine precursor L-dopa (59) and D1 and D2 agonist apomorphine did not affect P300 amplitude in healthy volunteers (60). Consistent with these findings, pretreatment with the D2 antagonist haloperidol did not affect ketamine’s effect on P300 (61). Acute depletion of precursors of dopamine and 5-hydroxytryptamine (5-HT) alone or in combination did not modulate MMN (62). Similarly, haloperidol failed to block perceptual changes induced by ketamine (63). These findings do not implicate the dopaminergic system as a primary modulator of ketamine’s effect on ERP or behavioral indices, but are in keeping with a disinhibited prefrontal network activity resulting from NMDA receptor antagonism (12–15, 64).
Our findings suggest that in humans, stimulation of the mGluR2/3 receptors by NAC does not enhance the NMDA receptor function impaired by ketamine. The dissociation between the animal and human data may represent differences primarily in the effects of NAC, since ketamine’s effects in humans paralleled those in rodents. It is possible that the distribution of the cystine-glutamate exchanger that differs between species and locally in the brain plays a role in this (31, 65). Interestingly, NAC supplementation in smaller doses (2000 mg /d vs 3000 mg/d in our study) was found to improve some symptoms in patients with schizophrenia (66). While this discrepancy may suggest a limitation of the ketamine model for schizophrenia, other explanations such as the effect of chronic NAC administration (6 months in the clinical trial vs single day pretreatment in our study) is difficult to rule out. In another study, 2000mg/d NAC treatment over 2 months was associated with increase in MMN in a small sample of schizophrenic patients (39), however, their MMN measurements were confounded by N1, and the subjects’ attention was not diverted away from the auditory channel that is typically done while studying MMN. Thus, their findings may have limited relevance to our findings.
Glutamatergic modulation of MMN has been studied in detail using intracortical recordings in primates (64) where PCP decreased MMN to the frequency and intensity deviants in a dose dependent fashion. Our findings in healthy humans parallel these data showing reduced MMN for the frequency and intensity deviants in response to ketamine. NAC, however, produced a reduction of the frequency deviant MMN amplitude. The divergence of NAC’s effects on pre-attentive processes (MMN) and attention-mediated processes orienting to novelty and detection of targets may be due to the differences in regional distribution/regulation of cellular mechanisms underlying generation of P300 (67–70) and MMN (16, 71, 72).
The fact that NAC enhanced P300 amplitude in healthy humans suggests that NAC further increases the capacity of normally functioning glutamatergic networks subserving this measure. This may be due to a transient increase in extrasynaptic Glu levels as NAC promotes uptake of cystine into the cells in exchange for Glu, suggesting a role for NAC via an extrasynaptic effect. Supporting this perspective, a recent study found that NAC treatment was associated with a decrease in the binding potential of a tracer with affinity for an allosteric site on mGluR5, which are extrasynaptically expressed (73). This suggestion would further necessitate an inverted U-shape relationship between extrasynaptic Glu levels and P300 amplitude, since ketamine induces potent increases in extrasynaptic Glu levels (74) and leads to reduced P300, shifting from optimal peak glutamate levels to excessive levels associated with the descending portion of the inverted-U function. Alternatively, the effect of NAC on P300 may be linked to stimulation of presynaptic autoreceptors leading to decreased Glu release, suggesting a synaptic effect as suggested by Baker and colleagues (2008). This possibility more readily fits our observation since ketamine leads to enhanced Glu release opposite to the effect of NAC, consistent with these agents’ divergent effects on P300. The effect of NAC may also involve additional/alternative mechanisms including increasing glutathione synthesis within the cells and/or its reducing properties as demonstrated earlier (75). Further preclinical electrophysiological studies are needed to clarify these mechanisms.
Regarding the methods, repeated measure designs as we have employed are sensitive to test effects, however, by randomizing subjects to active and placebo NAC, we have minimized this potential problem as our primary outcome measure was the effect of NAC pretreatment on ketamine-induced changes. Our P300 paradigm was not traditional as the standard stimuli were 20, 30 or 40 Hz click trains (500 ms) instead of typical higher frequency tones with shorter duration. However, this is unlikely to affect the results because the same paradigm was used for all drug conditions. Moreover, our results on P300 are consistent with other groups’ findings on ketamine using traditional P300 paradigms (12, 15, 61).
