Abstract
Independently, aging and stroke each have a significant negative impact on skeletal muscle, but the potential cumulative effects of aging and stroke have not been explored. Optimal interventions for individuals post-stroke may include those that specifically target skeletal muscle. Addressing changes in muscles may minimize activity limitations and enhance participation post-stroke. This paper reviews the impact of aging and stroke on muscle morphology and composition, including fiber atrophy, reductions in muscle cross-sectional area, changes in muscle fiber distributions, and increases in intramuscular fat. Relationships between changes in muscle structure, muscle function, and physical mobility are reviewed. Clinical recommendations that preserve and enhance skeletal muscle in the aging adult and individuals post-stroke are discussed. Future research directions that include systematic comparison of the differences in skeletal muscle between younger and older adults who have sustained a stroke are suggested.
Keywords: aging, stroke, sarcopenia, intramuscular fat
INTRODUCTION
Stroke is the leading cause of serious long-term neurological disability in the United States.1 According to 2007 statistics from the Centers for Disease Control and Prevention, approximately 700,000 strokes occur yearly in the United States, with three-fourths of these occurring in those 65 years and older.2 A myriad of impairments occur as a result of a stroke, including, but not limited to poor muscle activation,3 decreased sensation,4, 5 and incoordination.6, 7 Among older adults, muscle deterioration due to aging, decreased physical activity, and sub-optimal dietary intake,8 underlie and may compound the deleterious effects of a stroke. The purpose of this paper is to discuss the potential cumulative impact of aging and stroke on skeletal muscle. This paper reviews healthy, skeletal muscle structure and function and the effects of aging and a stroke on muscle size, composition, and performance. It presents relationships between muscle impairments and activity limitations and discusses current physical therapy treatments for age-related and post-stroke skeletal muscle changes. Suggestions are made for future research that compares younger and older individuals post-stroke to enhance our understanding of the impact of stroke in the presence of age-related changes.
SKELETAL MUSCLE: AN OVERVIEW
The motor unit is the smallest functional element within skeletal muscle that the central nervous system can control9 and consists of an alpha-motor neuron and the multiple muscle fibers the axon innervates. Each individual pre-synaptic nerve fiber innervates a single muscle fiber, which is classified as a Type I or Type II fiber. Type I fibers contain slow forms of myosin ATPase, resulting in slow twitch contractions.10, 11 High mitochondrial levels12 in conjunction with large capillary networks allow increased blood flow, enabling slow-twitch muscle fibers to produce sustained, low-load tension required by the postural muscles. Type II fibers have fewer mitochondria, smaller capillary networks, and rely on anaerobic forms of energy production.10–12 Anaerobic energy production allows faster contraction times and greater muscle tension, rendering Type II fibers beneficial during activities that require quick bursts of movement.11, 13–15
A single action potential results in a single muscle twitch; as the firing rate of the motor unit increases, multiple action potentials result in twitch summation, which leads to a synchronized, tetanic contraction of all muscle fibers innervated by a given motoneuron.16, 17 Motor units typically consist of a single muscle fiber type, with motor unit size dependent on fiber type and the number of innervated fibers. Motor units that innervate Type I fibers are smaller than those that innervate Type II fibers. During voluntary contractions, motor units innervating Type I fibers are preferentially recruited.9 Progressive force demands increase the firing rate of smaller motor units, termed rate coding, followed by recruitment of larger motor units.9
MUSCULAR CHANGES
Muscle Atrophy
Aging
Fiber-level changes within skeletal muscle are prevalent in the elderly population. Skeletal muscle biopsy studies performed on the vastus lateralis show a reduction in Type II fibers after 50 years of age.13, 14, 18, 19 Decrease in Type II fibers with progressive aging is further supported by evidence in both weight bearing and non-weight bearing muscles including the gastrocnemius, tibialis anterior, and biceps brachii.13, 14 Reduction in fiber number and size leads to a decline in overall muscle cross-sectional area. Cross-sectional area may be used to quantify the force production capabilities of a muscle20 and assess the loss of skeletal muscle due to sarcopenia, which is the age-related decline of skeletal muscle mass. In the elderly, loss of skeletal muscle cross-sectional area ranges from 21% to 40%, when compared to healthy, younger adults.21, 22 Cross-sectional area reductions are progressive, with increasing loss with advanced age.23 Further, reduced cross-sectional area has been associated with poorer physical performance in healthy, older adults.24 Consequently, fitness regimens for older adults should include resistance training exercises for both the upper and lower extremities, particularly in the later years of life, to combat Type II fiber changes, cross-sectional area reductions, and activity limitations.
In addition to fiber number loss, disuse may affect the cross-sectional area of individual fibers to a greater degree in older adults when compared to younger adults.25 Simulating disuse in a healthy population, Hvid et al found that two weeks of quadriceps immobilization resulted in greater deleterious effects on muscle fibers in older men when compared to younger men, with older individuals requiring increased recovery time post-immobilization.25 This study provides preliminary evidence for early rehabilitation in older adults post-immobilization and suggests longer episodes of care may be necessary to reestablish pre-morbid skeletal muscle status. Future studies should consider stratification of younger versus older individuals to allow comparisons of skeletal muscle response rates to immobilization and subsequent interventions.
Links between muscle size, physical activity, and disability in older adults are emerging.26, 27 Park et al found adults, ages 65 to 84 years, are less likely to have sarcopenia if they walk at least 7,000 to 8,000 steps per day and/or spend 15 to 20 minutes per day performing moderate physical activity (i.e. >3 metabolic equivalents).26 Moderate physical activity may include “brisk walking” at 3 to 4 miles per hour, cycling, house repair, and yardwork.28 Further, sarcopenia is associated with physical disability.27 For example, Chien et al found an odds ratio for physical disability of 3.03 (95% Confidence Interval: 1.21–7.61) for elderly men with sarcopenia when compared to healthy, older men with normal skeletal muscle mass indices.27 These emerging relationships require longitudinal, prospective investigations to determine whether sarcopenia precedes activity level decline and disability.