In summary, NMDA antagonism as modeled by systemic ketamine administration in healthy volunteers led to expected changes in behavioral/cognitive measures and reduction in ERP indices of pre-attentive reflections of sensory echoic memory (MMN), and top down (P3b) and bottom up (P3a) attentional processes. NAC alone was associated with a reduction in MMN for the frequency deviant and significant increases in P300 amplitude for both target and novel stimuli. Pretreatment with NAC did not affect the changes induced by ketamine. Our finding of interactive effects of NAC and ketamine for the ERP indices, but a lack thereof for the behavioral/cognitive measures suggest that electrophysiological indices lay more proximal to the biochemical processes induced by these agents and that further mechanisms play a role in modulating complex behavior. These findings also suggest that improvements in endophenotypes may not readily translate into functional improvement. The beneficial effect of NAC on P300, a measure of target detection, merits further investigation as a potential cognitive enhancing agent.
Supplementary Material
Acknowledgements
This study was supported by a NARSAD Young Investigator Award to Dr. Gunduz-Bruce.
We are thankful to the Biostudies Laboratory Nursing Staff, Angelina M. Genovese, RNC, MBA; Elizabeth O'Donnell, RN, Michelle Sanpedro RN; Brenda Breault RN, BSN for their outstanding nursing and Sonah Perry, R.Ph. for excellent Research Pharmacy support. We also thank all the research participants. This study was also partly supported by funding from the National Institute on Alcohol Abuse and Alcoholism, K05 AA 14906, National Institute on Alcohol Abuse and Alcoholism, 2P50 AA 012870, U.S. Department of Veterans Affairs Alcohol Research Center, National Center Post Traumatic Stress Disorder, Clinical Neurosciences Division, West Haven, CT to JHK, R01 MH076989 to DHM, VA Schizophrenia Biological Research Center, and grants from National Institute of Mental Health (MH40052, MH 58262, MH067967), and the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) to JMF.
Dr. D'Souza reports research grant support from Astra Zeneca, Abbott Laboratories, Eli Lilly Inc., Organon, Pfizer Inc., and Sanofi. Dr. Krystal reports serving as Consultant for the following companies: (The Individual Consultant Agreements listed below are less than $10,000 per year): Aisling Capital, LLC, AstraZeneca Pharmaceuticals, Brintnall & Nicolini, Inc., Easton Associates, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Inc., Merz Pharmaceuticals, MK Medical Communications, F. Hoffmann-La Roche Ltd, SK Holdings Co., Ltd, Takeda Industries, Teva Pharmaceutical Industries, Ltd. Dr. Krystal also serves for the Scientific Advisory Board of Abbott Laboratories, Bristol-Myers Squibb, Eisai, Inc., Eli Lilly and Co., Forest Laboratories, Inc., Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., Pfizer Pharmaceuticals, Shire Pharmaceuticals. Dr. Krystal’s Exercisable Warrant Options include Tetragenex Pharmaceuticals (value less than $150), and he serves as Board of Directors for the Coalition for Translational Research in Alcohol and Substance Use Disorders. Dr. Krystal is a President Elect for American College of Neuropsychopharmacology and receives Research Support to Department of Veterans Affairs and Janssen Research Foundation (Provided drug and some study support to the Department of Veterans Affairs). Dr. Krystal receives greater than $10,000 as the Editor of Biological Psychiatry. Patents and Inventions for Dr. Krystal include 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948. September 5, 1995, 2) Co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1). 3) Intranasal Administration of Ketamine to Treat Depression (pending).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of the data were previously presented as a poster at the 2007 annual meeting of the American College of Neuropsychopharmacology.
Financial Disclosures:
Dr. Gunduz-Bruce, Dr. Gueorguieva, Dr. Ford, Dr. Mathalon, Mr. Reinhart, Mr. Roach and Mr. Oliver report no biomedical financial interests or potential conflict of interests.