Decreased muscle cross-sectional area ultimately impacts the ability of the muscle to produce force. There is a direct relationship between increasing age and decreased force production.21, 23 The force per cross-sectional area of the vastus lateralis is reduced by approximately 1.5% per year and a total of 21% over 15 years.21 Frontera and colleagues reported similar results, with an annual decline in isokinetic muscle strength from 1.4 to 2.5% for the knee and elbow flexors and extensors over 12 years.23 Between the ages of 65 and 80 years, Jubrias and colleagues demonstrated a 39% drop in force capabilities during isokinetic knee extension.21 Given the overwhelming evidence for progressive loss, resistance training may preserve muscle function in the older adult enabling generation of forces required for everyday activities such as lifting, pulling, pushing, and stair negotiation.29, 30
Post-Stroke
Regardless of the age of the individual, muscles post-stroke demonstrate many similarities to aging muscle. After a stroke the number of Type II fibers progressively decrease, resulting in a loss of skeletal muscle cross-sectional area.11, 31, 32 Similar to age-related muscle atrophy, after stroke there is a progressive decrease in muscle fiber size bilaterally, with greater decreases on the more involved side.32 Stroke-induced muscle fiber reductions lead to a decrease in muscle cross-sectional area. In individuals post-stroke, diminished force production and contraction speed secondary to muscle atrophy may be observed as difficulty with sit to stand transfers,33, 34 limitations in stair negotiation,35 and impaired balance on compliant surfaces.36
When a stroke occurs in an older adult, the paretic muscles have an accelerated rate of the normal, detrimental skeletal muscle changes typically associated with aging alone.31, 37, 38 This decline is most often demonstrated by comparing the paretic and non-paretic sides of older adults post-stroke.31, 37–39 Hachisuka and colleagues reported an average difference in Type I fiber diameter of 8.5 microns when comparing muscles in the paretic and non-paretic limbs.31 At the whole muscle level, others have reported approximately a 20% reduction in the mid-thigh muscle area and muscle volume of the paretic limb compared to the non-paretic limb in older adults.37, 38 In the upper extremity, Ploutz-Snyder and colleagues reported the triceps brachii of the more affected side was 25% smaller than the less affected triceps brachii, with a corresponding strength deficit of 61% in individuals an average of 65 months post-stroke.39
Further, post-stroke skeletal muscle changes may occur in both limbs,40–43 therefore studies that evaluate inter-limb differences in the older adult may be underestimating the impact of a stroke on skeletal muscle. For example, Noskin et al reported that even at one year, ipsilateral impairments in grip strength and hand coordination, as measured by the 9-Hole Peg Test, persisted in individuals who sustained a first-time hemiparetic stroke.42 Thus, age-matched comparisons to healthy adults as opposed to inter-limb comparisons within patients post-stroke may be preferable. Age-matched comparisons may be essential due to the potential cumulative impact of stroke and aging. Enhanced understanding regarding the influence of stroke and aging on muscle fiber atrophy, decreased cross-sectional area, and decreased strength may be achieved through comparison studies of older versus younger individuals post-stroke.
Disuse post-stroke appears to be an important indicator of skeletal muscle loss and may influence strength44 and lean muscle mass.45 Andrews and Bohannon have shown that strength of eight paretic muscles were only 29 to 45% of age-matched subjects’ strength as tested with hand-held dynamometry, in individuals aged 44 to 85 years.44 Jorgensen and colleagues found that individuals who learned to walk within two months of their stroke had preservation of lean muscle mass.45 For those unable to walk at two months, lean muscle mass was lost in both lower limbs and regained only in the non-paretic limb at seven months.45 The gradual loss of muscle strength due to aging, coupled with the loss of strength due to paresis may cause a significant and progressive strength deficit in older individuals after stroke. Whether the loss of muscle strength is accelerated in the presence of advanced age has yet to be experimentally tested. Longitudinal, clinical trials evaluating younger versus older individuals post-stroke may assist with answering this question. Controlling for physical activity level may be critical given an association between muscle cross-sectional area and activity level in healthy, older adults.26 Paired comparisons are recommended that consider not only daily activity but physical capacity.
Muscle Composition Changes
In older adults, an even more important factor than decreased cross-sectional area in age-related strength loss may be muscle composition changes. Decreased muscle strength may be two to five times greater than the age-related loss of muscle size in healthy elders, implicating poorer muscle composition as a major factor in age-related strength decline.46 Motorneuron death and denervation-re-innervation influences overall muscle composition. With increased age, due to the death of motorneurons, the ratio of muscle fibers to alpha-motor axon increases.47 Newly denervated muscle fibers will significantly atrophy unless re-innervated.47 Denervated fibers may be re-innervated by the remaining healthy motorneurons, which preserves fiber cross-sectional area and results in larger motor units.9, 47, 48 In healthy, older adults, the natural remodeling processes of nerve re-sprouting and re-innervation occurs at a slower rate than in younger individuals.9, 47, 49 Re-innervation favors Type I fiber clusters.14, 18, 50 The morphological result of this denervation and re-innervation is a change in distribution of Type I and Type II fibers within older muscle.18, 50
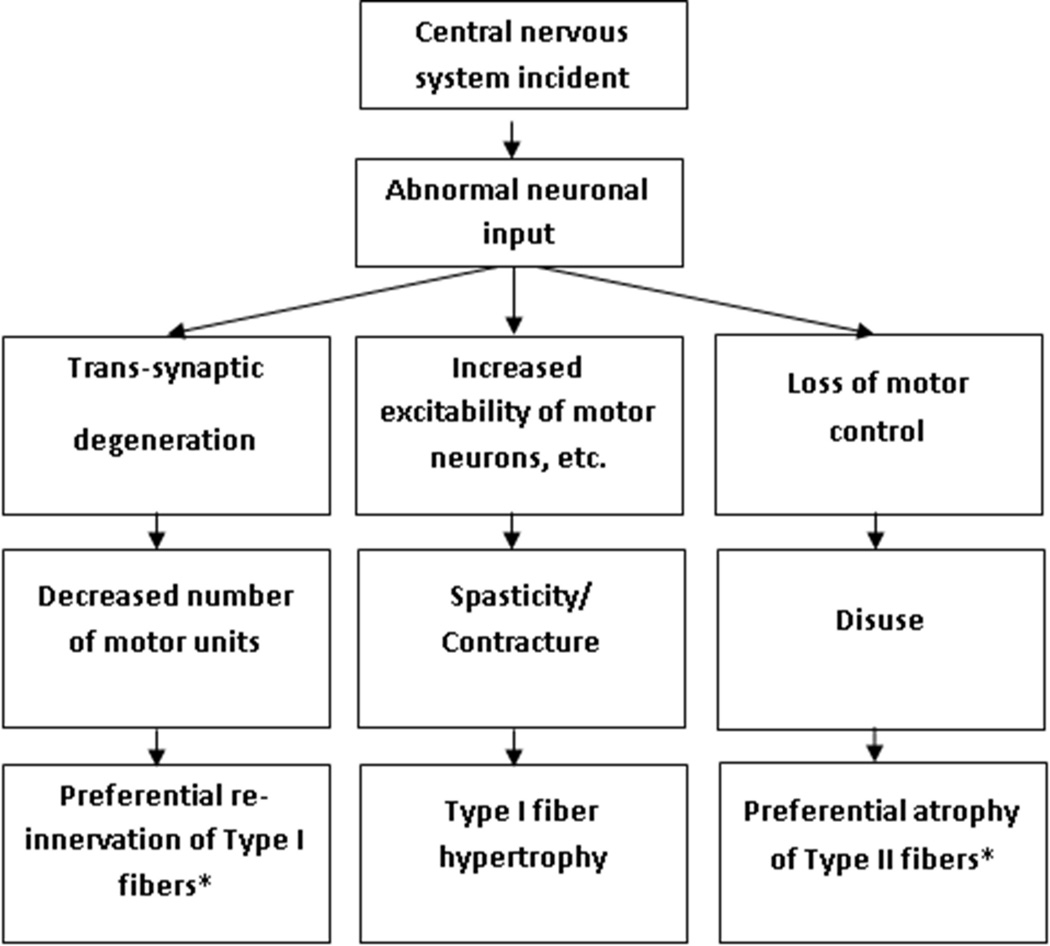
When investigating the effects of stroke on motorneurons, we find strikingly similar end results to aging (See Figure 1), despite different mechanisms in aging and stroke.51 Post-stroke, trans-synaptic degeneration may result from a lack of synaptic input and decreased lower motorneuron activation.12 An inability to activate higher threshold motor units post-stroke may partially explain the increased proportion of Type I fibers.11 Post-stroke, motorneuron function may be maintained for the first two months, but thereafter the number of functioning motorneurons is greatly reduced.12 In fact, researchers suggest that the number of motor units is significantly decreased as early as nine days after a stroke.52 Further, motor unit action potentials may be altered post-stroke. Lukacs et al propose that abnormal increases in action potential duration and amplitude signify dense, re-innervated motor units, which have expanded to replace lost motor units.53 Thus, the hypertrophy of Type I fibers post-stroke may be the result of increased alpha motorneuron excitability.31 Increased fiber density occurs in the first 10 months post-stroke and then remains stable.53
Figure 1.
Changes in skeletal muscle secondary to abnormal neuronal activity after stroke.
* end-results that parallel skeletal muscle changes that occur with aging.
Under the pathological conditions created by a stroke, in an older adult, the denervated fibers may or may not be re-innervated47 and the rate of re-innervation is slowed secondary to biological aging. This compounding effect may hinder recovery of the functional motor unit and ultimately impact force production. To date, no studies have evaluated post-stroke muscle fiber re-innervation in older versus younger skeletal muscle. Studies assessing re-innervation, while controlling for age, may help explain why an older individual, who experiences a stroke of the same magnitude and distribution as a younger individual, may require an extended episode of rehabilitative care.
Concurrent with age-related motorneuron changes, intramuscular fat, which is an increase in intramyocellular lipids within the muscle, is augmented in older adults.51, 54–59 Intramuscular fat, is highest in oxidative, Type I fibers and serves as a dynamic and immediate source of energy during sub-maximal physical activities.60–62 However, excessive intramuscular fat compromises muscle quality, affects muscle function, and is associated with reduced physical functioning.38, 63, 64 Taaffe et al reported that in the limbs of healthy, older adults, cessation of resistance training resulted in increased quadriceps intramuscular fat and decreased quadriceps strength (as evaluated using a one repetition maximum), without a significant reduction in muscle size.63 Visser et al reported that intramuscular fat independently predicted self-expressed mobility difficulties, including walking one-quarter of a mile and climbing 10 steps without resting.65 In the trunk of older adults, Hicks and colleagues reported greater intramuscular fat was associated with lower functional capacity and physical performance, including increased time with sit-to-stand transfers, decreased gait speed, and poorer balance.64 Future investigations may attempt to determine effective treatments to reduce intramuscular fat in older adults and whether or not reductions result in improvements in activities and participation.
In the older adult, an increasingly sedentary lifestyle coupled with inappropriate dietary intake may result in excessive intramuscular fat within skeletal muscle. With aging, there is a significant reduction in physical activity and a drop in resting metabolic rate, resulting in an overall reduction in caloric expenditure.66, 67 There is preferential oxidization of carbohydrates over fats,67 which may result in increased intramuscular fat stores in skeletal muscle, as free fatty acids reform into triglycerides as opposed to entering the mitochondria for energy metabolism.68, 69 Diets high in fat can further increase intramyocellular lipids.61, 70 Further, protein synthesis, which is necessary for building skeletal muscle mass, declines by 3.5% per decade71 and may be attenuated in the presence of inactivity.72 Thus, although older adults may consume less protein than younger individuals, they require more protein than their younger counterparts for maintenance of lean muscle mass.73 The net effect of the imbalance between energy expenditure and intake results in sarcopenic obesity, defined as decreased skeletal muscle mass and increased fat mass.74 Increased fat mass may appear in part as increased intramuscular fat affecting skeletal muscle function, as lower intramuscular fat is associated with greater muscle torque and force production.75
After a stroke, intramuscular fat proportion is further increased beyond age-related progression.37 Ryan and colleagues assessed patients with residual hemiparetic deficits at least six months post-cerebral infarction and found intramuscular fat of the involved thigh was significantly greater than the uninvolved side.37 More recently, using a rat model, de Castro Rodrigues et al reported that the intramuscular fat increase after denervation is greater than the age-related increase observed in healthy muscle, with greater fat invasion in slow-twitch muscles.76 The functional implications of increased intramuscular fat in individuals post-stroke remain unexplored.