References
- 1.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist ketamine in humans Psychotomimetic perceptual cognitive and neuroendocrine responses. Arch of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 3.Corlett PR, Honey GD, Aitken MR, Dickinson A, Shanks DR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition brain activity and psychosis. Arch of Gen Psychiatry. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- 4.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 5.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Duncan CC. Event-related brain potentials: a window on information processing in schizophrenia. Schizoph Bulletin. 1988;14:199–203. doi: 10.1093/schbul/14.2.199. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell BF, Faux SF, McCarley RW, Kimble MO, Salisbury DF, Nestor PG, Kikinis R, Jolesz FA, Shenton ME. Increased rate of P300 latency prolongation with age in schizophrenia Electrophysiological evidence for a neurodegenerative process. Arch of Gen Psychiatry. 1995;52:544–549. doi: 10.1001/archpsyc.1995.03950190026004. [DOI] [PubMed] [Google Scholar]
- 8.Turetsky BI, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: I Physiological evidence for gender and subtype specific differences in regional pathology. Biol Psychiatry. 1998;43:84–96. doi: 10.1016/S0006-3223(97)00258-8. [DOI] [PubMed] [Google Scholar]
- 9.Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36:153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 10.Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000;47:434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- 11.Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients paradigms and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- 12.Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 13.Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. The Intl J of Neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch of Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 15.Knott VJ, Millar AM, McIntosh JF, Shah DK, Fisher DJ, Blais CM, Ilivitsky V, Horn E. Separate and combined effects of low dose ketamine and nicotine on behavioural and neural correlates of sustained attention. Biol Psychol. 2011;88:83–93. doi: 10.1016/j.biopsycho.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Naatanen R. The mismatch negativity: a powerful tool for cognitive neuroscience. Ear and Hearing. 1995;16:6–18. [PubMed] [Google Scholar]
- 17.Isreal JB, Chesney GL, Wickens CD, Donchin E. P300 and tracking difficulty: evidence for multiple resources in dual-task performance. Psychophysiology. 1980;17:259–273. doi: 10.1111/j.1469-8986.1980.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Polich J. P300 from a passive auditory paradigm. Electroencephalography and Clinical Neurophysiology. 1989;74:312–320. doi: 10.1016/0168-5597(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 19.Soltani M, Knight RT. Neural origins of the P300. Critical Reviews in Neurobiology. 2000;14:199–224. [PubMed] [Google Scholar]
- 20.Donchin E. The P300 as a metric for mental workload. Electroencephalography and Clinical Neurophysiology. 1987;(Supplement 39):338–343. [PubMed] [Google Scholar]
- 21.Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 22.Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 23.Sutton S, Tueting P, Zubin J, John ER. Information delivery and the sensory evoked potential. Science. 1967;155:1436–1439. doi: 10.1126/science.155.3768.1436. [DOI] [PubMed] [Google Scholar]
- 24.Duncan-Johnson CC, Donchin E. On quantifying surprise: the variation of event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 25.Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 26.Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 27.Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- 28.Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects cortical glutamatergic function and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 30.Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1760–1772. doi: 10.1038/sj.npp.1301532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdo J, Dargusch R, Schubert D. Distribution of the cystine/glutamate antiporter system xc- in the brain kidney and duodenum. The Journal of Histochemistry and Cytochemistry : official journal of the Histochemistry Society. 2006;54:549–557. doi: 10.1369/jhc.5A6840.2006. [DOI] [PubMed] [Google Scholar]
- 32.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1990;4:1624–1633. [PubMed] [Google Scholar]
- 33.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of Neuroscience : the official journal of the Society for Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of Neuroscience : the official journal of the Society for Neuroscience. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilbride J, Huang LQ, Rowan MJ, Anwyl R. Presynaptic inhibitory action of the group II metabotropic glutamate receptor agonists LY354740 and DCG-IV. European Journal of Pharmacology. 1998;356:149–157. doi: 10.1016/s0014-2999(98)00526-3. [DOI] [PubMed] [Google Scholar]
- 36.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. The Journal of Pharmacology and Experimental Therapeutics. 2001;299:12–20. [PubMed] [Google Scholar]
- 37.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. The European Journal of Neuroscience. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 38.Yao JK, Keshavan MS. Antioxidants Redox Signaling and Pathophysiology in Schizophrenia: An Integrative View Antioxidants & Redox Signaling. 2011 doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, et al. Glutathione precursor N-acetyl-cysteine improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 40.Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- 41.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and clinical psychopharmacology. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 42.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. The British Journal of Psychiatry. 1989;(Supplement):59–67. [PubMed] [Google Scholar]