INTERVENTION
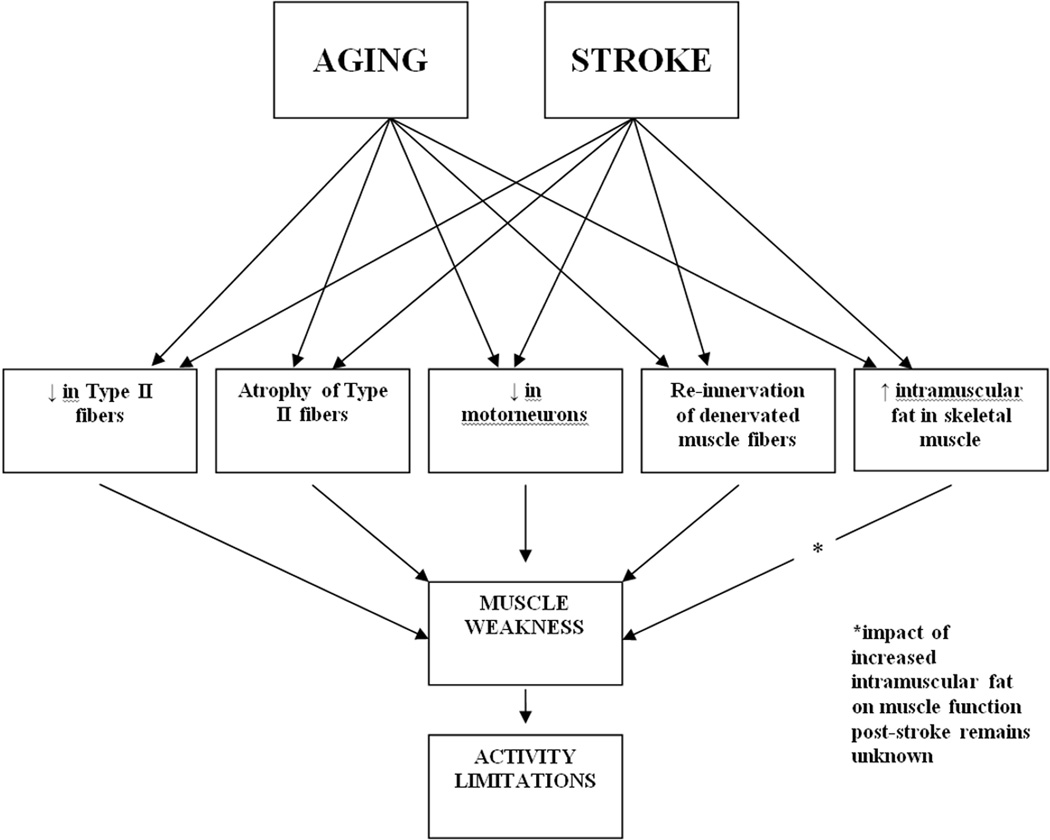
Concurrent negative changes occurring in skeletal muscle as a result of aging and stroke (See Figure 2), including atrophy and remodeling in the presence of pathology result in a weaker muscle. Understanding the potential for an additive effect of aging and stroke may help explain the differences in disability between a younger adult and an older adult, who present with a stroke of the same distribution and magnitude. To date, no studies have stratified persons with a stroke by age; therefore, clinical recommendations for addressing muscle structure and function deficits must be extrapolated by synthesizing two bodies of literature: (1) interventions for the aging adult and (2) interventions for individuals post-stroke.
Figure 2.
The potential cumulative impact of aging and stroke on skeletal muscle structure and function.
In the Aging Adult
Resistance training results in improved skeletal muscle size, composition, and performance in the elderly, as well as improvements in physical function.29, 63, 77–79 In 2006, Martel et al reported that nine weeks of heavy-resistance training of the quadriceps resulted in an increase in Type II fiber cross-sectional area in healthy, older adults.78 In 2008, Suetta and colleagues reported that 12 weeks of resistance training of 60 to 86 year-old individuals post unilateral hip arthroplasty resulted in Type I and Type II fiber area increases, increases of 29 to 30% in dynamic muscle strength, and improved stair climbing power.29 These increases were found to be related to greater Type II fiber area.29 A similar study of older individuals post-hip arthroplasty, found 12 weeks of resistance training increased cross-sectional area of the quadriceps by 12% and isokinetic muscle strength by 22 to 28%.77 Recently, Kukulijan et al reported improvements in thigh muscle cross-sectional area, lower extremity strength as assessed by a one-repetition maximum on the leg press, and gait speed after 18 months of weight-bearing resistance training of the lower extremities.80 Hanson and colleagues also reported improvements in lower extremity strength as assessed by a one-repetition maximum on the leg press and gait speed, as well as chair rise and stair climbing, following 22 weeks of upper and lower extremity resistance training in a group of sedentary older adults.81 In older adults, given age-related muscle changes and the potential benefits of resistance training, clinicians should encourage all older clients to consider gym memberships to continue resistance training post-physical therapy and work with community-centers to develop senior programs that incorporate resistance training.
Resistance training may also reduce intramuscular fat.63, 79 In 2008, Nakai et al demonstrated that a one-month walking program with training equipment that applied additional resistance to the lower extremities resulted in decreased intramuscular fat by 4% compared to walking without an external load.79 More recently, Taaffe and colleagues showed that cessation of upper and lower extremity resistance training in older adults resulted in increased intramuscular fat while resumption at twice weekly resulted in decreased fat.63 The ability to improve muscle composition is noteworthy as increased intramuscular fat in older adult muscle has been associated with a greater risk of reduced mobility-related function over time.64
Although assessing skeletal muscle size and composition is difficult, physical therapists can use dynamometers and one-repetition maximum tests to evaluate extremity strength in addition to "geriatric" clinical tests such as gait speed, timed repeated chair stands, and timed stair climbing. Strength and functional tests may allude to underlying skeletal muscle deficits that may be addressed through interventions targeting muscle morphology and composition. Rehabilitative ultrasound imaging is emerging as a reliable82 and valid83 non-invasive, clinical assessment tool to evaluate muscle and related soft tissue morphology and function.84 Unfortunately, most research to date has focused on younger populations. Future studies including adults over the age of 60 years may allow rehabilitative ultrasound imaging to be used for objective documentation of skeletal muscle size and function pre- and post-treatments in our geriatric patients.
Post-Stroke
Among older adults (ages 50 to 76 years) at least 6 months post-stroke, Ryan and colleagues recently published the first paper on the beneficial effects of resistance training on skeletal muscle.85 Participants of the 12-week resistance training program demonstrated increases in mid-thigh muscle cross-sectional area (13% paretic; 9% non-paretic); increases in one-repetition maximum strength for leg press (33% paretic side; 32% non-paretic) and knee extension (56% paretic; 31% non-paretic); and muscle attenuation indicative of a significant reduction in mid-thigh intramuscular fat for both limbs.85 This study supports bilateral resistance training of the lower extremities after a hemiparetic stroke to address both contralateral and ipsilateral changes in muscle size, function, and composition. Future investigations post-stroke may help determine the optimal time frame for initiation of resistance training and whether similar results are possible in upper extremity muscles, which differ from lower extremity muscles in their weight-bearing requirements.
Post-stroke, evidence regarding the impact of traditional resistance training on disability is inconclusive.86–89 For example, Weiss et al reported improvements in chair rise with 12 weeks of high-intensity resistance training at 70% of a 1 repetition-maximum in individuals post-stroke.87 In contrast, Ouellette et al reported a lack of significant improvements in chair rise with a similar program, although participants reported improvements in activity and participation domains.88 Furthermore, isokinetic resistance training of the affected side post-stroke has not resulted in substantial improvements in stair negotiation.89
Future investigations are necessary to understand the relationship between skeletal muscle changes and disability in older individuals post-stroke. Older individuals may need more vigorous strengthening than provided in these studies to address changes in body structure and function and to improve activity and participation domains due to the potential cumulative impact of aging and stroke. Unilateral strengthening may not address the baseline impairments in bilateral lower extremity strength as a result of biological aging and the stroke. Others argue that we should employ the principle of specificity, selecting exercises such as sit-to-stands, step-ups, and squats that mimic everyday activities to improve muscle structure and function and/or enhance activity and participation levels.86, 90 For example, Rose et al found that early post-stroke, a circuit training rehabilitation program addressing patient-specific, everyday activity limitations had superior results for improving gait speed when compared to a standard inpatient stroke rehabilitation program, consisting of gait retraining, transfer training, bed mobility, balance activities, range-of-motion exercises, and strengthening.91
Prolonged disuse post-stroke may result in skeletal muscle weakness beyond that expected due to biological aging, further impairing function.45 Therapists should intervene as soon as the patient is medically stabile to counter disuse.92 This may be particularly important, due to the overwhelming evidence for large scale loss of Type II fibers.1 Functionally relevant activities, such as sit-to-stand transfers and stair climbing, require large force production and therefore, may assist in ameliorating the reduction of Type II fibers. Use of neuromuscular electrical stimulation (NMES), which may recruit Type II fibers alongside Type I fibers, may assist with increasing motor unit recruitment when voluntary muscle activation is impaired.92–95 Functional electrical stimulation, a specific type of NMES, may be used as an adjunct to treadmill training to improve muscle performance in the lower extremities and assist with gait retraining in individuals with acute and chronic stroke-related deficits.92
Post-stroke, treadmill use is reserved for individuals without cardiac risk for treadmill training.92 Aerobic treadmill training has demonstrated superior results in 6-minute walk distance and self-reported walking mobility when compared to a low-intensity walking and stretching program in individuals post-stroke.96 Treadmill training, with use of partial body-weight support of up to 40% of the patient’s weight,97 is recommended to address lower extremity muscle impairments,92, 98 decreased gait speed,98, 99 and poor cardiovascular fitness.96, 99, 100 The mechanisms that have been suggested for body-weight support treadmill training’s success have focused mainly on neuroplasticity,101–103 but muscle size and composition changes may also be contributing to the beneficial effects of body-weight support treadmill training. Future investigations of early ambulation interventions may consider skeletal muscle measurements in addition to measures of physical performance. This is in line with current post-stroke clinical practice guidelines that recommend evaluations include assessments at the impairment level, i.e. muscle size and strength, as well as the activity level, i.e. performance in real-life or simulating daily activities.92
CONCLUSION
In summary, clinicians must be cognizant that post-stroke there may be an acceleration of negative skeletal muscle changes typically associated with aging,31, 37, 38, 76 resulting in a greater disadvantage for optimal muscle performance when compared to younger individuals post-stroke. Further, post-stroke skeletal muscle changes may occur in both limbs,40, 41, 43 warranting caution when comparing the paretic limb to the non-paretic limb. Comparisons with age-matched normative data may reveal bilateral skeletal muscle impairments and provide impetus for interventions targeting both the paretic and non-paretic limbs. Future investigations evaluating relationships between post-stroke skeletal muscle impairments and physical performance may support interventions that target skeletal muscle. Such interventions may improve the activity and participation levels in older adults post-stroke.
Acknowledgements
The work of Dr. Sions was supported by R21 HD057274 (NICHD) and the Promotion of Doctoral Studies I/II Scholarships from the Foundation for Physical Therapy. The work of Dr. Binder-Macleod was supported by NR010786 (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kirshner HS, Biller J, Callahan AS., 3rd Long-term therapy to prevent stroke. J Am Board Fam Pract. 2005;18(6):528–540. doi: 10.3122/jabfm.18.6.528. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and P. [Accessed November 19, 2008];Stroke facts and statistics. http://www.cdc.gov/stroke/stroke_facts.htm.