- 43.Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D'Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Archives of General Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 44.D'Souza DC, Gil RB, Zuzarte E, MacDougall LM, Donahue L, Ebersole JS, Boutros NN, Cooper T, Seibyl J, Krystal JH. gamma-Aminobutyric acid-serotonin interactions in healthy men: implications for network models of psychosis and dissociation. Bioll Psychiatry. 2006;59:128–137. doi: 10.1016/j.biopsych.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure C. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) Journal of Traumatic Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 46.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 47.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Critical Reviews in Neurobiology. 2000;14:1–21. [PubMed] [Google Scholar]
- 48.Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Current topics in behavioral neurosciences. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- 49.Naatanen R, Pakarinen S, Rinne T, Takegata R. The mismatch negativity (MMN): towards the optimal paradigm. Clinical Neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004;115:140–144. doi: 10.1016/j.clinph.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York: Wiley; 2002. [Google Scholar]
- 51.Todd J, Michie PT, Jablensky AV. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clinical Neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2003;114:2061–2070. doi: 10.1016/s1388-2457(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 52.Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Naatanen R. Deviant matters: duration frequency and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Bioll Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Michie PT. What has MMN revealed about the auditory system in schizophrenia? International Journal of Psychophysiology : official journal of the International Organization of Psychophysiology. 2001;42:177–194. doi: 10.1016/s0167-8760(01)00166-0. [DOI] [PubMed] [Google Scholar]
- 54.Michie PT, Budd TW, Todd J, Rock D, Wichmann H, Box J, Jablensky AV. Duration and frequency mismatch negativity in schizophrenia. Clinical Neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000;111:1054–1065. doi: 10.1016/s1388-2457(00)00275-3. [DOI] [PubMed] [Google Scholar]
- 55.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 56.Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia - therapeutic implications. Brain Res Brain Res Rev. 2000;31:342–349. doi: 10.1016/s0165-0173(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 57.Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. doi: 10.1016/j.brainresrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- 59.Oranje B, Gispen-de Wied CC, Westenberg HG, Kemner C, Verbaten MN, Kahn RS. No effects of l-dopa and bromocriptine on psychophysiological parameters of human selective attention. J Psychopharmacol. 2006;20:789–798. doi: 10.1177/0269881106061712. [DOI] [PubMed] [Google Scholar]
- 60.Luthringer R, Rinaudo G, Toussaint M, Bailey P, Muller G, Muzet A, Macher J. Electroencephalographic characterization of brain dopaminergic stimulation by apomorphine in healthy volunteers. Neuropsychobiology. 1999;39:49–56. doi: 10.1159/000026560. [DOI] [PubMed] [Google Scholar]
- 61.Oranje B, Gispen-de Wied CC, Westenberg HG, Kemner C, Verbaten MN, Kahn RS. Haloperidol counteracts the ketamine-induced disruption of processing negativity but not that of the P300 amplitude. The International Journal of Neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12:823–832. doi: 10.1017/S1461145708009814. [DOI] [PubMed] [Google Scholar]
- 62.Leung S, Croft RJ, Guille V, Scholes K, O'Neill BV, Phan KL, Nathan PJ. Acute dopamine and/or serotonin depletion does not modulate mismatch negativity (MMN) in healthy human participants. Psychopharmacology (Berl) 2010;208:233–244. doi: 10.1007/s00213-009-1723-0. [DOI] [PubMed] [Google Scholar]
- 63.Krystal JH, D'Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Jr, Vegso S, Heninger GR, et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- 64.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter system x(c)- in the mouse brain. The Journal of Neuroscience : the official journal of the Society for Neuroscience. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berk M, Copolov D, Dean O, Lu K, Jeavons, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind randomized placebo-controlled trial. Biological Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Smith ME, Halgren E, Sokolik M, Baudena P, Musolino A, Liegeois-Chauvel C, Chauvel P. The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalography and clinical neurophysiology. 1990;76:235–248. doi: 10.1016/0013-4694(90)90018-f. [DOI] [PubMed] [Google Scholar]
- 68.Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. NeuroImage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 69.Linden DE. The p300: where in the brain is it produced and what does it tell us? The Neuroscientist : a review journal bringing neurobiology neurology and psychiatry. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- 70.Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalography and clinical neurophysiology. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- 71.Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Research. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 72.Schall U, Johnston P, Todd J, Ward PB, Michie PT. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs. NeuroImage. 2003;20:729–736. doi: 10.1016/S1053-8119(03)00398-7. [DOI] [PubMed] [Google Scholar]
- 73.Miyake N, Skinbjerg M, Easwaramoorthy B, Kumar D, Girgis RR, Xu X, Slifstein M, Abi-Dargham A. Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [11C] ABP688 and N-acetylcysteine challenge. Bioll Psychiatry. 2011;69:822–824. doi: 10.1016/j.biopsych.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 74.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 75.Himi T, Ikeda M, Yasuhara T, Murota SI. Oxidative neuronal death caused by glutamate uptake inhibition in cultured hippocampal neurons. Journal of Neuroscience Research. 2003;71:679–688. doi: 10.1002/jnr.10510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.