- 3.Buurke JH, Hermens HJ, Erren-Wolters CV, Nene AV. The effect of walking aids on muscle activation patterns during walking in stroke patients. Gait Posture. 2005;22(2):164–170. doi: 10.1016/j.gaitpost.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005;293(19):2391–2402. doi: 10.1001/jama.293.19.2391. [DOI] [PubMed] [Google Scholar]
- 5.Zerwic JJ, Ennen K, DeVon HA. Stroke. Risks, recognition, and return to work. AAOHN J. 2002;50(8):354–359. [PubMed] [Google Scholar]
- 6.Ustinova KI, Fung J, Levin MF. Disruption of bilateral temporal coordination during arm swinging in patients with hemiparesis. Exp Brain Res. 2006;169(2):194–207. doi: 10.1007/s00221-005-0136-5. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GN, Perreault EJ. Side of lesion influences bilateral activation in chronic, post-stroke hemiparesis. Clin Neurophysiol. 2007;118(9):2050–2062. doi: 10.1016/j.clinph.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 9.Latash ML. Neurophysiological Basis of Movement. 2nd ed. Urbana, IL: Human Kinetics Publishers; 2008. [Google Scholar]
- 10.Kraemer WJ. Skeletal muscle physiology: plasticity and responses to exercise. Hormone Research. 2006;66(Supplement 1):2–16. [Google Scholar]
- 11.Toffola ED, Sparpaglione D, Pistorio A, Buonocore M. Myoelectric manifestations of muscle changes in stroke patients. Arch Phys Med Rehabil. 2001;82(5):661–665. doi: 10.1053/apmr.2001.22338. [DOI] [PubMed] [Google Scholar]
- 12.McComas AJ, Sica RE, Upton AR, Aguilera N. Functional changes in motoneurones of hemiparetic patients. J Neurol Neurosurg Psychiatry. 1973;36(2):183–193. doi: 10.1136/jnnp.36.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type IIA/B human skeletal muscle fibers. Clin Orthop Relat Res. 2006;450:231–237. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- 14.Brunner F, Schmid A, Sheikhzadeh A, Nordin M, Yoon J, Frankel V. Effects of aging on Type II muscle fibers: a systematic review of the literature. J Aging Phys Act. 2007;15(3):336–348. doi: 10.1123/japa.15.3.336. [DOI] [PubMed] [Google Scholar]
- 15.MacIntosh BR, Gardiner PF, McComas AJ. Skeletal Muscle: Form and Function. 2nd ed. Champaign, IL: Human Kinetics Publishers; 2006. [Google Scholar]
- 16.Kernell D. Principles of force gradation in skeletal muscles. Neural Plast. 2003;10(1–2):69–76. doi: 10.1155/NP.2003.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celichowski J. Mechanisms underlying the regulation of motor unit contraction in the skeletal muscle. J Physiol Pharmacol. 2000;51(1):17–33. [PubMed] [Google Scholar]
- 18.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34(11):1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 19.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 20.Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch. 1997;434(3):246–253. doi: 10.1007/s004240050392. [DOI] [PubMed] [Google Scholar]
- 22.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 23.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 25.Hvid LG, Aagaard P, Justesen L, et al. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol. 2010;109(6):1628–1634. doi: 10.1152/japplphysiol.00637.2010. [DOI] [PubMed] [Google Scholar]
- 26.Park H, Park S, Shephard RJ, Aoyagi Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol. 2010;109(5):953–961. doi: 10.1007/s00421-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 27.Chien MY, Kuo HK, Wu YT. Sarcopenia, cardiopulmonary fitness, and physical disability in community-dwelling elderly people. Phys Ther. 2010;90(9):1277–1287. doi: 10.2522/ptj.20090322. [DOI] [PubMed] [Google Scholar]
- 28.Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276(3):241–246. [PubMed] [Google Scholar]
- 29.Suetta C, Andersen JL, Dalgas U, et al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol. 2008;105(1):180–186. doi: 10.1152/japplphysiol.01354.2007. [DOI] [PubMed] [Google Scholar]
- 30.Holviala JH, Sallinen JM, Kraemer WJ, Alen MJ, Hakkinen KK. Effects of strength training on muscle strength characteristics, functional capabilities, and balance in middle-aged and older women. J Strength Cond Res. 2006;20(2):336–344. doi: 10.1519/R-17885.1. [DOI] [PubMed] [Google Scholar]
- 31.Hachisuka K, Umezu Y, Ogata H. Disuse muscle atrophy of lower limbs in hemiplegic patients. Arch Phys Med Rehabil. 1997;78(1):13–18. doi: 10.1016/s0003-9993(97)90003-4. [DOI] [PubMed] [Google Scholar]
- 32.Scelsi R, Lotta S, Lommi G, Poggi P, Marchetti C. Hemiplegic atrophy. Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol. 1984;62(4):324–331. doi: 10.1007/BF00687615. [DOI] [PubMed] [Google Scholar]
- 33.Bohannon RW. Knee extension strength and body weight determine sit-to-stand independence after stroke. Physiother Theory Pract. 2007;23(5):291–297. doi: 10.1080/09593980701209428. [DOI] [PubMed] [Google Scholar]
- 34.Cheng PT, Chen CL, Wang CM, Hong WH. Leg muscle activation patterns of sit-to-stand movement in stroke patients. Am J Phys Med Rehabil. 2004;83(1):10–16. doi: 10.1097/01.PHM.0000104665.34557.56. [DOI] [PubMed] [Google Scholar]
- 35.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83(1):49–57. [PubMed] [Google Scholar]
- 36.Marigold DS, Eng JJ, Tokuno CD, Donnelly CA. Contribution of muscle strength and integration of afferent input to postural instability in persons with stroke. Neurorehabil Neural Repair. 2004;18(4):222–229. doi: 10.1177/1545968304271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83(12):1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 38.Metoki N, Sato Y, Satoh K, Okumura K, Iwamoto J. Muscular atrophy in the hemiplegic thigh in patients after stroke. Am J Phys Med Rehabil. 2003;82(11):862–865. doi: 10.1097/01.PHM.0000091988.20916.EF. [DOI] [PubMed] [Google Scholar]
- 39.Ploutz-Snyder LL, Clark BC, Logan L, Turk M. Evaluation of spastic muscle in stroke survivors using magnetic resonance imaging and resistance to passive motion. Arch Phys Med Rehabil. 2006;87(12):1636–1642. doi: 10.1016/j.apmr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Sunderland A, Bowers MP, Sluman SM, Wilcock DJ, Ardron ME. Impaired dexterity of the ipsilateral hand after stroke and the relationship to cognitive deficit. Stroke. 1999;30(5):949–955. doi: 10.1161/01.str.30.5.949. [DOI] [PubMed] [Google Scholar]
- 41.Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the 'unaffected' upper extremity of elderly stroke patients. Stroke. 1996;27(9):1564–1570. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- 42.Noskin O, Krakauer JW, Lazar RM, et al. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2008;79(4):401–406. doi: 10.1136/jnnp.2007.118463. [DOI] [PubMed] [Google Scholar]
- 43.Baskett JJ, Marshall HJ, Broad JB, Owen PH, Green G. The good side after stroke: ipsilateral sensory-motor function needs careful assessment. Age Ageing. 1996;25(3):239–244. doi: 10.1093/ageing/25.3.239. [DOI] [PubMed] [Google Scholar]
- 44.Andrews AW, Bohannon RW. Distribution of muscle strength impairments following stroke. Clin Rehabil. 2000;14(1):79–87. doi: 10.1191/026921500673950113. [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone. 2001;28(6):655–659. doi: 10.1016/s8756-3282(01)00434-3. [DOI] [PubMed] [Google Scholar]
- 46.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2(1):21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 48.Kanda K, Hashizume K. Effects of long-term physical exercise on age-related changes of spinal motoneurons and peripheral nerves in rats. Neurosci Res. 1998;31(1):69–75. doi: 10.1016/s0168-0102(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 49.Kawabuchi M, Zhou CJ, Wang S, Nakamura K, Liu WT, Hirata K. The spatiotemporal relationship among Schwann cells, axons and postsynaptic acetylcholine receptor regions during muscle reinnervation in aged rats. Anat Rec. 2001;264(2):183–202. doi: 10.1002/ar.1159. [DOI] [PubMed] [Google Scholar]
- 50.Kirkendall DT, Garrett WE., Jr The effects of aging and training on skeletal muscle. Am J Sports Med. 1998;26(4):598–602. doi: 10.1177/03635465980260042401. [DOI] [PubMed] [Google Scholar]
- 51.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79(5):874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 52.Hara Y, Masakado Y, Chino N. The physiological functional loss of single thenar motor units in the stroke patients: when does it occur? Does it progress? Clin Neurophysiol. 2004;115(1):97–103. doi: 10.1016/j.clinph.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Lukacs M, Vecsei L, Beniczky S. Changes in muscle fiber density following a stroke. Clin Neurophysiol. 2009;120(8):1539–1542. doi: 10.1016/j.clinph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9(4):266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miljkovic-Gacic I, Wang X, Kammerer CM, et al. Fat infiltration in muscle: new evidence for familial clustering and associations with diabetes. Obesity (Silver Spring) 2008;16(8):1854–1860. doi: 10.1038/oby.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23(2):126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 58.Hadar H, Gadoth N, Heifetz M. Fatty replacement of lower paraspinal muscles: normal and neuromuscular disorders. AJR Am J Roentgenol. 1983;141(5):895–898. doi: 10.2214/ajr.141.5.895. [DOI] [PubMed] [Google Scholar]
- 59.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9(4):213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 60.van Loon LJ. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol. 2004;97(4):1170–1187. doi: 10.1152/japplphysiol.00368.2004. [DOI] [PubMed] [Google Scholar]
- 61.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes Res. 2005;13(12):2088–2094. doi: 10.1038/oby.2005.259. [DOI] [PubMed] [Google Scholar]
- 62.Hwang JH, Pan JW, Heydari S, Hetherington HP, Stein DT. Regional differences in intramyocellular lipids in humans observed by in vivo 1H-MR spectroscopic imaging. J Appl Physiol. 2001;90(4):1267–1274. doi: 10.1152/jappl.2001.90.4.1267. [DOI] [PubMed] [Google Scholar]
- 63.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55(2):217–223. doi: 10.1159/000182084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60(11):1420–1424. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 65.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 66.Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol. 2000;88(2):662–668. doi: 10.1152/jappl.2000.88.2.662. [DOI] [PubMed] [Google Scholar]
- 67.Johannsen DL, Ravussin E. Obesity in the elderly: is faulty metabolism to blame? Aging health. 2010;6(2):159–167. doi: 10.2217/ahe.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halloszy J, McArdle WD, Katch FI, Katch VL. Energy transfer in the body. Vol 5th. Baltimore, MD: Lippincott Williams & Wilkins; 2001. pp. 131–173. [Google Scholar]
- 69.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 70.White LJ, Ferguson MA, McCoy SC, Kim H. Intramyocellular lipid changes in men and women during aerobic exercise: a (1)H-magnetic resonance spectroscopy study. J Clin Endocrinol Metab. 2003;88(12):5638–5643. doi: 10.1210/jc.2003-031006. [DOI] [PubMed] [Google Scholar]
- 71.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 72.Guadagni M, Biolo G. Effects of inflammation and/or inactivity on the need for dietary protein. Curr Opin Clin Nutr Metab Care. 2009;12(6):617–622. doi: 10.1097/MCO.0b013e32833193bd. [DOI] [PubMed] [Google Scholar]
- 73.Chernoff R. Protein and older adults. J Am Coll Nutr. 2004;23(6 Suppl):627S–630S. doi: 10.1080/07315724.2004.10719434. [DOI] [PubMed] [Google Scholar]
- 74.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 75.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 76.de Castro Rodrigues A, Andreo JC, Rosa GM, Jr, dos Santos NB, Moraes LH, Lauris JR. Fat cell invasion in long-term denervated skeletal muscle. Microsurgery. 2007;27(8):664–667. doi: 10.1002/micr.20428. [DOI] [PubMed] [Google Scholar]
- 77.Suetta C, Magnusson SP, Rosted A, et al. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients--a controlled, randomized study. J Am Geriatr Soc. 2004;52(12):2016–2022. doi: 10.1111/j.1532-5415.2004.52557.x. [DOI] [PubMed] [Google Scholar]
- 78.Martel GF, Roth SM, Ivey FM, et al. Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp Physiol. 2006;91(2):457–464. doi: 10.1113/expphysiol.2005.032771. [DOI] [PubMed] [Google Scholar]
- 79.Nakai R, Azuma T, Sudo M, Urayama S, Takizawa O, Tsutsumi S. MRI analysis of structural changes in skeletal muscles and surrounding tissues following long-term walking exercise with training equipment. J Appl Physiol. 2008;105(3):958–963. doi: 10.1152/japplphysiol.01204.2007. [DOI] [PubMed] [Google Scholar]
- 80.Kukuljan S, Nowson CA, Sanders K, Daly RM. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol. 2009;107(6):1864–1873. doi: 10.1152/japplphysiol.00392.2009. [DOI] [PubMed] [Google Scholar]
- 81.Hanson ED, Srivatsan SR, Agrawal S, et al. Effects of strength training on physical function: influence of power, strength, and body composition. J Strength Cond Res. 2009;23(9):2627–2637. doi: 10.1519/JSC.0b013e3181b2297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koppenhaver SL, Hebert JJ, Fritz JM, Parent EC, Teyhen DS, Magel JS. Reliability of rehabilitative ultrasound imaging of the transversus abdominis and lumbar multifidus muscles. Arch Phys Med Rehabil. 2009;90(1):87–94. doi: 10.1016/j.apmr.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 83.Koppenhaver SL, Hebert JJ, Parent EC, Fritz JM. Rehabilitative ultrasound imaging is a valid measure of trunk muscle size and activation during most isometric sub-maximal contractions: a systematic review. Aust J Physiother. 2009;55(3):153–169. doi: 10.1016/s0004-9514(09)70076-5. [DOI] [PubMed] [Google Scholar]
- 84.Teyhen D. Rehabilitative Ultrasound Imaging Symposium San Antonio, TX, May 8–10, 2006. J Orthop Sports Phys Ther. 2006;36(8):A1–A3. [PubMed] [Google Scholar]
- 85.Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42(2):416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bohannon RW. Muscle strength and muscle training after stroke. J Rehabil Med. 2007;39(1):14–20. doi: 10.2340/16501977-0018. [DOI] [PubMed] [Google Scholar]
- 87.Weiss A, Suzuki T, Bean J, Fielding RA. High intensity strength training improves strength and functional performance after stroke. Am J Phys Med Rehabil. 2000;79(4):369–376. doi: 10.1097/00002060-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 88.Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35(6):1404–1409. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 89.Sharp SA, Brouwer BJ. Isokinetic strength training of the hemiparetic knee: effects on function and spasticity. Arch Phys Med Rehabil. 1997;78(11):1231–1236. doi: 10.1016/s0003-9993(97)90337-3. [DOI] [PubMed] [Google Scholar]
- 90.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence? Clin Rehabil. 2004;18(8):833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- 91.Rose D, Paris T, Crews E, et al. Feasibility and effectiveness of circuit training in acute stroke rehabilitation. Neurorehabil Neural Repair. 2011;25(2):140–148. doi: 10.1177/1545968310384270. [DOI] [PubMed] [Google Scholar]
- 92.VA/DOD Clinical practice guideline for the management of stroke rehabilitation. J Rehabil Res Dev. 2010;47(9):1–43. [PubMed] [Google Scholar]
- 93.Delitto A, Snyder-Mackler L. Two theories of muscle strength augmentation using percutaneous electrical stimulation. Phys Ther. 1990;70(3):158–164. doi: 10.1093/ptj/70.3.158. [DOI] [PubMed] [Google Scholar]
- 94.Petterson S, Snyder-Mackler L. The use of neuromuscular electrical stimulation to improve activation deficits in a patient with chronic quadriceps strength impairments following total knee arthroplasty. J Orthop Sports Phys Ther. 2006;36(9):678–685. doi: 10.2519/jospt.2006.2305. [DOI] [PubMed] [Google Scholar]
- 95.Marqueste T, Hug F, Decherchi P, Jammes Y. Changes in neuromuscular function after training by functional electrical stimulation. Muscle Nerve. 2003;28(2):181–188. doi: 10.1002/mus.10408. [DOI] [PubMed] [Google Scholar]
- 96.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005;36(10):2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 97.Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2005;(4):CD002840. doi: 10.1002/14651858.CD002840.pub2. [DOI] [PubMed] [Google Scholar]
- 98.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122–1128. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 99.Barbeau H, Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil. 2003;84(10):1458–1465. doi: 10.1016/s0003-9993(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 100.Ivey FM, Hafer-Macko CE, Macko RF. Task-oriented treadmill exercise training in chronic hemiparetic stroke. J Rehabil Res Dev. 2008;45(2):249–259. doi: 10.1682/JRRD.2007.02.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hesse S. Treadmill training with partial body weight support after stroke: a review. NeuroRehabilitation. 2008;23(1):55–65. [PubMed] [Google Scholar]
- 102.McCain KJ, Pollo FE, Baum BS, Coleman SC, Baker S, Smith PS. Locomotor treadmill training with partial body-weight support before overground gait in adults with acute stroke: a pilot study. Arch Phys Med Rehabil. 2008;89(4):684–691. doi: 10.1016/j.apmr.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 103.McCain KJ, Smith PS. Locomotor treadmill training with body-weight support prior to over-ground gait: promoting symmetrical gait in a subject with acute stroke. Top Stroke Rehabil. 2007;14(5):18–27. doi: 10.1310/tsr1405-18. [DOI] [PubMed] [Google Scholar]